Abstract
Transfusion-transmitted infections including hepatitis B virus (HBV) have been a major concern in transfusion medicine. Implementation of HBV nucleic acid testing (NAT) has revealed occult HBV infection (OBI) in blood donors. In the mid-1980s, hepatitis B core antibody (HBc) testing was introduced to screen blood donors in HBV non-endemic countries to prevent transmission of non-A and non-B hepatitis. That test remains in use for preventing of potential transmission of HBV from hepatitis B surface antigen (HBsAg)-negative blood donors, even though anti-hepatitis C virus tests have been introduced. Studies of anti-HBc-positive donors have revealed an HBV DNA positivity rate of 0%-15%. As of 2012, 30 countries have implemented HBV NAT. The prevalence of OBI in blood donors was estimated to be 8.55 per 1 million donations, according to a 2008 international survey. OBI is transmissible by blood transfusion. The clinical outcome of occult HBV transmission primarily depends on recipient immune status and the number of HBV DNA copies present in the blood products. The presence of donor anti-HBs reduces the risk of HBV infection by approximately five-fold. The risk of HBV transmission may be lower in endemic areas than in non-endemic areas, because most recipients have already been exposed to HBV. Blood safety for HBV, including OBI, has substantially improved, but the possibility for OBI transmission remains.
Keywords: Occult hepatitis B infection, Transfusion, Anti-hepatitis B core antibody, Nucleic acid testing, Blood service
Core tip: Hepatitis B surface antigen negative but hepatitis B virus (HBV) DNA positive blood products can evoke hepatitis in blood recipients. Anti-hepatitis B core and HBV nucleic acid testing screening tests are necessary to prevent occult HBV infection transmission by transfusion. Anti-HBs antibody in donors and recipients can protect against hepatitis B infection.
INTRODUCTION
Hepatitis B virus (HBV) infection via blood transfusion is a major concern in transfusion medicine[1-4]. Screening tests for hepatitis B surface antigens (HBsAg) and anti-hepatitis B core (HBc) antibodies detect HBV transmissible blood and prevent recipient HBV infection. After the introduction of HBV nucleic acid tests (NAT) in blood donor screening, the residual risk of HBV infection by transfusion decreased[5,6]. Implementation of this test revealed occult hepatitis B virus infection (OBI) in blood donors. OBI is defined as the presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals who tested negative for HBsAg[7]. The amount of viral DNA in the serum is typically very low in cases of true OBI. Because testing liver tissue is not always practical or possible, OBI is often diagnosed through serum HBV DNA and viral marker tests[8,9].
A positive OBI test may be found in blood donors as a result of various clinical conditions, including: (1) the incubation period of acute infections; (2) the tail-end stage of chronic hepatitis B; (3) low-level viral replication after recovery from hepatitis; and (4) escape mutants not detected by current HBsAg tests[10,11]. HBV transmission by blood transfusion from an OBI donor was first reported in 1978[12]. An increasing number of studies on OBI infectivity of blood products have recently been published[13,14]. In this review, we summarize the role of blood screening tests for HBV infections and update the known risks of OBI transfusion transmission.
ANTI-HBC ANTIBODY
Hepatitis B core antigen (HBcAg) appears in hepatocytes within 2 wk after HBV infection; infectious viremia including HBsAg and polymerase are present in the blood after 3 wk. Anti-HBc IgG forms during the recovery phase of infection and is persistent for life, thus, the presence of this antibody in blood indicates past HBV infection[15]. The analytic sensitivity of HBsAg tests in the 1980s was lower than that of current assays. In 1983, Nath et al[16] found that 1 of 16 samples with anti-HBc in the absence of anti-HBs was found to have HBsAg when tested with a more sensitive test. Therefore, additional screening for HBV and surrogate tests for non-A, non-B hepatitis were necessary in the 1980s until the anti-hepatitis C virus (HCV) antibody test became available[17]. The anti-HBc test was introduced in the mid-1980s for screening of blood donors in HBV non-endemic countries, such as the United States. Even after the introduction of the anti-HCV test in the early 1990s, the anti-HBc test continues to be used for donor screening in many countries to prevent potential transmission of HBV from HBsAg-negative donors[18,19]. Several studies have reported effective screening of blood for anti-HBc[20,21]. However, HBV endemic countries were unable to implement anti-HBc screening because many blood products would be discarded due to positive screening tests even though most of the blood would be safe for transfusion. Cases of posttransfusion hepatitis B from positive carrier blood and posttransfusion fulminant hepatitis B from blood containing precore-defective HBV mutants have been reported in Norway and Japan, respectively, countries that did not screen donors for anti-HBc[22,23]. In 1989, Japan introduced anti-HBc testing with a modified algorithm in which anti-HBc-reactive blood with titers < 1:32 or ≥ 1:32 with anti-HBs ≥ 200 mIU/mL were used for transfusion[24].
Anti-HBc prevalence is related to regional hepatitis B prevalence, and both are typically proportional to one another. The prevalence rates of anti-HBc in blood donors in the United States are 0.23%[25]; United Kingdom, 0.56%[21]; Denmark, 0.70%[26]; Japan, 1.1%[27]; Germany, 1.88%[28]; Italy, 4.85%[29]; India, 10.82%[30]; South Korea, 13.5%[31]; Egypt, 14.2%[32]; Greece, 14.9%[33]; and Pakistan, 17.28%[34] (Figure 1). O’Brien et al[35] reported 5585 (1.13%) anti-HBc repeat-reactive blood donors among 493344 blood donors in Canada, of which 29 (0.52%) were HBsAg-negative but HBV DNA-positive[35]. The anti-HBc test lacks specificity and reactivity of the test reagents varies by manufacturer. Therefore, comparison of anti-HBc positivity should be conducted with caution; it is better to describe general features rather than directly comparing studies. Efforts to improve test specificity have included the addition of reductants such as dithiothreitol and cysteine[36]. High donor HBsAg antibody (anti-HBs) levels (> 100 mIU/mL) are assumed to be putatively protective against the transfusion transmission of HBV[37]. Anti-HBc only or isolated anti-HBc are defined as anti-HBc positive without HBsAg and anti-HBs. An HBV DNA positivity of 0%-15% among those donors positive for anti-HBc only was reported in studies performed in Greece, China, Japan, and Germany[10]. Therefore, the presence of anti-HBc only does not necessarily indicate active viral replication and transmission potential.
Figure 1.
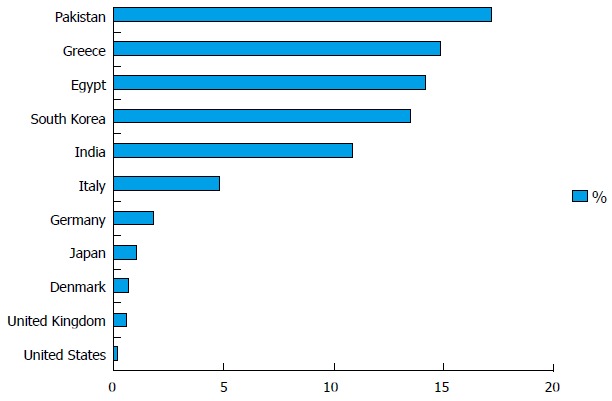
Prevalence of anti-hepatitis B core in blood donors.
NAT IN BLOOD SERVICE
Improved molecular methods for the detection of viral nucleic acids made it possible to introduce NAT for donor screening in the late 1990s[38]. By 1997, several countries in Europe had initiated voluntary screening of pooled plasma donations using NAT, and a directive was issued by the EU requiring HCV RNA testing for all plasma intended for fractionation in Europe by July 1, 1999. Routine NAT for HBV was first introduced in German blood transfusion services in January of 1997[39]. The United States began screening source plasma pools for HCV and human immunodeficiency virus (HIV)-1 RNA in early 1998 under the Food and Drug Administration’s Investigational New Drug (IND) program. Currently, more than 90% of whole blood and nearly all source plasma are screened for HCV and HIV by NAT. NAT for blood screening is typically conducted using a multiplex polymerase chain reaction method that simultaneously detects the presence of HIV, HCV and HBV[40]. And a multiplex reaction positive case requires discrimination testing[41]. NAT can be performed on pooled samples to reduce running costs[42]. In pooling test system, primary NAT positive cases require a secondary confirmation procedure for identification of positivity of individual donation samples. The American Red Cross implemented automated triplex NAT for HIV, HCV, and HBV in June of 2009[25]. Japan implemented NAT in November 1997 and all donations have been screened since October 1999. Japanese Red Cross Blood Centers serologically screened 500 samples that were pooled and tested for HBV, HCV and HIV-1. To increase HBV detection sensitivity in Japan, the sample pooling size was reduced to 50 in 2000, and 20 in 2004[43].
As of 2012, 30 countries have implemented HBV NAT[44,45], as shown in Table 1. According to international survey results, there were a total of 170 HBV NAT-only positive donors among 19887649 blood donations in 2008, and the prevalence of OBI among blood donors was an estimated 8.55 per 1 million donations[44]. In Taiwan, 8 (0.13%) HBV NAT-only positive donors were identified among 5,973 random donor samples[46]. In China, 22 among 165371 HBsAg-negative plasma samples were identified as OBI-positive; their alanine aminotransferase levels were normal and their viral loads low, with a median of 14 IU/mL[47]. In Iran, 4% of 1000 healthy blood donors were anti-HBc- and OBI-positive, and 8.23% of 11,240 volunteer donors were anti-HBc positive and OBI-positive in Mexico[48,49]. To detect OBI, the HBV DNA test is substantial, and minipool NAT can be used to reduce the cost of testing in developing countries. However, in HBV endemic areas, minipool NAT can result in many primary positive cases, which means that many blood products must be retained until the positivity of individual sample is resolved through a secondary confirmation test. Therefore, it is better to implement NAT for individual–donations rather than minipools for NAT in HBV endemic areas[50-53]. HBV replicates more slowly than HIV or HCV, and its doubling time during the ramp-up phase was estimated to be 2.56 d by Biswas et al[54]. Therefore, minipool NAT for HBV detection is less effective than for HIV or HCV detection. Each country should develop its own blood screening strategy based on HBV prevalence, yields of infectious units by different screening methods and cost-effectiveness of testing methods.
Table 1.
Introduction of hepatitis B virus nucleic acid testing in donor screening
| Year | Country |
| 1997 | Germany |
| 1999 | Austria, Japan |
| 2004 | Singapore, Spain |
| 2005 | Poland, France (OT + army), South Africa |
| 2006 | Greece, Italy, Portugal, Thailand |
| 2007 | Hong Kong, Kuwait, Malaysia, New Zealand, Slovenia, Switzerland |
| 2008 | Finland, Israel, Latvia, Netherlands, Taiwan |
| 2009 | United States, Denmark, Ireland, United Kingdom (England and Wales) |
| 2010 | Australia, United Kingdom (Scotland), Canada |
| 2012 | South Korea |
OBI TRANSMISSION BY BLOOD TRANSFUSION
Occult HBV is transmissible by blood transfusion, although the transmission rate is considered to be very low. The clinical outcome of OBI transmission mainly depends on the immune status and copies of HBV DNA in blood products of the recipient. A look-back program by the Japanese Red Cross showed that window period-derived blood components evoked 50% (11/22) seroconversion in recipients, but tail-end chronic hepatitis B infections caused only 3% (1/33) seroconversion in recipients[55]. Satake et al[55] concluded that the blood infectivity rate during the window period was 10-fold higher than the transmission rates from occult carriers with low-titer anti-HBc. In Canada, a look-back study identified 9.7% anti-HBc-positive recipients and 4 HBV DNA-positive, HBsAg-negative, anti-HBc-positive donors[35]. However, hepatitis cases were reported to have occurred as the result of transfusion of anti-HBc-positive, anti-HBs-positive (12 IU/L), HBV DNA-positive (180 IU/mL) blood product[56].
A study on blood component infectivity by Allain et al[14] reported that the presence of anti-HBs in donors reduces the risk of HBV infection by approximately five-fold, while therapeutic fresh frozen plasma over platelet concentrate increases the risk by approximately three-fold by logistic regression analysis[14]. A case of OBI transmission by plasma has been reported, but not by red blood cells[57,58]. Because the amount of plasma containing viral particles is very small in RBCs, they are considered to be less infective than plasma products. In a look-back study in Taiwan, Su et al[59] identified 12 (0.11%) OBI-positive donors among 10824 repository samples; they also identified no post-transfusion hepatitis cases among the recipients. They suggested that the risk of HBV transmission is lower in hyperendemic areas such as Taiwan than in non-endemic areas, because most recipients have already experienced an HBV infection. In a look-back study in Hyogo-prefecture, one of 12 recipients was diagnosed with post-transfusion hepatitis B. Of the remaining 11 recipients, 7 were lost to follow-up, and 4 were negative for HBV[60].
CONCLUSION
The life-saving role of blood transfusion makes it an essential component of modern medical practice. However, blood used for transfusion is not always free of transmissible diseases. According to the 2008 World Health Organization Global Database on Blood Safety, approximately 92 million blood donations are collected worldwide each year. Of these donations, 48% are collected in high-income countries. Blood products from these countries are screened for HBV, HCV, and HIV. However, 39 countries do not routinely test blood donations for transfusion-transmissible infections[61]. HBsAg-positive blood products, including OBI, could be transfused to patients in these counties more frequently than in the countries that screen blood. Although the prevalence of OBI in blood donors differs by country, a 2008 international survey estimated it to be 8.55 per 1 million donations. To reduce the risk of HBV transmission, HBsAg testing was introduced, followed by anti-HBc and HBV NAT in countries where additional testing was feasible. These approaches were also effective for the prevention of transfusion-transmission of OBI. A modified algorithm for anti-HBc screening of blood donors was implemented in intermediate HBV endemic areas, such as Japan, where the test alone could not be introduced because of the resultant high blood discard rates. Unlike anti-HBc screening, HBV NAT can detect infections during window periods. Therefore, despite higher costs, HBV NAT is more suitable for screening in areas with endemic HBV.
Although OBI infectivity depends on recipient immunity and blood product type, it is transmissible by transfusion. One study observed higher genetic diversity in occult HBV genotype B and C strains from South East Asian blood donors[62]. Epigenetic factors have also been identified in HBV cccDNA molecules, and studies to understand OBI immunopathogenesis are currently underway[63-68]. Even with HBV NAT screening, there is a risk of false-negative results. NAT using sample pooling systems in particular cannot detect low-level viremia. Pathogen inactivation technologies that destroy viral DNA or RNA in blood products using chemical agents and ultraviolet illumination have been introduced as alternatives in blood services[69,70]. HBV vaccination of potential recipients is also an important preventive measure. In conclusion, following the implementation of anti-HBc and HBV NAT screening, blood safety for HBV including OBI has improved substantially, but the potential for OBI transmission remains.
Footnotes
Conflict-of-interest: There are no conflicts of interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 24, 2014
First decision: November 3, 2014
Article in press: December 16, 2014
P- Reviewer: Arsenijevic N, Gherlan GS, Romeo R, Silva LD S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Korelitz JJ, Busch MP, Kleinman SH, Williams AE, Gilcher RO, Ownby HE, Schreiber GB. A method for estimating hepatitis B virus incidence rates in volunteer blood donors. National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study. Transfusion. 1997;37:634–640. doi: 10.1046/j.1537-2995.1997.37697335159.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang JT, Lee CZ, Chen PJ, Wang TH, Chen DS. Transfusion-transmitted HBV infection in an endemic area: the necessity of more sensitive screening for HBV carriers. Transfusion. 2002;42:1592–1597. doi: 10.1046/j.1537-2995.2002.00274.x. [DOI] [PubMed] [Google Scholar]
- 3.Candotti D, Allain JP. Transfusion-transmitted hepatitis B virus infection. J Hepatol. 2009;51:798–809. doi: 10.1016/j.jhep.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080–2099. doi: 10.1111/j.1537-2995.2010.02851.x. [DOI] [PubMed] [Google Scholar]
- 5.Roth WK, Weber M, Petersen D, Drosten C, Buhr S, Sireis W, Weichert W, Hedges D, Seifried E. NAT for HBV and anti-HBc testing increase blood safety. Transfusion. 2002;42:869–875. doi: 10.1046/j.1537-2995.2002.00128.x. [DOI] [PubMed] [Google Scholar]
- 6.Cable R, Lelie N, Bird A. Reduction of the risk of transfusion-transmitted viral infection by nucleic acid amplification testing in the Western Cape of South Africa: a 5-year review. Vox Sang. 2013;104:93–99. doi: 10.1111/j.1423-0410.2012.01640.x. [DOI] [PubMed] [Google Scholar]
- 7.Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652–657. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Brojer E, Grabarczyk P, Liszewski G, Mikulska M, Allain JP, Letowska M. Characterization of HBV DNA+/HBsAg- blood donors in Poland identified by triplex NAT. Hepatology. 2006;44:1666–1674. doi: 10.1002/hep.21413. [DOI] [PubMed] [Google Scholar]
- 9.Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48:1001–1026. doi: 10.1111/j.1537-2995.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 10.Allain JP. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 2004;86:83–91. doi: 10.1111/j.0042-9007.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 11.Gerlich WH, Wagner FF, Chudy M, Harritshoj LH, Lattermann A, Wienzek S, Glebe D, Saniewski M, Schüttler CG, Wend UC, et al. HBsAg non-reactive HBV infection in blood donors: transmission and pathogenicity. J Med Virol. 2007;79:S32–S36. [Google Scholar]
- 12.Hoofnagle JH, Seeff LB, Bales ZB, Zimmerman HJ. Type B hepatitis after transfusion with blood containing antibody to hepatitis B core antigen. N Engl J Med. 1978;298:1379–1383. doi: 10.1056/NEJM197806222982502. [DOI] [PubMed] [Google Scholar]
- 13.Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013;35:39–52. doi: 10.1007/s00281-012-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allain JP, Mihaljevic I, Gonzalez-Fraile MI, Gubbe K, Holm-Harritshøj L, Garcia JM, Brojer E, Erikstrup C, Saniewski M, Wernish L, et al. Infectivity of blood products from donors with occult hepatitis B virus infection. Transfusion. 2013;53:1405–1415. doi: 10.1111/trf.12096. [DOI] [PubMed] [Google Scholar]
- 15.Koziel MJ, Siddiqui A. Hepatitis B virus and hepatitis delta virus. In: Mandell , Douglas , and Bennett's principles and practice of infectious diseases, editors. 6th ed. Philadelphia: Elsevier; 2005. pp. 1864–1890. [Google Scholar]
- 16.Nath N, Pielech M, Dodd RY. Hepatitis-associated markers in the American Red Cross blood donor population. V. Prevalence of antibodies to core antigen in three blood services regions. Vox Sang. 1983;44:312–318. doi: 10.1111/j.1423-0410.1983.tb04488.x. [DOI] [PubMed] [Google Scholar]
- 17.Koziol DE, Holland PV, Alling DW, Melpolder JC, Solomon RE, Purcell RH, Hudson LM, Shoup FJ, Krakauer H, Alter HJ. Antibody to hepatitis B core antigen as a paradoxical marker for non-A, non-B hepatitis agents in donated blood. Ann Intern Med. 1986;104:488–495. doi: 10.7326/0003-4819-104-4-488. [DOI] [PubMed] [Google Scholar]
- 18.Kuhns MC, Kleinman SH, McNamara AL, Rawal B, Glynn S, Busch MP. Lack of correlation between HBsAg and HBV DNA levels in blood donors who test positive for HBsAg and anti-HBc: implications for future HBV screening policy. Transfusion. 2004;44:1332–1339. doi: 10.1111/j.1537-2995.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien SF, Yi QL, Fan W, Scalia V, Kleinman SH, Vamvakas EC. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47:316–325. doi: 10.1111/j.1537-2995.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 20.Mosley JW, Stevens CE, Aach RD, Hollinger FB, Mimms LT, Solomon LR, Barbosa LH, Nemo GJ. Donor screening for antibody to hepatitis B core antigen and hepatitis B virus infection in transfusion recipients. Transfusion. 1995;35:5–12. doi: 10.1046/j.1537-2995.1995.35195090661.x. [DOI] [PubMed] [Google Scholar]
- 21.Allain JP, Hewitt PE, Tedder RS, Williamson LM. Evidence that anti-HBc but not HBV DNA testing may prevent some HBV transmission by transfusion. Br J Haematol. 1999;107:186–195. doi: 10.1046/j.1365-2141.1999.01665.x. [DOI] [PubMed] [Google Scholar]
- 22.Larsen J, Hetland G, Skaug K. Posttransfusion hepatitis B transmitted by blood from a hepatitis B surface antigen-negative hepatitis B virus carrier. Transfusion. 1990;30:431–432. doi: 10.1046/j.1537-2995.1990.30590296376.x. [DOI] [PubMed] [Google Scholar]
- 23.Kojima M, Shimizu M, Tsuchimochi T, Koyasu M, Tanaka S, Iizuka H, Tanaka T, Okamoto H, Tsuda F, Miyakawa Y. Posttransfusion fulminant hepatitis B associated with precore-defective HBV mutants. Vox Sang. 1991;60:34–39. doi: 10.1111/j.1423-0410.1991.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 24.Tani Y, Aso H, Matsukura H, Tadokoro K, Tamori A, Nishiguchi S, Yoshizawa H, Shibata H. Significant background rates of HBV and HCV infections in patients and risks of blood transfusion from donors with low anti-HBc titres or high anti-HBc titres with high anti-HBs titres in Japan: a prospective, individual NAT study of transfusion-transmitted HBV, HCV and HIV infections. Vox Sang. 2012;102:285–293. doi: 10.1111/j.1423-0410.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 25.Stramer SL, Zou S, Notari EP, Foster GA, Krysztof DE, Musavi F, Dodd RY. Blood donation screening for hepatitis B virus markers in the era of nucleic acid testing: are all tests of value? Transfusion. 2012;52:440–446. doi: 10.1111/j.1537-2995.2011.03283.x. [DOI] [PubMed] [Google Scholar]
- 26.Christensen PB, Titlestad IL, Homburg KM, Georgsen J, Kristensen T. Hepatitis B core antibodies in Danish blood donors: a surrogate marker of risk behaviour. Vox Sang. 2001;81:222–227. doi: 10.1046/j.0042-9007.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- 27.Yotsuyanagi H, Yasuda K, Moriya K, Shintani Y, Fujie H, Tsutsumi T, Nojiri N, Juji T, Hoshino H, Shimoda K, et al. Frequent presence of HBV in the sera of HBsAg-negative, anti-HBc-positive blood donors. Transfusion. 2001;41:1093–1099. doi: 10.1046/j.1537-2995.2001.41091093.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M, Nübling CM, Scheiblauer H, Chudy M, Walch LA, Seifried E, Roth WK, Hourfar MK. Anti-HBc screening of blood donors: a comparison of nine anti-HBc tests. Vox Sang. 2006;91:237–243. doi: 10.1111/j.1423-0410.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 29.Manzini P, Girotto M, Borsotti R, Giachino O, Guaschino R, Lanteri M, Testa D, Ghiazza P, Vacchini M, Danielle F, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92:1664–1670. doi: 10.3324/haematol.11224. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri V, Nanu A, Panda SK, Chand P. Evaluation of serologic screening of blood donors in India reveals a lack of correlation between anti-HBc titer and PCR-amplified HBV DNA. Transfusion. 2003;43:1442–1448. doi: 10.1046/j.1537-2995.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 31.Seo DH, Whang DH, Song EY, Kim HS, Park Q. Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infections in Korean blood donors. Transfusion. 2011;51:1840–1846. doi: 10.1111/j.1537-2995.2010.03056.x. [DOI] [PubMed] [Google Scholar]
- 32.Said ZN, Sayed MH, Salama II, Aboel-Magd EK, Mahmoud MH, Setouhy ME, Mouftah F, Azzab MB, Goubran H, Bassili A, et al. Occult hepatitis B virus infection among Egyptian blood donors. World J Hepatol. 2013;5:64–73. doi: 10.4254/wjh.v5.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zervou EK, Dalekos GN, Boumba DS, Tsianos EV. Value of anti-HBc screening of blood donors for prevention of HBV infection: results of a 3-year prospective study in Northwestern Greece. Transfusion. 2001;41:652–658. doi: 10.1046/j.1537-2995.2001.41050652.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhatti FA, Ullah Z, Salamat N, Ayub M, Ghani E. Anti-hepatits B core antigen testing, viral markers, and occult hepatitis B virus infection in Pakistani blood donors: implications for transfusion practice. Transfusion. 2007;47:74–79. doi: 10.1111/j.1537-2995.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien SF, Fearon MA, Yi QL, Fan W, Scalia V, Muntz IR, Vamvakas EC. Hepatitis B virus DNA-positive, hepatitis B surface antigen-negative blood donations intercepted by anti-hepatitis B core antigen testing: the Canadian Blood Services experience. Transfusion. 2007;47:1809–1815. doi: 10.1111/j.1537-2995.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Y, Dubovoy N, Hayes-Rogers ME, Stewart J, Shah D. Detection of IgM to hepatitis B core antigen in a reductant containing, chemiluminescence assay. J Immunol Methods. 1999;230:29–35. doi: 10.1016/s0022-1759(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 37.Reesink HW, Allain JP. Management of donors and blood products reactive for hepatitis B virus DNA. Vox Sang. 2006;91:281. doi: 10.1111/j.1423-0410.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 38.Candotti D, Allain JP. Molecular virology in transfusion medicine laboratory. Blood Transfus. 2013;11:203–216. doi: 10.2450/2012.0219-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth WK, Weber M, Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus, and HIV-1 in a blood-bank setting. Lancet. 1999;353:359–363. doi: 10.1016/S0140-6736(98)06318-1. [DOI] [PubMed] [Google Scholar]
- 40.Margaritis AR, Brown SM, Seed CR, Kiely P, D’Agostino B, Keller AJ. Comparison of two automated nucleic acid testing systems for simultaneous detection of human immunodeficiency virus and hepatitis C virus RNA and hepatitis B virus DNA. Transfusion. 2007;47:1783–1793. doi: 10.1111/j.1537-2995.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 41.Charlewood R, Flanagan P. Ultrio and Ultrio Plus non-discriminating reactives: false reactives or not? Vox Sang. 2013;104:7–11. doi: 10.1111/j.1423-0410.2012.01624.x. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman SH, Strong DM, Tegtmeier GG, Holland PV, Gorlin JB, Cousins C, Chiacchierini RP, Pietrelli LA. Hepatitis B virus (HBV) DNA screening of blood donations in minipools with the COBAS AmpliScreen HBV test. Transfusion. 2005;45:1247–1257. doi: 10.1111/j.1537-2995.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- 43.Reesink HW, Engelfriet CP, Henn G, Mayr WR, Delage G, Bernier F, Krusius T, Assal A, Gallian P, Corbi C, et al. Occult hepatitis B infection in blood donors. Vox Sang. 2008;94:153–166. doi: 10.1111/j.1423-0410.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 44.Roth WK, Busch MP, Schuller A, Ismay S, Cheng A, Seed CR, Jungbauer C, Minsk PM, Sondag-Thull D, Wendel S, et al. International survey on NAT testing of blood donations: expanding implementation and yield from 1999 to 2009. Vox Sang. 2012;102:82–90. doi: 10.1111/j.1423-0410.2011.01506.x. [DOI] [PubMed] [Google Scholar]
- 45.Kang JW, Kwon SY, Seo YI, Lee MK, Huh K, Park SJ, Kim MH, Cho NS. HBV NAT implementation in Korea: 1 year experience. Vox Sang. 2014;107:150. [Google Scholar]
- 46.Lin KT, Chang CL, Tsai MH, Lin KS, Saldanha J, Hung CM. Detection and identification of occult HBV in blood donors in Taiwan using a commercial, multiplex, multi-dye nucleic acid amplification technology screening test. Vox Sang. 2014;106:103–110. doi: 10.1111/vox.12075. [DOI] [PubMed] [Google Scholar]
- 47.Zheng X, Ye X, Zhang L, Wang W, Shuai L, Wang A, Zeng J, Candotti D, Allain JP, Li C. Characterization of occult hepatitis B virus infection from blood donors in China. J Clin Microbiol. 2011;49:1730–1737. doi: 10.1128/JCM.00145-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaezjalali M, Rashidpour S, Rezaee H, Hajibeigi B, Zeidi M, Gachkar L, Aghamohamad S, Najafi R, Goudarzi H. Hepatitis B viral DNA among HBs antigen negative healthy blood donors. Hepat Mon. 2013;13:e6590. doi: 10.5812/hepatmon.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García-Montalvo BM, Farfán-Ale JA, Acosta-Viana KY, Puerto-Manzano FI. Hepatitis B virus DNA in blood donors with anti-HBc as a possible indicator of active hepatitis B virus infection in Yucatan, Mexico. Transfus Med. 2005;15:371–378. doi: 10.1111/j.1365-3148.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 50.Assal A, Barlet V, Deschaseaux M, Dupont I, Gallian P, Guitton C, Morel P, David B, De Micco P. Comparison of the analytical and operational performance of two viral nucleic acid test blood screening systems: Procleix Tigris and cobas s 201. Transfusion. 2009;49:289–300. doi: 10.1111/j.1537-2995.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 51.Vermeulen M, Lelie N, Sykes W, Crookes R, Swanevelder J, Gaggia L, Le Roux M, Kuun E, Gulube S, Reddy R. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion. 2009;49:1115–1125. doi: 10.1111/j.1537-2995.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang MH, Li L, Hung YS, Hung CS, Allain JP, Lin KS, Tsai SJ. The efficacy of individual-donation and minipool testing to detect low-level hepatitis B virus DNA in Taiwan. Transfusion. 2010;50:65–74. doi: 10.1111/j.1537-2995.2009.02357.x. [DOI] [PubMed] [Google Scholar]
- 53.Arora S, Doda V, Kirtania T. Sensitivity of individual donor nucleic acid testing (NAT) for the detection of hepatitis B infection by studying diluted NAT yield samples. Blood Transfus. 2014:Oct 23; Epub ahead of print. doi: 10.2450/2014.0048-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, Peddada L, Smith R, Schreiber GB, Epstein JS, et al. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43:788–798. doi: 10.1046/j.1537-2995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 55.Satake M, Taira R, Yugi H, Hino S, Kanemitsu K, Ikeda H, Tadokoro K. Infectivity of blood components with low hepatitis B virus DNA levels identified in a lookback program. Transfusion. 2007;47:1197–1205. doi: 10.1111/j.1537-2995.2007.01276.x. [DOI] [PubMed] [Google Scholar]
- 56.Levicnik-Stezinar S, Rahne-Potokar U, Candotti D, Lelie N, Allain JP. Anti-HBs positive occult hepatitis B virus carrier blood infectious in two transfusion recipients. J Hepatol. 2008;48:1022–1025. doi: 10.1016/j.jhep.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Coppola N, Loquercio G, Tonziello G, Azzaro R, Pisaturo M, Di Costanzo G, Starace M, Pasquale G, Cacciapuoti C, Petruzziello A. HBV transmission from an occult carrier with five mutations in the major hydrophilic region of HBsAg to an immunosuppressed plasma recipient. J Clin Virol. 2013;58:315–317. doi: 10.1016/j.jcv.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 58.Furuta RA, Kondo Y, Saito T, Tomita M, Oka K, Kishimoto Y, Tani Y, Shibata T. Transfusions of red blood cells from an occult hepatitis B virus carrier without apparent signs of transfusion-transmitted hepatitis B infection. Transfus Med. 2008;18:379–381. doi: 10.1111/j.1365-3148.2008.00898.x. [DOI] [PubMed] [Google Scholar]
- 59.Su TH, Chen PJ, Chen TC, Cheng HR, Li L, Lin KS, Kao JH, Chen DS, Liu CJ. The clinical significance of occult hepatitis B transfusion in Taiwan--a look-back study. Transfus Med. 2011;21:33–41. doi: 10.1111/j.1365-3148.2010.01036.x. [DOI] [PubMed] [Google Scholar]
- 60.Bouike Y, Imoto S, Mabuchi O, Kokubunji A, Kai S, Okada M, Taniguchi R, Momose S, Uchida S, Nishio H. Infectivity of HBV DNA positive donations identified in look-back studies in Hyogo-Prefecture, Japan. Transfus Med. 2011;21:107–115. doi: 10.1111/j.1365-3148.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization. Blood safety: Key global fact and figures in 2011. Available from: http://www.who.int/worldblooddonorday/media/who_blood_safety_factsheet_2011.pdf.
- 62.Candotti D, Lin CK, Belkhiri D, Sakuldamrongpanich T, Biswas S, Lin S, Teo D, Ayob Y, Allain JP. Occult hepatitis B infection in blood donors from South East Asia: molecular characterisation and potential mechanisms of occurrence. Gut. 2012;61:1744–1753. doi: 10.1136/gutjnl-2011-301281. [DOI] [PubMed] [Google Scholar]
- 63.Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998;27:1736–1742. doi: 10.1002/hep.510270638. [DOI] [PubMed] [Google Scholar]
- 64.Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 2005;42:302–308. doi: 10.1016/j.jhep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Lucifora J, Durantel D, Testoni B, Hantz O, Levrero M, Zoulim F. Control of hepatitis B virus replication by innate response of HepaRG cells. Hepatology. 2010;51:63–72. doi: 10.1002/hep.23230. [DOI] [PubMed] [Google Scholar]
- 66.Kaur P, Paliwal A, Durantel D, Hainaut P, Scoazec JY, Zoulim F, Chemin I, Herceg Z. DNA methylation of hepatitis B virus (HBV) genome associated with the development of hepatocellular carcinoma and occult HBV infection. J Infect Dis. 2010;202:700–704. doi: 10.1086/655398. [DOI] [PubMed] [Google Scholar]
- 67.Lucifora J, Arzberger S, Durantel D, Belloni L, Strubin M, Levrero M, Zoulim F, Hantz O, Protzer U. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol. 2011;55:996–1003. doi: 10.1016/j.jhep.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Pollicino T, Amaddeo G, Restuccia A, Raffa G, Alibrandi A, Cutroneo G, Favaloro A, Maimone S, Squadrito G, Raimondo G. Impact of hepatitis B virus (HBV) preS/S genomic variability on HBV surface antigen and HBV DNA serum levels. Hepatology. 2012;56:434–443. doi: 10.1002/hep.25592. [DOI] [PubMed] [Google Scholar]
- 69.McCullough J. Progress toward a pathogen-free blood supply. Clin Infect Dis. 2003;37:88–95. doi: 10.1086/375232. [DOI] [PubMed] [Google Scholar]
- 70.Schlenke P. Pathogen inactivation technologies for cellular blood components: an update. Transfus Med Hemother. 2014;41:309–325. doi: 10.1159/000365646. [DOI] [PMC free article] [PubMed] [Google Scholar]


