Abstract
AIM: To investigate the survival rates after transarterial embolization (TAE).
METHODS: One hundred third six hepatocellular carcinoma (HCC) patients [90 barcelona clinic liver cancer (BCLC) B] were submitted to TAE between August 2008 and December 2013 in a single center were retrospectively studied. TAE was performed via superselective catheterization followed by embolization with polyvinyl alcohol or microspheres. The date of the first embolization until death or the last follow-up date was used for the assessment of survival. The survival rates were calculated using the Kaplan-Meier method, and the groups were compared using the log-rank test.
RESULTS: The overall mean survival was 35.8 mo (95%CI: 25.1-52.0). The survival rates of the BCLC A patients (33.7%) were 98.9%, 79.0% and 58.0% at 12, 24 and 36 mo, respectively, and the mean survival was 38.1 mo (95%CI: 27.5-52.0). The survival rates of the BCLC B patients (66.2%) were 89.0%, 69.0% and 49.5% at 12, 24 and 36 mo, respectively, and the mean survival was 29.0 mo (95%CI: 17.2-34). The survival rates according to the BCLC B sub-staging showed significant differences between the groups, with mean survival rates in the B1, B2, B3 and B4 groups of 33.5 mo (95%CI: 32.8-34.3), 28.6 mo (95%CI: 27.5-29.8), 19.0 mo (95%CI: 17.2-20.9) and 13 mo, respectively (P = 0.013).
CONCLUSION: The BCLC sub-staging system could add additional prognosis information for post-embolization survival rates in HCC patients.
Keywords: Hepatocellular carcinoma, Barcelona clinic liver cancer, Transarterial embolization, Subclassification
Core tip: This is the first study to apply the barcelona clinic liver cancer (BCLC) B subclassification in a survival analysis for hepatocellular carcinoma patients after transarterial embolization. Were observed significant differences in the mean survival rates among B1, B2, B3 and B4 patients. The BCLC B sub-staging system could be an additional tool for accessing prognosis in the post-embolization survival rates of hepatocellular carcinoma patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third leading cause of cancer-related death[1,2]. Locoregional treatments, such as transarterial chemoembolization (TACE) and transarterial embolization (TAE), have been used for intermediate HCC patients, promoting an increase in overall survival[3-7]. An updated meta-analysis and a systematic review of randomized controlled trials comparing TACE with TAE did not proved significant difference in survival between this two techniques[6,7].
The intermediate stage of HCC affects a highly heterogeneous patient population and can present with varying tumor burdens and liver functionality that are usually staged in the same level as barcelona clinic liver cancer (BCLC) B[1,2,8].
Recently, some authors have proposed a sub-staging of BCLC B patients to facilitate therapeutic decisions, especially due to wide differences in response rates after transarterial treatments among intermediate HCC patients[8]. We analyzed the survival rates based on this sub-staging after TAE.
MATERIALS AND METHODS
We retrospectively analyzed a historical cohort of American Association for the Study of Liver Diseases diagnosis-based HCC patients[9] treated from June 2008 to December 2013 at the Gastroenterology Division of the Hepatology Unit of the Hospital de Clínicas de Porto Alegre.
TAE was indicated for patients with HCC BCLC A with nodules greater than 3 cm or without safe percutaneous access to ablative therapies and BCLC B with no signs of extra-hepatic disease[9].
BCLC D or BCLC C patients with evidence of extra-hepatic disease, portal vein thrombosis (or thrombosis of one of its branches), or hepatofugal portal flow were excluded. Patients with a definitive diagnosis of extra-hepatic metastasis, difficult to control ascites, other active malignant diseases or the following laboratorial anomalies were also excluded: serum creatinine above 1.5 mg/dL, total bilirubin above 3.0 mg/dL, platelets lower than 50000 mm3 or a prothrombin time less than 50%.
The BCLC B patients were sub-staged into four categories (Table 1). Group 1 comprised patients with Child-Pugh class A or B with a score of no more than 7, without current or previous decompensation, an Eastern Cooperative Oncology Group (ECOG) PS score of 0 and meeting the up-to-seven criteria. Group 2 comprised Child-Pugh A patients exceeding the up-to-seven criteria, without ascites or jaundice and an ECOG PS score of 0. Group 3 comprised patients with Child-Pugh class B exceeding the up-to-seven criteria and who had an ECOG PS score of 0. Group 4 comprised decompensated Child-Pugh B patients with severe ascites or jaundice, an ECOG PS score of 0 or 1 and who either did or did not exceed the up-to-seven criteria[8].
Table 1.
Barcelona clinic liver cancer B sub-stage categories
| BCLC sub staging | ||||
| B1 | B2 | B3 | B4 | |
| Child Pugh class | 5-6-7 | 5-6 | 7 | 8-9 |
| Beyond Milan and within up-to-7 | In | Out | Out | Any |
| ECOG PS | 0 | 0 | 0 | 0-1 |
BCLC: Barcelona clinic liver cancer; ECOG PS: The Eastern Cooperative Oncology Group performance status.
Procedure
TAE was performed by the same interventional radiologist through a common femoral access. Selective catheterization of the celiac trunk and the superior mesenteric artery were performed with a Cobra or Mikaelson 5F catheter to facilitate the liver blood flow study. The hepatic artery was selectively catheterized, followed by a superselective feeding branch tumor catheterization with a 2.8 F microcatheter (Progreat®, Terumo). In the PVA-TAE, a superselective injection of PVA (Cook, Bloomington, Indiana) was performed in the feeding artery as distal as possible. The ME-TAE injection was performed with ME embospheres (Biosphere medicals™, Rockland, MA, United States). The particle sizes were 100-300 microns for tumors up to 5 cm and 300-500 microns for tumors equal to or larger than 5 cm.
Statistical analysis
The categorical variables were described using frequency and percentages. The quantitative variables with symmetric distribution were expressed using their mean values and standard deviation; those with asymmetric distribution were described using the median and inter-quartile interval (25th percentile - 75th percentile). The χ2 test or Fisher’s Exact Test was used to compare the categorical variables. Quantitative variables with symmetric distribution between groups were compared using Student’s t test for the independent samples. Variables with asymmetric distribution were compared between the groups using the Mann-Whitney U test.
The date of the first embolization until death or the last follow-up date was used in the assessment of survival. The survival rates were calculated using the Kaplan-Meier method, and the groups were compared using the Log-Rank test.
Analyses were performed using SPSS software version 19.0 by a biomedical statician. The statistical level of significance was set at 0.05.
RESULTS
One hundred third six patients, 46 BCLC A (33.7%) and 90 BCLC B (66.25%), were treated with TAE. Among the BCLC B group, 48 (52.8%) patients were BCLC B1, 27 (30.2%) were BCLC B2, 13 (15.1%) were BCLC B3 and 2 (1.9%) was BCLC B4.
There were no significant differences at baseline in regard to demographic data, staging, laboratory or HCC characteristics according to the embolic agent. Table 2 summarizes the characteristics.
Table 2.
Baseline and tumoral characteristics
| Characteristics | B1 | B2 | B3 | B4 | P value1 |
| Age (yr) | 62 | 61 | 60 | 60 | 0.77 |
| Gender (male,%) | 66 | 73 | 76 | 50 | 0.48 |
| Caucasians (%) | 95 | 99 | 96 | 100 | 0.28 |
| HCV positive (%) | 81 | 77 | 76 | 100 | 0.79 |
| Alcohol (%) | 33 | 33 | 34 | 0 | 1.00 |
| PS (0) (%) | 100 | 95 | 95 | 50 | 0.27 |
| AST (U/L) | 35 | 36 | 37 | 38 | 0.90 |
| ALT (U/L) | 64 | 67 | 69 | 70 | 0.80 |
| GGT (U/L) | 133 | 136 | 144 | 155 | 0.79 |
| Platelets (× 1000/UL) | 110 | 110 | 105 | 90 | 0.35 |
| PT (%) | 73 | 73 | 77 | 79 | 0.72 |
| Albumin (U/L) | 3.5 | 3.4 | 3.3 | 3.3 | 0.28 |
| BT (mg/dL) | 1.2 | 1.3 | 1.3 | 1.4 | 0.22 |
| Creatinine | 0.93 | 0.94 | 0.95 | 0.97 | 0.73 |
| No. of TAE session | 1.7 | 1.9 | 1.9 | 1.9 | 0.20 |
P value analysis excluded B4 subgroup (only 2 patients). HCV: Hepatitis C virus; PS: Performance status; AST: Aspartate transaminase; ALT: Alanine transaminase; GGT: Gamma-glutamyl transferase; PT: Prothrombine time; BT: Total bilirubin; TAE: Transarterial embolization.
Throughout the analysis, the follow up time was 1 to 52 mo, with overall survival rates at 12, 24 and 36 mo of 98.5, 78.0 and 55.5%, respectively. The mean overall survival rate was 35.8 mo (95%CI: 25.1-52.0).
The survival rates of the BCLC A patients (33.7%) were 98.9%, 79.0% and 58.0% at 12, 24 and 36 mo, respectively, and the mean was 38.1 mo (95%CI: 25.0-52.0).
The survival rates of the BCLC B patients (66.2%) were 89%, 69% and 49.5% at 12, 24 and 36 mo, respectively, and the mean was 29.0 mo (95%CI: 17.2-34.3).
The survival rates according to BCLC B subclassification showed a significant difference between the groups, with mean survival rates for B1, B2, B3 and B4 of 33.6 mo (95%CI: 32.9-34.3), 28.6 mo (95%CI: 27.5-29.8), 19.0 mo (95%CI: 17.2-20.9) and 13 mo, respectively (P = 0.013). The median survival rates for B1, B2, B3 and B4 was 33, 28, 19 and 13 mo, respectively. Table 3 summarizes the survival rates, and Figure 1 shows the survival Kaplan-Meier curves according to BCLC B subclassification.
Table 3.
Survival rates according to barcelona clinic liver cancer classification and subclassification
| n (%) | Mean survival rate (mo) (95%CI) | |
| Overall | 136 (100) | 35.8 (25.0-52.0) |
| BCLC A | 46 (33.7) | 38.1 ( 25.0-52.0) |
| BCLC B | 90 (66.2) | 29.0 ( 17.2-34.3) |
| BCLC B1 | 48 (52.8) | 33.6 ( 32.9-34.3) |
| BCLC B2 | 27 (30.2) | 28.6 (27.5-29.8) |
| BCLC B3 | 13 (15.1) | 19.0 ( 17.2-20.9) |
| BCLC B4 | 2 (1.9) | 13.0 ( 25.0-52.0) |
BCLC: Barcelona clinic liver cancer.
Figure 1.
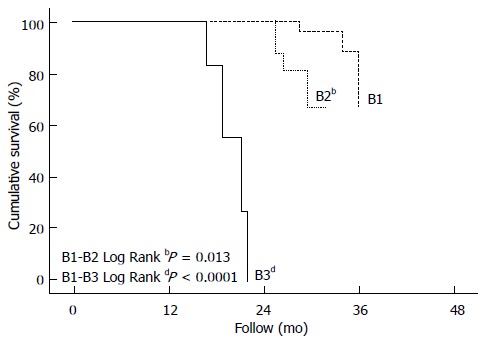
Kaplan-Meier curves.
There was no differences in the response rates according to mRECIST among the BCLC B substaging, as demonstrated in Table 4.
Table 4.
Barcelona clinic liver cancer B substaging response rates according to mRECIST n (%)
| CR | PR | PD | SD | |
| B1 | 12 (25) | 29 (60.4) | 3 (6.25) | 3 (6.25) |
| B2 | 6 (22.2) | 13 (48) | 1 (3.7) | 7 (26.1) |
| B3 | 1 (7.6) | 4 (30.7) | 1 (7.6) | 7 (53.8) |
| B4 | - | - | 1 (50) | 1 (50) |
CR: Complete response; PR: Partial response; PD: Progressive disease; SD: Stable disease.
DISCUSSION
The difficulty in finding an ideal treatment modality for BCLC B patients was been evaluated. Some authors showed a significant difference in survival between Child-Pugh B7 and B8 with survival means after TACE of 22 and 6 mo, respectively[10]. Survival differences between BCLC A and B patients after TACE were also demonstrated after drug-eluting beads chemoembolization (DEB-TACE), with survival rates after 12, 24 and 36 mo of 93.6%, 83.8% and 62%, respectively, in BCLC A, and 91.5%, 75% and 50.7% in BCLC B[11].
Therefore, Bolondi et al[8] proposed a refinement of intermediate HCC staging based on the Child-Pugh score, up-to-seven criteria, performance status and portal vein patency.
Our study also applied the BCLC B sub-staging, and significant survival differences were observed in subgroups, with mean survival rates for B1, B2, B3 and B4 of 33.6 mo (95%CI: 32.9-34.3), 28.6 mo (95%CI: 27.5-29.8), 19.0 mo (95%CI: 17.220.9) and 13 mo respectively (P = 0.013).
Some authors also have been validated that proposal, depicting significant differences in median survival time between B1 and B2 and B2 and B3[12]. Another recently study also showed differences in 5-year survival rates using sub-stages, and had proposed a modification of this system based on alpha-fetoprotein (AFP) levels[13]. According to AFP levels (200 ng/mL), B1 was classified into B1a and B1b and B2 into B2a and B2b, without differences in survival among this subgroups, but a re-classification into modified mB1 (B1a), mB2 (B1b + B2a) and mB3 (B2b + B3) provided better prognostic prediction[13]. Since AFP levels were available in a small number of our sample, we did not performed this modified evaluation.
Even not considering the AFP levels, this differences in survival rates among the B sub-staging patients stressed distinct clinical conditions and tumor characteristics, even though it could represent a measure of interaction between underlying liver disease and tumor burden.
The present study has some limitations. It is single-centered, retrospective, and the choice of the embolizing agent was not standardized. Nevertheless, the survival rates we found suggest that this issue warrants further investigation.
In conclusion, the BCLC sub-staging system could be an additional tool for accessing prognosis in the post-embolization survival rates, having potential to better select the therapeutic approach for intermediate HCC patients.
COMMENTS
Background
The intermediate stage of hepatocellular carcinoma is composed of a group of heterogeneous characteristics related to tumor burden and clinical aspects. Therefore, the standard embolization treatment proposed for intermediate stage could have different responses in this group.
Research frontiers
Given the potential differences in patients in stage B, is not yet known the survival rates of patients divided into four subcategories of barcelona clinic liver cancer (BCLC) stage B, that underwent transarterial embolization (TAE).
Innovations and breakthroughs
The TAE is a minimal invasive technique that has been applied to patients in intermediate stage hepatocellular carcinoma (HCC). In the literature however there is debate about its role in patients in stage B of BCLC, given heterogeneity at this stage. This study aims to better characterize these patients by determining survival in the different sub stages.
Applications
This approach aim to predict prognosis in terms of survival in an attempt to optimize the therapeutic approach in this group of patients.
Terminology
BCLC is a classification used to guide therapeutic for patients with hepatocellular carcinoma. One of the available treatments, especially for patients with intermediate stage HCC, TAE is a non invasive trans catheter technique in which are injected embolizing agents aimed at restricting arterial tumor supply.
Peer-review
This is an interesting manuscript where the authors have analysed the difference in survival between different subgroups in BCLC Stage B HCC after TAE.
Footnotes
Ethics approval: The study was reviewed and approved by the Hospital de Clínicas de Porto Alegre Review Board.
Informed consent: Retrospective study with no written informed consent.
Conflict-of-interest: The authors have no conflict of interest to disclosure.
Data sharing: Statistical code, and dataset available from the corresponding author at leandroscaffaro@yahoo.com.br.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 1, 2014
First decision: December 12, 2014
Article in press: February 10, 2015
P- Reviewer: Arias J, Kapoor S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Tsochatzis EA, Fatourou E, O’Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3069–3077. doi: 10.3748/wjg.v20.i12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sangro B. [Survival benefit with intraarterial techniques in hepatocellular carcinoma] Gastroenterol Hepatol. 2014;37 Suppl 2:95–101. doi: 10.1016/S0210-5705(14)70076-7. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 6.Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, Davies N, Williams E, Pereira SP, Hochhauser D, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer. 2013;108:1252–1259. doi: 10.1038/bjc.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 8.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piscaglia F, Terzi E, Cucchetti A, Trimarchi C, Granito A, Leoni S, Marinelli S, Pini P, Bolondi L. Treatment of hepatocellular carcinoma in Child-Pugh B patients. Dig Liver Dis. 2013;45:852–858. doi: 10.1016/j.dld.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, Marinis A, Kelekis A, Alexopoulou E, Chatziioannou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012;35:1119–1128. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]
- 12.Ha Y, Shim JH, Kim SO, Kim KM, Lim YS, Lee HC. Clinical appraisal of the recently proposed Barcelona Clinic Liver Cancer stage B subclassification by survival analysis. J Gastroenterol Hepatol. 2014;29:787–793. doi: 10.1111/jgh.12452. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Kee KM, Lin CY, Hung CH, Chen CH, Lee CM, Lu SN. Validation and modification of a proposed substaging system for patients with intermediate hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:358–363. doi: 10.1111/jgh.12686. [DOI] [PubMed] [Google Scholar]


