Abstract
The extent to which observed differences in emotion processing and regulation neural circuitry in adolescents with a history of suicide attempt are paralleled by structural differences is unknown. We measured brain cortical thickness and grey- and white-matter volumes in 100 adolescents: 28 with a history of suicide attempt and major depressive disorder (MDD); 31 with a history of MDD but no suicide attempt; and a healthy control group (n = 41). The first group compared with controls showed reduction in grey-matter volume in the right superior temporal gyrus (BA38), a region important for social emotion processing.
Suicide is the third leading cause of death in adolescence,1 and is associated with major depressive disorder (MDD),2 although most adolescents with depression never attempt suicide. We have described functional differences in emotion processing and regulation neural circuitry in adolescents with depression and past suicide attempt relative to other adolescents with depression and healthy controls.3 Specifically, we reported abnormal functioning of the salience and attention networks during emotion processing, with normal function in the absence of emotional content, which may represent markers of suicidal behaviour. Together, these findings suggest unique endophenotypes for suicidal behaviour distinct from MDD. What remains unknown is how these functional abnormalities are paralleled by structural abnormalities which could provide measures of neuropathophysiological processes underlying adolescent suicidal behaviour that are more accessible than measures of neural function.
Method
Participants comprised adolescents with a history of suicide attempt and MDD (n = 28); a history of MDD but no suicide attempt (n = 31); and no personal or family history of psychiatric disorder or suicide attempt (healthy controls, n = 41). Suicide attempts were defined by the Columbia Classification Algorithm of Suicide Assessment.4 Exclusion criteria included neurological disorders, anoxia history, head injuries, Wechsler verbal score <80,5 current use of sedative medication, pregnancy, ineligibility for magnetic resonance imaging (MRI), bipolar disorder, psychosis, substance misuse or positive urine drug/saliva alcohol screen, and left-handedness (owing to participant recruitment for concurrent functional neuroimaging studies). Axis I disorders were assessed using the Kiddie-SADS – Present and Lifetime version (K-SADS-PL).6 Suicide attempt was assessed with the Suicide Intent Scale and Suicide History Form.7,8 Depression, anxiety, suicidal ideation and pubertal status were assessed respectively with the Beck Depression Inventory (BDI), the Screen for Childhood Anxiety Related Emotional Disorders (SCARED), the Suicidal Ideation Questionnaire (SIQ) and the Petersen Pubertal Development Scale.9–12 Past trauma was determined by psychiatrist assessment and/or the Child Trauma Questionnaire.13 Positive trauma history included sexual or physical abuse, or trauma with risk of death or bodily harm.
Two MRI scanners were used: a 3 T Siemens Allegra for 49 scans and a 3 T Siemens Trio for 51 scans (Siemens, Erlangen, Germany). We acquired T1-weighted magnetisation-prepared rapid gradient echo (MPRAGE) structural images of 240 slices (thickness 0.8 mm): repetition time 1630 ms, echo time 2.48 ms, inversion time 800 ms, field of view 200 mm, flip angle 8°, matrix 256 × 256). Brain cortical thickness and grey- and white-matter volumes were measured using FreeSurfer version 5.1 for Linux (https://surfer.nrm.mgh.harvard.edu/). Topographical defects were automatically corrected and images normalised. Cortical thickness measures were computed as the distances between the grey/white-matter boundary and the pial surface.14 Cortical volumes were calculated from the surface mask.15 Two whole-brain surface-based analyses of covariance were completed in Qdec1.4 (FreeSurfer application) to examine the main effect of group on cortical thickness and volume respectively, with age, gender, total brain volume and scanner used as covariates. Groups were equally distributed across both scanners, and three participants were scanned in both scanners and compared. Monte Carlo simulation analyses were performed to correct for multiple voxel-wise comparisons in Qdec, and provided a cluster-wise significance threshold of P<0.05 for these analyses.
To examine the nature of between-group differences in cortical thickness and volume, values were extracted from all cortical regions identified in the above analysis of covariance (ANCOVA). Post hoc pairwise, between-group independent t-tests were conducted on these extracted values. Significance thresholds for comparisons were P<0.05 (two-tailed) and Bonferroni-corrected for the three post hoc comparisons. Exploratory correlational analyses to examine relationships between any cortical thickness and volume abnormalities and clinical variables were completed in SPSS version 20.0.
Results
Groups did not differ significantly in gender. The control group was significantly younger than the two groups with depression, but with no significant difference in pubertal status. The latter two groups did not differ significantly on BDI, SCARED or SIQ scores, trauma history or medication status (see online Table DS1). Results from three participants scanned in both scanners revealed no significant effect of scanner on reported volume (P = 0.75). One-way ANCOVA revealed a significant main effect of group on volume in the right superior temporal gyrus (rSTG) (F(2,98) = 3.74, P = 0.027; BA38, x = 35, y = 13, z = -41,vertex 160, cluster size 889.80 mm2, P<0.05 corrected) from the initial whole brain analyses. Post hoc pairwise t-tests revealed that adolescents who had attempted suicide showed significantly smaller rSTG cortical volume than controls (P = 0.016) (Fig. 1, online Table DS2). Exploratory analyses showed no significant relationship between BDI, SCARED and SIQ scores, past trauma, medication and rSTG volume in the suicide attempt group, using a statistical threshold of P = 0.05/5 (P = 0.01).
Fig. 1.
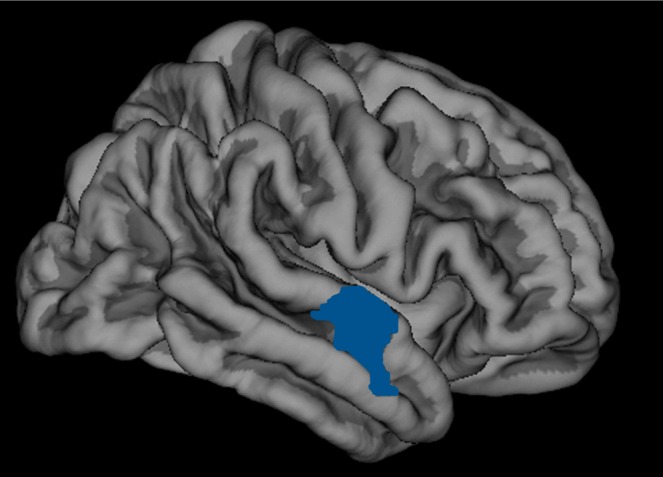
Diminished cortical volume in the right superior temporal gyrus in adolescents with a history of suicide attempt compared with a healthy control group (Brodmann area BA38, x = 35, y = 13, z = -41, vertex = 160, cluster size 889.80 mm2; P<0.05 corrected) with cortical volume post hoc t-tests, study group, covarying for age, gender, difference in scanners and total brain volume.
Discussion
The rSTG is associated with attention to emotion,16 spatial perception and exploration,17,18 and face processing;19 lesions result in difficulty discriminating others’ gaze,20 and this area is activated during Theory of Mind tasks.21 Studies have shown a relationship between lower bilateral STG volume and past suicide attempt in adult patients with psychotic disorders.22 Given our previous findings of salience network abnormalities in adolescents with a suicide attempt history during processing of socially relevant angry faces,3 our finding of abnormally decreased rSTG volume may be a structural neural marker of social-emotional information evaluation abnormalities in these young people. Furthermore, the absence of any significant relationship between this cortical volume abnormality and measures of present symptom severity, suicidal ideation severity, past trauma and medication in this group suggests that abnormally reduced rSTG volume may be a potential trait marker of suicide attempt in adolescence, although there are also findings of lower rSTG volume in schizophrenia.22
Limitations include the use of two scanners, despite balancing groups. Scanner type was a covariate in analyses, however, and volume measures of three participants scanned in both scanners did not differ. Sample size was relatively modest, with further study in larger samples indicated. It would be ideal to study adolescents with a history of suicide attempt without MDD.
In summary, our findings are the first to show cortical grey-matter abnormalities in adolescents with a history of suicide attempt, and indicate grey-matter volume reduction in a key cortical region important for social emotion processing. The extent to which this precedes or is a consequence of suicide attempt remains to be clarified.
Footnotes
Declaration of interest
None.
Funding
Research supported by the American Foundation for Suicide Prevention, the Klingenstein Third Generation Foundation Fellowship for Adolescent Depression, National Institute of Mental Health (NIMH) grants 1K23MH082884-01(L.P.), National Institutes of Health grants MH66775, MH65368, MH56612 and MH18951 (D.B.) and NIMH MH076971 (M.P.).
References
- 1. Zalsman G. Genetics of suicidal behavior in children and adolescents. In The Neurobiological Basis of Suicide (ed Y Dwivedi): 297–314 CRC Press, 2012. [PubMed] [Google Scholar]
- 2. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry 2006; 47: 372–94. [DOI] [PubMed] [Google Scholar]
- 3. Pan LA, Phillips ML. Toward identification of neural markers of suicide risk in adolescents. Neuropsychopharmacol Rev 2014; 39: 236–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Posner K, Oquendo M, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 2007; 164: 1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wechsler D. Manual for the Wechsler Intelligence Scale for Children. Psychological Corporation, 1974. [Google Scholar]
- 6. Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-SADS – Present and Lifetime Version (K-SADS-PL). J Am Acad Child Adolesc Psychiatry 1996; 36: 980–8. [DOI] [PubMed] [Google Scholar]
- 7. Beck AT, Schuyler D, Herman I. Development of suicidal intent scales. In The Prediction of Suicide (eds Beck AT, Resnick HLP, Lettieri DJ.): 45–56 Charles Press, 1974. [Google Scholar]
- 8. Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. In Standardized Evaluation in Clinical Practice (ed First MB.): 103–30 American Psychiatric Publishing, 2003. [Google Scholar]
- 9. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression (BDI). Arch Gen Psychiatry 1961; 4: 561–71. [DOI] [PubMed] [Google Scholar]
- 10. Birmaher B, Khetarpal S, Cully M, Brent DA, McKenzie S. Screen for Child Anxiety Related Disorders (SCARED) – parent form and child form (8 years and older). In Innovations in Clinical Practice: Focus on Children and Adolescents (eds VandeCreek L, Jackson T.): 99–104 Professional Resource Press/Professional Resource Exchange, 2003. [Google Scholar]
- 11. Reynolds WM. Suicidal Ideation Questionnaire (SIQ). Psychological Assessment Resources, 1987. [Google Scholar]
- 12. Petersen AC, Crockett L, Richards M. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988; 17: 117–33. [DOI] [PubMed] [Google Scholar]
- 13. Bernstein DP, Fink L, Handelsman L, Foote J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994; 151: 1132–6. [DOI] [PubMed] [Google Scholar]
- 14. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 2009; 19: 2001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain Res Cogn Brain Res 2001; 12: 225–31. [DOI] [PubMed] [Google Scholar]
- 17. Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain 2004; 127: 2307–15. [DOI] [PubMed] [Google Scholar]
- 18. Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 2011; 411: 950–3. [DOI] [PubMed] [Google Scholar]
- 19. Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998; 121: 47–57. [DOI] [PubMed] [Google Scholar]
- 20. Akiyama T, Kato M, Muramatsu T, Saito F, Nakachi R, Kashima H. A deficit in discriminating gaze direction in a case with right superior temporal gyrus lesion. Neuropsychologia 2006; 44: 161–70. [DOI] [PubMed] [Google Scholar]
- 21. Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage 2006; 29: 90–8. [DOI] [PubMed] [Google Scholar]
- 22. Aguilar EJ, Garcia-Marti G, Marti-Bonmati L, Lull JJ, Moratal D, Escarti MJ, et al. Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 1673–6. [DOI] [PubMed] [Google Scholar]


