Abstract
Objectives
Although mild stress hyperglycemia in pediatric illness is common, severe hyperglycemic responses (≥300 mg/dL [16.7 mmol/L]) to stress are unusual. We sought to determine the incidence and course of extreme stress hyperglycemia (ESH) in acute pediatric illness, including whether it is a marker of increased mortality or associated with subsequent development of diabetes mellitus (DM).
Methods
We retrospectively reviewed a cohort of 55,120 consecutive visits over 6 years to a pediatric emergency department at which blood glucose concentrations were measured and report on visits with laboratory glucose 300 mg/dL (16.7 mmol/L) or greater without DM.
Results
There were 72 cases of ESH (incidence of 0.13%). Median age was 8.8 years; 63% were male. The most common diagnoses were respiratory illness (49%), trauma (15%), and seizure (8%), and 65% of patients had received glucose-influencing interventions before evaluation. Eighty-five percent were ill appearing, 60% were admitted to the intensive care unit, and half had acidemic pH values. The overall mortality rate was 22%. Despite treatment of hyperglycemia in only 8 patients, glucose concentrations decreased to 150 mg/dL (8.3 mmol/L) or less within 48 hours in 67% and before discharge or death in 85% of patients. Preceding symptoms and concurrent laboratory results were helpful to exclude diabetes, and none of the surviving patients with follow-up available went on to develop type 1 or 2 DM.
Conclusions
Although rare, ESH (≥300 mg/dL [16.7 mmol/L]) does occur in acute pediatric illness, in most cases is at least partially iatrogenic, and is a marker of severe illness and high mortality. Normoglycemia is typically restored quickly with treatment of the primary illness. No association was found with a subsequent diagnosis of DM.
Keywords: hyperglycemia, stress hyperglycemia, diabetes, pediatric acute illness
Hyperglycemia is common in acute illness, even in the absence of known prior insulin resistance or diabetes mellitus (DM).1 This stress hyperglycemia is due to elevated cortisol, glucagon, growth hormone, catecholamines, and various cytokines, which stimulate glycogenolysis and gluconeogenesis, resulting in a transient increase in blood glucose concentration that typically normalizes when the stress abates.1–4 Relative insulin deficiency,1,5 peripheral insulin resistance,1,5 and dehydration causing decreased renal perfusion with limitation of urinary glucose excretion6 may also contribute, as can certain medications.2,7,8
Stress hyperglycemia greater than 150 mg/dL (8.3 mmol/L) occurs in up to 5% of patients presenting to pediatric emergency departments (EDs),9–11 and the incidence is as high as 25% to 60% in children with severe illness.12–14 Most patients with stress hyperglycemia experience mild to moderate elevations in blood glucose concentration between 150 and 299 mg/dL (8.3 and 16.6 mmol/L), but values of 300 mg/dL (16.7 mmol/L) or higher have also been reported in small numbers in some series9,11,15 and in case reports.2,6,14 However, because extreme elevations in glucose concentration in response to stress are unusual in patients without known DM when they present initially to the ED, such a laboratory finding may lead to confusion in diagnosis, management, and prognosis, including a potential misdiagnosis of concurrent new-onset DM or concern for an increased risk of developing diabetes later in life.9,10,16–19 Furthermore, even if extreme stress hyperglycemia (ESH) can reliably be differentiated from diabetes, it is unknown if this tends to resolve with the simultaneous initiation of treatment of the primary disease process, whether the initiation of insulin in the ED is necessary or beneficial, or if severity of illness is associated with the degree of stress hyperglycemia in the ED, as has been reported in other clinical settings.12,13,20–24
Our goal was to describe the incidence, significance, and course of ESH, which we defined as a blood glucose concentration of 300 mg/dL (16.7 mmol/L) or greater in the absence of DM. This threshold was chosen based on the relative paucity of literature available on stress hyperglycemic responses beyond 300 mg/dL (16.7 mmol/L) and thus the resulting difficulty acute care clinicians may experience in interpreting and managing such high glucose concentrations in the absence of diabetes, as well as the potential for misdiagnosis of new-onset DM. We hypothesized that hyperglycemia of 300 mg/dL (16.7 mmol/L) or greater is intermittently encountered as the far end of a spectrum of stress hyperglycemic responses in a variety of disease states, remains a transient physiological response that tends to improve with treatment of the primary illness, and is not associated with an increased risk for developing DM.
METHODS
Study Design and Setting
We conducted a retrospective review of the medical charts for all patients who presented to a pediatric ED from January 1, 1998, through December 31, 2003, and had a serum or whole-blood glucose concentration measured to identify patients with ESH. The study was conducted at an urban, academic pediatric hospital with an average of 52,000 ED visits per year and a level I trauma center. This study was approved by the institutional review board of Children’s Hospital Boston, Massachusetts, and a waiver of consent was granted to perform the chart review.
Selection of Participants
Eligible patient visits were identified through an electronic search algorithm based on ICD-9 (International Classification of Diseases, Ninth Revision) codes and was conducted by the hospital’s Information Services Department. All patient visits where the initial serum or whole-blood glucose value measured in the ED was 300 mg/dL (16.7 mmol/L) or greater were considered for inclusion. Measurements ascertained by glucometer or point-of-care testing were not included because of the lack of precision at elevated blood glucose concentrations. Patients with either known or correctly diagnosed new-onset DM, as determined by prior or subsequent visits with ICD-9 diabetes-associated billing codes or documentation of the diagnosis of DM by an endocrinologist during the patient’s visit confirmed on review of the medical chart, were excluded. Patients with erroneous glucose measurements (ie, immediate confirmatory glucose value <300 mg/dL [16.7 mmol/L] without intervening therapy) or documented hypoglycemia (<70 mg/dL [3.9 mmol/L]) at a transferring institution, medical office, or home glucometer reading immediately before initial blood glucose measurement in the ED were also excluded from the final analysis. Those patients with preceding hypoglycemia before their arrival in the ED were excluded because hyperglycemia was most likely the result of a glucose-containing rescue intervention.
Data Collection
A comprehensive review of the medical record was completed by a single author (S.L.W.) for all included patients. Data were recorded onto a standard abstraction form that was developed by collaborative input from all authors. One-half of the records were also independently reviewed for errors by a second author (J.A.) or a research assistant (who was trained for this investigation) to ensure accuracy of data extraction. Periodic meetings of the research team were held to resolve disputes in coded information and monitor the performance of the chart abstractors. Information obtained included demographics, historical data, general appearance (as documented in the ED physician’s physical assessment), treatment course, primary discharge diagnosis, length of stay (LOS), and hospital day on which a follow-up serum or whole-blood glucose concentration 150 mg/dL (8.3 mmol/L) or less was first documented, and survival status. Laboratory data included the initial serum or whole-blood glucose concentration drawn in the ED, and the initial blood gas analysis, electrolyte profile, complete blood count, urinalysis, percent glycosylated hemoglobin (HbA1c), and pancreatic autoantibody panel. (Pancreatic autoantibody panel includes autoantibodies to insulin, islet cells, and antiglutamic acid decarboxylase.) Patients were classified as nonsurvivors if they died during the ED visit or hospital course. All laboratory studies were performed in the hospital’s main clinical laboratory. Any intervention known to increase blood glucose as an adverse effect (eg, corticosteroids, inhaled β-agonists, epinephrine) was defined as a glucose-influencing medical intervention. Dextrose-containing intravenous fluids were included in this definition only if they were given as a bolus or contained greater than a 5% dextrose solution. Data were collected about any subsequent diagnosis of DM, hyperglycemia, or other endocrinologic illness. Duration of follow-up ranged from 4 to 10 years, depending on the timing of the initial ED visit, and was limited to patients who received subsequent care at the same institution as the initial ED visit.
Data Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS version 14.0; SPSS Inc, Chicago, Ill). Where demographic characteristics and laboratory values were not normally distributed, the median and interquartile range (IQR) were reported, and comparisons were generated using Wilcoxon rank sum for continuous variables and Fisher exact test or Χ2 analysis for categorical data. Strength of correlations was determined using Pearson correlation coefficients. P ≤ 0.05 was considered statistically significant. In cases where data (eg, laboratory value) were missing or unavailable, the patient visit was excluded from the relevant analysis.
RESULTS
There were a total of 308,639 ED visits over the 6-year study period; a serum or whole-blood glucose concentration was measured at 55,120 (17.9%) of these visits. Eighty-one patient visits had an initial glucose concentration of 300 mg/dL (16.7 mmol/L) or greater in the absence of known or correctly newly diagnosed DM. Of these, 9 patient visits were excluded from analysis—4 for erroneous laboratory values and 5 for previously documented hypoglycemia before arrival in the ED. Therefore, there were 72 patient visits with ESH, yielding an overall cumulative incidence of 0.13% (95% confidence interval [CI], 0.10%–0.16%) among all patients who had blood glucose concentration determined in the ED.
Blood glucose concentrations ranged from 300 to 1059 mg/dL (16.7–58.8 mmol/L), with a median concentration of 343 mg/dL (19.0 mmol/L) and IQR of 324 to 401 mg/dL (18.0–22.3 mmol/L). The median age of patients with ESH was 8.8 years (IQR, 2.6–13.9 years); 63% were male, and 56% were white (Table 1). For all patients with glucose concentrations measured in the ED during this same period, the median age was 5.7 years; 51% were male, and 54% were white. The proportion of male patients was significantly higher in patients with ESH than expected from the overall population (P = 0.047), but there was no difference in age (P = 0.067) or racial/ethnic diversity (P = 0.375).
TABLE 1.
Demographics
| All Patients (n = 72) |
Survivors (n = 56) |
Nonsurvivors (n = 16) |
P* | ||
|---|---|---|---|---|---|
| Age,† y | 8.8 (2.6–13.9) | 7.7 (2.0–13.9) | 9.7 (4.8–13.7) | 0.448 | |
| Sex, n (%) male | 45 (63) | 37 (66) | 8 (50) | 0.258 | |
| Race, n (%) | |||||
| White | 40 (56) | 32 (57) | 8 (50) | ||
| Black | 13 (18) | 10 (18) | 3 (19) | ||
| Hispanic | 6 (8) | 6 (11) | 0 (0) | 0.512 | |
| Asian | 2 (3) | 1 (2) | 1 (6) | ||
| Other | 2 (3) | 1 (2) | 1 (6) | ||
| Unknown | 9 (12) | 6 (10) | 3 (19) | ||
Comparison of survivors versus nonsurvivors.
Median (IQR).
The most common diagnosis in patients with ESH was respiratory illness, accounting for 35 (49%) of the patient visits. Asthma alone was diagnosed in 22 (31%) of patients, with 5 patients (7%) having pneumonia, one (1%) each with bronchiolitis and croup, and 6 (8%) with other respiratory illnesses. Trauma, mostly due to motor vehicle collisions, was the next most common diagnosis, representing 12 patients (17%). Seven patients presented with neurological disorders, with seizure accounting for 5 of these, or 7% of the total. Other diagnoses are reported in Table 2. Sixty-five percent of patients with ESH had received 1 or more glucose-influencing medical interventions before glucose measurement (Table 2). This was particularly true for patients with respiratory illness (31/35 patients; 89%), who were often treated with β-agonists and/or steroids, but was uncommon in trauma and neurological disorders.
TABLE 2.
Diagnoses, Glucose-Influencing Medical Therapy, and Mortality
| Diagnosis | Frequency, n (% of Total Cohort) |
Glucose-Influencing Therapy, n (% of Diagnostic Group) |
Disease-Specific Mortality, n (% of Diagnostic Group) |
|---|---|---|---|
| Respiratory illness | 35 (49) | 31 (89) | 2 (6) |
| Asthma | 22 (31) | 22 (100) | 1 (5) |
| Pneumonia | 5 (7) | 3 (60) | 1 (20) |
| Bronchiolitis | 1 (1) | 1 (100) | 0 (0) |
| Other respiratory illness | 7 (10) | 5 (71) | 0 (0) |
| Cardiovascular | 4 (5) | 4 (100) | 4 (100) |
| Gastrointestinal | 3 (4) | 1 (33) | 1 (33) |
| Dehydration | 2 (3) | 1 (50) | 0 |
| Infectious | 3 (4) | 2 (67) | 0 |
| Oncologic | 2 (3) | 2 (100) | 0 |
| Neurologic | 7 (10) | 2 (29) | 3 (43) |
| Seizure | 5 (7) | 1 (20) | 1 (20) |
| Other neurologic illness | 2 (3) | 1 (50) | 1 (50) |
| Trauma | 12 (17) | 3 (25) | 6 (50) |
| Toxic exposure | 4 (5) | 1 (25) | 0 (0) |
| Total | 72 (100) | 47 (65) | 16 (22) |
This cohort of patients with ESH exhibited a high severity of illness, with 85% described as “somewhat ill” or “toxic appearing” in the ED physician’s initial assessment. Excluding the 4 patients who died in the ED, 67 of 68 patients were admitted to the hospital, with 40 (60%) admitted to the intensive care unit (ICU). Median LOS in the hospital was 5.0 days (IQR, 3.0–8.0 days). Among the 49 patients for whom a blood gas analysis was performed, acidemia (arterial pH <7.35 or venous pH <7.30) was noted at 53% of the patient visits, with a median pH 7.30 (IQR, 7.16–7.39). Table 3 shows laboratory data for all patients, as well as for survivors and nonsurvivors separately. Only pH and platelet count were significantly different between survivors and nonsurvivors.
TABLE 3.
Laboratory Characteristics*
| All Patients (n = 72) | Survivors (n = 56) | Nonsurvivors (n = 16) | P† | |
|---|---|---|---|---|
| Glucose, mg/dL‡ | ||||
| n (% of total) | 72 (100) | 56 (100) | 16 (100) | |
| Median (IQR) | 343 (324–401) | 340 (323–385) | 386 (328–473) | 0.14 |
| pH | ||||
| n (% of total) | 49 (68) | 34 (61) | 15 (94) | |
| Median (IQR) | 7.30 (7.16–7.39) | 7.33 (7.25–7.39) | 7.17 (6.84–7.36) | 0.009 |
| tCO2, mmol/L | ||||
| n (% of total) | 64 (89) | 50 (89) | 14 (88) | |
| Median (IQR) | 18.5 (15–21) | 19 (15.8–21.0) | 16.5 (7.5–21.8) | 0.494 |
| PCO2, mm Hg | ||||
| n (% of total) | 48 (67) | 34 (61) | 14 (88) | |
| Median (IQR) | 42.5 (35.0–57.9) | 41.5 (34.8–54.5) | 56.0 (34.8–102.8) | 0.225 |
| WBC (×103/µL) | ||||
| n (% of total) | 66 (92) | 50 (89) | 16 (100) | |
| Median (IQR) | 12.6 (9.9–18.4) | 12.5 (9.9–19.7) | 13.0 (9.9–17.2) | 0.675 |
| Hematocrit, % | ||||
| n (% of total) | 66 (92) | 50 (89) | 16 (100) | |
| Median (IQR) | 35.2 (30.8–39.5) | 35.1 (31.9–39.2) | 35.7 (28.4–42.3) | 0.928 |
| Platelets, ×103/µL | ||||
| n (% of total) | 64 (89) | 48 (86) | 16 (100) | |
| Median (IQR) | 277 (195–385) | 285 (217–418) | 159 (105–301) | 0.010 |
| BUN,‡ mg/dL | ||||
| n (% of total) | 62 (86) | 48 (86) | 14 (88) | |
| Median (IQR) | 12 (9–19) | 11 (8–18) | 13 (11–21) | 0.307 |
| Creatinine,‡ mg/dL | ||||
| n (% of total) | 36 (50) | 27 (48) | 9 (56) | |
| Median (IQR) | 0.7 (0.5–1.0) | 0.7 (0.5–1.0) | 0.6 (0.6–0.9) | 0.927 |
| Urinalysis | ||||
| n (% of total) | 38 (53) | 30 (54) | 8 (50) | |
| Ketones ≥2+ | 13% | 13% | 13% | 0.721 |
| Glucose ≥2+ | 42% | 47% | 25% | 0.426 |
Values calculated from those patients who had laboratory values measured; not every patient had all laboratory tests.
Comparison of survivors versus nonsurvivors.
To convert glucose from mg/dL to mmol/L, multiply by 0.0555; to convert creatinine to µmol/L, multiply by 88.3; to convert BUN to mmol/L, multiply by 0.357.
tCO2 indicates total serum CO2 concentration; WBC, white blood cell count; PCO2, partial pressure of carbon dioxide; BUN, blood urea nitrogen.
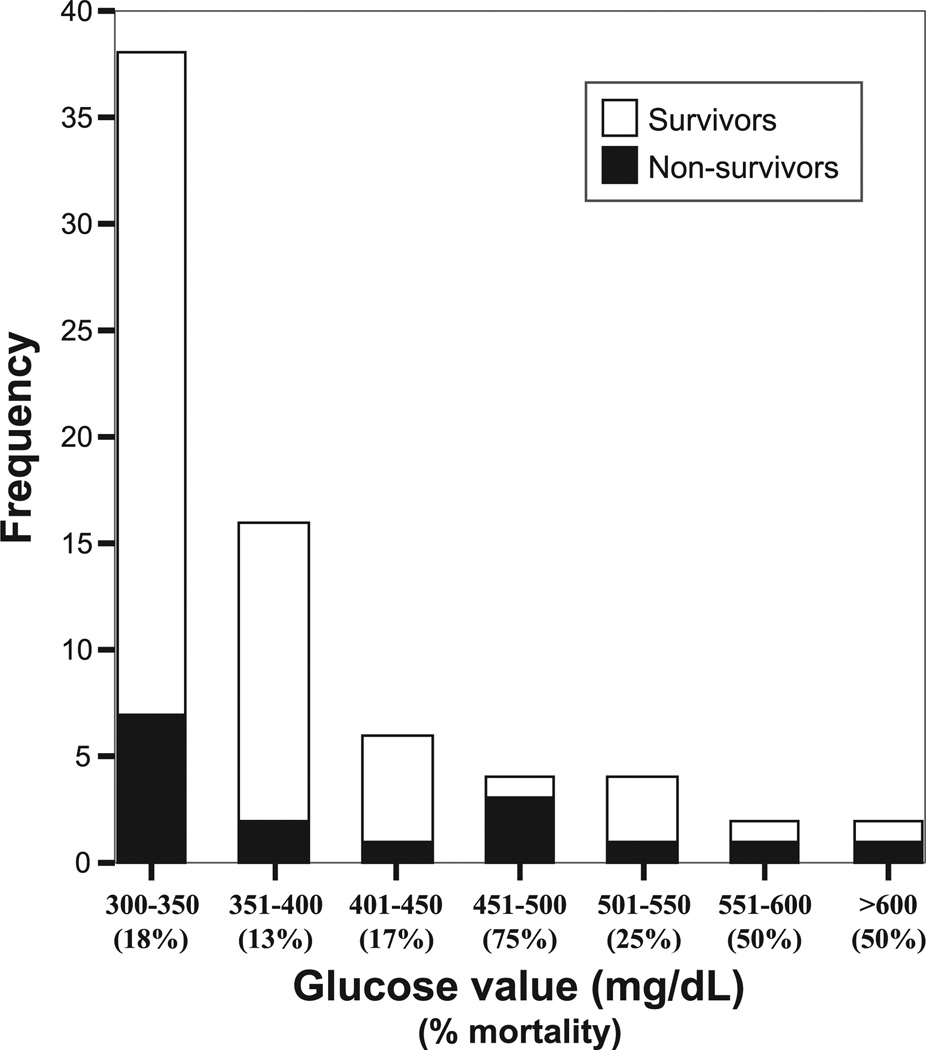
The mortality rate for all 72 patients with ESH was 22% (Table 2), with the highest rates in those with cardiac arrest (100%) and trauma (50%). Only 2 patients with respiratory illnesses died, although 64% of asthmatics were admitted to the ICU, suggesting that severity of illness remained high. Four patients died in the ED, with the other 12 deaths occurring after admission to the ICU. Blood glucose concentration was significantly correlated with mortality (r = 0.245, P = 0.04), with incrementally higher glucose values associated with increased risk of death (Fig. 1). Compared with the 17% mortality rate (9/54) in patients with glucose concentrations of 300 to 399 mg/dL (16.7–22.1 mmol/L), those with glucose concentrations 400 mg/dL (22.2 mmol/L) or greater had a mortality rate of 39% (7/18; odds ratio, 3.2; 95% CI, 1.0–10.4).
FIGURE 1.
Distribution of glucose concentrations with number of nonsurvivors (shaded portion of each bar). Note the higher proportion of nonsurvivors as glucose concentration increased (mortality rate of 17% for 300–399 mg/dL vs 39% for ≥400 mg/dL, P = 0.05).
Thirty-eight patients (53%) had a urinalysis performed. Despite the extreme hyperglycemia, only 42% of these patients had glucosuria, and 13% had ketonuria (Table 3). Furthermore, biochemical characteristics diagnostic for diabetic ketoacidosis (DKA) were also rare despite acidemia being common. (Biochemical criteria for DKA were defined as hyperglycemia with concurrent arterial pH <7.35 or venous pH <7.30 or total serum carbon dioxide concentration <15 mmol/L and at least 2+ ketonuria.25) Of the 37 patients who had a blood gas analysis or total serum carbon dioxide concentration and a urinalysis available, only one of these patients—diagnosed and treated for asthma—would have met all laboratory criteria for DKA. This patient reported no preceding symptoms of diabetes, such as polyuria, polydipsia, or weight loss, and ultimately was not diagnosed with DM. Overall, no patients were incorrectly diagnosed with DM in the ED.
Only 8 patients (11%) received therapy specifically directed at controlling hyperglycemia—6 were treated with insulin, and 2 others were given intravenous fluids without dextrose. Despite infrequent treatment, 32 (67%) of the 48 patients who had serial serum or whole-blood glucose measurements during their hospitalization had normalization of glucose concentration to 150 mg/dL (8.3 mmol/L) or less within 2 days. All 15 patients in whom hyperglycemia of greater than 150 mg/dL (8.3 mmol/L) persisted beyond 2 days received corticosteroids—11 for acute bronchospasm, and 4 were continued on long-term corticosteroid therapy for chronic illness. Three of the 6 patients who received insulin developed hypoglycemia (range, 44–68 mg/dL [2.4–3.7 mmol/L]) after initiation of insulin.
Forty-seven (84%) of the surviving 56 patients were seen for at least 1 subsequent visit, and 7 (13%) were seen in the institution’s outpatient endocrine clinic. Twenty-three percent experienced at least 1 episode of hyperglycemia greater than 150 mg/dL (8.3 mmol/L) at a future visit; 4 had recurrent hyperglycemia greater than 300 mg/dL (16.7 mmol/L). HbA1c levels were measured in 3 patients during their hospitalization and were 5.9%, 8.2%, and 9.7% (laboratory reference range, 4.0%–6.0%). The 2 patients with elevated HbA1c were both receiving chronic medications known to alter glucose metabolism, resulting in prolonged hyperglycemia. Pancreatic autoantibodies were measured at 2 ED visits and were positive in one patient who was thought to have “insulinitis” or “pre-DM” given the presence of pancreatic autoantibodies in the setting of hyperglycemia without meeting diagnostic criteria for DM.26 No patients underwent an oral glucose tolerance test.
Over the 4 to 10 years since their ED visit, only 1 of the 47 patients followed up at this institution was subsequently diagnosed with DM, and this was determined to be steroid induced rather than type 1 or 2 DM. Five patients received other endocrinologic diagnoses at follow-up visits: 1 patient each with insulinitis or pre-DM (without progression to DM after 4 years of follow-up), insulin resistance (elevated insulin level with normal glucose concentration) in the setting of obesity, diabetes insipidus, hypothyroidism with diabetes insipidus, and benign pubertal gynecomastia.
DISCUSSION
Although stress hyperglycemia is well recognized in clinical practice, extreme elevations of glucose in the absence of diabetes are unusual.9–11,15 This is the first study to examine ESH, defined here as blood glucose concentration of 300 mg/dL (16.7 mmol/L) or greater, in a nonreferral, heterogeneous population of patients presenting to a pediatric ED. This threshold was chosen based on the relative paucity of literature available on stress hyperglycemic responses beyond 300 mg/dL (16.7 mol/L) in the ED setting and the resulting difficulty for acute care clinicians in deciding how best to interpret and manage this laboratory finding, as well as exclude the diagnosis of a novel presentation of diabetes. Our primary goal was to document the occurrence of extreme elevations in glucose concentration during acute illness, understand its significance, and describe its natural history. We found that, although rare, ESH does occur in the pediatric ED setting, with an incidence of 0.13% of the 55,120 patients who had a blood glucose concentration measured.
Consistent with prior studies of stress hyperglycemia,2,9–11,15,19 ESH was documented to occur in a wide variety of pediatric diagnoses routinely seen in the ED, with respiratory illness, seizure, and trauma being the most common. Although it was not feasible to differentiate between stress-mediated and iatrogenic hyperglycemia in this study as timing of laboratory assessment and therapeutic interventions was not controlled, preceding medical therapy is likely to have influenced the degree of hyperglycemia in most cases. This was especially true in patients with respiratory illness, who are routinely treated with corticosteroids and β-agonists, both of which stimulate glycogenolysis and gluconeogenesis.1,7,8 However, given that most patients with severe asthma exacerbations do not experience ESH despite similar medical therapies,27 there were likely also significant contributions from each individual’s stress response to their illness, underlying glucose homeostatic mechanisms, relative insulin reserve or sensitivity, and degree of dehydration and renal perfusion. Surprisingly, a disproportionate percentage of patients with ESH were male. To our knowledge, an association of male sex with stress hyperglycemia has not been reported previously. However, childhood asthma28 and trauma29 both have a male predominance, and the large number of patients with asthma and trauma (61% of whom were male) in our study may have contributed to the sex discrepancy observed with ESH.
We found that ESH, like more mild levels of stress hyperglycemia,9–11 remains a transient physiological response that tends to improve rapidly with treatment of the primary illness. Although very few of the patients in this study were treated with insulin or other therapies directed at controlling hyperglycemia, glucose concentrations decreased to 150 mg/dL (8.3 mmol/L) or less within 2 days of hospitalization in two thirds of patients who had serial glucose measurements determined. Only 15 patients had persistent hyperglycemia beyond the second day of hospitalization, all of whom were treated with corticosteroids. This is consistent with the findings of a prior study by Gupta et al,11 who reported that all pediatric ED patients with stress hyperglycemia experienced a decrease in glucose concentration to less than 150 mg/dL (8.3 mmol/L) within 24 hours without receiving insulin. Therefore, patients with ESH who are not on prolonged corticosteroid treatment may not require any therapy other than rehydration and adequate treatment of the primary disease process to lower blood glucose concentration to more acceptable levels. Furthermore, the addition of insulin for the purpose of restoration of normoglycemia seems unnecessary in most patients and, given that half of those treated with insulin in our study experienced at least 1 episode of hypoglycemia (glucose <70 mg/dL [3.9 mmol/L]), is not without risk. Whether initiation of insulin therapy could have improved clinical outcomes is an area of current controversy20,21,30–33—particularly in pediatrics23,34—and was beyond the scope of this study.
Although we hypothesized that ESH would occur simply as the far end of a spectrum of stress hyperglycemic responses, it is noteworthy that the severity of illness was also particularly high in these patients, as illustrated by the large percentage with “ill” or “toxic” clinical appearance, high admission rate to the ICU, frequency of acidemia, long LOS, and high overall mortality rate of 22%. These findings are in agreement with prior studies reporting hyperglycemia to be common in critically ill children in the ICU.13,14,25 However, reports on the association of stress hyperglycemia with severity of illness in the more heterogeneous ED population have been less robust10,11 and have not shown a correlation with mortality. Bhisitkul et al10 reported a higher prevalence of glucose concentrations 150 mg/dL (8.3 mmol/L) or greater in pediatric ED patients who had fever greater than 39.5°C, required intravenous fluids, and were admitted to the ICU, but did not report on mortality. In contrast, Gupta et al11 found that hyperglycemia was not associated with severity of illness in pediatric ED patients with glucose concentrations 150 mg/dL (8.3 mmol/L) or greater, and although those with stress hyperglycemia had a 2-fold higher mortality rate than nonhyperglycemic patients, the difference was not statistically significant (odds ratio, 2.17; 95% CI, 0.81–5.82). Our study suggests that, even in the ED, extreme elevations in glucose concentration greater than 300 mg/dL (16.7 mmol/L) can identify a subgroup of patients with severe illness and may be an early indicator of increased risk of mortality. Furthermore, we found a correlation between degree of hyperglycemia and likelihood of death, with disproportionate mortality observed at glucose values greater than 400 mg/dL (22.2 mmol/L). Such an increase in mortality rate with progressive degrees of stress hyperglycemia in pediatric patients has also been reported by others.12,13,24 Notably, for patients with respiratory illnesses, ESH was associated with a high severity of illness but not increased mortality—perhaps because of the higher likelihood of an iatrogenic component. Thus, there may be fewer implications of ESH for mortality risk in this patient group than for other presenting illnesses.
No patients in this study were misdiagnosed with new-onset DM or DKA despite diabetes being more common in patients with extreme hyperglycemia overall. One reason for this may have been that, despite glucose concentrations 300 mg/dL (16.7 mmol/L) or greater, few patients with ESH exhibited ketonuria or glucosuria. This is consistent with a prior study that found ketonuria and glucosuria in only 12.5% of pediatric ED patients with stress hyperglycemia 150 mg/dL (8.3 mmol/L) or greater11 and suggests that limitation of urinary glucose excretion due to dehydration might be an important factor in the development of ESH.6 In addition, only 1 patient in our study met criteria for a diagnosis of DKA based on laboratory evaluation, and no patients had preceding clinical symptoms of DM. Therefore, although severe hyperglycemia should continue to raise suspicion for diabetes, this study suggests that ESH may be differentiated from concurrent new-onset DM on the basis of clinical symptoms and supporting laboratory values.
Finally, although glucose metabolism is known to be transiently altered during acute illness,1–4 some have raised concern that stress hyperglycemia could also represent the initial manifestation of pancreatic β-cell dysfunction that may herald the subsequent development of DM.16,17 In our study, a quarter of patients experienced a subsequent episode of transient hyperglycemia. However, no patients were diagnosed with type 1 or 2 DM on follow-up, although 1 patient did develop corticosteroid-induced diabetes and remained insulin dependent. This is consistent with several prior studies that have reported the risk of subsequent DM to be low after an episode of transient hyperglycemia in the presence of severe systemic illness9,10,18,19 For instance, in a prospective study by Herskowitz-Dumont et al,9 32% of children with hyperglycemia in the absence of a serious illness developed subsequent DM, whereas only 2.3% (1 of 44 children) of those with stress-related hyperglycemia did so. Furthermore, all those who developed DM did so within 18 months of their initial hyperglycemic episode,9 signifying that our follow-up duration of 4 to 10 years in the current study was appropriate to detect those who were likely to go on to develop DM. Thus, our results further support that even ESH is unlikely to increase risk for the development of DM.
Several important limitations of our study are worth noting. First is that because only one fifth of the ED patients had a glucose concentration measured, the 0.13% incidence is representative of only a subset of the ED patient population over the entire study period. Second, our use of the initial glucose value may have underestimated the true incidence of ESH in acute illness, as it has been observed that maximum glucose values within 24 hours of presentation often exceed the initial glucose value.12 However, the utility of the initial glucose level as an early marker of mortality has implications for triage and patient disposition, especially in the ED where serial laboratory values are typically not available. In the absence of a control group without ESH, our findings also cannot be used to demonstrate causality and do not differentiate between an association of ESH with severity of illness versus mortality. Finally, only 84% of our patients had follow-up data available because chart review was limited to a single institution, and we did not have approval to contact patients for research purposes alone. It is therefore possible that some of the patients received a diagnosis of DM at a separate institution that is not reflected in our chart review or will go on to develop DM at a time beyond the interval of follow-up of this study.
In summary, acute care physicians should be aware that, although a rare occurrence, extreme hyperglycemic responses of 300 mg/dL (16.7 mmol/L) or greater do occur in acute pediatric illness in the absence of diabetes. This is most common in respiratory illness, trauma, and neurological disorders and, in most cases, is at least partially influenced by preceding medical therapy. Notably, ESH is associated with a high severity of illness and may be an early marker for increased risk of mortality. While consideration for DM is important, the absence of preceding symptoms suggestive of DM and further laboratory testing should be reassuring to exclude this diagnosis. The natural history of ESH is a return to normoglycemia, usually within 24 to 48 hours of presentation, and, in most patients, this occurs with hydration and treatment of the primary disease process alone without the need for insulin. Importantly, there does not seem to be an increased risk for the subsequent development of DM, although a study with more definitive follow-up is necessary to fully answer this question. Further study is also necessary to determine if ESH is an independent determinant of mortality and whether early initiation of insulin therapy would improve clinical outcomes.
ACKNOWLEDGMENTS
The authors thank Garry Steil, PhD, for his comments on the preparation of this article.
Financial support was provided by the Medicine Critical Care Program, Department of Medicine, Children’s Hospital Boston, Harvard Medical School, Boston, MA.
REFERENCES
- 1.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–756. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 2.Chernow B, Rainey T, Heller R, et al. Marked stress hyperglycemia in a child. Crit Care Med. 1982;10:696–697. doi: 10.1097/00003246-198210000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Rayfield EJ, Curnow RT, George DT, et al. Impaired carbohydrate metabolism during a mild viral illness. N Engl J Med. 1973;289:618–620. doi: 10.1056/NEJM197309202891207. [DOI] [PubMed] [Google Scholar]
- 4.Rocha DM, Santeusanio F, Faloona GR, et al. Abnormal pancreatic alpha-cell function in bacterial infections. N Engl J Med. 1973;288:700–703. doi: 10.1056/NEJM197304052881402. [DOI] [PubMed] [Google Scholar]
- 5.Black PR, Brooks DC, Bessey PQ, et al. Mechanisms of insulin resistance following injury. Ann Surg. 1982;196:420–433. doi: 10.1097/00000658-198210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware SD, Tamborlane WV. Not all severe hyperglycemia is diabetes. Pediatrics. 1992;89:330–332. [PubMed] [Google Scholar]
- 7.Smith AP, Bank J, Buchana K, et al. Mechanisms of abnormal glucose metabolism during the treatment of acute severe asthma. Q J Med. 1992;82:71–80. [PubMed] [Google Scholar]
- 8.Dawson KP, Penna AC, Manglick P. Acute asthma, salbutamol and hyperglycaemia. Acta Paediatr. 1995;84:305–307. doi: 10.1111/j.1651-2227.1995.tb13633.x. [DOI] [PubMed] [Google Scholar]
- 9.Herskowitz-Dumont R, Wolfsdorf JI, Jackson RA, et al. Distinction between transient hyperglycemia and early insulin-dependent diabetes mellitus in childhood: a prospective study of incidence and prognostic factors. J Pediatr. 1993;123:347–354. doi: 10.1016/s0022-3476(05)81731-7. [DOI] [PubMed] [Google Scholar]
- 10.Bhisitkul DM, Morrow AL, Vinik AI, et al. Prevalence of stress hyperglycemia among patients attending a pediatric emergency department. J Pediatr. 1994;124:547–551. doi: 10.1016/s0022-3476(05)83132-4. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, Natarajan G, Agarwal KN. Transient hyperglycemia in acute childhood illnesses: to attend or ignore. Indian J Pediatr. 1997;64:205–210. doi: 10.1007/BF02752447. [DOI] [PubMed] [Google Scholar]
- 12.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 13.Wintergerst KA, Buckingham B, Gandrud L, et al. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatr. 2006;118:173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 14.Latorre HA, Drash A. Stress hyperglycemia re development of diabetes. J Pediatr. 1991;118:827–828. doi: 10.1016/s0022-3476(05)80065-4. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson RE, Bowyer FP. Hyperglycemia with hyperosmolal dehydration in nondiabetic infants. J Pediatr. 1970;77:818–823. doi: 10.1016/s0022-3476(70)80241-4. [DOI] [PubMed] [Google Scholar]
- 16.Herskowitz RD, Wolfsdorf JI, Ricker AT, et al. Transient hyperglycemia in childhood: identification of a subgroup with imminent diabetes mellitus. Diabetes Res. 1988;9:161–167. [PubMed] [Google Scholar]
- 17.Vardi P, Shehade N, Etzioni A, et al. Stress hyperglycemia in childhood: a very high risk group for the development of type I diabetes. J Pediatr. 1990;117:75–77. doi: 10.1016/s0022-3476(05)82447-3. [DOI] [PubMed] [Google Scholar]
- 18.Schatz DA, Kowa H, Winter W, et al. Natural history of incidental hyperglycemia and glycosuria of childhood. J Pediatr. 1989;115:676–680. doi: 10.1016/s0022-3476(89)80641-9. [DOI] [PubMed] [Google Scholar]
- 19.Shehadeh N, On A, Kessel R, et al. Stress hyperglycemia and the risk for the development of type I diabetes. J Pediatr Endocr Metab. 1997;10:283–286. doi: 10.1515/jpem.1997.10.3.283. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 22.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–38. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- 23.Klein GW, Hojsak JM, Schmeidler J, et al. Hyperglycemia and outcome in the pediatric intensive care unit. J Pediatr. 2008;153:379–384. doi: 10.1016/j.jpeds.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan F, Spinella PC, Drott HR, et al. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 25.Wolfsdorf J, Craig ME, Daneman D, et al. ISPAD Clinical Practice Consensus Guidelines 2006–2007: diabetic ketoacidosis. Pediatr Diabetes. 2007;8:28–43. [Google Scholar]
- 26.Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 27.Meert KL, Clark J, Sarnaik AP. Metabolic acidosis as an underlying mechanism of respiratory distress in children with severe acute asthma. Pediatr Crit Care Med. 2007;8:519–523. doi: 10.1097/01.PCC.0000288673.82916.9D. [DOI] [PubMed] [Google Scholar]
- 28.Weiss ST, Gold DR. Gender differences in asthma. Pediatr Pulmonol. 1995;19:153–155. doi: 10.1002/ppul.1950190302. [DOI] [PubMed] [Google Scholar]
- 29.Bowman SM, Bird TM, Aitken ME, et al. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatr. 2008;122:988–993. doi: 10.1542/peds.2007-3511. [DOI] [PubMed] [Google Scholar]
- 30.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 31.NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. NEJM. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 32.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control with insulin in the ICU: facts and controversies. Chest. 2007;132:267–278. doi: 10.1378/chest.06-3121. [DOI] [PubMed] [Google Scholar]
- 33.Weiner RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 34.Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]