Abstract
Introduction
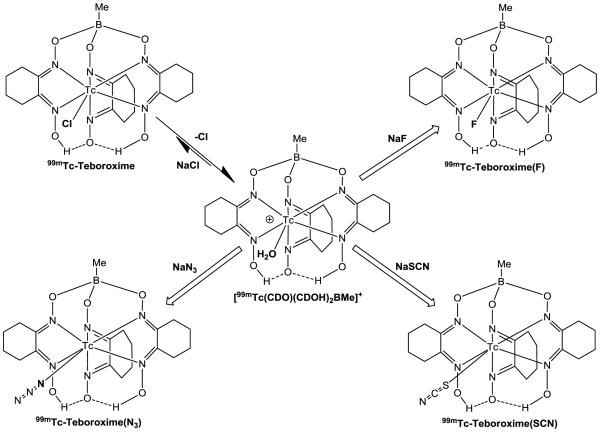
99mTc-Teboroxime ([99mTcCl(CDO)(CDOH)2BMe]) is a member of the BATO (boronic acid adducts of technetium dioximes) class of 99mTc(III) complexes. This study sought to explore the impact of co-ligands on solution stability, heart uptake and myocardial retention of [99mTc(L)(CDO)(CDOH)2BMe] (99mTc-Teboroxime: L = Cl; 99mTc-Teboroxime(F): L = F; 99mTc-Teboroxime(SCN): L = SCN; and 99mTc-Teboroxime(N3): L = N3).
Methods
Radiotracers 99mTc-Teboroxime(L) (L = F, SCN and N3) were prepared by reacting 99mTc-Teboroxime with NaF, NaSCN and NaN3, respectively. Biodistribution and imaging studies were carried out in Sprague-Dawley rats. Image quantification was performed to compare their heart retention and liver clearance kinetics.
Results
Complexes 99mTc-Teboroxime(L) (L = F, SCN and N3) were prepared in high yield with high radiochemical purity. All new radiotracers were stable for >6 h in the kit matrix. In its HPLC chromatogram, 99mTc-Teboroxime showed one peak at ~15.5 min, which was shorter than that of 99mTc-Teboroxime(F) (~16.4 min). There were two peaks for 99mTc-Teboroxime(SCN) at 16.5 and 18.3 min. 99mTc-Teboroxime(N3) appeared as a single peak at 18.4 min. Their heart retention and liver clearance curves were best fitted to the bi-exponential decay function. The half-times of fast/slow components were 1.6 ± 0.4/60.7±8.9 min for 99mTc-Teboroxime, 0.8±0.2/101.7±20.7 min for 99mTc-Teboroxime(F), 1.2±0.3/84.8±16.6 min for 99mTc-Teboroxime(SCN), and 2.9±0.9/51.6±5.0 min for 99mTc-Teboroxime(N3). The 2-min heart uptake followed the order of 99mTc-Teboroxime (3.00±0.37%ID/g) > 99mTc-Teboroxime(N3) (2.66±0.01 %ID/g) ≈ 99mTc-Sestamibi (2.55±0.46 %ID/g) > 99mTcN-MPO (2.38±0.15 %ID/g). 99mTc-Teboroxime remains the best in first-pass extraction. The best image acquisition window is 0 – 5 min for 99mTc-Teboroximine and 0 – 15 min for 99mTc-Teboroximine(N3).
Conclusion
Co-ligands had significant impact on the heart uptake and myocardial retention of complexes [99mTc(L)(CDO)(CDOH)2BMe] (L = Cl, F, SCN and N3). Future studies should be directed towards minimizing the liver uptake and radioactivity accumulation in the blood vessels while maintaining their high heart uptake.
Keywords: 99mTc-Teboroxime derivatives, 99mTc radiotracers, heart imaging
INTRODUCTION
Myocardial perfusion imaging (MPI) with radiotracers is an integral component in evaluation of patients with known or suspected coronary artery disease (CAD) [1-10]. MPI with SPECT (single photon-emission computed tomography) or PET (positron emission tomography) radiotracers remains the only imaging modality available for assessment of physiological consequence of coronary stenosis or myocardial infarction [9]. More than 8-9 million SPECT MPI studies performed in the United States alone 2010 [10]. Both 99mTc-Sestamibi ([99mTc(MIBI)6]+; MIBI = 2-methoxy-2-methylpropylisonitrile) and 99mTc-Tetrofosmin ([99mTcO2(tetrofosmin)2]+, tetrofosmin = 1,2-bis[bis(2-ethoxyethyl)phosphino]ethane)) are most widely used radiotracers for MPI [11-18]. The overwhelming success of SPECT MPI is, in large part, attributed to their widespread clinical applications. It is believed that SPECT MPI will continue to play a major role for nuclear cardiology for many years ahead.
A significant drawback of 99mTc-Sestamibi and 99mTc-Tetrofosmin is their low first-pass extraction [15-18]. In contrast, 99mTc-Teboroxime (Figure 1: [99mTcCl(CDO)(CDOH)2BMe]; CDOH2 = cyclohexanedione dioxime), is a member of the BATO (boronic acid adducts of technetium dioximes) class of 99mTc(III) complexes [19-21], has the highest first-pass extraction fraction among all 99mTc perfusion radiotracers [22-30]. There is an excellent linear relationship between its heart uptake and blood flow over a wide range (0 – 4.5 mL/min/g) [24,25,27,28]. High first-pass extraction and linear relationship between the blood flow and radiotracer heart uptake permit better detection of the presence and extent of coronary disease, and more precise delineation of perfusion defects, which is of considerable benefit in the management of patients with known or suspected CAD and assessing risk of future cardiac events (e.g. myocardial infarction and sudden death). Despite this advantage, its initial clinical experience was disappointing due to its short myocardial residence time. More than 60% of the initial heart radioactivity is washed out within 5 min post-injection (p.i.) [28-30], which is too fast for standard SPECT cameras to acquire high-quality images. As a result, 99mTc-Teboroxime was withdrawn from the market even though it was the first FDA-approved radiotracer for MPI. However, the recent developments in high-speed cardiac SPECT cameras make it possible to use 99mTc-Teboroxime for SPECT MPI due to its high first-pass extraction fraction [31].
Figure 1.
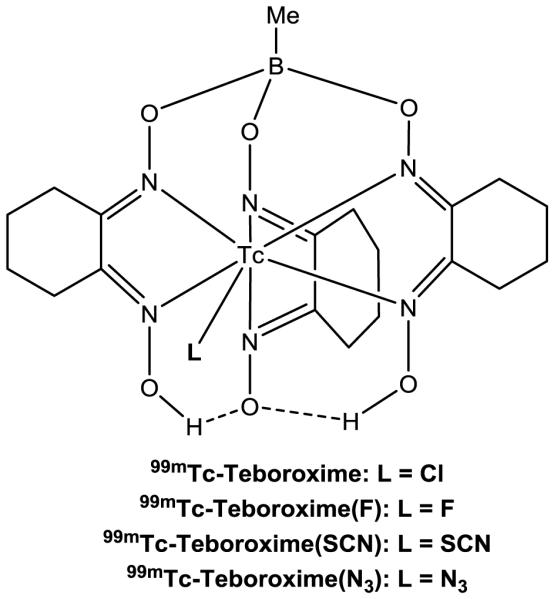
Structures of 99mTc(III) radiotracers [99mTc(L)(CDO)(CDOH)2BMe] (99mTc-Teboroxime: L = Cl; 99mTc-Teboroxime(F): L = F; 99mTc-Teboroxime(SCN): L = SCN; and 99mTc-Teboroxime(N3): L = N3). 99mTc-Teboroxime was the first FDA-approved radiotracer for MPI; but it was later withdrawn from the market due to its fast washout from heart. 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3) are new radiotracers evaluated in this study.
The short myocardial retention of 99mTc-Teboroxime has been attributed to rapid dissociation of the Cl ligand and its hydrolysis in vivo [19,20]. It was reported that the half-life of Cl− anion was ~13 min under physiological conditions (pH = 7.4) [20]. If this is true, then the myocardial retention of a radiotracer might be improved by increasing its solution stability. With this in mind, we prepared new 99mTc(III) complexes [99mTc(L)(CDO)(CDOH)2BMe] (99mTc-Teboroxime(F): L = F; 99mTc-Teboroxime(SCN): L = SCN; and 99mTc-Teboroxime(N3): L = N3). Thiocyanate and azide anions are used since soft donors (S and N) are expected to form strong bonding with 99mTc(III). F− is known to form stable bonds with trivalent metals, such as Al(III) [32-39]. Our hypothesis was that the formation of stable 99mTc(III)-L bonds would prevent hydrolysis of 99mTc(III) radiotracers. In this report, we present the synthesis and evaluation of 99mTc(III) complexes [99mTc(L)(CDO)(CDOH)2BMe] (L = F, SCN and N3) for their potential as heart imaging agents. The objective was to explore the impact of co-ligands on both heart uptake and myocardial retention times of 99mTc(III) radiotracers. Our long-term goal is to develop a 99mTc radiotracer with a longer myocardial retention than that of 99mTc-Terboroxime while maintaining the high heart uptake. The high heart uptake in combination with long myocardial retention makes possible to collect sufficient radioactivity counts using both the standard and ultrafast cardiac SPECT cameras for MPI studies in the future.
EXPERIMENTAL METHODS
Materials
Citric acid, γ-cyclodextrin (γ-CD), cyclohexanedione dioxime (CDOH), diethylenetriaminepentaacetic acid (DTPA), 1,2-diaminopropane-N,N,N’,N’-tetraacetic acid (PDTA), methylboronic acid, sodium chloride, sodium fluoride, sodium azide, sodium thiocyanate, and stannous chloride dihydrate (SnCl2·2H2O) were purchased from Sigma/Aldrich (St. Louis, MO), and were used without further purification. 99mTcN-MPO([99mTcN(mpo)(PNP5)]+: PNP5 = N-ethoxyethyl-N,N-bis[2-(bis(3-methoxypropyl)phosphino)ethyl]amine)) was prepared according to literature methods [40-44], and its radiochemical purity (RCP) was >95% before being used for biodistribution study. Na99mTcO4 were obtained from Cardinal HealthCare® (Chicago, IL). Cardiolite® vials were obtained as a gift from Lantheus Medical Imaging (formerly Bristol Myers Squibb Medical Imaging, N. Billerica, MA). 99mTc-Sestamibi was prepared according to the manufacturer’s package insert.
Radio-HPLC Method
The radio-HPLC method for routine analysis of 99mTc(III) complexes [99mTc(L)(CDO)(CDOH)2BMe] (L = F, SCN and N3) used the Agilent HP-1100 HPLC system (Agilent Technologies, Santa Clara, CA) equipped with a β-ram IN/US detector (Tampa, FL) and Zorbax C8 column (4.6 mm × 250 mm, 300 Å pore size; Agilent Technologies, Santa Clara, CA). The flow rate was 1 mL/min. The mobile phase was isocratic with 30% solvent A (10 mM NH4OAc buffer, pH = 6.8) and 70% solvent B (methanol) between 0 and 5 min, followed by a gradient from 70% solvent B at 5 min to and 90% solvent B at 15 min and continued till 20 min. The instant thin layer chromatography (ITLC) used Gelman Sciences silica-gel strips and a 1:1 mixture of acetone and saline as the mobile phase. 99mTc(III) complexes and 99mTcO4− migrated to solvent front while [99mTc]colloid stayed at the origin. [99mTc]colloid was reported as the percentage of radioactivity at the origin over the total radioactivity on each strip.
99mTc-Teboroxime
99mTc-Teboroxime was prepared using the kit formulation. Each lyophilized vial contains 2 mg of CDOH, 2 mg of methylboronic acid, 50 μg of SnCl2·2H2O, 9 mg of citric acid, 2 mg of DTPA, 100 mg of NaCl and 20 mg of γ-cyclodextrin. To a lyophilized vial was added 1.0 mL 99mTcO4− solution (10 – 30 mCi). The reconstituted vial was then heated at 100 °C for 10 – 15 min. After radiolabeling, the vial was allowed to stand at room temperature for 5 min. A sample of the resulting solution was analyzed by radio-HPLC. The RCP was 95 – 98% with minimal amount of [99mTc]colloid (<0.5%).
99mTc-Teboroxime(L) (L = F, SCN and N3). 99mTc-Teboroxime was prepared using the procedure above. After radiolabeling, part of the solution (0.3 – 0.5 mL) containing 5 – 15 mCi of 99mTc-Teboroxime was transferred into a sealed 5 mL vial, which contains sodium fluoride (2 – 5 mg), sodium azide (2 – 3 mg) or sodium thiocyanate (2 – 3 mg) dissolved in dissolved in 0.5 mL of 0.2 M phosphate buffer (pH = 7.4). The reconstituted vial was heated for 5 – 10 min at 100 °C. After the reaction, a sample of the resulting solution was diluted with saline containing ~20% propylene glycol to 500 μCi/mL, and was analyzed by radio-HPLC and ITLC. Their solution stability was monitored by HPLC at 0, 2, 4, and 6 h post-labeling.
Doses Preparation
Doses for biodistribution were prepared by dissolving radiotracer kit solution to ~1.1 MBq/mL with saline containing 20% propylene glycol. Propylene glycol was used to prevent extensive absorption of 99mTc radiotracers on the surfaces of glass vials or plastic syringe. The injection volume was 0.1 mL for each animal for biodistribution studies. Doses for imaging studies were made by dissolving the 99mTc radiotracer solution to ~370 MBq/mL with saline containing 20% propylene glycol. All dose solutions were filtered with a 0.20 micron filter unit to eliminate foreign particles before being injected into animals. The injection volume was ~0.1 mL per animal for biodistribution and 0.2 – 0.5 mL per animal for imaging studies.
Animal Preparation
Animal studies were conducted in compliance with the NIH animal experiment guidelines (Principles of Laboratory Animal Care, NIH Publication No. 86-23, revised 1985). The protocols for biodistribution and imaging studies were approved by the Purdue University Animal Care and Use Committee (PACUC). The SD rats (200 – 250 g) were purchased from Harlan (Indianapolis, IN), and were acclimated for more than 24 h. Animals were anesthetized with intramuscular injection of a mixture of ketamine (80 mg/kg) and xylazine (19 mg/kg) before being used for biodistribution and planar imaging studies.
Biodistribution
The SD rats (8 – 12 females and 8 – 12 males) were used for each 99mTc radiotracer. Each animal was administered with 100 – 111 KBq of 99mTc radiotracer dissolved in 0.1 mL saline containing 20% propylene glycol via the tail vein. Animals (4 to 6) were sacrificed by sodium pentobarbital overdose (100 – 200 mg/kg) at 2, 15, 30 and 60 min p.i. Blood was withdrawn from the heart. Organs of interest (heart, brain, lungs, liver, spleen, kidneys, muscle and intestines) were excised, rinsed with saline, dried with absorbent tissues, weighed and counted on a Perkin Elmer Wizard – 1480 γ-counter (Shelton, CT). Six extra doses were also weighed and counted before and after organ tissue samples. Organ uptake was calculated and reported as the percentage of injected dose per gram of wet mass (%ID/g).
Planar Imaging
Five SD rats (200 – 250 g) were used for planar imaging. Each animal was administered with 99mTc radiotracer (50 – 80 MBq) via the tail vein. The animal was placed prone on a single head mini γ-camera (Diagnostic Services Inc., NJ) equipped with a parallel-hole, low-energy, and high-resolution collimator. A standard radiation source was placed beside animal. Static images were acquired at 15, 30 and 60 min p.i. and were stored digitally in a 128 × 128 matrix. The count limits were set at 300 K. For dynamic imaging during first 5 min, the 1-min static images were acquired, followed by the 2-min static images at 6 – 30, 40, 50 and 60 min p.i.
After imaging, animals were returned to a lead-shielded cage for radiation decay. The images were analyzed by drawing regions of the heart and standard radiation source. The results were corrected for background. The results were expressed as a percentage of the initial activity. The exponential fit of the heart retention and liver clearance were determined using GraphPad Prim 5.0 (GraphPad Software, Inc., San Diego, CA). The imaging quantification data were reported as an average ± standard deviation on the basis of results from 5 animals in each formulation group.
SPECT Imaging
SPECT images of SD rats (n = 3) were obtained using a u-SPECT-II/CT scanner (Milabs, Utrecht, The Netherlands) equipped with a 1.0 mm multi-pinhole collimator. The animal was placed into a shielded chamber connected to an isoflurane anesthesia unit (Univentor, Zejtun, Malta). Anesthesia was induced using an air flow rate of 350 mL/min and ~3.0% isoflurane, and maintained using the air flow rate of ~250 mL/min with ~2.5% isoflurane during the whole time of preparation and image data acquisition (6 frames: 75 projections over 5 min per frame).
The animal was administered with the 99mTc radiotracer (120 – 180 MBq) in 0.5 mL saline containing ~20% propylene glycol through a catheter, followed with 0.5 mL saline solution flash. Rectangular scan in regions of interest (ROIs) from SPECT and CT were selected one the basis of the orthogonal X-ray images provided by the CT. After SPECT acquisition, the animal was allowed to recover in a shielded cage.
Image Reconstruction and Data Processing
SPECT reconstruction was performed using a POSEM (pixelated ordered subsets by expectation maximization) algorithm with 6 iterations and 16 subsets. CT data were reconstructed using a cone-beam filtered back-projection algorithm (NRecon v1.6.3, Skyscan). After reconstruction, the SPECT and CT data were automatically co-registered according to the movement of the robotic stage, and then re-sampled to equivalent voxel sizes. Co-registered images were further rendered and visualized using the PMOD software (PMOD Technologies, Zurich, Switzerland). A 3D-Guassian filter (1.2 mm FWHM) was applied to smooth noise, and the LUTs (look up tables) were adjusted for good visual contrast. The images were visualized as both orthogonal slices and maximum intensity projections.
Data and Statistical Analysis
Biodistribution data, T/B ratios and imaging quantification data were reported as an average ± standard deviation based on the results from four to six SD rats at each time point. Comparison between two radiotracers was made using a one-way ANOVA test. The level of significance was set at p < 0.05.
RESULTS
Synthesis of 99mTc-Teboroxime(L) (L = Cl, F, SCN and N3)
99mTc-Teboroxime was prepared according to the literature method [20,22]. Its RCP is 95 – 98% without purification. Once it was prepared, 99mTc-Teboroxime was stable for more than 6 h in kit matrix (Figure SI1). In the literature [20,22], excess NaCl (~100 mg per vial) was used to maintain the solution stability of 99mTc-Teboroxime. We found that NaCl was not needed since the product showed identical HPLC profiles without NaCl (Figure SI2). Complexes 99mTc-Teboroxime(L) (L = F, SCN and N3) were prepared (Chart I) by reacting 99mTc-Teboroxime with NaF, NaSCN and NaN3, respectively.
The RCP was 85 – 90% for 99mTc-Teboroxime(F), 90 – 93% for 99mTc-Teboroxime(SCN), and >95% for 99mTc-Teboroxime(N3). The reaction between 99mTc-Teboroxime and F−, SCN− or N3− was very slow with RCP being <5% after 2 hours of reaction at room temperature (Figure SI3). Heating at 100 °C was required to complete the ligand exchange. The pH value in reaction mixture was controlled using 0.2 M phosphate buffer (pH = 7.4). When the pH was <5.0 in the reaction mixture, the RCP for 99mTc-Teboroxime(F) and 99mTc-Teboroxime(SCN) was less than 90%. However, the pH value (5.0 – 8.0) had little impact of the RCP of 99mTc-Teboroxime(N3). The optimal amount of NaF was 5 mg/vial while it was ~2 mg/vial for NaSCN and NaN3.
HPLC Characterization and Solution Stability
Figure 2 shows HPLC chromatograms of 99mTc-Teboroxime, 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3). There was only one peak at ~15.5 min for 99mTc-Teboroxime (Figure 2A). The HPLC retention time of 99mTc-Teboroxime(F) was ~16.4 min (Figure 2B), which was almost 1 min longer than that of 99mTc-Teboroxime. Co-injection of these two radiotracers further confirmed this observation (Figure SI4). 99mTc-Teboroxime(SCN) showed two peaks at 16.5 and 18.3 min, likely due to the asymmetric nature of SCN in binding to 99mTc(III). In contrast, 99mTc-Teboroxime(N3) appeared as a single peak at 18.4 min, which was ~3 min longer than that of 99mTc-Teboroxime. It was surprising that 99mTc-Teboroxime(F) was stable for >6 h at room temperature (Figure SI5). Both 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3) remained stable in the kit matrix for more than 6 h at room temperature (Figure SI6).
Figure 2.
Radio-HPLC chromatograms of 99mTc-Teboroxime (RCP >95%), 99mTc-Teboroxime(F) (RCP = 85 – 90%), 99mTc-Teboroxime(SCN) (RCP = 90 – 93% for the sum of two radiometric peaks at 16.4 and 18.4 min) and 99mTc-Teboroxime(N3) (RCP >95%).
Dynamic Planar Imaging
Dynamic imaging studies were performed in SD rats. The purpose of these studies was to explore the heart retention and liver clearance rates of 99mTc radiotracers; and to select appropriate 99mTc(III) radiotracer(s) for biodistribution. Figure 3 shows the selected planar images of the SD rats administered with 99mTc-Teboroxime(L) (L = Cl, F, SCN and N3) over the first 5 min. All images acquired at 0 – 1 min showed high heart and liver uptake; but their lung radioactivity accumulation was low. 99mTc-Teboroxime(F) showed a lower heart uptake along with a faster heart washout than other three radiotracers. 99mTc-Teboroxime(N3) had the longest heart retention among four 99mTc radiotracers evaluated in this study. Planar image quantification was performed to compare heart retention and liver clearance kinetics of 99mTc-Teboroxime, 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3) in the SD rats. It was found that their heart retention curves were best fitted to a bi-exponential function (Figure 4). Approximately two-thirds of the heart radioactivity cleared within 5 min for 99mTc-Teboroxime through the fast component. The half-times of the fast/slow clearance components were calculated to be 1.6 ± 0.4/60.7 ± 8.9 min for 99mTc-Teboroxime, 0.8 ± 0.2/101.7 ± 20.7 min for 99mTc-Teboroxime(F), 1.2 ± 0.3/84.8 ± 16.6 min for 99mTc-Teboroxime(SCN), and 2.9 ± 0.9/51.6 ± 5.0 min for 99mTc-Teboroxime(N3). The fast-phase myocardial retention time followed the order of 99mTc-Teboroxime(N3) > 99mTc-Teboroxime > 99mTc-Teboroxime(SCN) > 99mTc-Teboroxime(F).
Figure 3.
Planar images of the SD rats administered with 99mTc-Teboroxime, 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3). Animals were anesthetized with intramuscular injection of a mixture of ketamine (80 mg/kg) and xylazine (19 mg/kg). Each animal was injected with 1.0 – 1.5 mCi of 99mTc radiotracer. The 1-min static images were acquired over the first 5 min post-injection, followed by the 2-min static images at 6 – 30, 40, 50 and 60 min p.i. Arrows indicate the presence of radioactivity accumulation in the heart. White circles indicate the radioactivity accumulation in the blood vessels. 99mTc-Teboroxime(F) had a very fast washout from the heart. 99mTc-Teboroxime and 99mTc-Teboroxime(N3) showed significant radioactivity accumulation in blood vessels.
Figure 4.
Image quantification data to compare the heart retention times of 99mTc-Teboroxime, 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3). The experimental data were expressed as the total heart uptake (%ID). The heart retention curve was best fitted to a bi-exponential function using the individual animal time/activity data.
By 20 min p.i., the heart radioactivity almost disappeared for all four radiotracers. The liver clearance curves were also best fitted to the two-phase bi-exponential decay function (Figure 5). Despite their differences in the heart uptake and myocardial retention time, all four radiotracers shared similar liver clearance kinetics with a prolonged liver uptake over the 60-min period (Figure 5). It was interesting to note that there was a significant radioactivity accumulation above the heart (Figure 3) in the SD rats administered with 99mTc-Teboroxime or 99mTc-Teboroxime(N3). The residual time of this radioactivity accumulation was significantly longer in the SD rats administered with 99mTc-Teboroxime(N3) than that with 99mTc-Teboroxime (Figure 3). Another important observation is that 99mTc-Teboroxime and 99mTc-Teboroxime(N3) had very little excretion via both renal and hepatobiliary routes during the 60-min study period. Many attempts to collect the urine and feces samples for metabolism studies after planar imaging (60 min p.i.) were made without any success because of the limited amount of radioactivity (<5 μCi collected from each animal).
Figure 5.
Image quantification data to compare the liver clearance kinetics of 99mTc-Teboroxime, 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3). These data were expressed as % of the initial liver uptake at 0 – 1 min. The liver clearance curve was best fitted to a bi-exponential function using the individual animal time/radioactivity data. It is impossible to quantify the “absolute liver radioactivity level” because there was no clear separation between liver and surrounding organs.
Biodistribution Properties
The selected biodistribution data and heart/background ratios for 99mTc-Teboroxime and 99mTc-Teboroxime(N3) are summarized in Tables 1 and 2, respectively. 99mTc-Teboroxime(N3) was of our interest because (1) it exists in solution as a single isomer, (2) its initial heart uptake was close to that of 99mTc-Teboroxime on the basis of planar imaging, and (3) it had longer myocardial retention than 99mTc-Teboroxime (Figure 4). The main objective was to compare their heart uptake, myocardial retention and liver clearance kinetics. We found that 99mTc-Teboroxime(N3) had significantly lower uptake than 99mTc-Teboroxime in most organs over the 60 min study period (Tables 1 and 2). For example, 99mTc-Teboroxime(N3) showed the blood radioactivity levels of 0.30 ± 0.05, 0.14 ± 0.01, 0.13 ± 0.00 and 0.07 ± 0.02 %ID/g at 2, 15, 30 and 60 min p.i., respectively. The blood activity levels for 99mTc-Teboroxime were 0.47 ± 0.05, 0.23 ± 0.05, 0.31 ± 0.09 and 0.22 ± 0.05 %ID/g, respectively, at the same time points. However, the heart uptake of 99mTc-Teboroxime (3.00 ± 0.37, 1.25 ± 0.09, 0.86 ± 0.17 and 0.58 ± 0.04 %ID/g at 2, 15, 30 and 60 min p.i., respectively) was higher than that 99mTc-Teboroxime(N3) (2.66 ± 0.01, 0.90 ± 0.10, 0.51 ± 0.02 and 0.29 ± 0.02 %ID/g at 2, 15, 30 and 60 min p.i., respectively) over the 60-min period. The initial liver uptake of 99mTc-Teboroxime(N3) (3.68 ± 0.09 %ID/g at 2 min p.i.) was slightly higher than that of 99mTc-Teboroxime (2.87 ± 0.67%ID/g), but their liver uptake values were almost identical at >15 min p.i., which was consistent with the data from the planar image quantification (Figure 4).
Table 1.
Selected biodistribution data for 99mTc-Teboroxime in SD rats (n = 4).
| Organ | 2 min | 15 min | 30 min | 60 min |
|---|---|---|---|---|
| Blood | 0.47 ± 0.05 | 0.23 ± 0.05 | 0.31 ± 0.09 | 0.22 ± 0.05 |
| Brain | 0.13 ± 0.02 | 0.05 ± 0.01 | 0.08 ± 0.04 | 0.05 ± 0.01 |
| Heart | 3.00 ± 0.37 | 1.25 ± 0.09 | 0.86 ± 0.17 | 0.58 ± 0.04 |
| Intestines | 1.04 ± 0.20 | 1.23 ± 0.35 | 1.84 ± 0.32 | 0.81 ± 0.09 |
| Kidneys | 3.21 ± 0.38 | 2.18 ± 0.80 | 1.97 ± 0.17 | 0.68 ± 0.45 |
| Liver | 2.67 ± 0.47 | 2.40 ± 0.67 | 2.22 ± 0.17 | 0.95 ± 0.16 |
| Lungs | 2.90 ± 0.35 | 1.64 ± 0.28 | 1.41 ± 0.25 | 0.94 ± 0.25 |
| Muscle | 0.45 ± 0.10 | 0.33 ± 0.01 | 0.27 ± 0.07 | 0.21 ± 0.05 |
| Spleen | 2.33 ± 0.58 | 1.38 ± 0.11 | 1.14 ± 0.04 | 0.42 ± 0.10 |
| Heart/Blood | 6.42 ± 1.26 | 5.55 ± 0.95 | 2.93 ± 0.90 | 2.88 ± 1.04 |
| Heart/Lung | 1.07 ± 0.12 | 0.78 ± 0.12 | 0.61 ± 0.04 | 0.66 ± 0.21 |
| Heart/Liver | 1.18 ± 0.21 | 0.56 ± 0.16 | 0.39 ± 0.09 | 0.62 ± 0.12 |
| Heart/Muscle | 6.12 ± 2.03 | 3.82 ± 0.19 | 3.57 ± 1.49 | 2.90 ± 0.67 |
Table 2.
Selected biodistribution data for 99mTc-Teboroxime(N3) in SD rats (n = 6).
| Organ | 2 min | 15 min | 30 min | 60 min |
|---|---|---|---|---|
| Blood | 0.30 ± 0.05 | 0.14 ± 0.01 | 0.13 ± 0.00 | 0.07 ± 0.02 |
| Brain | 0.16 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.01 | 0.06 ± 0.01 |
| Heart | 2.66 ± 0.01 | 0.90 ± 0.10 | 0.51 ± 0.02 | 0.29 ± 0.02 |
| Intestines | 0.73 ± 0.05 | 0.76 ± 0.02 | 0.92 ± 0.06 | 0.76 ± 0.23 |
| Kidneys | 2.49 ± 0.13 | 0.91 ± 0.08 | 0.73 ± 0.10 | 0.41 ± 0.05 |
| Liver | 3.68 ± 0.09 | 2.30 ± 0.08 | 1.57 ± 0.08 | 0.70 ± 0.12 |
| Lungs | 1.77 ± 0.21 | 0.57 ± 0.04 | 0.45 ± 0.01 | 0.25 ± 0.04 |
| Muscle | 0.19 ± 0.06 | 0.23 ± 0.00 | 0.19 ± 0.04 | 0.16 ± 0.01 |
| Spleen | 1.35 ± 0.04 | 0.45 ± 0.01 | 0.38 ± 0.07 | 0.21 ± 0.01 |
| Vessels | 1.29 ± 0.51 | 1.20 ± 0.67 | 1.10 ± 0.42 | 0.82 ± 0.34 |
| Heart/Blood | 9.02 ± 0.52 | 6.41 ± 1.33 | 3.99 ± 0.31 | 4.14 ± 0.57 |
| Heart/Lung | 1.53 ± 0.18 | 1.60 ± 0.29 | 1.12 ± 0.06 | 1.16 ± 0.09 |
| Heart/Liver | 0.72 ± 0.02 | 0.39 ± 0.06 | 0.32 ± 0.00 | 0.41 ± 0.04 |
| Heart/Muscle | 15.56 ± 5.15 | 3.85 ± 0.44 | 2.79 ± 0.71 | 1.78 ± 0.06 |
Since the first-pass takes place within the first 2 min after administration of the radiotracer, the higher radiotracer heart uptake at this time point suggests a better first-pass extraction fraction. In this study, we performed biodistribution to compare the 2-min heart uptake of 99mTc-Teboroxime, 99mTc-Teboroxime(N3), 99mTcN-MPO and 99mTc-Sestamibi in SD rats (Figure 6). 99mTc-Sestamibi is the most successful radiotracer for SPECT MPI. 99mTcN-MPO is currently under clinical evaluation as a new radiotracer with faster liver clearance kinetics than that 99mTc-Sestamibi.47 It was found that the 2-min heart uptake values followed the general order of 99mTc-Teboroxime (3.00 ± 0.37%ID/g) > 99mTc-Teboroxime(N3) (2.66±0.01 %ID/g) ≈ 99mTc-Sestamibi (2.55±0.46 %ID/g) > 99mTcN-MPO (2.38±0.15 %ID/g). These results suggested that 99mTc-Teboroxime remains the best in first-pass extraction fraction.
Figure 6.
Direct comparison of the 2-min heart uptake (%ID/g) for 99mTc-Sestamibi, 99mTcN-MPO, 99mTc-Teboroximine and 99mTc-Teboroxime(N3).
Radioactivity Accumulation in Blood Vessels
In order to determine where the radioactivity accumulation above the heart was located (Figure 3), we isolated the blood vessels around the heart in SD rats administered with 99mTc-Teboroxime(N3). It was found that the uptake was 1.29 ± 0.51, 1.20 ± 0.67, 1.10 ± 0.42 and 0.82 ± 0.34 %ID/g at 2, 15, 30 and 60 min p.i., respectively. In fact, the blood vessel uptake was higher than the heart uptake of 99mTc-Teboroxime(N3) at >15 min p.i. At this moment, it is not clear why 99mTc-Teboroxime(N3) has such a high uptake in the blood vessels. This type of radioactivity accumulation was not seen in SD rats administered with 99mTc-Sestamibi and 99mTcN-MPO [42-44].
SPECT Imaging
Figure 9A shows the coronal views of SPECT images from the SD rats administered with 99mTc-Teboroximine or 99mTc-Teboroximine(N3). The right and left ventricular walls were clearly delineated. Despite intense liver uptake, as indicated by activity accumulation at the apex of heart, high quality SPECT images could be acquired for both 99mTc-Teboroximine and 99mTc-Teboroximine(N3) due to their high first-pass extraction fractions (Figure 6). We also found that the best image data acquisition window is 0 – 5 min for 99mTc-Teboroximine (Figure SI7).
Longer acquisition time did not improve the image quality due to its fast myocardial washout and prolonged liver radioactivity accumulation. In contrast, high quality SPECT images was obtained at 0 – 15 min for 99mTc-Teboroximine(N3) due to its longer heart retention (Figure SI7). In the sagittal and transaxial SPECT images, there was a significant overlap between the heart and liver activity. This was particularly true in the images obtained at 0 – 15 min (Figure SI7). Figure 7B compares the 3-D SPECT/CT images from the SD rats administered with 99mTc-Teboroximine or 99mTc-Teboroximine(N3) to show the relative location of radioactivity accumulation. The activity in blood vessels was seen in the SD rat administered with 99mTc-Teboroximine(N3) (Figure SI7), which is consistent with the biodistribution data (Table 2).
Figure 7.
A: Coronal views of SPECT images of the SD rats administered with 80 – 90 MBq of 99mTc-Teboroximine (upper panel) or 99mTc-Teboroximine(N3) (lower panel). Anesthesia was induced using an air flow rate of 350 mL/min and ~3.0% isoflurane. SPECT images were obtained over the first 5 min with camera being focused in the heart region. Arrows indicate the presence of the liver radioactivity. Despite intense liver uptake, high quality SPECT images could be acquired. B: The 3D views of SPECT/CT images of the SD rats administered with 80 – 90 MBq of 99mTc-Teboroximine or 99mTc-Teboroximine(N3) to illustrate the radioactivity accumulation in the heart region. The radioactivity in the blood vessels (above the heart) was clearly seen in the SPECT/CT image of the SD rat administered with 99mTc-Teboroximine(N3).
DISCUSSION
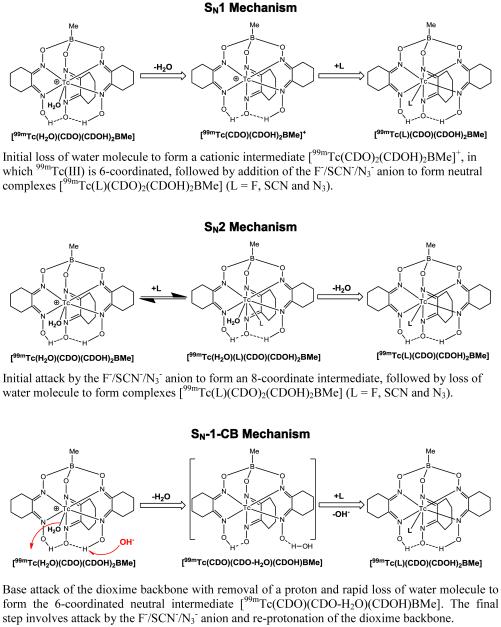
Over the last 30 years, 99mTc-Teboroxime has been believed as a neutral 99mTc(III) complex with a formula of [99mTcCl(CDO)(CDOH)2BMe]. In solid state, the Tc(III) center in [TcCl(CDO)(CDOH)2BMe] is indeed seven-coordinated with six imine-N donors and a monodentate chloride in a trigonally-capped prismatic geometry [19,21]. However, its solution structure remains unknown because the chloride ligand is very labile. Considering its extremely low concentration (10-8 – 10-6 M), it is hard to imagine that the chloride ligand in [99mTcCl(CDO)(CDOH)2BMe] remains attached the 99mTc(III) center. Even though excess NaCl (~100 mg per vial or 1.0 – 2.0 M) is often used in the kit formulation to prevent dissociation of chloride ligand, this hardly compares the high concentration of water (~55.6 M). It has been reported that 99mTc-Teboroxime undergoes rapid the chloro-hydroxy exchange with a half-life of ~13 min under physiological conditions [20]. However, we believe that the actual exchange is between the chloride anion and water. As a result, 99mTc-Teboroxime most likely exists in solution as its cationic form [99mTc(H2O)(CDO)(CDOH)2BMe]+ (Chart II). This statement is further supported by the fact that the HPLC retention time of 99mTc-Teboroxime is ~1 min shorter than that of 99mTc-Teboroxime(F) (Figure 2B). If 99mTc-Teboroxime were to exist in solution as its neutral form [99mTcCl(CDO)(CDOH)2BMe], it would have had the same HPLC retention time as that of 99mTc-Teboroxime(F) due to the similarity between F− and Cl− anions. Because of the rapid equilibrium between [99mTc(H2O)(CDO)(CDOH)2BMe]+ and [99mTc(OH)(CDO)(CDOH)2BMe] (Chart I), 99mTc-Teboroxime appeared as a single radiometric peak at 15.5 min in its HPLC chromatogram (Figure 2A). If this equilibrium were to be slow, they should have been detected as two separate radiometric peaks due to the difference in their overall molecular charges.
It is well-accepted that the F− anion is a “hard base”, and the bonding between F− and metal ions is manly ionic. It has been reported that the Al(NOTA) (NOTA = 1,4,7-triazacyclononane-1,4,7-triacetic acid) chelate is an efficient prosthetic group for 18F-labeling of biomolecules [32-39]. Due to the strong binding between F− and Al(III), the Al(NOTA)18F chelate remains stable under the physiological conditions. In this study, we were surprised to see that 99mTc-Teboroxime(F) could be readily prepared in high yield (85 – 90%) in the presence of excess NaCl, its HPLC retention time was about 1 min longer than that of 99mTc-Teboroxime (Figure 2C), and it was able to maintain its solution stability for more than 6 h in the kit matrix (Figure SI5). If the F− anion were to be dissociated, 99mTc-Teboroxime(F) would have shared the same radio-HPLC profile with 99mTc-Teboroxime under identical chromatographic conditions. Thus, we strongly believe that the F− anion remains attached to the 99mTc(III) center in solution. It is not clear why the 99mTc-F bond is so strong, and 99mTc-Teboroxime(F) is able to maintain its stability in aqueous solution. However, these results suggest that the Tc(III)/Re(III) or Tc(V)-oxo/Re(V)-oxo chelates with appropriate chelators might be useful as prosthetic groups for the 18F-labeling of small biomolecules.
The SCN− anion is quite unique because it is able to bond to Tc(III) via its both S and N atoms. That might explain why 99mTc-Teboroxime(SCN) exists in solution as two isomers, as indicated by the presence of two radiometric peaks at 16.5 and 18.4 min in its radio-HPLC chromatogram (Figure 2C). In contrast, N3− forms the same complex 99mTc-Teboroxime(N3) regardless of which end of N3− is attached to 99mTc(III). As a result, 99mTc-Teboroxime(N3) shows only one radiometric peak in its HPLC chromatogram (Figure 2D). The radiometric peak at ~18.4 min from 99mTc-Teboroxime(SCN) is almost identical to that from 99mTc-Teboroxime(N3), suggesting that SCN− bonds to 99mTc(III) mainly via the S-end. Since they have longer HPLC retention times than that of 99mTc-Teboroxime (Figure 2A), it is reasonable to believe that 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3) exist in solution as their neutral forms [99mTc(L)(CDO)2(CDOH)2BMe] (L = SCN and N3) with the 99mTc(III) center being 7-coordinated. This statement is supported by their high solution stability (Figure SI6) due to the capability SCN− and N3− anions to form stronger bonds with 99mTc(III) via both σ- and π-bonding.
Three possible reaction mechanisms (SN1, SN2 and SN-l-CB) have been proposed for the chloro-hydroxide exchange [20]. While 99mTc-Teboroxime might exist in small portion as its neutral form [99mTcCl(CDO)2(CDOH)2BMe] in the original vial (pH = 3.0 – 4.0), we believe that the exchange at pH ~ 7.0 is actually between H2O in [99mTc(H2O)(CDO)(CDOH)2BMe]+ and F−, SCN− or N3− anion. This statement is supported by the fact that increasing the chloride concentration to >1 M does not affect the ligand exchange rate. Chart II shows three possible mechanisms (SN1, SN2 and SN-l-CB) for the ligand exchange between [99mTc(H2O)(CDO)(CDOH)2BMe]+ and L (L = F−, SCN− and N3−). In the SN1 mechanism, the first step is dissociation of H2O to form the 6-coordinated [99mTc(CDO)(CDOH)2BMe]+ intermediate, followed by addition of incoming ligand (F−, SCN− or N3−). However, this mechanism hardly explains the reaction rate dependence on pH, temperature and concentration of F−, SCN− and N3− anion. The reaction between 99mTc-Teboroxime and SCN− was slow at room temperature and the RCP for [99mTc(SCN)(CDO)2(CDOH)2BMe]) was <5% (Figure SI2). The SN2 mechanism involves initial attack by the F−/SCN−/N3− anion to form an 8-coordinate intermediate, followed by loss of water molecule to afford neutral complexes [99mTc(L)(CDO)(CDOH)2BMe] (L = F, SCN and N3). However, the SN2 mechanism is not consistent with the pH dependence of ligand exchange. In SN-l-CB mechanism, the rate controlling step is the base-catalyzed removal of a nearby proton preceding elimination of water molecule to form the 6-coordinated intermediate [99mTc(L)(CDO)(CDO-H2O)(CDOH)BMe]. Since there is no HPLC evidence for the presence of this intermediate, we believe that this intermediate is transient, with the rapid conversion back to a neutral, 7-coordinate complex cation [99mTc(H2O)(CDO)(CDOH)2BMe]+. As illustrated in Chart II, it is possible that the proton eliminated from the BAT backbone during the SI-CB axial ligand exchange process may originate from a free oxime group.
The short myocardial retention times of 99mTc-Teboroxime and 99mTc-Teboroxime(N3) are definitely one of their drawbacks when imaging is performed using the standard SPECT cameras, even though 99mTc-Teboroxime(N3) has longer heart retention than 99mTc-Teboroxime (Figure 4). This drawback can be overcome using ultra-fast cardiac SPECT cameras, in which the cadmium-zinctelluride (CZT) solid-state detectors are used to replace traditional NaI(Tl) detectors. The CZT-based cardiac camera allows a more than five-fold reduction in the scan time using same amount of radiotracer and provides clinical information equivalent to that from the standard SPECT MPI [46-52]. The increased sensitivity could also allow the use of lower radiotracer dose to reduce the radiation exposure to patient without loss of image quality. Another drawback of 99mTc-Teboroxime and 99mTc-Teboroxime(N3) is their high liver uptake over the 60-min period (Figure 6). High liver radioactivity accumulation may interfere with visualization of the inferior wall of the heart using the standard SPECT cameras. For ultra-fast cardiac SPECT cameras, it is possible to position the patient upright during image acquisition, which would be helpful in minimizing the interference from the liver radioactivity because liver tends to drop downward in this position, allowing better separation of the cardiac and hepatic radioactivity [9,51,52]. As a result of their fast heart washout and prolonged liver radioactivity accumulation, we believe that 99mTc-Teboroxime and 99mTc-Teboroxime(N3) are better suited for early image acquisition with ultra-fast cardiac SPECT cameras.
Dynamic planar imaging is an important screening tool to evaluate the heart retention and liver clearance kinetics of 99mTc radiotracers without sacrificing a large number of animals. Image quantification could be achieved by using an external radiation source with the known amount of radioactivity. While it is possible to calculate the percentage of the injected radioactivity (% ID) in the heart, it is much more difficulty to do the same for the liver radioactivity due to its larger size and difficulty to identify the boundary between liver and other organs in the region. Thus, the relative liver radioactivity has to be expressed as the percentage of the initial uptake.
Another important finding of this study is that 99mTc-Teboroxime and 99mTc-Teboroxime(N3) show high radioactivity accumulation in blood vessels (Figure 3). This is particularly obvious in the 3D SPECT/CT image of the SD rat administered with 99mTc-Teboroxime(N3) (Figure 7B). As a matter of fact, the blood vessel uptake values of 99mTc-Teboroxime(N3) is significantly (p < 0.05) higher than that in the heart at >15 min p.i. (Table 2). It is not clear why 99mTc-Teboroxime(N3) have such a high uptake in the blood vessels around the heart. This type of radioactivity accumulation has not been seen in the SD rats administered the cationic 99mTc radiotracers, such as 99mTc-Sestamibi and 99mTcN-MPO [42-44].
The heart localization mechanism of 99mTc-Teboroxime and 99mTc-Teboroxime(N3) is not known. It is also unknown whether they actually enter myocytes or simply attach to phospholipid layers of the cell membrane. It has been suggested that [99mTc(H2O)(CDO)(CDOH)2BMe]+ is responsible for the high heart uptake of 99mTc-Teboroxime [20]. This assumption seems consistent with the heart localization mechanism of most cationic 99mTc and 18F radiotracers [9,53-64], and supported by the low heart uptake and fast heart washout of 99mTc-Teboroxime(F). However, this hardly explains the fact that 99mTc-Teboroxime(N3), also a neutral 99mTc(III) complex, has a relatively high initial heart uptake (2.66±0.01 %ID/g at 2 min p.i.) with the longer myocardial retention time (Figure 5: T1/2 = 2.9±0.9 min) as compared to that of 99mTc-Teboroxime (Figure 5: T1/2 = 1.6±0.4 min). Therefore, there must be an alternative mechanism for the high heart uptake of 99mTc-Teboroxime and 99mTc-Teboroxime(N3). Like 99mTcN-NOET ([99mTcN(NOET)2: NOET = N-ethoxy-N-ethyldithiocarbamato) [65-68], 99mTc-Teboroxime(N3) may bind to the L-type calcium channels in the open configuration without entering myocytes, its cellular uptake mechanism is not energy-dependent. It must be noted that this explanation remains largely speculation in the absence of more experimental data.
CONCLUSIONS
In this study, we found that all new radiotracers [99mTc(L)(CDO)2(CDOH)2BMe] (L = F, SCN and N3) are neutral in their overall molecular charge and remain stable in the reaction mixture for >6 h. The co-ligands in [99mTc(L)(CDO)(CDOH)2BMe] (L = Cl, F, SCN and N3) had significant impact on their solution stability, heart uptake and myocardial retention. 99mTc-Teboroxime(N3) had a longer myocardial retention than 99mTc-Teboroxime despite of its relatively lower heart uptake. The results from SPECT studies suggest that the best image data acquisition window is 0 – 5 min for 99mTc-Teboroximine, and 0 – 15 min for 99mTc-Teboroximine(N3) due to its longer myocardial retention. Future studies should be directed towards minimizing the liver uptake and radioactivity accumulation in blood vessels while maintaining their high heart uptake.
Supplementary Material
Chart I.
Schematic Illustration for Radiosynthesis of 99mTc-Teboroxime, 99mTc-Teboroxime(F), 99mTc-Teboroxime(N3) and 99mTc-Teboroxime(SCN).
Chart II.
Three Possible Mechanisms for Ligand Exchange Reaction.
Acknowledgement
This work was supported, in part, by Purdue University, the Indiana Clinical and Translational Sciences Institute funded in part by grant Number TR000006 (Clinical and Translational Award) from the National Institutes of Health, the National Center for advancing Translational Science, and R21 EB017237-01 (S.L.) from the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
ABBRIVIATIONS
- BATO
boronic acid adducts of technetium dioximes
- CAD
coronary artery disease
- DTPA
diethylenetriaminepentaacetic acid (or pentetic acid)
- MPI
myocardial perfusion imaging
- RCP
radiochemical purity
- SPECT
single photon-emission computed tomography
- 99mTcN-MPO
[99mTcN(mpo)(PNP5)]+ (mpo = 2-mercaptopyridine oxide, and PNP5 = N-ethoxyethyl-N,N-bis[2-(bis(3-methoxypropyl)phosphino)ethyl]amine)
- 99mTcN-NOET
99mTcN(NOET)2 (NOET = N-ethoxy-N-ethyldithiocarbamato)
- 99mTc-Sestamibi
[99mTc(MIBI)6]+ (MIBI = 2-methoxy-2-methylpropylisonitrile)
- 99mTc-Tetrofosmin
[99mTcO2(tetrofosmin)2]+ (tetrofosmin = 1,2-bis[bis(2-ethoxyethyl)phosphino]ethane)
- 99mTc-Teboroxime
[99mTcCl(CDO)(CDOH)2BMe] (CDOH2 = cyclohexanedione dioxime)
- 99mTc-Teboroxime(F)
[99mTc(F)(CDO)(CDOH)2BMe]
- 99mTc-Teboroxime(SCN)
[99mTc(SCN)(CDO)(CDOH)2BMe]
- 99mTc-Teboroxime(N3)
[99mTc(N3)(CDO)(CDOH)2BMe]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Authors declare that they have no conflict of interest.
Supporting Information Available: HPLC chromatograms of 99mTc-Teboroxime prepared with excess sodium chloride at T = 0 and 6 h post-labeling (Figure SI1), HPLC chromatograms of 99mTc-Teboroxime prepared without excess sodium chloride at T = 0 and 6 h post-labeling (Figure SI2), HPLC chromatograms of the reaction solution containing 99mTc-Teboroxime and excess sodium salt of co-ligands (F−, SCN− and N3−) after 2 h reaction at room temperature (Figure SI3), HPLC chromatogram obtained by co-injecting 99mTc-Teboroxime with 99mTc-Teboroxime(F), 99mTc-Teboroxime(SCN) and 99mTc-Teboroxime(N3) (Figure SI4), HPLC chromatograms of 99mTc-Teboroxime(F) at T = 0, 2.5 and 6 h post-labeling (Figure SI5), HPLC chromatograms of 99mTc-Terboximine(SCN) and 99mTc-Terboximine(N3) at 6 h p.i., and selected SPECT images from the SD rats administered with 80 – 90 MBq of 99mTc-Teboroximine or 99mTc-Teboroximine(N3), are all in word document.
References
- 1.Acampa W, Di Benedetto C, Cuocolo A. An overview of radiotracers in nuclear cardiology. J Nucl Cardiol. 2000;7:701–7. doi: 10.1067/mnc.2000.109969. [DOI] [PubMed] [Google Scholar]
- 2.Dilsizian V. The role of myocardial perfusion imaging in vascular endothelial dysfunction. J Nucl Cardiol. 2000;7:180–4. doi: 10.1016/s1071-3581(00)90042-4. [DOI] [PubMed] [Google Scholar]
- 3.Beller GA, Zaret BL. Contributions of nuclear cardiology to diagnosis and prognosis of patients with coronary artery disease. Circulation. 2000;101:1465–8. doi: 10.1161/01.cir.101.12.1465. [DOI] [PubMed] [Google Scholar]
- 4.Parker JA. Cardiac nuclear medicine in monitoring patients with coronary heart disease. Semin Nucl Med. 2001;31:223–7. doi: 10.1053/snuc.2001.23529. [DOI] [PubMed] [Google Scholar]
- 5.Kapur A, Latus KA, Davies G, Dhawan RT, Eastick S, Jarritt PH, et al. A comparison of three radionuclide myocardial perfusion tracers in clinical practice: the ROBUST study. Eur J Nucl Med Mol Imaging. 2002;29:1608–16. doi: 10.1007/s00259-002-0998-8. [DOI] [PubMed] [Google Scholar]
- 6.Henneman MM, Schuijf JD, van der Wall EE, Bax JJ. Non-invasive anatomical and functional imaging for the detection of coronary artery disease. Br Med Bull. 2006;79-80:187–202. doi: 10.1093/bmb/ldl014. [DOI] [PubMed] [Google Scholar]
- 7.Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009;2:412–24. doi: 10.1161/CIRCIMAGING.109.854893. [DOI] [PubMed] [Google Scholar]
- 8.Stirrup J, Wechalekar K, Maenhout A, Anagnostopoulos C. Cardiac radionuclide imaging in stable coronary artery disease and acute coronary syndromes. Br Med Bull. 2009;89:63–78. doi: 10.1093/bmb/ldp004. [DOI] [PubMed] [Google Scholar]
- 9.Gaemperli O, Kaufmann PA. Lower dose and shorter acquisition: pushing the boundaries of myocardial perfusion SPECT. J Nucl Cardiol. 2011;18:830–2. doi: 10.1007/s12350-011-9410-z. [DOI] [PubMed] [Google Scholar]
- 10.Perrone-Filardi P, Costanzo P, Dellegrottaglie S, Gargiulo, P, Ruggiero D, Savarese G, et al. Prognostic role of myocardial single photon emission computed tomography in the elderly. J Nucl Cardiol. 2010;17:310–5. doi: 10.1007/s12350-009-9182-x. [DOI] [PubMed] [Google Scholar]
- 11.Nunn AD. Radiopharmaceuticals for imaging myocardial perfusion. Semin Nucl Med. 1990;20:111–8. doi: 10.1016/s0001-2998(05)80164-3. [DOI] [PubMed] [Google Scholar]
- 12.Saha GB, Go RT, MacIntyre WJ. Radiopharmaceuticals for cardiovascular imaging. Int J Rad Appl Instrum B. 1992;19:1–20. doi: 10.1016/0883-2897(92)90179-3. [DOI] [PubMed] [Google Scholar]
- 13.Opie LH, Hesse B. Radionuclide tracers in the evaluation of resting myocardial ischemia and viability. Eur J Nucl Med. 1997;24:1183–93. doi: 10.1007/BF01254255. [DOI] [PubMed] [Google Scholar]
- 14.Jain D. Technetium-99m labeled myocardial perfusion imaging agents. Semin Nucl Med. 1999;29:221–36. doi: 10.1016/s0001-2998(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S, Pillai MR, Ramamoorthy N. Evolution of Tc-99m in diagnostic radiopharmaceuticals. Semin Nucl Med. 2001;31:260–77. doi: 10.1053/snuc.2001.26205. [DOI] [PubMed] [Google Scholar]
- 16.Kailasnath P, Sinusas AJ. Comparison of Tl-201 with Tc-99m-labeled myocardial perfusion agents: technical, physiologic, and clinical issues. J Nucl Cardiol. 2001;8:482–98. doi: 10.1067/mnc.2001.115078. [DOI] [PubMed] [Google Scholar]
- 17.Llaurado JG. The quest for the perfect myocardial perfusion indicator…still a long way to go. J Nucl Med. 2001;42:282–4. [PubMed] [Google Scholar]
- 18.Baggish AL, Boucher CA. Radiopharmaceutical agents for myocardial perfusion imaging. Circulation. 2008;118:1668–74. doi: 10.1161/CIRCULATIONAHA.108.778860. [DOI] [PubMed] [Google Scholar]
- 19.Treher EN, Francesconi LC, Gougoutas JZ, Malley MF, Nunn AD. Monocapped Tris(dioxime) complexes of technetium(III): synthesis and structural characterization of TcX(dioxime)3B-R (X = Cl, Br; dioxime = dimethylglyoxime, cyclohexanedione dioxime; R = CH3, C4H9) Inorg Chem. 1989;28:3411–6. [Google Scholar]
- 20.Jurisson SS, Hirth W, Linder KE, Di Rocco RJ, Narra RK, Nowotnik DP, et al. Chloro → hydroxy substitution on technetium BATO [TcCl(dioxime)3 BR] complexes. Int J Rad Appl Instrum B. 1991;18:735–44. doi: 10.1016/0883-2897(91)90012-a. [DOI] [PubMed] [Google Scholar]
- 21.Jurisson SS, Francesconi L, Linder KE, Treher E, Malley MF, Gougoutas JZ, et al. Synthesis, characterization, and reactivity of manganese and rhenium dioxime complexes. X-ray crystal structures of [MnII(CDO)(CDOH)2(BPh(OCH3))2], an unusual pseudoclathrochelate complex [ReIIICl(CDO)(CDOH)2BPh] Inorg Chem. 1991;30:1820–7. [Google Scholar]
- 22.Seldin DW, Johnson LL, Blood DK, Muschel MJ, Smith KF, Wall RM, et al. Myocardial perfusion imaging with technetium-99m SQ30217: comparison with thallium-201 and coronary anatomy. J Nucl Med. 1989;30:312–319. [PubMed] [Google Scholar]
- 23.Leppo JA, Meerdink DJ. Comparative myocardial extraction of two technetium-labeled BATO derivatives (SQ30217, SQ32014) and thallium. J Nucl Med. 1990;31:67–74. [PubMed] [Google Scholar]
- 24.Marshall RC, Leidholdt EM, Jr, Zhang DY, Barnett CA. The effect of flow on technetium-99m-teboroxime (SQ30217) and thallium-201 extraction and retention in rabbit heart. J Nucl Med. 1991;32:1979–88. [PubMed] [Google Scholar]
- 25.McSherry BA. Technetium-99m-Teboroxime: a new agent for myocardial perfusion imaging. J Nucl Med Technol. 1991;19:22–6. [Google Scholar]
- 26.Iskandrian AS, Heo J, Nguyen T, Mercuro J. Myocardial imaging with Tc-99m teboroxime: technique and initial results. Am Heart J. 1991;121:889–94. doi: 10.1016/0002-8703(91)90204-u. [DOI] [PubMed] [Google Scholar]
- 27.Fleming RM, Kirkeeide RL, Taegtmeyer H, Adyanthaya A, Cassidy DB, Goldstein RA. Comparison of technetium-99m teboroxime tomography with automated quantitative coronary arteriography and thallium-201 tomographic imaging. J Am Coll Cardiol. 1991;17:1297–302. doi: 10.1016/s0735-1097(10)80139-1. [DOI] [PubMed] [Google Scholar]
- 28.Rumsey WL, Rosenspire KC, Nunn AD. Myocardial extraction of teboroxime: effects of teboroxime interaction with blood. J. Nucl. Med. 1992(33):94–101. [PubMed] [Google Scholar]
- 29.Williams KA, Taillon LA, Draho JM, Foisy MF. First-pass radionuclide angiographic studies of left ventricular function with technetium-99m-teboroxime, technetium-99m-sestamibi and technetium-99m-DTPA. J Nucl Med. 1993;34:394–9. [PubMed] [Google Scholar]
- 30.Johnson LL. Myocardial perfusion imaging with technetium-99m-teboroxime. J Nucl Med. 1994;35:689–92. [PubMed] [Google Scholar]
- 31.Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013;54:83–89. doi: 10.2967/jnumed.112.111476. [DOI] [PubMed] [Google Scholar]
- 32.McBride WJ, Sharkey RM, Karacay H, D'Souza CA, Rossi EA, Laverman P, et al. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991–8. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 33.Laverman P, McBride WJ, Sharkey RM, Eek A, Joosten L, et al. A novel facile method of labeling octreotide with 18F-fluorine. J Nucl Med. 2010;51:454–61. doi: 10.2967/jnumed.109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Liu H, Jiang H, Xu Y, Zhang H, Cheng Z. One-step radiosynthesis of 18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. Eur J Nucl Med Mol Imaging. 2011;38:1732–41. doi: 10.1007/s00259-011-1847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Souza CA, McBride WJ, Sharkey RM, Todaro LJ, Goldenberg DM. High-yielding aqueous 18F-labeling of peptides via Al18F chelation. Bioconjugate Chem. 2011;22:1793–1803. doi: 10.1021/bc200175c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBride WJ, D'Souza CA, Sharkey RM, Karacay H, Rossi EA, Chang CH, et al. Improved 18F labeling of peptides with a fluoride-aluminum-chelate complex. Bioconjugate Chem. 2010;21:1331–40. doi: 10.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang L, Li W, Guo N, Ma Y, Zhu L, Kiesewetter DO, et al. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjuate Chem. 2011;22:2415–22. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBride WJ, D'Souza CA, Karacay H, Sharkey RM, Goldenberg DM. New lyophilized kit for rapid radiofluorination of peptides. Bioconjugate Chem. 2012;23:538–47. doi: 10.1021/bc200608e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laverman P, D'Souza CA, Eek A, McBride WJ, Sharkey RM, Oyen WJ, et al. Optimized labeling of NOTA-conjugated octreotide with F-18. Tumor Biol. 2012;33:427–34. doi: 10.1007/s13277-011-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, He Z, Hsieh WY, Kim YS. Evaluation of novel cationic 99mTc-nitrido complexes as radiopharmaceuticals for heart imaging: improving liver clearance with crown ether groups. Nucl Med Biol. 2006;33:419–32. doi: 10.1016/j.nucmedbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, He Z, Hsieh WY, Liu S. Impact of bidentate chelators on lipophilicity, stability, and biodistribution characteristics of cationic 99mTc-nitrido complexes. Bioconjugate Chem. 2007;18:929–36. doi: 10.1021/bc0603182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YS, Wang J, Broisat A, Glover DK, Liu S. Tc-99m-N-MPO: novel cationic Tc-99m radiotracer for myocardial perfusion imaging. J Nucl Cardiol. 2008;15:535–46. doi: 10.1016/j.nuclcard.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Kim YS, Shi J, Zhai S, Hou G, Liu, S. Mechanism for myocardial localization and rapid liver clearance of Tc-99m-N-MPO: a new perfusion radiotracer for heart imaging. J Nucl Cardiol. 2009;16:571–9. doi: 10.1007/s12350-009-9068-y. [DOI] [PubMed] [Google Scholar]
- 44.Bu L, Li R, Jin Z, Wen X, Liu S, Yang B, et al. Evaluation of 99mTcN-MPO as a new myocardial perfusion imaging agent in normal dogs and in an acute myocardial infarction canine model: comparison with 99mTc -Sestamibi. Mol Imaging Biol. 2011;13:121–7. doi: 10.1007/s11307-010-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao S, Zhao G, Wen Q, Bai L, Chen B, Ji T, et al. Pharmacokinetics and biodistribution of 99mTc N-MPO in healthy human volunteers. Clin Nucl Med. 2014;39:e14–e19. doi: 10.1097/RLU.0b013e3182872a8c. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann PA, Gaemperli O. Combining CT and nuclear: a winning hybrid team. J Nucl Cardiol. 2009;16:170–2. doi: 10.1007/s12350-008-9048-7. [DOI] [PubMed] [Google Scholar]
- 47.Esteves FP, Raggi P, Folks RD, Keidar Z, Askew JW, Rispler S, et al. Novel solid-state-detector dedicated cardiac camera for fast myocardial perfusion imaging: multicenter comparison with standard dual detector cameras. J Nucl Cardiol. 2009;16:927–34. doi: 10.1007/s12350-009-9137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buechel RR, Pazhenkottil AP, Herzog BA, Husmann L, Nkoulou RN, Burger IA, et al. Real-time breath-hold triggering of myocardial perfusion imaging with a novel cadmium-zinc-telluride detector gamma camera. Eur J Nucl Med Mol Imaging. 2010;37:1903–8. doi: 10.1007/s00259-010-1480-7. [DOI] [PubMed] [Google Scholar]
- 49.Duvall WL, Croft LB, Godiwala T, Ginsberg E, George T, Henzlova MJ. Reduced isotope dose with rapid SPECT MPI imaging: initial experience with a CZT SPECT camera. J Nucl Cardiol. 2010;17:1009–14. doi: 10.1007/s12350-010-9215-5. [DOI] [PubMed] [Google Scholar]
- 50.Pazhenkottil AP, Buechel RR, Herzog BA, Nkoulou RN, Valenta I, Fehlmann U, et al. Ultrafast assessment of left ventricular dyssynchrony from nuclear myocardial perfusion imaging on a new high-speed gamma camera. Eur J Nucl Med Mol Imaging. 2010;37:2086–92. doi: 10.1007/s00259-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 51.Schillaci O, Danieli R. Dedicated cardiac cameras: a new option for nuclear myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2010;37:1706–9. doi: 10.1007/s00259-010-1526-x. [DOI] [PubMed] [Google Scholar]
- 52.Fiechter M, Ghadri JR, Kuest SM, Pazhenkottil AP, Wolfrum M, Nkoulou RN, et al. Nuclear myocardial perfusion imaging with a novel cadmium-zinc-telluride detector SPECT/CT device: first validation versus invasive coronary angiography. Eur J Nucl Med Mol Imaging. 2011;38:2025–30. doi: 10.1007/s00259-011-1877-y. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava PC, Knapp FF., Jr [(E)-1-[123I]Iodo-1-penten-5-yl]triphenylphosphonium iodide: convenient preparation of a potentially useful myocardial perfusion agent. J Med Chem. 1984;27:978–81. doi: 10.1021/jm00374a007. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava PC, Hay HG, Knapp FF., Jr Effects of alkyl and aryl substitution on the myocardial specificity of radioiodinated phosphonium, arsonium, and ammonium cations. J Med Chem. 1985;28:901–4. doi: 10.1021/jm00145a009. [DOI] [PubMed] [Google Scholar]
- 55.Krause BJ, Szabo Z, Becker LC, Dannals RF, Scheffel U, Seki C, et al. Myocardial perfusion with [11C]methyl triphenyl phosphonium: measurements of the extraction fraction and myocardial uptake. J Nucl Biol Med. 1994;38:521–6. [PubMed] [Google Scholar]
- 56.Madar I, Ravert HT, Du Y, Hilton J, Volokh L, Dannals RF, et al. Characterization of uptake of the new PET imaging compound 18F-fluorobenzyltriphenyl phosphonium in dog myocardium. J Nucl Med. 2006;47:1359–66. [PubMed] [Google Scholar]
- 57.Kim DY, Kim HS, Le UN, Jiang SN, Kim HJ, Lee KC, et al. Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl)triphenylphosphonium cation, in a rat myocardial infarction model. J Nucl Med. 2012;53:1779–85. doi: 10.2967/jnumed.111.102657. [DOI] [PubMed] [Google Scholar]
- 58.Kim DY, Kim HJ, Yu KH, Min JJ. Synthesis of [18F]-labeled (6-fluorohexyl)triphenylphosphonium cation as a potential agent for myocardial imaging using positron emission tomography. Bioconjugate Chem. 2012;23:431–437. doi: 10.1021/bc2004439. [DOI] [PubMed] [Google Scholar]
- 59.Kim DY, Kim HJ, Yu KH, Min JJ. Synthesis of [18F]-labeled (2-(2-fluoroethoxy)ethyl)triphenylphosphonium cation as a potential agent for myocardial imaging using positron emission tomography. Bioorg Med Chem Lett. 2012;22:319–22. doi: 10.1016/j.bmcl.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Madar I, Ravert H, Nelkin B, Abro M, Pomper M, Dannals R, et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging. 2007;34:2057–65. doi: 10.1007/s00259-007-0500-8. [DOI] [PubMed] [Google Scholar]
- 61.Kim DY, Yu KH, Bom HS, Min JJ. Synthesis of (4-[18F]fluorophenyl)triphenylphosphonium as a mitochondrial voltage sensor for PET. Nucl Med Mol Imaging. 2007;41:561–5. [Google Scholar]
- 62.Madar I, Ravert H, Dipaula A, Du Y, Dannals RF, Becker L. Assessment of severity of coronary artery stenosis in a canine model using the PET agent 18F-fluorobenzyl triphenyl phosphonium: comparison with 99mTc-tetrofosmin. J Nucl Med. 2007;48:1021–30. doi: 10.2967/jnumed.106.038778. [DOI] [PubMed] [Google Scholar]
- 63.Higuchi T, Fukushima K, Rischpler C, Isoda T, Javadi MS, Ravert H, et al. Stable delineation of the ischemic area by the PET perfusion tracer 18F-fluorobenzyl triphenyl phosphonium after transient coronary occlusion. J Nucl Med. 2011;52:965–9. doi: 10.2967/jnumed.110.085993. [DOI] [PubMed] [Google Scholar]
- 64.Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 2005;70:222–30. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 65.Pasqualini R, Duatti A. Synthesis and characterization of the new neutral myocardial imaging agent [99mTcN(noet)2](noet =N-ethyl-N-ethoxydithiocarbamato) J Chem Soc Chem Commun. 1992;18:1354–5. [Google Scholar]
- 66.Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, et al. Bis(dithiocarbamato) nitrido technetium-99m radiopharmaceuticals: a class of neutral myocardial imaging agents. J Nucl Med. 1994;35:334–41. [PubMed] [Google Scholar]
- 67.Riou LM, Ghezzi C, Mouton O, Mathieu JP, Pasqualini R, Comet M, et al. Cellular uptake mechanisms of 99mTcN-NOET in cardiomyocytes from newborn rats: calcium channel interaction. Circulation. 1998;98:2591–7. doi: 10.1161/01.cir.98.23.2591. [DOI] [PubMed] [Google Scholar]
- 68.Riou LM, Ghezzi C, Vanzetto G, Broisat A, Mathieu JP, Bontronl R, et al. Verapamil does not inhibit 99mTcN-NOET uptake in situ in normal or ischemic canine myocardium. J Nucl Med. 2003;44:981–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.