Abstract
Reward comparison in the brain is thought to be achieved through the use of a ‘common currency’, implying that reward value representations are computed on a unique scale in the same brain regions regardless of the reward type. Although such a mechanism has been identified in the ventro-medial prefrontal cortex and ventral striatum in the context of decision-making, it is less clear whether it similarly applies to non-choice situations. To answer this question, we scanned 38 participants with fMRI while they were presented with single cues predicting either monetary or erotic rewards, without the need to make a decision. The ventral striatum was the main brain structure to respond to both cues while showing increasing activity with increasing expected reward intensity. Most importantly, the relative response of the striatum to monetary vs erotic cues was correlated with the relative motivational value of these rewards as inferred from reaction times. Similar correlations were observed in a fronto-parietal network known to be involved in attentional focus and motor readiness. Together, our results suggest that striatal reward value signals not only obey to a common currency mechanism in the absence of choice but may also serve as an input to adjust motivated behaviour accordingly.
Keywords: reward value, motivation, striatum, fMRI, common currency
INTRODUCTION
The ability to estimate the value of expected rewards is crucial for adaptive behaviour. How this operation is implemented in the brain is a key question, which has been extensively studied in Decision Neuroscience. Modern theories suggest that efficient decision-making relies on the computation of a ‘common neural currency’ allowing the value of different rewards to be compared on a single scale (Sugrue et al., 2005; Levy and Glimcher, 2012). The concept of a common currency implies two important hypotheses at the brain level. First, reward value should be represented centrally in the brain, meaning that increasing levels of anticipated reward should elicit increasing activity in a unique set of brain regions regardless of reward type. Second, reward value should be encoded along a common reference scale with respect to other available options, in such a way that the relative brain activity elicited by two different rewards should be directly proportional to their relative expected utility. A wealth of fMRI studies in the field of decision-making has provided evidence supporting these two hypotheses. These studies have shown that, regardless of reward type, the computation of decision values systematically engages two key brain regions, namely the ventral striatum and ventro-medial prefrontal cortex (vmPFC) (Peters and Büchel, 2009; Levy and Glimcher, 2012; Clithero and Rangel, 2013). Moreover, brain activity in these regions was found to correlate with desirability ratings (Knutson et al., 2007; Hare et al., 2009), willingness-to-pay (Hare et al., 2008; Plassmann et al., 2010) and choice preferences (Chib et al., 2009; FitzGerald et al., 2009), suggesting that decision values are represented along a common scale regardless of reward type. The consistency of those results has been well illustrated in several recent meta-analyses (Peters and Buchel, 2010; Bartra et al., 2013).
In the present study, we investigate whether the concept of common currency similarly applies to motivational values and not just decision values. Motivational values are computed when there is a variety of reward-predicting cues in the environment, while no explicit choice is required. This happens for instance when browsing a Christmas catalogue or walking down a busy street and being exposed to a multitude of shop signs. Are the motivational values derived from these shop signs encoded with a common currency, or are they computed independently of one another? Given that the absence of choice eliminates the need to perform explicit comparisons between potential rewards, it is unclear whether the use of a common frame of reference is maintained, or whether these reward cues are treated in isolation.
Thus, we are addressing two main questions. First, does the computation of expected reward value based on incentive cues recruit the same brain regions as observed during decision-making? In the absence of choice, this question has been examined using cue-reactivity or conditioning protocols in which participants passively anticipate rewards. However, most of these protocols have focused on one type of reward only. Yet, comparing different expected rewards within the same individuals is necessary to test the hypothesis of a common currency. Only a handful of brain imaging studies have directly addressed this question in humans. Two studies comparing monetary and social incentive cues have reported increasing activity in the striatum in response to increasing amounts of both rewards (Spreckelmeyer et al., 2009; Rademacher et al., 2010). Another study comparing money- vs juice-predicting cues found overlapping activity solely in the vmPFC (Kim et al., 2011). More studies using similar direct comparisons between rewards are needed to strengthen those results.
Second, an open question is whether motivational values are represented on a common scale in the absence of choice. Even when overt choices are not required, adjusting energy expenditure and attention level according to relative preferences is important (Montague and King-Casas, 2007; Vlaev et al., 2011). For example, one might invest minimal effort in obtaining reward A if a preferred reward B is known to be available at a later time. To achieve such optimal tuning of motivated behaviour, it is crucial to encode expected reward value on a common frame of reference. In line with this idea, several studies have shown that brain activity in the striatum and vmPFC keeps track of ordinal preferences and reflects the most or the least desirable reward in a given context (Tremblay and Schultz, 1999; Cromwell et al., 2005; Elliott et al., 2008). In healthy individuals, relative responses to food vs monetary cues in the ventral striatum were found to predict individual differences in the relative motivation for these rewards (Clithero et al., 2011). Recent results from our laboratory have further shown a differential reactivity of the striatum to monetary vs non-monetary cues in pathological gambling, a behavioural addiction in which the urge to procure money overrides the incentive value of alternative rewards (Sescousse et al., 2013). This effect was accompanied by a similar difference in the motivation to obtain those rewards, as reflected by reaction times. These findings suggest that striatal cue reactivity might thus represent a meaningful index of relative motivation, used to adjust behaviour accordingly.
The present study investigated the use of a common neural currency for representing expected reward value in the absence of choice. To this end, we used fMRI and an incentive delay protocol manipulating monetary and erotic cues independently (Sescousse et al., 2010). Monetary rewards have been widely studied and are now considered as a benchmark for reward processing. In contrast, much less is known about sexual stimuli, which are yet highly pervasive in our modern societies and have a crucial biological value (Georgiadis and Kringelbach, 2012). Based on our question, we focused our analyses on the cue-related phase (see Sescousse et al., 2010 for an analysis of the reward outcome phase). The hypothesis of a common currency leads to two main predictions: the incentive value of monetary and erotic cues should be represented in the same brain region(s), and any difference in their subjective valuation should be expressed in relative brain activity levels. Based on previous literature, we expected those conditions to be met in the ventral striatum, and possibly in the vmPFC. We measured reward value both in terms of motivation (‘wanting’) and pleasure (‘liking’) by collecting reaction times (RTs) and subjective ratings, respectively.
EXPERIMENTAL PROCEDURES
Participants
Two groups of healthy right-handed participants totalizing 38 individuals (mean age = 27.5 ± 6.8 years) were included in this study. All participants were heterosexual males because men are generally more responsive to visual sexual stimuli than women (Hamann et al., 2004). Data from these two groups (18 and 20 participants, respectively) were previously reported in two separate studies using the same protocol (Sescousse et al., 2010; Sescousse et al., 2013). These studies focused on different questions than the one currently at stake, namely the comparison of primary vs secondary reward outcomes in healthy controls, and the comparison of reward processing between healthy controls and pathological gamblers. The results reported in the current study are therefore entirely original, while benefiting from the statistical power provided by pooling those two groups. All participants gave written informed consent to be part of the experiment, which was approved by the local ethics committee and performed in accordance with the principles of the Declaration of Helsinki.
Sexual arousability was assessed at intake using the Sexual Arousability Inventory (SAI; Hoon and Chambless, 1998). The mean SAI score was 91.1 ± 12.0, which is comparable with the score reported in the reference population (Hoon and Chambless, 1998: 90.6 ± 14.7). Depressive symptoms were measured with the Beck Depression Inventory (BDI; Beck and Beck, 1972) in group 1 (mean score: 1.4 ± 2.0) and the Hospital Anxiety and Depression scale (Zigmond and Snaith, 1983) in group 2 (mean score: 3.4 ± 2.3). Participants in group 2 also underwent a psychiatric interview and were screened for psychiatric disorders (as part of the matching with pathological gamblers). In both groups, participants reporting no interest whatsoever in erotica or showing low sexual arousability (cut-off SAI: 69) were excluded at intake. Moreover, participants showing depressive symptoms (as assessed by the psychiatric interview in group 2 or based on a cut-off of 6 on the BDI in group 1) were excluded.
To further ensure that all participants would be in a similar state of motivation to see erotic stimuli, we asked them to avoid any sexual contact during a period of 24 h before the scanning session. We also sought to enhance the motivation for money by telling the participants that the financial compensation for their participation would amount to the winnings accumulated in one of the runs of the study. For ethical reasons though, and unbeknownst to the participants, they all received a fixed amount at the end of the experiment.
Task
The task is the same as described by Sescousse et al. (2010; 2013). Each trial consisted of an anticipation phase, a discrimination task and an outcome phase (Figure 1). During anticipation, participants saw 1 of 12 explicit cues announcing the type (monetary/erotic), probability (25/50/75%) and intensity (low/high) of an upcoming reward (2.5 s). An additional control cue was associated with a null reward probability. After a variable delay period (question mark representing a pseudorandom draw, 1.5–4.5 s), participants were asked to perform a target discrimination task. If they answered correctly within <1 s, they were then allowed to view the outcome of the pseudorandom draw. RTs were later used as an index of motivation. In rewarded trials, outcomes took the form of an erotic image or a sum of money displayed on a safe (1.5 s), whose intensity was high or low depending on the preceding cue (see below). Following each reward outcome, participants had 2.5 s to provide a hedonic rating by moving a cursor along a 1–9 scale (1 = very little pleased and 9 = very highly pleased). In non-rewarded and control trials, participants were presented with ‘scrambled’ pictures. A fixation cross was finally used as an inter-trial interval of variable length (2–5 s).
Fig. 1.
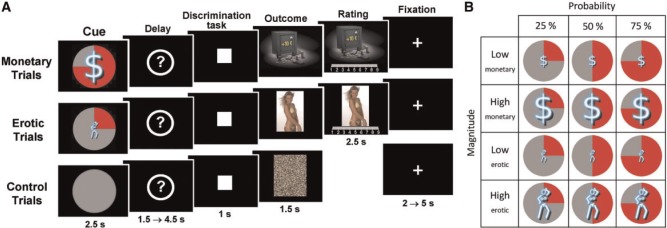
(A) Incentive delay task. Subjects first saw a cue informing them about the type (blue pictogram), intensity (size of pictogram) and probability (pie chart) of an upcoming reward. Following a delay period, participants had to perform a target discrimination task within <1 s. The target was either a triangle (left button press required) or a square (right button press required). Both their performance and the result of the pseudorandom draw determined the nature of the outcome. In rewarded trials, subjects saw a monetary amount displayed on a safe (top) or an erotic picture (middle) and had to provide a hedonic rating on a continuous scale. In non-rewarded and control trials, subjects saw a scrambled picture (bottom). (B) Overview of monetary and erotic cues used across the experiment.
The task was divided into several runs of 57 trials each. Participants from group 1 performed four runs (i.e. 228 trials), whereas participants from group 2 performed three runs (i.e. 171 trials, because of time constraints). To correct this imbalance in task length and avoid any bias, the data from the fourth run in group 1 were discarded from all analyses. Each run included four repetitions of each cue, with the exception of the control condition, repeated nine times. Within each run the order of the different conditions was pseudorandomized and optimized for further signal deconvolution. The order of the runs was counterbalanced between participants. Before scanning, all subjects were given oral instructions and familiarized with the cognitive task in a short training session.
Task stimuli
Two categories (high and low intensity) of erotic pictures and monetary gains were used. Nudity being the main criteria driving the reward value of erotic stimuli, we separated them into a ‘low intensity’ group displaying females in underwear or bathing suits and a ‘high intensity’ group displaying naked females in an inviting posture. Each erotic picture was presented only once during the course of the task to avoid habituation. A similar element of surprise was introduced for the monetary rewards by randomly varying the amounts at stake: the low amounts were either 1, 2 or 3 € and the high amounts were either 10, 11 or 12 €. The pictures displayed in non-rewarded and control trials were scrambled versions of the pictures used in rewarded trials and hence contained the same information in terms of chromaticity and luminance.
fMRI data acquisition
Imaging was performed on a 1.5 T Siemens Sonata scanner, using an eight-channel head coil. Each of the functional runs comprised 296 volumes. Twenty-six interleaved slices parallel to the AC-PC line were acquired per volume (field of view = 220 mm, matrix 64 × 64, voxel size = 3.4 × 3.4 × 4 mm, gap 0.4 mm), using a gradient-echo echoplanar (EPI) T2*-weighted sequence (repetition time = 2500 ms, echo time = 60 ms, flip angle = 90°). To improve the local field homogeneity and hence minimize susceptibility artefacts, a manual shimming was performed within a rectangular region including the OFC and the basal ganglia. A high-resolution T1-weighted structural scan was also acquired in each participant.
fMRI data analysis
Preprocessing and statistical analyses of fMRI data were conducted with SPM2 (www.fil.ion.ucl.ac.uk/spm/software/spm2), to ensure direct comparability with our previous studies. The first four functional volumes of each run were removed, and the remaining images were corrected for slice-timing artefacts, and spatially realigned to the first image of each time series. We then searched for residual artefacts in the time series with the tsdiffana utility (http://imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics) and modelled them with dummy regressors in our general linear model. The functional images were then normalized to the MNI stereotaxic space using SPM2 EPI template, and spatially smoothed with a 10 mm full width at half maximum isotropic Gaussian kernel. Anatomical scans were normalized to the MNI space using the icbm152 template brain and averaged across all participants.
We then ran a first-level analysis modelling brain responses to reward anticipation and outcome. Anticipation-related responses were modelled as 2.5 s box-car functions time locked to the onset of the cue. Monetary and erotic cues were modelled separately and modulated by two orthogonal parametric regressors accounting for reward probability and intensity. The control condition was modelled in a separate regressor. Outcome-related responses were modelled as events time locked to the appearance of the reward. Monetary and erotic outcomes were modelled separately, as well as rewarded vs non-rewarded outcomes, leading to four different regressors. Two covariates linearly modelling reward probability and hedonic ratings were further added to each rewarded condition, while another covariate modelling probability was added to each of the non-rewarded conditions. A final regressor modelled the appearance of a scrambled picture in the control condition. All these regressors were subsequently convolved with the canonical hemodynamic response function. In addition, the six motion parameters estimated during realignment were included as regressors of no interest. A high-pass filter with a cut-off of 128 s was applied to the time series. Contrast images were calculated based on the parameter estimates output by the general linear model, and were then entered in a second-level group analysis.
Brain regions recruited by the anticipation of monetary and erotic rewards were first identified using the contrasts ‘monetary cue > control’ and ‘erotic cue > control’. Modulation of brain activity by reward probability and intensity was further assessed with the corresponding parametric regressors. Monetary and erotic cues were directly contrasted, and resulting brain activity was further correlated with RTs using a simple regression analysis. All results are reported at a cluster-level P < 0.05 corrected for multiple comparisons across the whole brain, combined with a voxel-level uncorrected P < 0.001 or less. Anatomical localization of functional clusters was performed based on a probabilistic atlas (Hammers et al., 2003).
Additional brain-behaviour correlations across participants were performed within striatal regions of interest (ROIs). Percent signal change was extracted using MarsBaR (http://marsbar.sourceforge.net/), within functional ROIs defined from independent whole-brain analyses. For a given condition in a given ROI, it was calculated as the effect size of that condition (beta value) divided by the mean activity of that ROI and multiplied by 100.
RESULTS
Behaviour
RTs on the discrimination task and hedonic ratings were analysed in two separate three-way ANOVAs including reward type, probability and intensity as within-subject factors. RT data were accidentally lost for one participant, and hedonic ratings could not be fully collected for another participant owing to technical problems. Therefore, analyses of both RTs and ratings were restricted to 37 participants. RT analyses were performed on successful trials (excluding control trials), which accounted for 85–100% of all trials depending on participants (mean hit rate = 97.1 ± 3.5).
Participants showed similar RTs following monetary and erotic cues (main effect of reward type: F(1,36) = 0.79, P = 0.41), suggesting that those cues had similar incentive values across the whole group. Participants were also faster for high compared with low rewards (main effect of intensity: F(1,36) = 51.89, P < 0.001), but did not show any difference between monetary and erotic cues (intensity × reward interaction: F(1,36) = 0.44, P = 0.51, Figure 2A). This suggests that our manipulation of reward intensity was perceived equally well for both rewards and confirms that participants were motivated. There was also a general trend for decreasing RTs with increasing probability (main effect of probability: F(2,72) = 3.18, P = 0.05), but this effect was essentially driven by monetary cues (probability × reward interaction: F(2,72) = 9.12, P < 0.001).
Fig. 2.
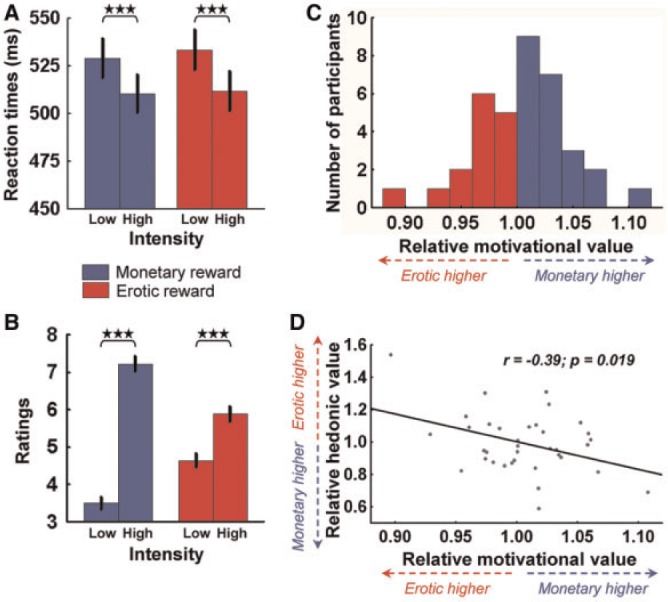
Behavioural results. (A) Plot of mean RTs according to reward type and intensity in the discrimination task. There is a main effect of intensity, but no main effect of reward or intensity × reward interaction across the whole group. (B) Plot of mean hedonic ratings according to reward type and intensity. There is a robust main effect of intensity on the ratings, demonstrating that the high vs low categories were well perceived by the participants. (C) Distribution of relative motivational value across participants, defined as the ratio of RTs following erotic cues vs monetary cues. (D) Plot of relative hedonic value (Ratingserotic/Ratingsmonetary) as a function of the relative motivational value (RTerotic/RTmonetary). Those two measures showed a significant negative correlation. Error bars indicate SEM. Asterisks denote significance of Tukey’s HSD tests (***P < 0.001).
Similarly as for RTs, mean hedonic ratings were not different between monetary and erotic rewards (main effect of reward type: F(1,36) = 0.21, P = 0.65). High rewards were perceived as more pleasant than low rewards (main effect of intensity: F(1,36) = 180.82, P < 0.001), and this effect was even more pronounced for monetary rewards (intensity × reward interaction: F(1,36) = 123.60, P < 0.001, Figure 2B). Moreover, hedonic ratings linearly increased when reward probability decreased (main effect of probability: F(2,72) = 8.28, P < 0.001), probably reflecting a prediction error-like computation for both rewards (probability × reward interaction: F(2,72) = 2.71, P = 0.07).
Finally, we built two behavioural indices of relative reward value, which were used to study inter-individual differences. First, we computed an index of relative motivational value (RMV), based on the ratio of mean RTs following erotic compared with monetary cues, i.e. RTerotic/RTmonetary (Figure 2C). A value >1 reflects a higher relative value of monetary incentives and vice-versa. We then computed a similar ratio with the ratings (Ratingerotic/Ratingmonetary), reflecting relative hedonic value (RHV). In this case, a value >1 reflects a higher relative value of erotic rewards and vice-versa. Similar approaches have been used in recent papers (e.g. Carter et al., 2009; Clithero et al., 2011). The RMV index had a mean of 1.01 (SD = 0.04) across participants, whereas the RHV index had a mean of 0.99 (SD = 0.18), indicating no particular skewness towards either reward. Both indices appeared to be normally distributed (Shapiro–Wilk test for normality, P > 0.36). Interestingly, the two indices showed a negative correlation (Figure 2D), suggesting that the relative value of the two rewards was consistently reflected at the motivational (RTs) and hedonic (ratings) levels.
fMRI results
We first examined the brain responses to each reward cue separately, using the control condition as a baseline. Similar patterns of activity were observed throughout the whole brain (Figure 3A). In particular, we found robust activations in the bilateral striatum for both monetary (x,y,z = −9, 12, −15, T = 7.89; 9, 6, 0, T = 9.17) and erotic cues (x,y,z = −9, 12, −12, T = 6.09; 6, 15, 3, T = 6.86). Other foci, including the vmPFC, thalamus and visual areas, are reported in Supplementary Tables S1 and S2.
Fig. 3.
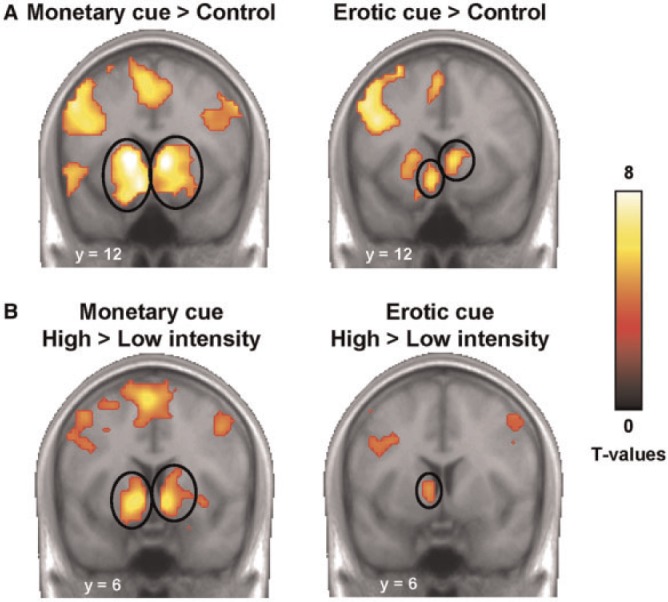
Brain responses to reward cues. (A) Activation of the striatum in response to monetary and erotic cues as compared with the control condition. Activations are overlaid on an average anatomical scan of all subjects (display threshold: P < 0.0001 voxel-level uncorrected and P < 0.05 cluster-level corrected). (B) Higher response to high vs low reward cues in the ventral striatum, for both monetary and erotic cues (display threshold: P < 0.0005 voxel-level uncorrected and P < 0.05 cluster-level corrected).
Then, we contrasted cues predicting high vs low reward, for each reward type separately (Figure 3B). This analysis revealed focal activity in the ventral striatum, for both monetary (x,y,z = −12, 6, −6, T = 6.55; 9, 9, −9, T = 7.02) and erotic cues (x,y,z = −9, 6, 0, T = 4.60), suggesting that this region is sensitive to expected reward intensity regardless of reward type. Other foci are reported in Supplementary Tables S3 and S4. We performed the same analysis for expected reward probability, using the corresponding parametric regressors. However, we did not observe any correlation between expected probability and brain activity in the reward system, for either monetary or erotic cues (at P < 0.001 uncorrected). This is consistent with our behavioural results showing that RTs are more sensitive to expected reward intensity than to expected probability, and further suggests that reward value representation in the ventral striatum is essentially driven by expected intensity.
Finally, we performed a direct comparison between monetary and erotic cues (Figure 4). This analysis revealed that, despite responding to both monetary and erotic cues, the bilateral ventral striatum responded more strongly to monetary cues (x,y,z = −12, 12, −9, T = 4.74; 15, 6, −6, T = 5.84). No brain region was found to respond more strongly to erotic cues. To investigate whether this differential cue reactivity was related to relative reward value, we examined it as a function of our RMV index. We extracted the mean percent signal change for monetary and erotic cues, computed the difference and then plotted it as a function of RTerotic/RTmonetary. In both the left and right ventral striatum, we observed a significant positive correlation, reflecting that the differential reactivity of the striatum to monetary vs erotic cues is predictive of the relative vigour exerted to obtain those rewards. We investigated the same correlation at the whole-brain level, using the RMV index as a between-subject covariate. This analysis also revealed activity in the bilateral striatum (although not surviving a proper correction for multiple comparisons), as well as in the dorso-medial prefrontal cortex (dmPFC), premotor cortex and intra-parietal sulcus (IPS) (Figure 5).
Fig. 4.
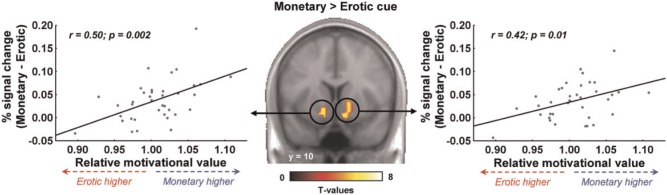
Differential cue reactivity in the ventral striatum. The ventral striatum responds more strongly to monetary than to erotic cues across the whole group. Activations are overlaid on an average anatomical scan of all subjects (display threshold: P < 0.0001 voxel-level uncorrected and P < 0.05 cluster-level corrected). This differential reactivity to monetary vs erotic cues is correlated with the relative motivational value of these rewards as indexed by reaction time ratio (RTerotic/RTmonetary).
Fig. 5.
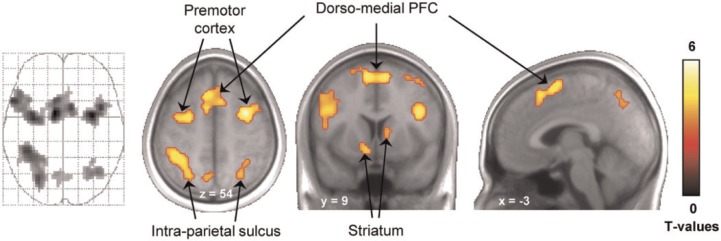
Brain regions where differential cue reactivity are correlated with relative motivation for monetary and erotic rewards. This T-map originates from a simple regression across participants between the contrast ‘monetary > erotic cue’ and relative motivational value as indexed by reaction time ratio (RTerotic/RTmonetary). Activations are overlaid on an average anatomical scan of all subjects (display threshold: P < 0.001 voxel-level uncorrected, cluster size ≥38). Activations in dorso-medial PFC, premotor cortex and left IPS survive a cluster-level P < 0.05 corrected for multiple comparisons across the whole brain. Note that this simple regression is conceptually identical to the plots presented in Figure 4, but statistically independent.
DISCUSSION
The brain activity pattern observed in the ventral striatum confirms the two predictions made in the introduction. First, we showed that, regardless of reward type, expected reward value was represented centrally in this region. This is in line with a wealth of previous studies showing common value signals in the striatum for primary and secondary rewards (Izuma et al., 2008; Valentin and O'Doherty, 2009; Izuma et al., 2010), for gains and losses (Tom et al., 2007) or for magnitude, delay and probability (Dreher et al., 2006; Kable and Glimcher, 2007; Tobler et al., 2007; Peters and Buchel, 2009; Prevost et al., 2010; Dreher, 2013). Second, we found that the relative response of the striatum to monetary vs erotic cues was correlated with the relative motivational value of these rewards as indexed by RTs. Together, these results are consistent with a common currency mechanism for the representation of motivational value in the ventral striatum in the absence of choice.
Our results indicate that visual erotic stimuli are powerful motivators of behaviour, which can elicit robust anticipatory brain responses. This is important given the increasing pervasiveness of these stimuli in our daily environment, in particular through advertisement (Reichert, 2002). It is also consistent with prior work showing that people are willing to wait or exert effort to gain extended access to visual erotic stimuli (Prevost et al., 2010). Even though the ventral striatum responded to both monetary and erotic cues, responses to monetary cues were stronger. A similar difference was reported in other studies comparing monetary with food or social cues (Daniel and Pollmann, 2010; Rademacher et al., 2010; Clithero et al., 2011). One possibility is that the dollar sign used for monetary cues has a universal and automatic meaning, in contrast to the somewhat abstract pictogram used for erotic cues. As a result, the acquisition of incentive value through conditioning might have been more immediate and efficient for monetary compared with erotic cues.
Importantly though, the differential striatal reactivity to monetary vs erotic cues varied substantially between individuals and covaried with relative levels of motivation. Previous studies have shown that reward value is flexibly and dynamically encoded in the brain, ultimately contributing to adaptive behaviour. Cue- and outcome-related value signals encoded by midbrain and vmPFC neurons are scaled according to the local distribution of reward intensities, allowing for an optimal exploitation of the limited firing range of neurons (Tobler et al., 2005; Padoa-Schioppa, 2009; Kobayashi et al., 2010). In the context of gambling or learning, feedback-related value signals in the striatum are often computed relatively to a meaningful reference point, typically the mean of all possible outcomes or the value of an unchosen option (Breiter et al., 2001; Kuhnen and Knutson, 2005; Nieuwenhuis et al., 2005; Lohrenz et al., 2007). Similarly, decision-value signals were found to be encoded in a relative fashion in various choice paradigms. For instance, striatal and vmPFC activity correlates with the difference in value between available options (FitzGerald et al., 2009) or between attended and unattended items (Lim et al., 2011). Here, we extend those findings to a non–decision-making context. We show that, when single cues are presented in isolation, corresponding value signals in the striatum are not computed in isolation, but relatively to other cues known to be available in the same environment. This is in line with prior work showing that, in the vmPFC and ventral striatum, relative responses to individually presented pleasant stimuli can predict later choices between those stimuli (Lebreton et al., 2009; Smith et al., 2010; Levy et al., 2011). Together, these findings suggest that, even in the absence of choice, implicit and automatic comparison mechanisms are occurring. As suggested in the introduction, those mechanisms might be used for optimal effort allocation.
Several experiments have suggested that the ventral striatum plays a role in translating appetitive value signals triggered by external cues into motor behaviour. In an fMRI task requiring cognitive or physical efforts to obtain a reward, cue-elicited striatal activity was found to predict variations in effort allocation across participants (Schmidt et al., 2012). Similarly in rats, the firing of nucleus accumbens neurons in response to a reward-predictive tone was causally correlated with the vigour of subsequent approach behaviour (McGinty et al., 2013). This relationship was found to be particularly stable across time, as demonstrated by a recent fMRI study in which individual differences in striatal cue reactivity to food and erotic pictures were shown to predict weight gain and sexual activity 6 months later (Demos et al., 2012). Complementing those findings, our study shows that the relative speed with which participants react following monetary or erotic cues is in direct proportion to the relative striatal activity evoked by those cues. This suggests that the striatal signals observed in the current experiment do not merely reflect the pavlovian value of anticipated rewards, but carry an incentive value that further drives behavioural performance. This idea is consistent with the location of the striatum at the crossroads of various cortico-subcortical loops, which places it in an ideal position to implement the interface between motivation and action (Delgado, 2007; Knutson and Greer, 2008; Haber and Knutson, 2010).
In addition to the ventral striatum, activity in the dmPFC, IPS and premotor cortex was found to correlate with our RMV index. The dmPFC is known to be sensitive to anticipated efforts (Kurniawan et al., 2013), while the IPS and premotor cortex are involved in attentional focus and motor preparation (Corbetta and Shulman, 2002). Thus, these regions likely play a role in mediating the effect of value representations on RTs in the present context, through increased attention and motor readiness. This interpretation is consistent with recent accounts showing that the dmPFC, IPS and motor areas are conjointly involved in transforming stimulus value signals into motor commands during binary choices (Hare et al., 2011).
Overall, our results show that striatal value signals elicited by incentive cues reflect both the intensity of expected rewards as well as their relative motivational value compared with other rewards. Remarkably, these signals are computed regardless of reward type and the need to make a decision. These observations are compatible with reinforcement learning accounts of ventral striatal function. In this framework, the ventral striatum is described as a critic module generating cached, model-free, predictions indexing reward value in the form of an abstract common currency (McDannald et al., 2012; Dolan and Dayan, 2013). These model-free value representations are blind to the specific features of rewards and mostly useful to guide habitual behaviour. Our results further reveal that these signals are shared with other regions involved in attention (IPS) and motor preparation (dmPFC and premotor cortex), supporting the hypothesis that they serve as an input to adjust motivated behaviour.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank the staff of CERMEP–Imagerie du Vivant for helpful assistance with data collection. This work was performed within the framework of the LABEX ANR-11-LABEX-0042 of Université de Lyon, within the program ‘Investissements d'Avenir’ (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR). This work was also supported by grants from the Fyssen foundation and ANR EMCO to J.-C.D. and a grant from the MILDT/INSERM (MIL0805) to J.-C.D. and G.S. G.S. was funded by a PhD fellowship from the French Ministry of Research and the Fondation pour la Recherche Médicale. Y.L. was supported by a PhD fellowship from Pari Mutuel Urbain (PMU).
REFERENCES
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–27. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technic. Postgraduate Medicine. 1972;52(6):81–5. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30(2):619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Carter RM, Macinnes JJ, Huettel SA, Adcock RA. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in Behavioral Neuroscience. 2009;3:21. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O'Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. The Journal of Neuroscience. 2009;29(39):12315–20. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst106. nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, Huettel SA. Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Frontiers in Human Neuroscience. 2011;5:87. doi: 10.3389/fnhum.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Experimental Brain Research. 2005;162(4):520–5. doi: 10.1007/s00221-005-2223-z. [DOI] [PubMed] [Google Scholar]
- Daniel R, Pollmann S. Comparing the neural basis of monetary reward and cognitive feedback during information-integration category learning. The Journal of Neuroscience. 2010;30(1):47–55. doi: 10.1523/JNEUROSCI.2205-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of New York Academy of Science. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience. 2012;32(16):5549–52. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Dayan P. Goals and habits in the brain. Neuron. 2013;80(2):312–25. doi: 10.1016/j.neuron.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC. Neural coding of computational factors affecting decision making. Progress in Brain Research. 2013;202:289–320. doi: 10.1016/B978-0-444-62604-2.00016-2. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex. 2006;16(4):561–73. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Medial orbitofrontal cortex codes relative rather than absolute value of financial rewards in humans. European Journal of Neuroscience. 2008;27(9):2213–8. doi: 10.1111/j.1460-9568.2008.06202.x. [DOI] [PubMed] [Google Scholar]
- FitzGerald TH, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. The Journal of Neuroscience. 2009;29(26):8388–95. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis JR, Kringelbach ML. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Progress in Neurobiology. 2012;98(1):49–81. doi: 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7(4):411–6. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping. 2003;19(4):224–47. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. The Journal of Neuroscience. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Schultz W, Camerer CF, O'Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proceedings of the National Academy of Sciences USA. 2011;108(44):18120–25. doi: 10.1073/pnas.1109322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon E, Chambless D. Sexual arousability inventory and sexual arousability inventory—expanded. In: Davis C, Yarber W, Bauserman R, Schreer R, Davis S, editors. Handbook of Sexuality-Related Measures. Thousand Oaks, CA: Sage; 1998. pp. 71–4. [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2010;22(4):621–31. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex. 2011;21(4):769–776. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1511):3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53(1):147–56. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Pinto de Carvalho O, Schultz W. Adaptation of reward sensitivity in orbitofrontal neurons. The Journal of Neuroscience. 2010;30(2):534–44. doi: 10.1523/JNEUROSCI.4009-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, Dayan P, Dolan RJ. Effort and valuation in the brain: the effects of anticipation and execution. The Journal of Neuroscience. 2013;33(14):6160–69. doi: 10.1523/JNEUROSCI.4777-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64(3):431–9. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Current Opinion in Neurobiology. 2012;22(6):1027–38. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Lazzaro SC, Rutledge RB, Glimcher PW. Choice from non-choice: predicting consumer preferences from blood oxygenation level-dependent signals obtained during passive viewing. The Journal of Neuroscience. 2011;31(1):118–25. doi: 10.1523/JNEUROSCI.3214-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SL, O'Doherty JP, Rangel A. The decision value computations in the vmPFC and striatum use a relative value code that is guided by visual attention. The Journal of Neuroscience. 2011;31(37):13214–23. doi: 10.1523/JNEUROSCI.1246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrenz T, McCabe K, Camerer CF, Montague PR. Neural signature of fictive learning signals in a sequential investment task. Proceedings of the National Academy of Sciences USA. 2007;104(22):9493–8. doi: 10.1073/pnas.0608842104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Takahashi YK, Lopatina N, Pietras BW, Jones JL, Schoenbaum G. Model-based learning and the contribution of the orbitofrontal cortex to the model-free world. European Journal of Neuroscience. 2012;35(7):991–6. doi: 10.1111/j.1460-9568.2011.07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78(5):910–22. doi: 10.1016/j.neuron.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, King-Casas B. Efficient statistics, common currencies and the problem of reward-harvesting. Trends in Cognitive Sciences. 2007;11(12):514–9. doi: 10.1016/j.tics.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, Alting von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005;25(4):1302–9. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. The Journal of Neuroscience. 2009;29(44):14004–14. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. The Journal of Neuroscience. 2009;29(50):15727–34. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Neural representations of subjective reward value. Behavioural Brain Research. 2010;213(2):135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of Neuroscience. 2010;30(32):10799–808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost C, Pessiglione M, Metereau E, Clery-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. The Journal of Neuroscience. 2010;30(42):14080–90. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Reichert T. Sex in advertising research: a review of content, effects, and functions of sexual information in consumer advertising. Annual Review of Sex Research. 2002;13(1):241–73. [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Clery-Melin ML, Daunizeau J, Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biology. 2012;10(2):e1001266. doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Barbalat G, Domenech P, Dreher JC. Imbalance in the sensitivity to different types of rewards in pathological gambling. Brain. 2013;136:2527–38. doi: 10.1093/brain/awt126. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Redouté J, Dreher JC. The architecture of reward value coding in the orbitofrontal cortex. The Journal of Neuroscience. 2010;30:13095–104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. The Journal of Neuroscience. 2010;30(7):2490–5. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4(2):158–65. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nature Reviews Neuroscience. 2005;6(5):363–75. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O'Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. Journal of Neurophysiology. 2007;97(2):1621–32. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–8. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Valentin VV, O'Doherty JP. Overlapping prediction errors in dorsal striatum during instrumental learning with juice and money reward in the human brain. Journal of Neurophysiology. 2009;102(6):3384–91. doi: 10.1152/jn.91195.2008. [DOI] [PubMed] [Google Scholar]
- Vlaev I, Chater N, Stewart N, Brown GD. Does the brain calculate value? Trends in Cognitive Sciences. 2011;15(11):546–54. doi: 10.1016/j.tics.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


