Abstract
The neural and cognitive mechanisms by which primed constructs can impact on social behavior are poorly understood. In the present study, we used functional magnetic resonance imaging (fMRI) to explore how scrambled sentence priming can impact on mimicry behavior. Sentences involving pro/antisocial events from a first/third-person point of view were presented in short blocks, followed by a reaction-time assessment of mimicry. Behavioral results showed that both prosociality and viewpoint impact on mimicry, and fMRI analysis showed this effect is implemented by anterior medial prefrontal cortex (amPFC). We suggest that social primes may subtly modulate processing in amPFC in a manner linked to the later behavior, and that this same region also implements the top-down control of mimicry responses. This priming may be linked to processing of self-schemas in amPFC. Our findings demonstrate how social priming can be studied with fMRI, and have important implications for our understanding of the underlying mechanisms of prime-to-behavior effects as well as for current theories in social psychology.
Keywords: social priming, fMRI, mimicry, medial prefrontal cortex, top-down control, self-schema
INTRODUCTION
The question of whether and how primed constructs can impact on social behavior is currently a controversial one (Bower, 2012). Early studies in social psychology showed that individuals exposed to words related to the elderly stereotype tended to walk more slowly and those exposed to words related to rudeness behaved more impolitely (Bargh et al., 1996). Similar priming effects were reported later on a wide range of behaviors such as social attitude/judgment, cooperation decisions and helping behavior (Bargh et al., 2012), but not all findings were robust and replicable (Doyen et al., 2012; Pashler et al., 2012). The neural and cognitive mechanisms of any influence of priming on behavior are also unclear (Bargh et al., 2012; Powers and Heatherton, 2013).
This article builds on our recent demonstration of a robust prime-to-behavior effect in the domain of mimicry (Wang and Hamilton, 2013) and a recent neurocognitive model of the control of mimicry behavior (Wang and Hamilton, 2012). We aim to use functional magnetic resonance imaging (fMRI) to localize the neural implementation of this behavioral priming effect and thus advance our neurocognitive models of this phenomenon.
First, we provide a little background about how social priming impacts on mimicry. Mimicry occurs when a participant copies another person’s action without awareness and is considered a prosocial behavior (Lakin et al., 2003). This behavior is sensitive to social contexts, and increases following priming with abstract prosocial scrambled sentences (Lakin and Chartrand, 2003; Leighton et al., 2010). We recently demonstrated that this effect depends on the viewpoint present in the scrambled sentences (Wang and Hamilton, 2013). Specifically, participants mimic more following priming with prosocial behaviors presented in first-person (‘I share sweets with Lola and her friends’) or antisocial behaviors presented in third-person (‘Eric plays loud music to interrupt Sarah studying’), but mimic less when priming with prosocial behaviors in third-person (‘John gives Laura a warm and affectionate hug’) or antisocial behaviors in first-person (‘I cruelly bullied Stephanie about her weight problem’). In this article, we investigate the neurocognitive mechanisms responsible for this effect.
Different predictions for the brain systems involved in social priming of mimicry can be drawn from different literatures. Mimicry itself is likely to involve the human mirror neuron system in the inferior frontal and inferior parietal cortex (Heyes, 2011). However, the control of mimicry involves medial prefrontal cortex (mPFC) (Brass et al., 2009; Wang et al., 2011). A recent model called ‘STORM’ (social top-down response modulation) suggests that automatic imitation behaviors (like mimicry) can be implemented by the mirror neuron system but are subject to top-down control from prefrontal cortex (Wang and Hamilton, 2012). As mPFC has a particular role in implementing top-down control of mimicry based on social cues such as eye gaze (Wang et al., 2011), we test here whether mPFC plays a similar role when social cues come from implicit priming.
Turning to previous neuroimaging studies of implicit priming, a variety of results have been reported. For example, priming with individualism/collectivism self-construal was associated with activations in mPFC and middle frontal cortex (Chiao et al., 2010; Sui and Han, 2007). Eddington et al. (2007) primed participants with an approach/avoidance goal and found engagement of orbital prefrontal cortex. Bengtsson et al. (2011) primed participants with high or low self-esteem (being clever or stupid) and found anterior cingulate cortex (ACC) was modulated by the primes when participants performed a memory task. Negative results have also been reported. Powers and Heatherton (2013) used the classic scrambled sentence task to prime participants with social exclusion and found no changes at the neural level, even with a large sample. However, the positive results have all linked to prefrontal regions, including mPFC to implicit priming. A recent neurocomputation model also suggests mPFC as a central node for information processing during prime-to-behavior effects (Schröder and Thagard, 2013).
One limitation of the above studies is that few have examined both the priming phase and the response phase of a task during fMRI. Participants in these studies often completed priming procedures outside the scanner and then responded to new stimuli during an fMRI scan. Therefore, this experimental design only examined neural responses during the ‘behavior phase’ of prime-to-behavior effect (i.e. how the primed brain processes stimuli and regulates behavior), but lost the opportunity to explore the earlier neural responses during the ‘priming phase’ (i.e. how the brain processes the prime and how the prime changes one’s mindset).
This study aims to use fMRI to investigate the neural mechanism of social priming of mimicry. We adopted a paradigm similar to Wang and Hamilton (2013), where each participant alternated between completing a scrambled sentence task that primed different concepts and a finger tapping task that measured mimicry. We aim to address two key questions. First, what neural mechanism implements the control of mimicry responses by social primes? Second, what is the relationship between brain activity during the priming phase of the task and activity during the response phase?
Considering the first question, we hypothesize that mPFC could be a likely neural substrate for social priming of mimicry. Previous studies found mPFC as a key region in regulation of prosocial behavior (Raine et al., 2006; Masten et al., 2011; Rameson et al., 2012; Zaki and Mitchell, 2013). This is also the region highlighted in the STORM model and previous studies of mimicry as a center for top-down control (Wang and Hamilton, 2012). Finally, mPFC is linked to implicit priming in a number of previous studies, as highlighted above.
Considering the second question, there are two ways in which brain activity during priming might relate to activity during responding. One possibility is that prime-to-behavior effects result from passive carry over of earlier processing (Bargh, 2006). In this case, strong engagement during the priming phase might continue in a weaker fashion during the behavioral response and thus bias responding. Conversely, if prime-to-behavior effects reflect a more active control process, then weak signals during the priming phase might be amplified during responding. This study will examine these two questions.
METHODS
Participants
Twenty paid volunteers from the University of Nottingham (9 males and 11 females; mean ± s.d. age, 19.15 ± 1.15 years) participated in this experiment. All participants were right-handed native English speakers with no history of neurological problems. They gave their written informed consent to complete the experiment in accord with the local ethics board.
Stimuli and experimental design
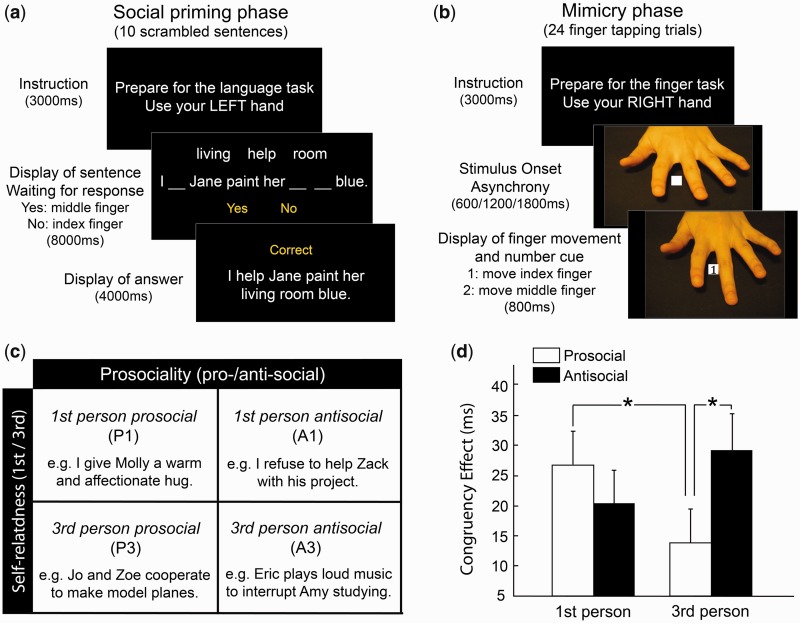
The task was presented with two alternating phases—a scrambled sentence priming task and a mimicry task. For the priming task, 64 sentences were adapted from our previous study of social priming of mimicry (Wang and Hamilton, 2013) (see details in Supplementary Materials). Four different categories of sentences were presented, in a 2 × 2 factorial design (prosociality × self-relatedness) (Figure 1c). These were first-person-prosocial sentences (P1, ‘I helped Jane paint her living room blue’), third-person-prosocial sentences (P3, ‘Tina and Arthur bake cakes for their friends’), first-person-antisocial sentences (A1, ‘I blame the project failure on Carmen harshly’) and third-person-antisocial sentences (A3, ‘Jerry snatched the strawberry lollipop from Mary’s hand’). Scrambled sentences were presented in blocks of 10 trials. Each block contained six prime sentences from the appropriate category, two ungrammatical sentences (which were created based on prime sentences) and two neutral (non-social) sentences. In each trial, the sentence was presented with three words missing, and the three filler words were presented above (Figure 1a). Participants were asked to judge if the words could be placed in the blanks to make a grammatical sentence. Following each judgment, feedback was given showing the complete sentence (for grammatical sentences) to reinforce the priming effect. Each trial lasted 12 s in total with up to 8 s available for the response and at least 4 s of feedback when the correct answer was displayed. As there was no intertrial interval for the sentence priming task, each sentence block lasted 120 s (10 sentences × 12 s each).
Fig. 1.
Behavioral tasks and results. In each session, participants completed two tasks: a sentence priming task and a mimicry task. In the priming task (a), they were provided with (c) four types of scrambled sentences manipulated by prosociality and self-relatedness; they had to decide if three filler words can make the scrambled sentence below grammatically correct. In the mimicry task (b), participants had to respond to a number cue (1 or 2) by pressing down their index or middle finger while observing congruent or incongruent finger movements on the background. The analysis of CE in the mimicry task suggested (d) an interaction between prosociality × self-relatedness (asterisks: statistically significant difference between two bars; vertical bars: standard error).
Following each block of sentence priming, participants performed the mimicry task. Five stimulus images were prepared. A preparatory stimulus showed a human hand above a black table. Four cue stimuli showed the same hand with either the index figure or middle finger touching the table, and the number 1 or 2 in a white box between the two fingers (Figure 1b). On each trial, the preparatory stimulus was presented for a variable duration (600/1200/1800 ms), followed by the number cue. When a response was recorded, the hand image vanished and a blank screen remained until the next trial. Intertrial intervals for the mimicry task ranged from 500 to 3000 ms, and each mimicry block lasted ∼96 s.
Participants were instructed to respond to number 1 with an index finger button press, and to number 2 with a middle finger button press. This therefore gives a 2 × 2 factorial design (observed finger movement × number cue) in which trials could be congruent (the observed finger movement matches performed movement) or incongruent (the observed finger movement does not match the performed movement). As before, mimicry was calculated as the reaction time (RT) difference between congruent and incongruent trials (the ‘Congruency effect’, Wang and Hamilton, 2013; Heyes, 2011). Mimicry trials were presented in blocks of 25 (six repetitions of each possible trial plus one), with the first trial dropped from analysis to remove task switching effects.
Procedure
Participants were told that they would complete two independent tests, a language test and a motor test, which would be alternated in the scanner to reduce fatigue (Bengtsson et al., 2011). Instructions for the sentence task were slightly modified from Wang and Hamilton (2013) to meet fMRI requirement and were presented as follows: ‘You will see three words and a sentence structure. Please judge if these words could make the structure into a grammatically correct sentence. Use your left hand to respond. Please make your decision fast and correct’. Instructions for the mimicry task were as follows: ‘You will see a hand and a white box in the middle, then a number will appear on the box. 1 means press your right index finger. 2 means press your right middle finger. Use your right hand to respond. Please press the key as fast as you can’.
Participants completed the study in two sessions, each with four blocks of scrambled sentences task (‘P1’, ‘P3’, ‘A1’ and ‘A3’) alternated with four blocks of mimicry task. The order of sessions, the order of priming blocks, the order of sentences in each block and the order of trials in mimicry task were all randomized across participants. Before going to the scanner, they completed a practice session with 10 neutral scrambled sentences followed by 25 mimicry trials to ensure they understood both tasks.
fMRI data acquisition
Imaging was performed using a 3T Phillips Achieva scanner, equipped with an eight-channel phased-array head coil. Thirty-eight axial slices (field of view: 192 × 192 × 116 mm3, matrix: 64 × 64; slice thickness: 3.5 mm) parallel to bicommissural line (AC-PC, i.e. the line joining the anterior and posterior commissure) were acquired using a T2*-weighted dual-echo planar imaging sequence (TR: 2250 ms; TE1: 20 ms; TE2: 45 ms; flip angle: 80°). After the functional runs, structural images were also acquired for each participant using high-resolution T1-weighted MPRAGE sequence.
fMRI data analysis
First, dual-echo images were combined using a weighted summation based on the signal strength in each brain region (Marciani et al., 2006). From this point onward, only the combined images were analyzed further and were treated like data from typical single-echo fMRI. To remove sources of noise and artifact, functional data were realigned, unwarped, corrected for slice timing, normalized to the MNI template with a resolution of 3 mm × 3 mm × 3 mm and spatially smoothed (8 mm) using SPM8 (Statistical Parametric Mapping, University College London) software. A design matrix was fitted for each participant, with six regressors for each priming sentence type (i.e. P1, P3, A1, A3, ‘non-grammatical’, ‘non-social’) and eight regressors for each mimicry type primed by that specific sentence type (i.e. CP1, CP3, CA1, CA3, IP1, IP3, IA1 and IA3, where C = congruent and I = incongruent). Each sentence/mimicry type was modeled as a boxcar with the duration of that single event convolved with the standard hemodynamic response function. Specifically, for the sentence priming task, each sentence trial was modeled with 8 s duration because the participant spent at least 8 s considering that sentence, in either its scrambled or unscrambled form. For the mimicry task, we model the onset at the start of the preparatory stimulus. We model the duration for each trial as ‘SOA (600/1200/1800ms) + 800ms’ because 800 ms was the upper limit for RT data (see Results). This boxcar modeling is similar to Wang et al. (2011) where we used another type of mimicry task.
Our primary analyses here focus on the interaction between prosociality, self-relatedness and mimicry. Taking data from the priming phase of the study, we calculated contrasts for the two-way interaction between prosociality and self-relatedness in sentence blocks [(P1 + A3) > (A1 + P3)]. In the mimicry phase of the task, we calculated contrasts for three-way interaction between prosociality, self-relatedness and congruency [‘(IP1 − CP1) + (IA3 − CA3)’ > ‘(IA1 − CA1) + (IP3 − CP3)’]. Other main effects and interactions are described in Supplementary Materials. Contrast images for all participants were then taken to the second level for a random-effects analysis in SPM8. Brain regions were initially thresholded at a voxel-level threshold of P < 0.001 and 10 voxels. Only regions that survive a cluster-level FDR (false discovery rate) correction of P < 0.05 over the whole brain are reported and discussed.
In addition, two exploratory analyses were conducted based on a region-of-interest (ROI) in anterior medial prefrontal cortex (amPFC) identified in this analysis. The ROI was constructed based on the three-way interaction of prosociality, self-relatedness and congruency in the mimicry phase of the study (Figure 2a). Parameter estimates were extracted from this ROI in each condition of the priming phase in each participant and averaged over the ROI voxels to get a measure of priming-phase activation. A two-way ANOVA was then conducted on these data with factors of prosociality and self-relatedness. Also, the prime-modulated BOLD (Blood Oxygenation Level Dependent) signal in this area was calculated as [P1 + A3] − [A1 + P3] for each participant. Correlation analysis was used to determine if the prime-modulated BOLD during the priming phase could predict the RT modulation during the mimicry phase, which was calculated as the RT for [(IP1 − CP1) + (IA3 − CA3)] − [(IA1 − CA1) + (IP3 − CP3)]). Equivalent analyses could not be carried out for the BOLD signal during the mimicry phase because this would be double-dipping (i.e. circular analysis).
Fig. 2.
amPFC as the neural substrates for social priming of mimicry. (a) amPFC was the only brain region found in the three-way interaction contrast. Sagittal and transverse view of the cluster suggested its precise location in the anterior-most portion of the mPFC (Brodmann area 10). Parameter estimates (SPM betas) for amPFC activations were shown separately in (b) sentence priming phase and (c) mimicry phase (I = Incongruent, C = Congruent). (d) Scatterplot showing the relationship between prime-modulated amPFC responses during priming phase (in units of weighted BOLD parameter estimates) and prime-modulated CEs during mimicry phase (in units of milliseconds). Each point represents the data from a single participant.
RESULTS
Behavioral performance
To remove trials where participants did not attend to the number cues, incorrect responses were excluded from the analysis, as were all RTs <100 ms or >800 ms (0.07%). To minimize the effect of outliers, we also excluded RTs that were >2 s.d. from the conditional means of each participant (0.64%) (Wang and Hamilton, 2013).
To examine whether participants’ mimicry responses were influenced by social primes, a three-way repeated measures ANOVA was conducted on participants’ mean RT, with congruency (congruent/incongruent), prosociality (prosocial/antisocial) and self-relatedness (third-person/first-person) as variables. The three-way ANOVA analysis revealed a significant main effect of congruency [F(1,19) = 27.15, P < 0.001, partial η2 = 0.588] and a significant three-way interaction: congruency × prosociality × self-relatedness [F(1,19) = 12.48, P = 0.002, partial η2 = 0.396]. Second, the congruency effect (CE) for each participant was calculated by subtracting RT in congruent trials from RT in incongruent trials (Figure 1d). Post hoc t-test showed that CE in P3 was significantly smaller than the CE in A3 [t(19) = 4.05, P = 0.001, d = 0.581] and the CE difference between P1 and P3 was also marginally significant [t(19) = 2.02, P = 0.054, d = 0.556]. These results are consistent with our previous studies (Wang and Hamilton, 2013) and show that the effect of social priming on mimicry can be replicated in the fMRI environment.
fMRI results
To examine the sentence priming phase, we tested for an interaction between prosociality and self-relatedness. No brain regions were engaged in either the forward or reverse contrast (i.e. no region survived the whole-brain cluster-corrected threshold). To examine the mimicry phase, we tested for the three-way interaction ‘self-relatedness × prosociality × congruency’. The only region activated was located in amPFC (BA10) (Figure 2a, MNI coordinates: 12, 65, 25, t = 6.00, P < 0.05 FDR cluster-corrected with two sub-peaks at 21, 59, 31 and 27, 50, 37). Plots of the parameter estimates (i.e. SPM betas) for this region in both the sentence priming phase and the mimicry phase are shown in Figure 2b and c. The plot of mimicry phase (Figure 2c) revealed greater activation when participants must inhibit mimicry (i.e. incongruent trials) in the first-person-prosocial and third-person-antisocial conditions. Post hoc t-tests confirmed stronger engagement of amPFC in ‘IP1’ than in ‘IP3’ [t(19) = 2.432, P = 0.025, d = 0.798] and stronger engagement in ‘IA3’ than in ‘IP3’ [t(19) = 2.468, P = 0.023, d = 0.760].
We then used this region as the ROI for our exploratory analysis of activations during the priming phase. Although the whole-brain analysis did not reveal any activations in the priming phase, the two-way ANOVA conducted directly on the extraction of parameter estimates in amPFC during priming phase revealed a significant interaction between prosociality and self-relatedness [F(1,19) = 4.63, P = 0.044, partial η2 = 0.242]. There were stronger responses to first-prosocial and third-antisocial than to first-antisocial and third-prosocial (Figure 2b). This suggests that amPFC did respond differently to different primes during priming phase, in a similar pattern to the mimicry phase. We note, however, that effect sizes were much smaller during the priming phase (partial η2 = 0.242) than during mimicry phase (partial η2 = 0.768).
We also tested whether the activation of amPFC during the priming phase was related to behavioral CEs in the mimicry phase. We found that the prime-modulated amPFC responses during priming phase [(P1 + A3) − (A1 + P3)] was positively correlated with the prime-modulated CE [(IP1 − CP1) + (IA3 − CA3)] − [(IA1 − CA1) + (IP3 − CP3)] with Pearson r = 0.552, P = 0.012 (Figure 2d).
DISCUSSION
This study used fMRI to investigate the neural mechanisms by which social primes impact on mimicry behavior. We aimed to address two questions: what brain regions implement prime-to-behavior effects, and are these regions more strongly engaged during the priming phase or the behavior phase of the task? First, behavioral results replicated our previous findings (Wang and Hamilton, 2013) and demonstrated that mimicry was enhanced only by first-person-prosocial and third-person-antisocial primes (Figure 1d). Second, the fMRI data suggest that amPFC is the most likely neural substrate of this behavioral effect. amPFC was the only brain region engaging in the three-way interaction between prosociality, self-relatedness and congruency in the mimicry phase (Figure 2a). amPFC activation in the priming phase showed a similar interaction between prosociality and self-relatedness, with a smaller effect size (Figure 2b). Critically, the amPFC activation during priming phase was positively correlated with the behavioral priming effects (Figure 2c), suggesting that in participants whose amPFC was strongly modulated by primes during priming phase, there were also stronger priming effects during the later mimicry phase. We now discuss the specific role of amPFC in each phase and its implications for our understanding of the underlying mechanisms of prime-to-behavior effects.
Brain mechanisms of social priming on mimicry
Here, we consider in detail the cognitive and computational role of amPFC in social priming of mimicry. Our description here is based on the STORM hypothesis (Wang and Hamilton, 2012), in which we suggested that medial prefrontal regions have an important role in the top-down control of mimicry responses. However, we acknowledge that other models or theories of this data may be possible.
First, we examine the mimicry phase, in which participants must respond to the number on the screen and ignore the moving finger on the background. In congruent trials, this is a relatively easy task, but in incongruent trials, the participant must inhibit the prepotent tendency to imitate and instead respond to the number. Previous studies suggest when participant must inhibit imitation (Brass et al., 2001, 2005, 2009) or control the tendency to mimic (Wang et al., 2011), there is strong engagement of medial prefrontal regions. Our data are consistent with this. In amPFC, we found a three-way interaction between prosociality, self-relatedness and congruency during mimicry behavior. This interaction was primarily driven by strongest activations in the first-person-prosocial incongruent and third-person-antisocial incongruent conditions (Figure 2c). These are the two conditions where participants showed the largest CE (Figure 1d), indicating that substantial control was required to inhibit the prepotent tendency to imitate. Thus, we suggest that the engagement of amPFC during the mimicry phase of our study mainly reflects the need to control imitation.
What might drive this substantial demand for top-down control of mimicry in first-person-prosocial and third-person-antisocial conditions? To understand this, we turn to the priming phase. The whole-brain analysis in the priming phase did not reveal any significant clusters, but an ROI analysis on amPFC revealed a reliable interaction between prosociality and self-relatedness in this region. This suggests that the implicit priming in this task might not be driven by an independent brain region outside mPFC, but is rather integral to amPFC. We note that main effects analysis showed that prosocial priming (compared with antisocial) engaged anterior cingulate and angular gyrus, while first-person priming (compared with third-person) engaged precuneus (see Supplementary Materials). Thus, the separate processing of prosociality and perspective in these regions may feed forward to and combine in amPFC to contribute to the priming effect.
In amPFC, there was stronger activation on exposure to first-person-prosocial primes and third-person-antisocial primes than third-person-prosocial and first-person-antisocial primes. We suggest that exposures to the former primes (1P and 3A) may activate a prosocial self-schema stored in amPFC and lead participants to perceive themselves as a prosocial individual. This is in line with an ‘active-self’ model of priming (Wheeler et al., 2007) described in more detail below. When feeling prosocial, the participant is more inclined to mimic in future, and engages the amPFC control systems that are needed to enhance mimicry. However, in incongruent trials, the participant must suppress this inclination to prosocially mimic. This means that incongruent trials following first-person-prosocial or third-person-antisocial priming demand more top-down control and greater activation of amPFC.
To summarize, we suggest that the subtle engagement of amPFC during priming reflects the activation of top-down control ready to ‘enhance’ mimicry, while the strong engagement of amPFC during responding reflects the battle between this enhancement and the task requirement to ‘inhibit’ mimicry. We note that the BOLD signal may not reliably differentiate between activation of excitatory neurons and activation of inhibitory neurons, but rather reflects the overall level of neural activity. As amPFC exerts both excitatory and inhibitory impacts on mimicry (particularly in incongruent trials of first-person-prosocial and third-person-antisocial conditions), amPFC activations during mimicry phase might just reflect the superposition of these two opposite top-down control processes (see a similar interpretation in Wang et al., 2011). This account is coherent with the STORM model (Wang and Hamilton, 2012), which suggests that mPFC has a key role in top-down control of mimicry. We believe this model gives a plausible account of our data, though other models may also be possible.
Further evidence to link amPFC activation during priming to subsequent mimicry behavior can be found in our correlation analysis. We found that prime-modulated amPFC activations during priming phase predict prime-modulated CEs during mimicry phase. This means that participants who showed stronger modulation of amPFC in response to the primes also showed a stronger behavioral effect of priming. This finding supports the claim that amPFC activation during priming, though subtle, is a driving factor in the modulation of mimicry behavior. Overall, our first hypotheses—that medial prefrontal regions have a key role in social priming—is confirmed.
The second question we set out in our hypotheses concerned the relationship between the priming phase of the study and the mimicry phase. Few previous studies have been able to examine both. One possibility is that prime-to-behavior effects might result from passive carry over of a large activation in the priming phase to a smaller activation in the behavior phase. Conversely, if prime-to-behavior effects reflect a more active control process, then weak signals during the priming phase might be amplified during responding.
Our data clearly support the latter position. The three-way interaction between prosociality, self-relatedness and congruency in amPFC during mimicry phase has a large effect size (partial η2 = 0.768). The two-way interaction of prosociality and self-relatedness during priming phase was also robust, but with a much smaller effect size (partial η2 = 0.242) and smaller BOLD signal (Figure 2b). As the scrambled sentences were presented for more time and modeled with longer boxcars than the mimicry trials (both factors should boost the power for the analysis of the scrambled sentences), it is unlikely that the smaller amPFC activations for sentence priming phase are due to a weak design or lack of power. Instead, the finding that the weak activation of amPFC during priming phase correlates with the later behavioral effect suggests that modest activation of amPFC during priming may then lead to stronger engagement of the same region when the opportunity for controlling a relevant behavior arises.
Together, these discussions lead us to suggest a model in which, during the priming phase, amPFC integrates different social cues (e.g. prosociality and self-relatedness) and prepares to control mimicry should the opportunity arise. This control signal is reflected in the stronger activations in conditions that make the participant feel prosocial (i.e. first-person-prosocial and third-person-antisocial conditions) but remains modest during priming phase. During the mimicry phase, amPFC is then engaged in the top-down control of responses, with the largest signal when the prosocial desire to mimic conflicts with the task demand to inhibit mimicry. Overall, this model gives amPFC a central role in the control of social responding, in particular for self-related and prosocial contexts.
Relation to other priming studies
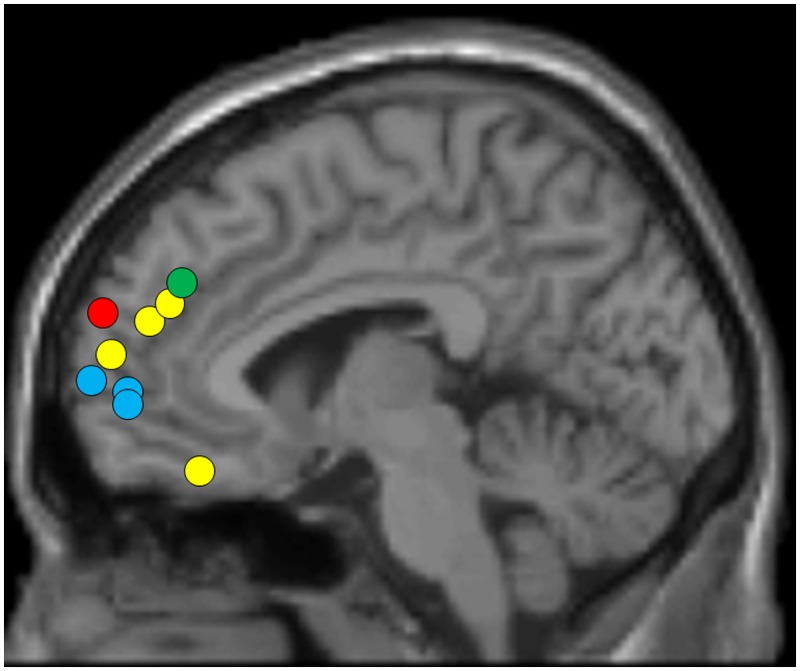
To interpret our data, it is also helpful to consider precisely how the coordinates we report relate to other studies of implicit priming using fMRI. Previous studies examining the behavior phase of implicit priming tasks reported neural activations in different parts of mPFC (e.g. anterior, posterior, orbital, dorsal, see Figure 3). For example, Chiao et al. (2010) primed participants with individualism/collectivism self-construals and then examined the priming effects by implementing a self-judgment task in the scanner. They found the changed self-judgment was strongly correlated with activations in anterior mPFC. Bengtsson et al. (2011) found that priming with ‘clever’ self-esteem concept reduces error rates in a memory task and this effect originates from posterior mPFC (i.e. ACC). Eddington et al. (2007) primed participants with approach/avoidance goal and found correlated activations in orbital PFC. Powers et al. (2013) primed participants with social exclusion (an approach to boost one’s motivation to positively engage with others) and found stronger engagement of dorsal mPFC when observing social photos. These results thus suggest that there is no single brain mechanism for implicit priming; rather the type of priming (i.e. the specific primed construct) determines the brain region engaged (Figure 3): priming of self-judgment is linked to anterior mPFC; priming of error control is linked to ACC; priming of goal pursuit to orbitofrontal cortex; and priming of social engagement to dorsal mPFC.
Fig. 3.
Mapping of mPFC activations in previous studies involving implicit priming and inhibition of mimicry. Yellow circles represent four studies of implicit priming: Bengtsson et al. (2011) [10, 50, 30], Powers et al. (2013) [6, 54, 21], Chiao et al. (2010) [−6, 62, 14] and Eddington et al. (2007) [−38, 42, −27]. Blue circles represent three studies of inhibition of mimicry using the same mimicry task as the present study: Brass et al. (2001) [8, 66, 7], Brass et al. (2005) [2, 56, 0] and Spengler et al. (2009) [−4, 56, 3]. The green circle represents a recent study on social control of mimicry primed by eye contact (Wang et al., 2011, [6, 44, 34]). The red circle represents the result of current study ([12, 65, 25]). All coordinates were MNI coordinates.
Our data also fit this pattern, though the precise coordinates we report do not overlap with previous studies (Figure 3). We primed participants with self/other-related pro/antisocial concepts and found engagement of amPFC during both priming and behavior phase. This anterior region is associated with representations of self-concepts and self-schemas (Chao et al., 2010; Northoff and Bermpohl, 2004; Forbes and Grafman, 2010; Heatherton, 2011) and plays an important role in implicit self-judgment, perspective-taking and self-control (David et al., 2006; D’Argembeau et al., 2007; Rameson et al., 2010; Raposo et al., 2010; Bengtsson et al., 2011). Other studies link amPFC to the regulation of prosocial behaviors (Rameson et al., 2012) and inhibition of mimicry (Brass et al., 2009). Thus, the localization we report in amPFC is consistent with the role of this region in self-related processing, prosociality processing and the control of mimicry behavior. Overall, the distribution of mPFC engagement in different implicit priming paradigms suggests that there is no one mPFC region which is the ‘control center’ of implicit priming. Rather, different regions within mPFC are acting in a prime-specific manner to control responses.
Implications for theories in social psychology
These results can also be understood in relation to theories from social psychology. Several different theories have been proposed to account for automatic and unintentional prime-to-behavior effects. For example, Bargh et al. (2001) suggested that a given prime can directly activate a behavioral goal, which then leads to pursuit of that goal (‘goal-activation theory’). Wheeler et al. (2007) suggested that primed constructs influence behavior by changing one’s understanding of the self and the changed self-schema then regulates the behavior (‘active-self theory’). More general models attempt to integrate a number of different types of priming in terms of activation of different categories of mental content. For example, Loersch and Payne (2011) suggested that primes change the accessibility of prime-related mental content and make people use a biased mental resource to react to the environment (‘situated inference theory’). Schröder and Thagard (2013) propose a neurocomputational model in which the situated self is one of the components that can be activated by primes.
The ‘active-self’ model (Wheeler et al., 2007) is particularly relevant to the current study because the self-relatedness of the primes had a dramatic effect on the prime-to-behavior mapping. This model suggests that primes activate particular components of a participant’s self-schema, which in turn influences behavior. Our behavioral data are coherent with this model because we found that both first-person-prosocial primes (i.e. context of ‘I am doing affiliative deeds’) and third-person-antisocial primes (i.e. context of ‘other people are doing disaffiliative behaviors’) result in a strong mimicry effect. The ‘active-self’ model suggests these two primes evoke a mental state of ‘me as a prosocial person’, in a way that first-person antisocial primes or third-person prosocial primes do not. The model then suggests that the active self-schema of ‘me as a prosocial person’ drives and regulates the mimicry behavior. This is also compatible with our neuroimaging findings. amPFC is a critical brain region that stores, organizes and selects self-schemas (Rameson et al., 2010; Northoff and Bermpohl, 2004; Forbes and Grafman, 2010). Thus, engagement of amPFC following first-person-prosocial and third-person-antisocial priming may reflect the way that these primes implicitly invoke one’s prosocial self-schema in amPFC and use that prosocial self-schema to guide and regulate future behavior.
SUMMARY
Overall, this study revealed that amPFC may provide a neural basis for the effect of social primes on mimicry behavior. Our data suggest that priming with first-person-prosocial or third-person-antisocial scrambled sentences engaged an active self-concept of ‘me as prosocial’, reflected in modest activation of amPFC. When there is a conflict between this active prosocial self-schema and the task requirement to suppress mimicry, this region was strongly engaged in top-down control of the mimicry response. Thus, amPFC has a central role in controlling social responding based on subtle self-related priming of prosocial behavior.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by Economic and Social Research Council (ESRC) Small Research Grant ES/J006793/1 (to A.H.).
References
- Bargh JA. What have we been priming all these years? On the development, mechanisms, and ecology of nonconscious social behavior. European Journal of Social Psychology. 2006;36(2):147–68. doi: 10.1002/ejsp.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh JA, Chen M, Burrows L. Automaticity of social behavior: direct effects of trait construct and stereotype activation on action. Journal of Personality and Social Psychology. 1996;71(2):230–44. doi: 10.1037//0022-3514.71.2.230. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Gollwitzer PM, Lee-Chai A, Barndollar K, Trotschel R. The automated will: nonconscious activation and pursuit of behavioral goals. Journal of Personality and Social Psychology. 2001;81:1014–27. [PMC free article] [PubMed] [Google Scholar]
- Bargh JA, Schwader KL, Hailey SE, Dyer RL, Boothby EJ. Automaticity in social-cognitive processes. Trends in Cognitive Science. 2012;16(12):593–605. doi: 10.1016/j.tics.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Raymond JD, Passingham RE. Priming for self-esteem influences the monitoring of one's own performance. Social Cognitive and Affective Neuroscience. 2011;6(4):417–25. doi: 10.1093/scan/nsq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower B. The hot and the cold of priming. Science News. 2012;181 [Google Scholar]
- Brass M, Derrfuss J, von Cramon DY. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005;43:89–98. doi: 10.1016/j.neuropsychologia.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Brass M, Ruby P, Spengler S. Inhibition of imitative behaviour and social cognition. Philosophical Transactions of Royal Society London B Biological Sciences. 2009;364:2359–67. doi: 10.1098/rstb.2009.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Zysset S, von Cramon DY. The inhibition of imitative response tendencies. Neuroimage. 2001;14:1416–23. doi: 10.1006/nimg.2001.0944. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda. H, et al. Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience. 2010;22(1):1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–44. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- David N, Bewernick B, Cohen MX, et al. Neural representations of self versus other: visual-spatial perspective-taking and agency in a virtual ball-tossing game. Journal of Cognitive Neuroscience. 2006;18(6):898–10. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- Doyen S, Klein O, Pichon CL, Cleeremans A. Behavioral priming: it’s all in the mind, but whose mind? PloS one. 2012;7:e29081. doi: 10.1371/journal.pone.0029081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington KM, Dolcos F, Cabeza R, Krishnan KR, Strauman TJ. Neural correlates of promotion and prevention goal: an fMRI study using an idiographic approach. Journal of Cognitive Neuroscience. 2007;19:1152–62. doi: 10.1162/jocn.2007.19.7.1152. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annual Review of Neuroscience. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C. Automatic imitation. Psychological Bulletin. 2011;137:463–83. doi: 10.1037/a0022288. [DOI] [PubMed] [Google Scholar]
- Lakin JL, Chartrand TL. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychological Science. 2003;14:334–9. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- Lakin JL, Jefferis VE, Cheng CM, Chartrand TL. The chameleon effect as social glue: evidence for the evolutionary significance of nonconscious mimicry. Journal of Nonverbal Behavior. 2003;27:145–62. [Google Scholar]
- Leighton J, Bird G, Orsini C, Heyes C. Social attitudes modulate automatic imitation. Journal of Experimental Social Psychology. 2010;46:905–10. [Google Scholar]
- Loersch C, Payne KB. The situated inference model: an integrative account of the effects of primes on perception, behavior, and motivation. Perspectives on Psychological Science. 2011;6:234–52. doi: 10.1177/1745691611406921. [DOI] [PubMed] [Google Scholar]
- Marciani L, Pfeiffer JC, Hort J, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. Journal of Neuroscience Methods. 2006;158(2):186–94. doi: 10.1016/j.jneumeth.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for ‘social pain' and subsequent prosocial behavior. Neuroimage. 2011;55(1):381–8. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cognitive Science. 2004;8(3):102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pashler H, Coburn N, Harris CR. Priming of Social Distance? Failure to Replicate Effects on Social and Food Judgments. PLoS One. 2012;7(8):e42510. doi: 10.1371/journal.pone.0042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Heatherton TF. Implicitly priming the social brain: failure to find neural effects. PLoS One. 2013;8(2):e56596. doi: 10.1371/journal.pone.0056596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Social, Cognitive and Affective Neuroscience. 2013;8:151–7. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience. 2006;1:203–13. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50:701–8. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience. 2012;24:235–45. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Raposo A, Vicens L, Clithero JA, Dobbins IG, Huettel SA. Contributions of frontopolar cortex to judgments about self, others, and relations. Social, Cognitive, and Affective Neuroscience. 2010;6:260–9. doi: 10.1093/scan/nsq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler S, von Cramon DY, Brass M. Control of shared representations relies on key processes involved in mental state attribution. Human Brain Mapping. 2009;30:3704–18. doi: 10.1002/hbm.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Han S. Self-construal priming modulates neural substrates of self-awareness. Psychological Science. 2007;18(10):861–6. doi: 10.1111/j.1467-9280.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- Schröder T, Thagard P. The affective meanings of automatic social behaviors: three mechanisms that explain priming. Psychological Review. 2013;120:255–80. doi: 10.1037/a0030972. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hamilton A. Understanding the role of the ‘self’ in the social priming of mimicry. PLoS One. 2013;8(4):e60249. doi: 10.1371/journal.pone.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hamilton A. Social top-down response modulation (STORM): a model of the control of mimicry in social interaction. Frontiers in Human Neuroscience. 2012;6:153. doi: 10.3389/fnhum.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramsey R, Hamilton A. The control of mimicry by eye contact is mediated by the meidal prefrontal cortex. The Journal of Neuroscience. 2011;31:12001–10. doi: 10.1523/JNEUROSCI.0845-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SC, DeMarree KG, Petty RE. Understanding the role of the self in prime-to-behavior effects: the active-self account. Personality and Social Psychology Review. 2007;11:234–61. doi: 10.1177/1088868307302223. [DOI] [PubMed] [Google Scholar]
- Zaki J, Mitchell JP. Intuitive prosociality. Current Directions in Psychological Science. 2013;22:466. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.