Abstract
Well-being and subjective experience of a coherent world depend on our sense of ‘self’ and relations between the self and the environment (e.g. people, objects and ideas). The ventromedial prefrontal cortex (vMPFC) is involved in self-related processing, and disrupted vMPFC activity is associated with disruptions of emotional/social functioning (e.g. depression and autism). Clarifying precise function(s) of vMPFC in self-related processing is an area of active investigation. In this study, we sought to more specifically characterize the function of vMPFC in self-related processing, focusing on two alternative accounts: (i) assignment of positive subjective value to self-related information and (ii) assignment of personal significance to self-related information. During functional magnetic resonance imaging (fMRI), participants imagined owning objects associated with either their perceived ingroup or outgroup. We found that for ingroup-associated objects, vMPFC showed greater activity for objects with increased than decreased post-ownership preference. In contrast, for outgroup-associated objects, vMPFC showed greater activity for objects with decreased than increased post-ownership preference. Our findings support the idea that the function of vMPFC in self-related processing may not be to represent/evaluate the ‘positivity’ or absolute preference of self-related information but to assign personal significance to it based on its meaning/function for the self.
Keywords: self-related processing, ventromedial prefrontal cortex (vMPFC), personal significance, subjective value, self
INTRODUCTION
As William James (1890/1983, p. 289) noted, individuals place an “altogether unique kind of interest” on aspects of the world that one can call ‘me or mine’ compared to those that are ‘not me or not mine’. Neuroimaging studies consistently show that contemplating the ‘me or mine’ aspects of the world (i.e. self-related processing) such as one’s personality traits recruits the ventromedial prefrontal cortex (vMPFC) (Northoff et al., 2006; Lieberman, 2010; Wagner et al., 2012). Furthermore, hyper- or hypoactivity in vMPFC during self-related processing is one of the characteristic features of patients with disorders in social and/or emotional processing (e.g. depression: Johnson et al., 2009; Sheline et al., 2009; autism: Lombardo et al., 2010). Yet, despite substantial evidence implicating vMPFC in self-related processing, there is as yet no consensus about the precise function of this region in such processing. Understanding the specific function subserved by this region during self-related processing should contribute to illuminating not only healthy but also dysfunctional mechanisms of self-related processing.
Across various domains, vMPFC activity has been associated with the ‘positivity’ of experiences (e.g. fear extinction: Milad et al., 2007; positive reappraisal success: Wager et al., 2008). In particular, vMPFC has been shown to reflect subjective value of various types of stimuli ranging from food to social reward (Peters and Büchel, 2010; Rangel and Hare, 2010). Thus, one possibility is that vMPFC activity during self-related processing reflects assignment of positive subjective value to self-related information by virtue of its self-relatedness. An alternative is that vMPFC assigns personal significance to information by evaluating its meaning/function for the self (D’Argembeau, 2013). In this case, vMPFC activity would not necessarily always be associated with positive subjective value of self-related information. Some support for this view comes from evidence that vMPFC activity tracks the level of self-relevance of items rather than the positive valence of items per se. For example, Phan et al. (2004) found that participants’ appraisal of personal relatedness of pictures varying in emotional valence engaged vMPFC, and the amount of vMPFC activity tracked individuals’ reported level of personal relatedness to both positive and negative affective pictures. Similarly, using positive and negative trait adjectives, Moran et al. (2006) found that activity in vMPFC was greater for trait adjectives that people judged as more self-descriptive compared to less self-descriptive regardless of the valence of the traits. However, these findings cannot conclusively rule out the possibility that vMPFC activity during self-related processing reflects positive subjective valuation of information by virtue of its self-relatedness. That is, vMPFC activity could, in part, reflect assignment of greater positivity to both positive and negative stimuli as a result of their associations with the self. To dissociate activity reflecting assignment of personal significance from activity reflecting assignment of greater positivity arising from self-relatedness, it is necessary to examine changes in the subjective value of items from before and after they acquire self-relevance.
We tested these alternatives (i.e. positive subjective valuation vs assignment of personal significance) by contrasting contexts in which self-relevance of stimuli is likely to yield positive vs negative subjective values. We started with the idea that ‘mineness’ accrues to aspects of the environment or experiences relevant to oneself, ‘expanding’ the self beyond the physical body (Belk, 1988; Aron et al., 2004). We based our study on recent findings (Kim and Johnson, 2012, 2013) that vMPFC activates when people assign ‘mineness’ to everyday objects by imagining owning those objects, and that vMPFC activity is associated with more favorable evaluation of ‘self-owned’ objects compared to identical objects that are not owned (i.e. the mere ownership effect; Beggan, 1992). In the current study, during functional magnetic resonance imaging (fMRI), participants imagined owning objects that were described to be ‘currently owned and highly valued by’ either their perceived ingroup or outgroup.
The classic literature on social categorization, in particular on social identity (Wilder and Allen, 1978; Branscombe et al., 1999; Brewer, 1991, 1999), suggests that individuals are motivated to assimilate with their ingroups (‘I’m like us’) and to differentiate themselves from outgroups (‘I’m not like them’). Furthermore, recent findings show that non-verbal behaviors of others typically associated with feeling of interpersonal warmth (i.e. mimicry) can trigger the experience of physical coldness when displayed by one’s outgroup (i.e. an individual from a different racial group than one’s own; Leander et al., 2012) and that a neurophysiological index of spontaneous mirroring of others’ movement (suppression of mu rhythm) significantly decreases when the movement is delivered by someone with whom an individual exhibits no or minimal desire for social connection (Aragón et al., 2013). Such findings provide further evidence of individuals’ assimilation vs dissimilation motivations toward ingroups vs outgroups. Based on such results, we predicted that individuals’ motivation to assimilate with the ingroup and to differentiate themselves from the outgroup would produce more favorable evaluation of ingroup-associated objects and less favorable evaluation of outgroup-associated objects from before to after ownership imagination.
Our design allowed us to ask whether vMPFC is differentially responsive when people form associations with ingroup- vs outgroup-associated objects through ownership and, more important, how vMPFC activity relates to preference changes from pre- to post-ownership. If vMPFC assigns positive subjective value to information by virtue of its self-relatedness, then post-ownership preference increase should be related to greater vMPFC activity regardless of an object’s association with one’s ingroup or outgroup. Alternatively, if vMPFC assigns personal significance to self-related information by evaluating its meaning/function for the self, then the relationship between vMPFC activity and post-ownership preference change should depend on whether objects are associated with an ingroup or outgroup. That is, the personal significance of assimilating with one’s ingroup should be reflected in greater vMPFC activity associated with preference increases from pre- to post-ownership. In contrast, the personal significance of differentiating from one’s outgroup should be reflected in greater vMPFC activity associated with preference decreases from pre- to post-ownership.
METHODS
Participants
Twenty-four healthy, right-handed adults (14 female; mean age = 21.67 ± 3.23) participated. Participants provided informed consent and were compensated in accordance with the Yale University School of Medicine Human Investigation Committee.
Experimental procedure
There were three within-subjects conditions defined by whether the objects were associated with (i.e. ‘currently owned and highly valued by’) a person perceived to be one’s ingroup or outgroup (Ingroup and Outgroup conditions), determined post-experimentally based on participants’ rating of their similarity to each person and their performance on the Implicit Association Test (IAT; Greenwald et al., 1998), or whether the objects were associated with a computer (Computer condition). Participants were told that the purpose of this study was to explore whether people process objects differently according to whether objects are assigned to them by human agents or by a non-human agent (computer), and whether having some basic information about individuals assigning them objects affects brain activity as they imagine owning the objects. We told participants that to prepare for this study we ran an online survey involving many people where we showed pictures of everyday objects of various price ranges and asked them to pick out items within each price range that they currently own and highly value. Participants were told that they would see pictures of objects that were picked out by two of these individuals of the same gender as them, along with other objects picked out randomly by a computer.
Before entering the scanner, participants rated how much they liked each of the 126 objects on a 1 (lowest preference) to 9 (highest preference) scale. Then, they read descriptions of two fictitious individuals (ADAM/MIKE for male participants and NINA/RUTH for female participants). One person was described as having liberal and the other as having conservative sociopolitical views, with both engaging in activities reflecting these views (e.g. electoral campaigning for a Republican; modified from descriptions used in Mitchell et al., 2006). Participants were asked to learn as much as possible about and form an impression of each person and given as much time as needed. The order of presentation (liberal or conservative person first) was counterbalanced across participants.
During scanning, participants performed an ‘Ownership Imagination’ task. On each trial, following a 400 ms fixation period, an object picture and a basket labeled ‘MINE’ appeared at the top and bottom of the screen, respectively. Two seconds later, a cue signaling who assigned the object to the participant appeared in between the object and the basket (first names for Ingroup and Outgroup conditions and ‘COM’ for Computer condition). Participants then pressed a button with their right index finger to move the object to the basket and were asked to imagine all the items as belonging to them regardless of who had assigned the objects. The whole trial lasted for 7 s. Trials were separated by jittered intertrial fixation intervals (10.6-16.6 s). There were 18 trials (6 trials per condition) in each of the 7 functional runs, and the trials within each run were randomly ordered.
After scanning, participants performed a source memory test where they indicated for each object whether it was assigned to them by ADAM/NINA, MIKE/RUTH or the computer. Then, participants re-rated their preference for the same 126 objects. Finally, participants completed the IAT designed to measure how strongly they associated the concept of ‘Us’ with each of the two individuals. On each trial, participants categorized an exemplar from one of four categories of stimuli: (i) the name of the liberal, (ii) the name of the conservative, (iii) an ingroup-designating pronoun (e.g. ‘us’) or (iv) an outgroup-designating pronoun (e.g. ‘them’). In one block, participants were to press the same button for both ingroup pronouns and the name of the liberal (liberal-with-ingroup), and in another block, ingroup pronouns required the same responses as the name of the conservative (conservative-with-ingroup). The difference in the mean response time between the two blocks indexed the degree to which each participant perceived the liberal or conservative to belong to his/her ingroup. In a post-experimental questionnaire, participants indicated how similar they were to the liberal and conservative individuals separately on a 1 (not at all) to 9 (very much) scale.
Localizer ‘self-referencing’ task
To locate regions of interest (ROI) involved in self-referential processing, independently from our main task, we asked participants to rate how well trait adjectives describe themselves (self-referent) or former president George W. Bush (other-referent) on a four-point scale. Each block consisted of five sequential presentations of adjectives (2.7 s word presentation, 500 ms interstimulus interval). Ten blocks for each reference condition were alternated and separated by an 8 s fixation period. A total of 100 trait adjectives were divided into two lists and were matched for number of syllables, word length and desirability (Anderson, 1968) and assigned to the self- and other-referent conditions in a counterbalanced manner.
Stimuli
The stimuli were 126 pictures (250 × 250 pixels) of items available for purchase from a large offline/online market (e.g. stationary, clothing). They were divided into 3 sets of 42 objects matched for mean preference level, estimated cost, masculinity/femininity and ease of identification based on data from a separate pilot study. The assignment of stimulus sets to liberal, conservative and computer conditions was counterbalanced across participants.
Image acquisition and preprocessing
Data were acquired using a 3T Siemens TimTrio scanner with a 12-channel head coil. For each run of the ownership imagination task, 188 functional volumes were acquired using an echo-planar pulse sequence (TR = 2 s, TE = 25 ms, flip angle = 90°, FOV = 240 mm, matrix = 64 × 64, slice thickness = 3.5 mm, 34 slices). For the localizer run, 254 volumes were acquired with the same imaging parameters as the main functional runs. Two sets of structural images were acquired for registration: (i) coplanar images, using T1 FLASH sequence (TR = 300 ms, TE = 2.47 ms, flip angle = 60°, FOV = 240 mm, matrix = 256 × 256, slice thickness = 3.5 mm, 34 slices) and (ii) high-resolution images, using 3D MP-RAGE sequence (TR = 2530 ms, TE = 3.34 ms, flip angle = 7°; FOV = 256 mm, matrix = 256 × 256, slice thickness = 1 mm, 160 slices).
Image preprocessing was performed using the FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl). The first four volumes (8 s) of each functional dataset were discarded to allow for MR equilibration. Preprocessing included skull-stripping, slice-timing correction, motion correction using MCFLIRT, spatial smoothing (Gaussian, FWHM 5 mm) and high-pass temporal filtering (cutoff = 50 s). Registration was conducted through a three-step procedure: functional images were registered to coplanar images, which were then registered to high-resolution images and normalized to the Montreal Neurological Institute’s 152 template.
fMRI data analysis
Whole-brain voxel-wise regression analyses were performed using FSL’s FEAT. First-level analyses were performed using a separate explanatory variable (EV) for each trial type. For the main ownership imagination task contrast, the model included three EVs corresponding to the Ingroup, Outgroup and Computer conditions. The preference contrast was modeled using six EVs defined by condition and post- compared to pre-ownership preference change: (i) Ingroup Higher, (ii) Ingroup Lower, (iii) Outgroup Higher, (iv) Outgroup Lower, (v) Computer Higher and (vi) Computer Lower. Each trial type was modeled for the entire 7 s trial duration with a boxcar function, convolved with a single-gamma hemodynamic response function. Subject-level analyses combining multiple runs were conducted using a fixed effects model. Contrasts comparing parameter estimates obtained from the regression analyses were defined at the subject level to identify brain regions showing trial-type and condition-specific effects. The contrasts of particular interest were the comparisons between the Ingroup and Outgroup conditions.1
Group-level analyses were performed on the parameter estimates obtained from each of the contrasts calculated at the subject level using a mixed effects model, with the random effects component of variance estimated using FSL’s FLAME 1 + 2 procedure (Beckmann et al., 2003). For significance testing, voxels were thresholded at an entry level of Z > 2.3 and the significance of the resulting cluster was then evaluated at a cluster probability P < 0.05 using a Gaussian random field theory approach to correct for multiple comparisons (Worsley et al., 1996).
ROI definition and analysis
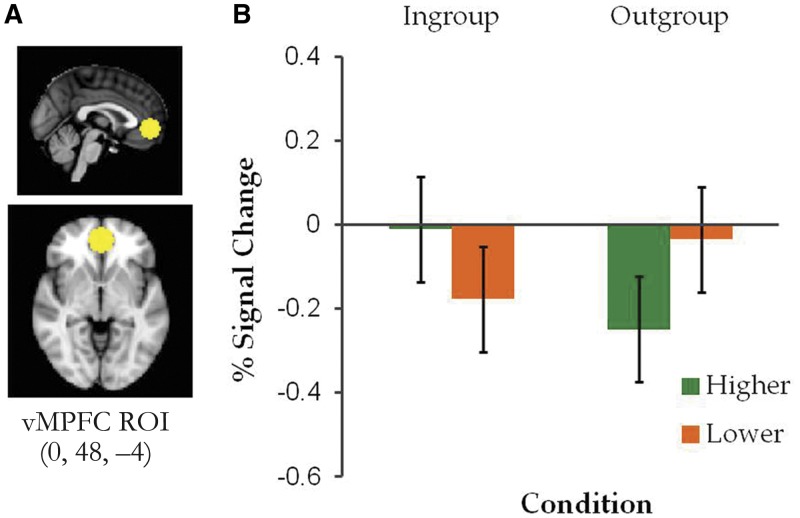
The localizer task was analyzed using the same approach of preprocessing, subject- and group-level analyses as described for the main task. The EVs consisted of self- and other-referent conditions. The group-level contrast map for self-referent > other-referent contrast (Z > 2.7, cluster probability P < 0.05) identified two clusters in the vMPFC/anterior cingulate gyrus and posterior temporal fusiform cortex. For the current analysis, we created a spherical ROI with a 10 mm radius centered at the voxel showing the maximal BOLD contrast effect within vMPFC (0, 48, −4; Figure 4A).
Fig. 4.
(A) vMPFC ROI derived from the self-referent > other-referent contrast in an independent trait descriptiveness rating task and (B) mean percent signal change in the vMPFC ROI as a function of condition (Ingroup or Outgroup) and post- vs pre-ownership preference change (higher or lower). Error bars represent 95% within-subject confidence intervals (Loftus and Masson, 1994).
The fMRI signal from each voxel in each participant’s functional data was calculated across peri-events created separately for Ingroup and Outgroup conditions in each contrast of interest. The fMRI signals were then converted to percent signal change relative to an intertrial baseline and averaged over the voxels contained in our ROI for four time points (epochs) of interest expected to show the maximal BOLD effect (2-8 s after the onset of the cue signaling who assigned the object).
RESULTS
Behavioral results
IAT performance
Participants were faster to categorize stimuli in the liberal-with-ingroup block (M = 557 ms, s.d. = 81.77) than the conservative-with-ingroup block (M = 607 ms, s.d. = 81.85), t(23) = −3.12, P = 0.005, d = 0.90, demonstrating that they more strongly associated the liberal with their ingroup than the conservative. Out of 24 participants, only 2 showed faster responses in the conservative-with-ingroup block than the liberal-with-ingroup block.
Post-experimental similarity ratings
The similarity rating for the liberal (M = 7.08, s.d. = 1.10) was significantly higher than that for the conservative (M = 2.96, s.d. = 1.71), t(23) = 8.29, P < 0.001, d = 2.41. Difference scores (liberal minus conservative ratings) were positive for 22 of 24 participants (M = 4.73, s.d. = 1.39). Difference scores for the remaining two participants (the same individuals who showed faster responses on the IAT in the conservative-with-ingroup block than the liberal-with-ingroup block) were negative (M = −2.5, s.d. = 0.71). The assignment of the liberal- and conservative-associated object trials to the Ingroup or Outgroup conditions was determined for each participant based on the IAT results: liberal as Ingroup for 22 participants and conservative as Ingroup for 2 participants.
Imagined ownership
A repeated-measures analysis of variance (ANOVA) with condition (Ingroup, Outgroup or Computer) as a factor performed on the mean response times (i.e. a button press to move objects into the ‘MINE’ basket after the onset of the cue signaling who assigned the object to the participant) revealed a marginally significant effect of condition, F(2, 46) = 2.76, P = 0.074, 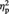 = 0.11. Participants showed a tendency to be faster at pressing the button to assign objects from the Computer condition (M = 1557 ms, s.d. = 480) compared with objects from the Ingroup (M = 1641 ms, s.d. = 540), F(1, 23) = 3.57, P = 0.072,
= 0.11. Participants showed a tendency to be faster at pressing the button to assign objects from the Computer condition (M = 1557 ms, s.d. = 480) compared with objects from the Ingroup (M = 1641 ms, s.d. = 540), F(1, 23) = 3.57, P = 0.072, 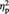 = 0.13 or Outgroup (M = 1626 ms, s.d. = 536) conditions, F(1, 23) = 3.34, P = 0.081 and
= 0.13 or Outgroup (M = 1626 ms, s.d. = 536) conditions, F(1, 23) = 3.34, P = 0.081 and 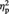 = 0.13. There was no significance difference in mean response time between the Ingroup and Outgroup conditions, P > 0.6.
= 0.13. There was no significance difference in mean response time between the Ingroup and Outgroup conditions, P > 0.6.
Source memory
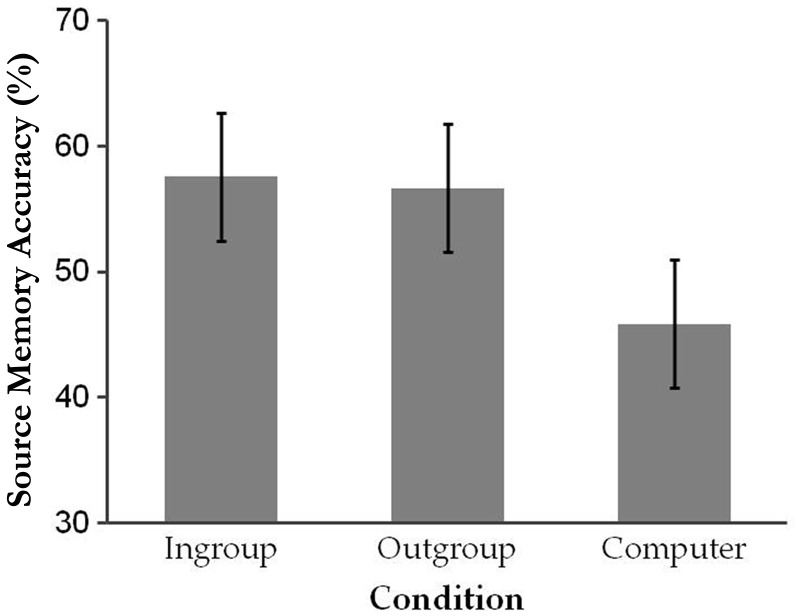
Source memory accuracy was calculated by dividing the number of correct source assignments to each condition by the total number of items for that condition and entered into repeated-measures ANOVA with condition (Ingroup, Outgroup or Computer) as a factor (Figure 1). There was a significant main effect of condition, F(2, 36.914) = 8.34, P = 0.002, 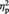 = 0.27 (Greenhouse–Geisser corrected). Participants were less successful at remembering an object’s source for items from the Computer condition (M = 45.83%, s.d. = 15.13) compared with items from the Ingroup (M = 57.54%, s.d. = 14.42), F(1, 23) = 9.01, P = 0.006,
= 0.27 (Greenhouse–Geisser corrected). Participants were less successful at remembering an object’s source for items from the Computer condition (M = 45.83%, s.d. = 15.13) compared with items from the Ingroup (M = 57.54%, s.d. = 14.42), F(1, 23) = 9.01, P = 0.006, 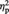 = 0.28, or Outgroup (M = 56.65%, s.d. = 15.51) conditions, F(1, 23) = 17.31, P < 0.001,
= 0.28, or Outgroup (M = 56.65%, s.d. = 15.51) conditions, F(1, 23) = 17.31, P < 0.001, 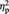 = 0.43. Source memory did not significantly differ between the Ingroup and Outgroup conditions, P > 0.7.
= 0.43. Source memory did not significantly differ between the Ingroup and Outgroup conditions, P > 0.7.
Fig. 1.
Source memory accuracy as a function of condition. Error bars represent 95% within-subject confidence intervals (Loftus and Masson, 1994).
Preference ratings
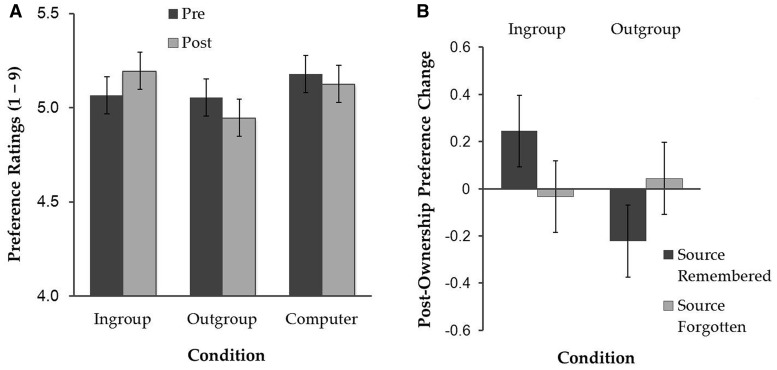
Post-ownership preference ratings from one participant were not collected due to computer malfunction (thus, N = 23). A 3 (condition: Ingroup, Outgroup or Computer) × 2 (time of rating: pre- or post-ownership) repeated-measures ANOVA revealed only a significant two-way interaction, F(2, 44) = 3.28, P = 0.047, 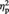 = 0.13. As shown in Figure 2A, simple effects analyses indicated that Ingroup-associated objects were given higher preference post-ownership (M = 5.20, s.d. = 0.61) than pre-ownership (M = 5.07, s.d. = 0.64), F(1, 22) = 7.36, P = 0.013,
= 0.13. As shown in Figure 2A, simple effects analyses indicated that Ingroup-associated objects were given higher preference post-ownership (M = 5.20, s.d. = 0.61) than pre-ownership (M = 5.07, s.d. = 0.64), F(1, 22) = 7.36, P = 0.013, 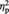 = 0.25. In contrast, the Outgroup-associated objects showed a trend for decreased preference post-ownership (M = 4.95, s.d. = 0.77) than pre-ownership (M = 5.05, s.d. = 0.72), F(1, 22) = 3.87, P = 0.062,
= 0.25. In contrast, the Outgroup-associated objects showed a trend for decreased preference post-ownership (M = 4.95, s.d. = 0.77) than pre-ownership (M = 5.05, s.d. = 0.72), F(1, 22) = 3.87, P = 0.062, 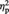 = 0.15. No significant difference in preference ratings between pre- (M = 5.18, s.d. = 0.79) and post-ownership (M = 5.13, s.d. = 0.85) was found for the Computer-associated objects, P > 0.5. Pre-ownership ratings did not significantly differ among the conditions, Ps > 0.4. Of the 42 Ingroup-associated objects, on average, participants’ preference increased for 20.65 items, decreased for 11.30 items and remained the same for 10.05 items from pre- to post-ownership. Of the 42 Outgroup-associated objects, participants’ preference increased for 12.26 items, decreased for 18.09 items and remained the same for 11.65 items. Of the 42 Computer-associated objects, participants’ preference increased for 14.96 items, decreased for 12.78 items and remained the same for 14.26 items.
= 0.15. No significant difference in preference ratings between pre- (M = 5.18, s.d. = 0.79) and post-ownership (M = 5.13, s.d. = 0.85) was found for the Computer-associated objects, P > 0.5. Pre-ownership ratings did not significantly differ among the conditions, Ps > 0.4. Of the 42 Ingroup-associated objects, on average, participants’ preference increased for 20.65 items, decreased for 11.30 items and remained the same for 10.05 items from pre- to post-ownership. Of the 42 Outgroup-associated objects, participants’ preference increased for 12.26 items, decreased for 18.09 items and remained the same for 11.65 items. Of the 42 Computer-associated objects, participants’ preference increased for 14.96 items, decreased for 12.78 items and remained the same for 14.26 items.
Fig. 2.
(A) Pre- and post-ownership preference ratings as a function of condition and (B) post-ownership preference change as a function of source memory status (source-remembered or source-forgotten) and condition (Ingroup or Outgroup). Error bars represent 95% within-subject confidence intervals (Loftus and Masson, 1994).
To examine whether participants’ source memory was related to their tendency to overvalue objects associated with the Ingroup and to devalue those associated with the Outgroup, we calculated preference changes separately for the source-remembered and source-forgotten items from the Ingroup and Outgroup conditions (Figure 2B). A 2 (condition: Ingroup or Outgroup) × (source memory status: remembered or forgotten) repeated-measures ANOVA showed a main effect of condition, F(1, 22) = 8.10, P = 0.009, 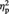 = 0.27, which was qualified by a two-way interaction, F(1, 22) = 13.78, P = 0.001,
= 0.27, which was qualified by a two-way interaction, F(1, 22) = 13.78, P = 0.001, 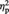 = 0.39. Simple effects analyses indicated that for the Ingroup condition, participants showed greater preference increase for objects when they remembered the source than when they did not (M = 0.24 and M = −0.03 for source-remembered vs source-forgotten Ingroup objects, respectively), F(1, 22) = 7.40, P = 0.012,
= 0.39. Simple effects analyses indicated that for the Ingroup condition, participants showed greater preference increase for objects when they remembered the source than when they did not (M = 0.24 and M = −0.03 for source-remembered vs source-forgotten Ingroup objects, respectively), F(1, 22) = 7.40, P = 0.012, 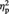 = 0.25. In contrast, for the Outgroup condition, participants showed greater preference decrease for objects when they remembered the source than when they did not (M = −0.22 and M = 0.04 for source-remembered vs source-forgotten Outgroup objects, respectively), F(1, 22) = 7.00, P = 0.015,
= 0.25. In contrast, for the Outgroup condition, participants showed greater preference decrease for objects when they remembered the source than when they did not (M = −0.22 and M = 0.04 for source-remembered vs source-forgotten Outgroup objects, respectively), F(1, 22) = 7.00, P = 0.015, 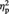 = 0.24.
= 0.24.
fMRI Results
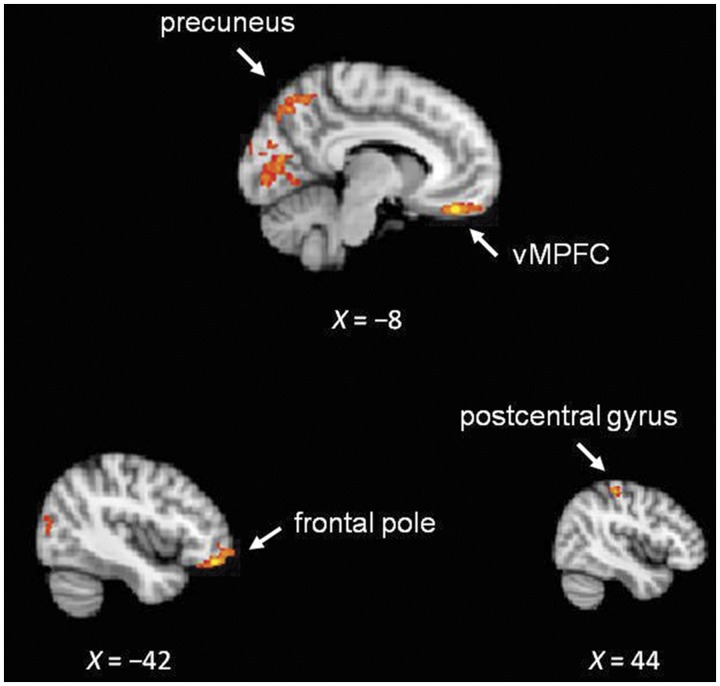
The Ingroup > Outgroup whole-brain contrast identified activation clusters, with peak voxels located in vMPFC, precuneus, frontal pole and postcentral gyrus (Figure 3 and Table 1). The reverse Outgroup > Ingroup contrast did not identify any significant cluster.
Fig. 3.
Activation map from whole-brain regression analysis for Ingroup > Outgroup contrast.
Table 1.
Peak coordinates of the significant clusters from Ingroup > Outgroup whole-brain contrast
| Region | Hemisphere | Montreal Neurological Institute’s coordinates |
Z-score | Number of voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| vMPFC | Left | −8 | 42 | −22 | 3.57 | 764 |
| Precuneus | Left | −22 | −60 | 6 | 3.70 | 2999 |
| Lingual gyrus | Right | 26 | −62 | 0 | 3.40 | |
| Lateral occipital cortex | Right | 32 | −80 | 26 | 3.32 | |
| Intracalcarine cortex | Right | 6 | −82 | 2 | 3.21 | |
| Frontal pole | Left | −42 | 44 | −16 | 3.41 | 374 |
| Postcentral gyrus | Right | 44 | −28 | 56 | 3.32 | 377 |
Note. Where the number of voxels is not listed, the activation occurred within subclusters of the preceding cluster.
In our independently defined vMPFC ROI (Figure 4A), a 2 (condition: Ingroup or Outgroup) × 2 (post-ownership preference change: higher or lower) repeated-measures ANOVA performed on percent signal changes showed a significant two-way interaction, F(1, 22) = 9.94, P = 0.005, 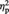 = 0.31. As shown in Figure 4B, simple effects analyses revealed that the Ingroup Higher items showed greater activation (i.e. less deactivation) than the Ingroup Lower items, F(1, 22) = 11.19, P = 0.003,
= 0.31. As shown in Figure 4B, simple effects analyses revealed that the Ingroup Higher items showed greater activation (i.e. less deactivation) than the Ingroup Lower items, F(1, 22) = 11.19, P = 0.003, 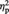 = 0.34. In contrast, the Outgroup Higher items showed less activation than the Outgroup Lower items, F(1, 22) = 5.90, P = 0.024,
= 0.34. In contrast, the Outgroup Higher items showed less activation than the Outgroup Lower items, F(1, 22) = 5.90, P = 0.024, 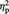 = 0.21. No significant difference was observed between the Ingroup Higher and Outgroup Lower items and between the Ingroup Lower and Outgroup Higher items, Ps > 0.1.2 While no cluster survived cluster correction for the whole-brain contrasts involving preference change between the Ingroup and Outgroup conditions, the same patterns of results as found for the independently defined vMPFC ROI were obtained when an analysis was performed on percent signal changes within the vMPFC cluster derived from the Ingroup > Outgroup whole-brain contrast shown in Figure 3.
= 0.21. No significant difference was observed between the Ingroup Higher and Outgroup Lower items and between the Ingroup Lower and Outgroup Higher items, Ps > 0.1.2 While no cluster survived cluster correction for the whole-brain contrasts involving preference change between the Ingroup and Outgroup conditions, the same patterns of results as found for the independently defined vMPFC ROI were obtained when an analysis was performed on percent signal changes within the vMPFC cluster derived from the Ingroup > Outgroup whole-brain contrast shown in Figure 3.
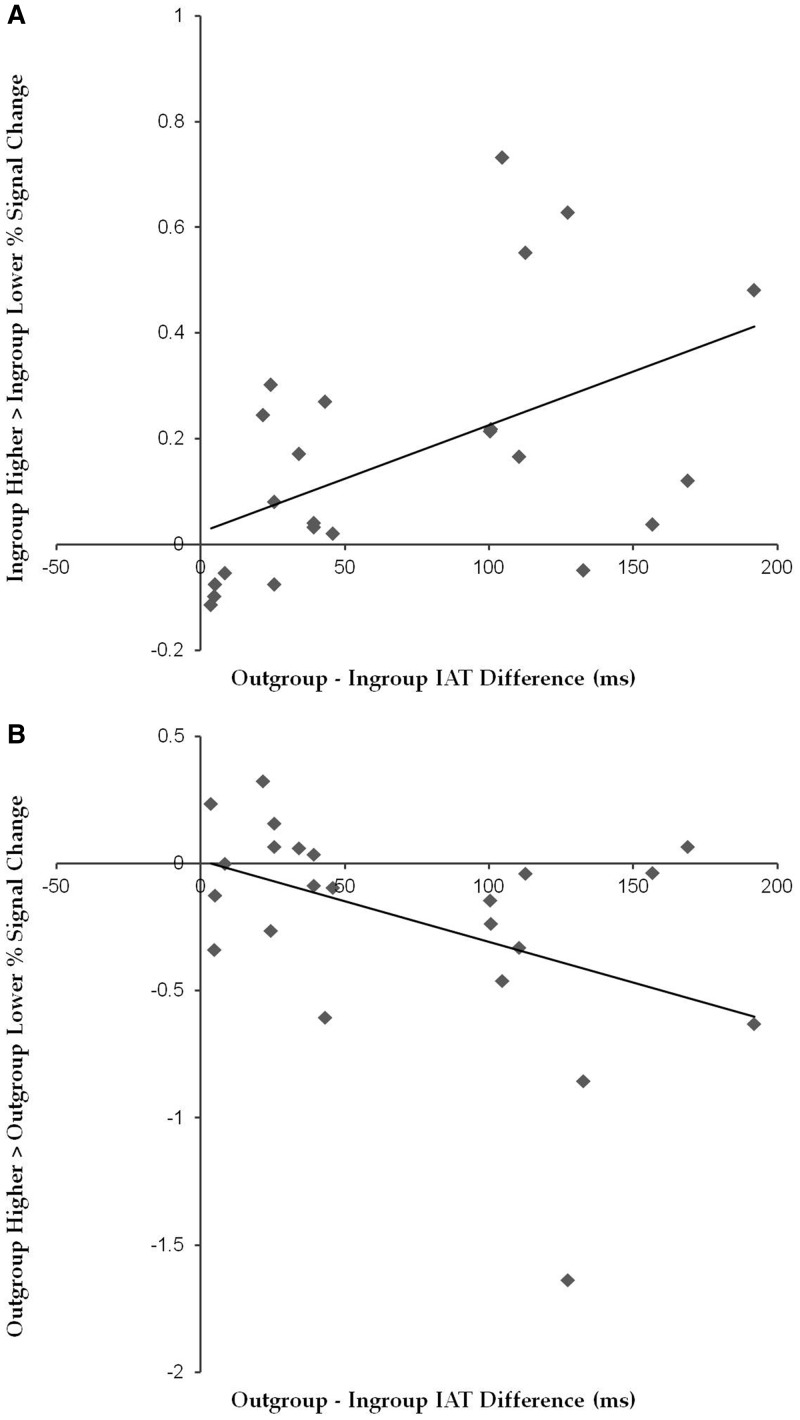
To examine the relationship between the degree to which participants implicitly associated either the liberal or conservative with their ingroup and the percent signal change for objects with higher vs lower post-ownership preference, we correlated IAT response time differences (mean response time for Outgroup minus mean response time for Ingroup) with the percent signal change difference between objects with higher post-ownership preference and those with lower post-ownership preference, separately for the Ingroup- and Outgroup-associated objects. As shown in Figure 5A, for Ingroup-associated objects, IAT difference scores were positively correlated with percent signal difference between objects with higher vs lower post-ownership preference, r(21) = 0.50, P = 0.016. In contrast, for Outgroup-associated objects (Figure 5B), IAT difference scores were negatively correlated with percent signal difference between objects with higher vs lower post-ownership preference, r(21) = −0.44, P = 0.034.
Fig. 5.
Mean percent signal change difference in the vMPFC ROI shown in Figure 4A between objects with higher vs lower post-ownership preference in relation to IAT difference scores: (A) Ingroup-associated objects and (B) Outgroup-associated objects.
DISCUSSION
In this study, we sought to characterize the function of vMPFC in self-related processing, focusing on two alternative hypotheses: (i) vMPFC evaluates or represents positive value of self-related information or assigns positive value to information by virtue of its self-relatedness and (ii) vMPFC assigns personal significance to self-related information by evaluating or representing its meaning/function for the self. Within an imagined ownership paradigm, we introduced and contrasted two different contexts in which people form associations between themselves and objects that were associated with their ingroup vs outgroup. We found that vMPFC was more active when people imagined owning objects associated with their ingroup compared to owning objects associated with their outgroup. Most important, for ingroup-associated objects, vMPFC showed greater activity for objects with increased post-ownership preference than those with decreased preference. In contrast, for outgroup-associated objects, vMPFC showed greater activity for objects with decreased post-ownership preference than those with increased preference.
Activity in vMPFC previously has been found to be greater for an ingroup member (i.e. someone similar to oneself) than for an outgroup member (i.e. someone dissimilar to oneself), and the strength of activation in vMPFC to the ingroup relative to the outgroup was positively related to the strength of positivity participants felt toward the ingroup but not to the overall amount of affect associated with ingroup/outgroup (e.g. no modulation of vMPFC activation by negativity participants felt toward the outgroup) (Mitchell et al., 2006). Do the current findings simply reflect a similar effect of ingroup vs outgroup? The fact that activity in vMPFC was modulated not only by post-ownership preference increases for ingroup-associated objects but also by preference decreases for outgroup-associated objects indicates that a simple main effect of ingroup vs outgroup cannot solely explain the present findings. One might also ask whether the present findings can be explained by participants being less willing to or successful at imagining owning objects or otherwise less engaged in processing them when the objects were associated with their outgroup compared to their ingroup. If this were the case, one would expect to see ownership-induced preference changes for ingroup-associated objects but no such changes for outgroup-associated objects. Yet, we found that the participants tended not only to increase their preference for ingroup-associated objects but also to decrease their preference for outgroup-associated objects. In addition, given that encoding information in reference to self produces a memorial advantage (i.e. self-reference effect, Rogers et al., 1977), our finding of no significant difference in source memory accuracy for ingroup- vs outgroup-associated objects further suggests that the participants in our study were likely to be equally successful at imagining both ingroup- and outgroup-associated objects as belonging to them. Thus, the overall pattern of current findings does not provide strong support for an alternative interpretation of obtained results as reflecting simply differences in success in imagining ownership of objects and/or differences in the extent to which objects from different social sources were processed.
The current findings are consistent with the idea that the role of vMPFC in self-related processing is not simply to assign positive subjective value to a self-related experience. The different patterns of vMPFC activity for ingroup- vs outgroup-associated objects are more in line with recent proposals suggesting that the function of vMPFC may be to generate a sense of significance (i.e. affective meaning) of incoming stimuli for an organism’s well-being and future prospects (Roy et al., 2012) and to evaluate or represent the personal significance of self-related contents in particular (D’Argembeau et al., 2012; D’Argembeau, 2013). In this view, vMPFC activity is involved in assigning significance to self-relevant experiences based on individuals’ motivations (needs, goals) that are salient at a given moment. In the present study, the nominally ‘same’ experience of owning a particular object likely had different functional significance for the participants according to whether the objects were associated with their ingroup or outgroup: the former would accentuate the motivation to assimilate to the ingroup and the latter would accentuate the motivation to differentiate from the outgroup (Brewer, 1991).
Additional insights about the function of vMPFC should come from future studies exploring how vMPFC activity patterns associated with seemingly the same event may differ across situational contexts that are likely to activate or induce different values, attitudes and goals. In a related vein, the self-related objects, people and ideas that individuals view as personally significant may depend on which of ‘multiple’ selves (Markus and Wurf, 1987; Deaux, 1992) is salient at a given moment. In this study, by having ‘ownership status’ constant across experimental conditions (i.e. no objects were other-owned), we made the social aspect of oneself more salient than the personal self. Thus, the self-enhancement motive at the level of personal self, suggested to underlie overvaluation of self-associated objects (Beggan, 1992), might have been less important than the need for differentiation at the level of social self when to-be-owned objects were associated with one’s outgroup. Given that various aspects of self can be associated with different motives, goals or needs, future work would be useful that explores whether these factors are differentially weighted in terms of their significance for oneself, as reflected in vMPFC activity, based on relative saliency of different aspects of the self.
Our findings suggest an intriguing possibility that altered vMPFC activity during self-related processing in patients with various clinical disorders (Johnson et al., 2009; Sheline et al., 2009; Lombardo et al., 2010) may reflect disruption of mental operations related to assessment and assignment of personal significance. Whether in these populations alterations of self-boundaries (Feinberg, 2011) or rigid focus on dysfunctional self-views (Lemogne et al., 2012) arise, in part, from disrupted assignment of personal significance to ‘me or mine’ aspects of the world, awaits future investigation.
Acknowledgments
We thank Jason Mitchell for sharing descriptions for the perceived similarity/dissimilarity manipulation and Erich Greene and Su Mei Lee for helpful discussions.
This work was supported by the National Institutes of Health [grant number R37 AG009253] and the Yale University FAS Imaging Fund.
Footnotes
1 Comparisons between the Computer and other two conditions were in line with the role of MPFC in social cognition in general. The Person (Ingroup and Outgroup conditions combined) > Computer contrast showed greater activation in both dorsal (0, 22, 52) and ventral (6, 38, −18) MPFC as well as in thalamus (2, −12, 4) and posterior cingulate gyrus (0, −48, 24). For brevity, the results from contrasts involving the Computer condition are not further reported.
2 Participants tended to increase and decrease their preference for Ingroup- and Outgroup-associated objects that were later associated with correct source memory. Given that vMPFC shows greater activity at encoding for self-referenced items that are subsequently remembered than forgotten (e.g. Macrae et al., 2004), our findings potentially reflected not only the effect of ownership-induced preference changes but also the effect of source memory performance. Importantly, we obtained essentially the same pattern of results when we only included objects that were later associated with correct source memory in the 2 (condition) × 2 (post-ownership preference change) repeated-measures ANOVA.
REFERENCES
- Anderson NH. Likableness ratings of 555 personality trait words. Journal of Personality and Social Psychology. 1968;9:272–9. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Aragón OR, Sharer EA, Bargh JA, Pineda JA. Social Cognitive and Affective Neuroscience. Advance online publication; 2013. Modulations of mirroring activity by desire for social connection and relevance of movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, McLaughlin-Volpe T, Mashek D, Lewandowski G, Wright SC, Aron EN. Including others in the self. European Review of Social Psychology. 2004;15:101–32. [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beggan JK. On the social nature of nonsocial perception: the mere ownership effect. Journal of Personality and Social Psychology. 1992;62:229–37. [Google Scholar]
- Belk RW. Possessions and the extended self. Journal of Consumer Research. 1988;15:139–68. [Google Scholar]
- Branscombe NR, Ellemers N, Spears R, Doosje B. The context and content of social identity threats. In: Ellemers N, Spears R, Doosje B, editors. Social Identity: Context, Commitment, Content. Oxford, UK: Blackwell; 1999. pp. 35–58. [Google Scholar]
- Brewer MB. The social self: on being the same and different at the same time. Personality and Social Psychology Bulletin. 1991;17:475–82. [Google Scholar]
- Brewer MB. The psychology of prejudice: ingroup love or outgroup hate? Journal of Social Issues. 1999;55:429–44. [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in Human Neuroscience. 2013;7:1–13. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E. Valuing one’s self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cerebral Cortex. 2012;22:659–67. doi: 10.1093/cercor/bhr144. [DOI] [PubMed] [Google Scholar]
- Deaux K. Personalizing identity and socializing self. In: Breakwell GM, editor. Social Psychology of Identity and the Self-concept. London, UK: Academic Press; 1992. pp. 9–33. [Google Scholar]
- Feinberg TE. Neuropathologies of the self: clinical and anatomical features. Consciousness and Cognition. 2011;20:75–81. doi: 10.1016/j.concog.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. Journal of Personality and Social Psychology. 1998;74:1464–80. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Cambridge, MA: Harvard University Press; 1890/1983. [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection, and depression. Social Cognitive and Affective Neuroscience. 2009;4:313–27. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Johnson MK. Extended self: medial prefrontal activity during transient association of self and objects. Social Cognitive and Affective Neuroscience. 2012;7:199–207. doi: 10.1093/scan/nsq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Johnson MK. Social Cognitive and Affective Neuroscience. Advance online publication; 2013. Extended self: spontaneous activation of medial prefrontal cortex by objects that are ‘mine’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander NP, Chartrand TL, Bargh JA. You give me the chills: embodied reactions to inappropriate amounts of behavioral mimicry. Psychological Science. 2012;23:772–9. doi: 10.1177/0956797611434535. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders. 2012;136:e1–11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5th edn. New York, NY: McGraw-Hill; 2010. pp. 143–93. [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin and Review. 1994;1:476–90. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. Atypical neural self-representation in autism. Brain. 2010;133:611–24. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Markus H, Wurf E. The dynamic self-concept: a social psychological perspective. Annual Review of Psychology. 1987;38:299–337. [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Neural representations of subjective reward value. Behavioral Brain Research. 2010;213:135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010;20:262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35:677–88. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16:147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3:451–70. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder DA, Allen VL. Group membership and preference for information about others. Personality and Social Psychology Bulletin. 1978;4:106–10. [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]