Abstract
Animal studies reveal that the amygdala promotes attention and emotional memory, in part, by driving activity in downstream target regions including the prefrontal cortex (PFC) and hippocampus. Prior work has demonstrated that the amygdala influences these regions directly through monosynaptic glutamatergic signaling, and indirectly by driving activity of the cholinergic basal forebrain and subsequent downstream acetylcholine release. Yet to date, no work has addressed the functional relevance of the cholinergic basal forebrain in facilitating signaling from the amygdala in humans. We set out to determine how blood oxygen level-dependent signal within the amygdala and cholinergic basal forebrain interact to predict neural responses within downstream targets. Here, we use functional connectivity analyses to demonstrate that the cholinergic basal forebrain moderates increased amygdala connectivity with both the PFC and the hippocampus during the processing of biologically salient stimuli in humans. We further demonstrate that functional variation within the choline transporter gene predicts the magnitude of this modulatory effect. Collectively, our results provide novel evidence for the importance of cholinergic signaling in modulating neural pathways supporting arousal, attention and memory in humans. Further, our results may shed light on prior association studies linking functional variation within the choline transporter gene and diagnoses of major depression and attention-deficit hyperactivity disorder.
Keywords: amygdala, basal forebrain, choline transporter, functional connectivity, imaging genetics
INTRODUCTION
Survival is dependent on adaptively shifting attention toward biologically salient stimuli and remembering the contexts in which these stimuli were encountered. Shifting attention functions to marshal resources in service of reactive responses while remembering contexts allows for proactive responses. Evidence from animal models suggests that the amygdala enables these processes directly through glutamatergic projections, and indirectly by promoting the release of acetylcholine (ACh) within downstream target regions including the hippocampus and prefrontal cortex (PFC).
Specifically, the basolateral amygdala (BLA) provides glutamatergic efferents to the PFC and hippocampus (Orozco-Cabal et al., 2006; Felix-Ortiz and Tye, 2014). The BLA additionally projects to cholinergic nuclei in the basal forebrain, including the nucleus basalis of Meynert, which in turn provide cholinergic efferents to both PFC and hippocampus (Price and Amaral, 1981; Russchen et al., 1985). Release of ACh in the hippocampus and PFC functions to increase responsiveness of target neurons by lowering their action potential thresholds, rendering them more sensitive to glutamatergic signaling (Sawaguchi and Matsumura, 1985; Nakajima et al., 1986). Consistent with this evidence, electrical stimulation of the BLA results in increased activity in both the PFC and hippocampus, which can be blocked by cholinergic–muscarinic receptor antagonists (Dringenberg and Vanderwolf, 1996).
Abundant research in animal models has established the critical importance of these pathways in facilitating attention and memory; however, scant evidence exists in support of similar pathways in humans. While multiple studies in humans have examined how systemic pharmacologic augmentation of ACh globally affects function of the PFC and hippocampus (Bentley et al., 2003, 2008, 2011), none to date have directly examined the moderating role of cholinergic nuclei on neural pathways supporting shifting attention to and increasing memory for biologically salient stimuli. Here, we apply psycho-physio–physiological interaction analyses (McLaren et al., 2012) to blood oxygen level-dependent functional magnetic resonance imaging (BOLD fMRI) data collected from 410 young adults during the processing of emotionally expressive facial expressions to directly examine the moderating role of activity in the basal forebrain cholinergic nuclei on the functional connectivity between the amygdala and downstream target regions including the hippocampus and PFC. Within this manuscript, we refer to basal forebrain moderation of task-dependent amygdala connectivity in the statistical sense, based on this three-way interaction. Based on the existing literature, we hypothesized that functional connectivity between the BLA and both hippocampus and PFC would increase during the processing of salient facial stimuli and that greater relative activity in the basal forebrain cholinergic nuclei would facilitate task-modulated functional connectivity. We further hypothesized that such increased functional connectivity between the amygdala and PFC would be associated with faster reaction times on our task consistent with the pro-attentional effect of ACh (Bentley et al., 2003, 2008, 2011).
To implicate cholinergic signaling specifically in the modulation of these pathways, we additionally used an imaging genetics strategy (Hariri, 2009) in a subset of 142 non-Hispanic Caucasians targeting a nonsynonymous single nucleotide polymorphism (SNP) (rs1013940) within the human choline transporter gene (CHT1). Focusing the imaging genetics analyses on this subsample reduces the potential confounding effects of broad differences in genetic background associated with racial and ethnic ancestry. The choline transporter is a presynaptic mechanism through which choline is transported into neurons and subsequently made available for de novo ACh biosynthesis (Sarter and Parikh, 2005). As such, the choline transporter is a major rate-limiting mechanism in cholinergic signaling, and it has been suggested that choline transporter function is critical for cognitive processes (Sarter and Parikh, 2005). The valine (89Val) allele of rs1013940 results in a 40–50% decrease in choline uptake in mammalian cell lines in comparison with the isoleucine (Iso89) allele (Okuda et al., 2002). Thus, we further hypothesized that carriers of the Val allele, who presumably have relatively decreased capacity for de novo ACh biosynthesis and subsequent signaling, would exhibit decreased cholinergic basal forebrain modulation of task-dependent functional connectivity between the amygdala and both hippocampus and PFC.
EXPERIMENTAL PROCEDURES
An extended description of the experimental procedures is included in the Supporting Experimental Procedures (Supplementary Material).
Participants
Data were available from 470 participants who had successfully completed the ongoing Duke NeuroGenetics Study (DNS), which assesses a wide range of behavioral and biological traits among nonpatient, 18- to 22-year-old university students. All participants provided informed consent in accordance with Duke University guidelines prior to participation. BOLD fMRI analyses were limited to 410 participants (237 females, mean age = 19.71 ± 1.27 s.d.), with fMRI data passing our quality control procedures. Imaging genetics analyses were conducted on a further subset of 142 non-Hispanic Caucasians with overlapping fMRI and genotype data.
All participants were in good general health and free of the following study exclusions:
medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease or lifetime history of psychotic symptoms;
use of psychotropic, glucocorticoid or hypolipidemic medication; and
conditions affecting cerebral blood flow and metabolism (e.g. hypertension).
As the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology, we did not exclude participants based on a diagnosis of any past or current Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) Axis I or select Axis II (antisocial and borderline personality) disorders as identified through clinical interviews using the electronic MINI (Sheehan et al., 1998). However, as stated earlier, no subjects were taking psychotropic medication at a minimum of 10 days prior to study participation, and we explicitly control for current or past diagnosis in our analyses as described below.
Neuroimaging
Our paradigm, which has been used widely to elicit robust amygdala reactivity (Nikolova and Hariri, 2012; Carré et al., 2013), consists of four blocks of a perceptual face-matching task interleaved with five blocks of a shape-matching sensorimotor control. The paradigm was administered while acquiring BOLD signal across a series of 34 interleaved axial slices on a research-dedicated GE MR750 3 T scanner equipped with an eight-channel head coil for parallel imaging.
Genetics
Genomic DNA was extracted from saliva, and rs1013940 genotyping was performed at the National Genetics Institute, a Clinical Laboratory Improvement Amendments certified clinical laboratory and subsidiary of Laboratory Corporation of America, using the Illumina Omni Express Plus chip augmented with a custom array of ∼300, 000 SNPs (Eriksson et al., 2010; Do et al., 2011; Tung et al., 2011). Genotype differences in 142 were evaluated between participants homozygous for the Iso89 allele (N = 118) and those carrying the 89Val allele (N = 24).
Statistics
Task-dependent activity
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used to conduct fMRI data analyses. Following preprocessing, linear contrasts using canonical hemodynamic response functions were used to estimate main effects of the experimental paradigm (e.g. Faces > Shapes) for each individual. Individual contrast images were then used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine areas engaged by the paradigm using one-sample t-tests with a voxel-level statistical threshold of P < 0.05, family wise error (FWE) corrected for multiple comparisons across the entire brain and a cluster threshold of 10 contiguous voxels.
Psycho-physio–physiological interaction
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used to conduct fMRI data analyses using the generalized psycho-physiological interaction (gPPI) toolbox (McLaren et al., 2012). Owing to the small sizes of the amygdala and basal forebrain regions of interest (ROIs) and their relative proximity to one another, spatial smoothing was conducted in a manner that has previously been used to successfully delineate the functional connectivity of similarly situated ROIs (Roy et al., 2009). Roy et al. effectively demonstrated differences in functional connectivity between basolateral and central/medial amygdala ROIs using 3 × 3 × 3 mm3 voxel acquisition and 6 mm full width at half maximum (FWHM) spatial smoothing. Here, we used the same probabilistic BLA ROIs used by Roy et al. and a probabilistic basal forebrain ROI from the same toolbox (Eickhoff et al., 2005). Moreover, we used similar acquisition parameters (3.75 × 3.75 × 4 mm voxels) and identical smoothing (6 mm FWHM) on our data as Roy et al. Thus, we are confident in our ability to distinguish signal from these ROIs, particularly given that our basal forebrain ROI was of comparable size and more distant than the central/medial amygdala ROI used by Roy et al. (Amunts et al., 2005; Zaborszky et al., 2008).
Following preprocessing, deconvolved time courses averaged across the BLA (tc1) and the CH4 region of the basal forebrain (tc2) were separately extracted, and entered into first-level statistical models, which also included a psychological regressor corresponding to the faces and shapes matching task, as well as all interaction terms (tc1*tc2, tc1*task, tc2*task and tc1*tc2*task). Individual beta images corresponding to the three-way interaction term (tc1*tc2*task) were then used in a second-level random effects model accounting for scan-to-scan and participant-to-participant variability to determine correlates of the amygdala time course that vary as a function of basal forebrain BOLD signal and experimental condition using one-sample t-tests with a voxel-level statistical threshold of P < 0.05, FWE corrected for multiple comparisons across the entire brain and a cluster threshold of 10 contiguous voxels.
Imaging genetics
BOLD parameter estimates corresponding to the ‘BLA time course-by-cholinergic basal forebrain ROI time course-by-experimental task’ three-way interaction term were extracted from the medial PFC (mPFC) and hippocampus. These extracted parameter estimates represent the effect of activity in cholinergic basal forebrain on the task-dependent functional connectivity between the BLA and ipsilateral mPFC and hippocampus. Analysis of covariance controlling for gender and past or current DSM-IV diagnosis was conducted to assess the effects of CHT1 rs1013940 on these parameter estimates in a subsample of 142 non-Hispanic Caucasian.
Performance
To determine if the patterns of cholinergic basal forebrain moderation of task-dependent functional connectivity predicted behavioral performance, namely, reaction times, BOLD parameter estimates corresponding to the ‘BLA time course-by-cholinergic basal forebrain ROI time course-by-experimental task’ three-way interaction term from the mPFC cluster were entered into a multiple regression model predicting the difference between reaction times derived from correctly identified trials from task and control blocks. Gender as well as past or current DSM-IV diagnosis were entered as covariates.
RESULTS
Task-dependent activity
Consistent with prior studies (Hariri et al., 2002; Nikolova and Hariri, 2012; Ahs et al., 2013; Carré et al., 2013; Prather et al., 2013; Wellman et al., 2013), analyses in the full sample (N = 410) revealed robust (P < 0.05, whole-brain FWE corrected) activity in bilateral amygdala, hippocampus and posterior fusiform gyrus as well as medial and lateral PFC in response to salient biological stimuli, namely, novel facial expressions (Supplementary Figure 1).
Activity of cholinergic basal forebrain moderates amygdala connectivity
Psycho-physio–physiological interaction analyses in the full sample (N = 410) revealed that the magnitude of task-dependent functional connectivity between the BLA and downstream targets was significantly modulated by activity in the cholinergic basal forebrain. Specifically, activity within the right cholinergic basal forebrain ROI moderated task-dependent functional connectivity between the right BLA and distributed areas of the cortical mantle, the medial temporal lobes, striatum and cerebellum (see Table 1 for coordinates and voxel statistics). Similar results were observed in the left hemisphere (Table 2). Of specific interest, the magnitude of activity in cholinergic basal forebrain ROIs predicted larger increases in task-dependent functional connectivity between the BLA and both hippocampus and mPFC in the right (Figure 1) and left hemispheres.
Table 1.
Brain regions exhibiting increased task-dependent functional connectivity with the right BLA as a function of activity in the right cholinergic basal forebrain as identified using psycho-physio–physiological interaction analyses of BOLD fMRI data in the full sample of 410 participants
| Anatomical location | T statistic | Z statistic | MNI coordinates | P < (FWE) | Cluster size (kE) |
|---|---|---|---|---|---|
| Frontal lobe | |||||
| Medial prefrontal cortex | 6.38 | 6.22 | 0, 58, −4 | 0.001 | 774 |
| Bilateral precentral gyrus | 6.3 | 6.15 | 16, −28, 68 | 0.001 | 1053 |
| Left lateral frontal orbital gyrus | 5.47 | 5.37 | −28, 32, −14 | 0.001 | 60 |
| Left superior frontal gyrus | 5.26 | 5.17 | −8, 52, 40 | 0.003 | 25 |
| Right middle frontal gyrus | 5.22 | 5.13 | 26, 32, 42 | 0.004 | 161 |
| Left inferior frontal gyrus | 5.15 | 5.07 | −48, 36, 12 | 0.005 | 26 |
| Left middle frontal gyrus | 5.14 | 5.06 | −26, 38, 42 | 0.005 | 69 |
| Left superior frontal gyrus | 5.13 | 5.05 | −2, 38, 50 | 0.006 | 18 |
| Right inferior frontal gyrus | 5.07 | 4.99 | 32, 62, 2 | 0.008 | 16 |
| Parietal lobe | |||||
| Right parietal lobe/angular gyrus | 5.31 | 5.22 | 40, −64, 30 | 0.003 | 149 |
| Precuneus/superior parietal lobule | 5.09 | 5.01 | 0, −60, 46 | 0.007 | 115 |
| Left parietal lobe | 5.00 | 4.93 | −26, −82, 36 | 0.01 | 32 |
| Posterior cingulate | 4.78 | 4.72 | 4, −50, 16 | 0.025 | 21 |
| Temporal lobe | |||||
| Left superior temporal sulcus | 6.73 | 6.55 | −58, −18, −8 | 0.001 | 551 |
| Left fusiform gyrus | 6.16 | 6.02 | −42, −50, −22 | 0.001 | 227 |
| Left middle temporal gyrus | 5.64 | 5.53 | −44, −78, 18 | 0.001 | 435 |
| Right middle temporal gyrus | 5.58 | 5.48 | 56, −8, −20 | 0.001 | 75 |
| Right inferior temporal gyrus | 5.21 | 5.12 | 46, −50, −18 | 0.004 | 20 |
| Right inferior temporal gyrus | 5.15 | 5.07 | 58, 52, −16 | 0.005 | 109 |
| Occipital lobe | |||||
| Right lingual gyrus | 5.64 | 5.53 | 18, −90, −6 | 0.001 | 43 |
| Left lingual gyrus | 5.42 | 5.32 | −22, −94, −10 | 0.002 | 143 |
| Right occipital lobe | 5.05 | 4.97 | 36, −70, −20 | 0.008 | 24 |
| Right middle occipital gyrus | 4.9 | 4.83 | 40, −78, 12 | 0.015 | 22 |
| Subcortical | |||||
| Bilateral hippocampus, central amygdala, | 8.9 | Inf | 22, 0, −14 | 0.000 | 2442 |
| Putamen, caudate head | |||||
| Left caudate head | 4.85 | 4.78 | −14, 22, 4 | 0.019 | 19 |
| Right extra-nucleur thalamus | 5.08 | 5.00 | 2, −4, 8 | 0.007 | 30 |
| Pons | 5.06 | 4.98 | −6, −30, −38 | 0.008 | 13 |
| Left culmen/anterior cerebellum | 4.99 | 4.91 | −18, −40, −22 | 0.011 | 12 |
| Right culmen/anterior cerebellum | 5.33 | 5.24 | 10, −36, −26 | 0.002 | 17 |
Table 2.
Brain regions exhibiting increased task-dependent functional connectivity with the left BLA as a function of activity in the left cholinergic basal forebrain as identified using psycho-physio–physiological interaction analyses of BOLD fMRI data in the full sample of 410 participants
| Anatomical location | T statistic | Z statistic | MNI coordinates | P < (FWE) | Cluster size (kE) |
|---|---|---|---|---|---|
| Frontal lobe | |||||
| Left superior frontal gyrus | 6.05 | 5.91 | −18, 58, 28 | 0.001 | 200 |
| Right superior frontal gyrus | 5.82 | 5.7 | 24, 48, 38 | 0.001 | 62 |
| Left superior frontal gyrus | 5.8 | 5.68 | −24, 40, 42 | 0.001 | 83 |
| Medial frontal gyrus | 5.31 | 5.22 | −2, 56, −2 | 0.003 | 313 |
| Left inferior frontal gyrus | 5.27 | 5.18 | −34, 28, −16 | 0.003 | 19 |
| Left middle frontal gyrus | 5.11 | 5.02 | −4, 26, 60 | 0.007 | 26 |
| Parietal lobe | |||||
| Precuneus | 5.37 | 5.27 | −2, −52, 44 | 0.002 | 106 |
| Left posterior cingulate | 5.17 | 5.08 | −14, −28, 30 | 0.005 | 14 |
| Temporal lobe | |||||
| Left temporal lobe | 6.64 | 6.47 | −50, −36, 0 | 0.001 | 906 |
| Right superior temporal gyrus | 5.94 | 5.81 | 58, −56, 14 | 0.001 | 156 |
| Bilateral parahippocampal gyrus, posterior cingulate | 5.93 | 5.8 | −6, −40, 4 | 0.001 | 410 |
| Left superior temporal pole | 5.71 | 5.6 | −36, 12, −24 | 0.001 | 11 |
| Right middle temporal gyrus | 5.55 | 5.45 | 54, −22, −6 | 0.001 | 69 |
| Occipital lobe | |||||
| Right lingual gyrus | 5.63 | 5.52 | 16, −90, −6 | 0.001 | 64 |
| Subcortical | |||||
| Left central amygdala | 6.54 | 6.37 | −18, −2, −16 | 0.001 | 64 |
| Left hippocampus | 6.25 | 6.1 | −26, −28, −24 | 0.001 | 197 |
| Right central amygdala | 5.7 | 5.59 | 18, −2, −16 | 0.001 | 40 |
| Cerebellar vermis | 5.25 | 5.16 | 6, −44, 0 | 0.004 | 51 |
Fig. 1.
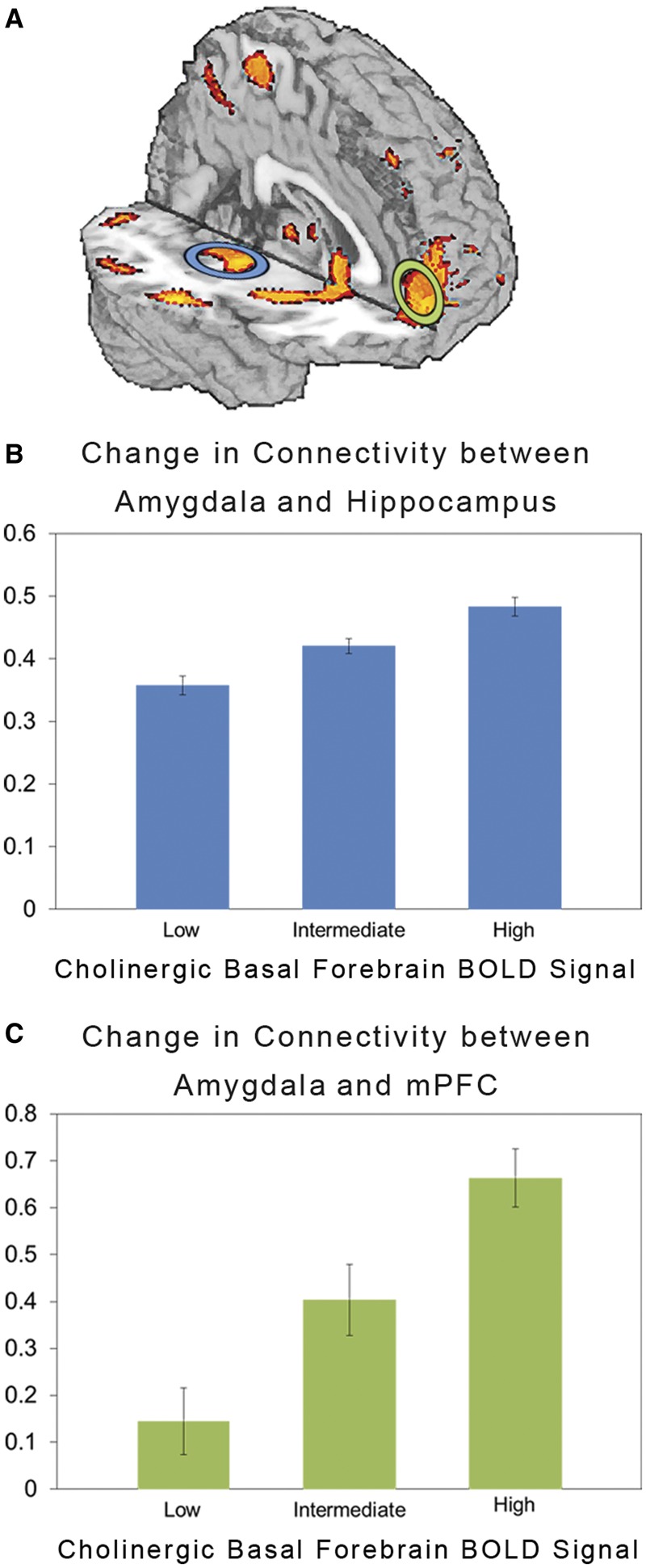
Functional connectivity predicted by interaction of experimental task, BLA BOLD signal and cholinergic basal forebrain BOLD signal (N = 410). (A) Statistical parametric map of brain regions exhibiting changes in task-dependent functional connectivity as a function of activity in the cholinergic basal forebrain. Bar graphs depicting this moderated change in functional connectivity between (B) BLA and hippocampus and (C) BLA and mPFC in the right hemisphere. The y-axis represents the two-way BLA time course-by-experimental task interaction term (mean ± SEM) at low (−1 s.d.), intermediate (mean) and high (+1 s.d.) levels of activity in cholinergic basal forebrain.
CHT1 rs1013940 predicts cholinergic moderation of amygdala connectivity
Analyses revealed that in comparison with individuals homozygous for the Iso89 allele, the magnitude of cholinergic modulation of task-dependent functional connectivity between the right BLA and both hippocampus (F = 5.44, P < 0.05) and mPFC (F = 5.43, P < 0.05) was significantly decreased in carriers of the 89Val allele (Figure 2). The effects of CHT1 rs1013940 genotype on these patterns in the left hemisphere were not significant (P’s > 0.1).
Fig. 2.
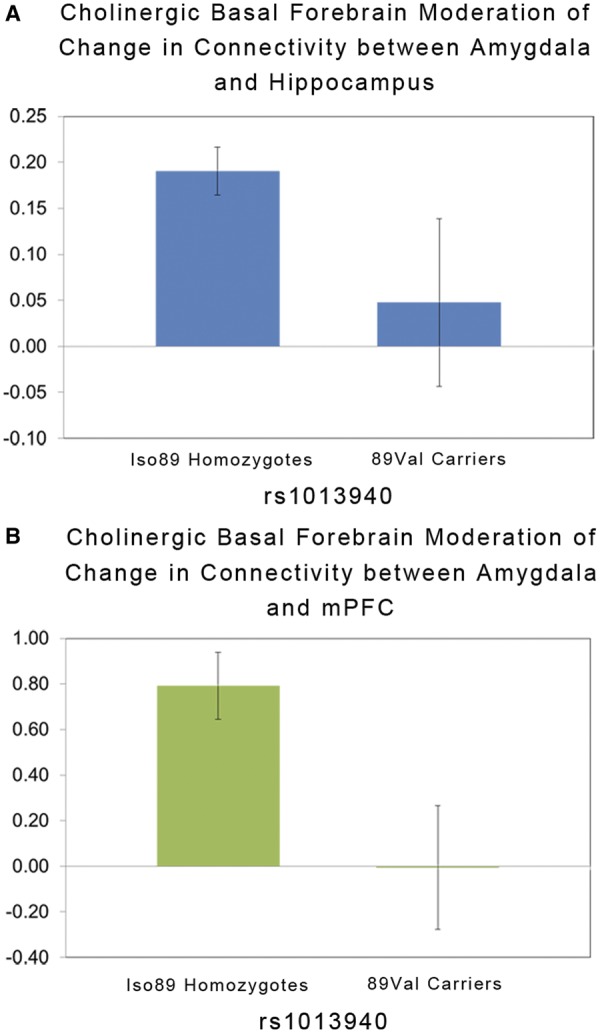
CHT1 rs1013940 genotype effects on the moderated functional connectivity between (A) BLA and hippocampus and (B) BLA and mPFC from Figure 1. The y-axis in (A) and (B) refers to the three-way BLA time course-by-basal forebrain time course-by-experimental task interaction term (mean ± SEM).
Effects on task performance
Parameter estimates extracted from the mPFC cluster were not associated with reaction times (all P’s > 0.1). To assess whether connectivity with other cortical regions involved with attention or sensory information processing were associated with reaction times, parameter estimates between the right BLA and dorsolateral PFC as well as right BLA and posterior fusiform gyrus were entered into multiple regression models with reaction time differences as the outcome. Although not statistically significant after correction for multiple comparisons, the patterns were consistent with a facilitating effect of increasing functional connectivity on faster reaction times (left dorsolateral PFC: β = −18.25, P = 0.010; right dorsolateral PFC: β = −9.53, P = 0.229; left posterior fusiform gyrus: β = −17.54, P = 0.094; right posterior fusiform gyrus: β = −14.51, P = 0.145).
The effect of CHT1 rs1013940 genotype on reaction time differences was not significant after controlling for the effects of gender and past or current DSM-IV diagnosis (β = −14.51, P = 0.145). However, in light of the striking effect of CHT1 rs1013940 genotype, we explored associations with reaction time differences in only the 118 non-Hispanic Caucasian participants homozygous for the Iso89 allele for whom there was significant moderation of connectivity. Again, parameter estimates extracted from the mPFC cluster were not associated with reaction times (all P’s > 0.1). In contrast, parameter estimates from other cortical regions were significantly associated with faster reaction time differences (left dorsolateral PFC: β = −23.48, P = 0.074; right dorsolateral PFC: β = −32.84, P = 0.036; left posterior fusiform gyrus: β = −47.20, P = 0.020; right posterior fusiform gyrus: β = −45.66, P = 0.025).
DISCUSSION
Our results provide novel evidence for the importance of human cholinergic basal forebrain activity in modulating the functional connectivity of the amygdala and downstream targets including the hippocampus and PFC in response to biologically salient stimuli. Such cholinergic modulation of corticolimbic circuitry is consistent with the potentiating effects of ACh on attentional and mnemonic processes supported by the PFC and hippocampus, respectively. Our data further highlight neuroanatomical pathways in the human brain consistent with the importance of the amygdala in promoting reactive and proactive information processing in the service of adaptively responding to challenges emerging in our environments (Davis and Whalen, 2001; Sander et al., 2003; LaBar and Cabeza, 2006).
Increased attention to biologically salient events is important for generating appropriate responses to environmental challenge, and the amygdala supports the survival of an organism by directing attention toward relevant stimuli in the environment. Increased attention is elicited by potentially threatening or ambiguous stimuli and often results in such behaviors as searching or scanning activity (Yang et al., 2004), which, in ecological contexts, is associated with increased stimulus detection (Caine, 1984) and is positively associated with survival rates for organisms in areas with high predation risk (Sansom et al., 2009). Further, research in humans has demonstrated that participants can more quickly identify threatening stimuli during threatening contexts, and that increased connectivity between the amygdala and mPFC predicts this recognition advantage (Robinson et al., 2012). Our results provide a plausible neuroanatomical basis from such adaptive responding in humans that is highly consistent with those identified in animal models (Voytko et al., 1994; Baxter and Chiba, 1999; Parikh et al., 2013).
Contextual information is likewise necessary for adaptive behavior, as it allows organisms to predict and proactively respond to future environmental demands. Previous research has demonstrated that interactions between the amygdala and hippocampus during encoding predict better subsequent memory for emotional events (Dolcos et al. 2004). Moreover, contextual conditioning affects ACh release within the hippocampus and results in increased connectivity between the amygdala and hippocampus (Nail-Boucherie et al., 2000; Alvarez et al., 2008). Our results demonstrate that the human cholinergic basal forebrain functions to facilitate communication between the amygdala and hippocampus during the processing of salient stimuli, which may promote subsequent memory for the stimuli.
Our imaging genetics analyses further implicate cholinergic signaling pathways in the task-dependent modulation of functional connectivity between the amygdala and both hippocampus and PFC. Specifically, we demonstrate that the 89Val allele of CHT1 rs1013940 is associated with relatively decreased cholinergic basal forebrain modulation of functional connectivity. Previous research has demonstrated that the 89Val allele results in a 40–50% decrease in choline uptake (Okuda et al., 2002), which would presumably decrease de novo ACh biosynthesis and subsequent cholinergic signaling. Interestingly, the 89Val allele has been linked to increased incidence of attention-deficit hyperactivity disorder (English et al., 2009). Our current results suggest that this risk may be mediated by decreased functional connectivity between the amygdala and mPFC necessary for target detection, sustained attention and response inhibition (Strange et al., 2000; Kiehl et al., 2001; Ousdal et al., 2008). This relative reduction in task-dependent functional connectivity between the amygdala and mPFC may further inform associations between the 89Val allele and increased risk for depression (Hahn et al., 2008). Cognitive reappraisal is linked to reductions in negative affect, and increased functional coupling of the mPFC and amygdala predict the level of reduction in negative affect (Banks et al., 2007), processes that are impaired in depressed individuals and predict depressive symptoms (Erk et al., 2010).
It is unclear why we observe effects of rs1013940 in the right, but not the left hemisphere. However, it is possible that this laterality is an artifact of our experimental paradigm. Previous research has suggested that the right amygdala is responsible for the immediate detection of salient stimuli, while the left amygdala is responsible for more elaborative stimulus processing (Whalen et al., 1998; Phillips et al., 2001; Wright et al., 2001). Our perceptual matching task with multiple novel trials is likely biased toward the former processing pattern resulting in preferential reactivity and subsequent connectivity of the right amygdala. This possibility is supported by the observation of nominally larger effects of connectivity for the right amygdala, especially with clusters in the frontal lobe. Thus, it is possible that effects of rs1013940 within the left hemisphere would be observed with experimental paradigms involving more elaborative processing. Additional research is needed to evaluate this possibility.
There are important limitations to our current work deserving careful consideration. First, our work cannot establish a direct link between activity in the cholinergic basal forebrain measured with BOLD fMRI and ACh release. However, studies in primates suggest that 90% of neurons within our anatomical ROIs are cholinergic (Mesulam et al., 1983), and the results of our imaging genetics further implicate cholinergic signaling in pathways characterized by dense cholinergic projections (Mesulam et al., 1983, 1986; Okuda et al., 2002). Nevertheless, explicit demonstration that the modulation of functional connectivity we describe using BOLD fMRI is associated with variation in ACh release in target regions as may be possible with neuroreceptor positron emission tomography (Frey et al., 1992; Ding et al., 1996; Shinotoh et al., 2000) would be welcome.
Second, we lack clear evidence that the cholinergic modulation of task-dependent functional connectivity is expressed as improvement in either attentional or mnemonic processes as would be predicted. Specifically, increased functional connectivity between the BLA and mPFC as a function of cholinergic basal forebrain activity did not significantly predict faster differential reaction times between our task and control conditions. Although cholinergic modulation of functional connectivity between the BLA and other cortical regions including the dorsolateral PFC and fusiform gyrus did nominally predict faster reaction times, these effects did not reach statistical significance after correcting for multiple comparisons. However, we used a simple perceptual matching task that does not tax attentional resources with participants performing near ceiling for accuracy (Faces mean % = 97.3 ± 6.1 s.d.; Shapes mean % = 96.6 ± 7.2 s.d.), and exhibiting little inter-individual variability in reaction time differences between conditions (mean = 236 ms ± 151 s.d.). Thus, even a nominal effect on reaction times consistent with cholinergic potentiation of attention is noteworthy. Likewise, we could not examine potential effects of these modulatory pathways on mnemonic function, as we did not probe subsequent memory for our task stimuli. Thus, we lack the necessary dependent variables to examine the possible pro-mnemonic effects of the observed cholinergic modulation of functional connectivity between the BLA and hippocampus. Future work using more demanding tasks (e.g. attentional conflict) or explicit tests of episodic memory could reveal that these modulatory pathways do, in fact, promote reactive and proactive behavioral processes associated with adaptive responses to the environment.
These limitations notwithstanding, our current results are the first to demonstrate that the human cholinergic basal forebrain contributes to changes in the functional connectivity of the amygdala and downstream targets including the PFC and hippocampus in response to biologically salient stimuli. Further, our results provide novel evidence that a putatively functional genetic polymorphism in the human choline transporter gene (rs1013940) is associated with alterations in cholinergic basal forebrain modulation of corticolimbic circuit connectivity. Thus, our findings shed light on a fundamental neural mechanism for the promotion of attention and memory in humans and also inform possible neurogenetic pathways of risk for psychopathology associated with cholinergic dysfunction.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Bartholomew Brigidi, Kelly Faig, Spenser Jacobson and Yuliya Nikolova for their assistance in DNS data collection and analysis.
The DNS is supported by Duke University and the National Institute on Drug Abuse R01DA033369. A.R.H. receives additional support through the National Institute on Drug Abuse R01DA031579.
REFERENCES
- Ahs F, Davis CF, Gorka AX, Hariri AR. Feature-based representations of emotional facial expressions in the human amygdala. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. The Journal of Neuroscience. 2008;28(24):6211–19. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210(5-6):343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Current Opinion in Neurobiology. 1999;9(2):178–83. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain. 2008;131(2):409–24. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Progress in Neurobiology. 2011;94(4):360–88. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. Journal of Neurophysiology. 2003;90(2):1171–81. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Caine NG. Visual scanning by tamarins. Folia Primatologica. 1984;43(1):59–67. [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Social Neuroscience. 2013;8(2):122–35. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Ding Y, Gatley SJ, Fowler JS, et al. Mapping nicotinic acetylcholine receptors with PET. Synapse. 1996;24(4):403–7. doi: 10.1002/(SICI)1098-2396(199612)24:4<403::AID-SYN8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Do CB, Tung JY, Dorfman E, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genetics. 2011;7(6):e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–63. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Experimental Brain Research. 1996;108(2):285–96. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- English BA, Hahn MK, Gizer IR, et al. Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. Journal of Neurodevelopmental Disorders. 2009;1(4):252–63. doi: 10.1007/s11689-009-9033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson N, Macpherson JM, Tung JY, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genetics. 2010;6(6):e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience. 2010;30(47):15726–34. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. The Journal of Neuroscience. 2014;34(2):586–95. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey KA, Koeppe RA, Mulholland GK, et al. In vivo muscarinic cholingeric receptor imaging in human brain with [11C]scopolamine and positron emission tomography. Journal of Cerebral Blood Flow and Metabolism. 1992;12(1):147–54. doi: 10.1038/jcbfm.1992.18. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blackford JU, Haman K, et al. Multivariate permutation analysis associates multiple polymorphisms with subphenotypes of major depression. Genes, Brain and Behavior. 2008;7(4):487–95. doi: 10.1111/j.1601-183X.2007.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Review of Neuroscience. 2009;32:225–47. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, et al. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, et al. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38(1):133–42. [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. The Journal of Comparative Neurology. 1983;214(2):170–97. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Wainer BH. Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Research. 1986;367(1–2):301–8. doi: 10.1016/0006-8993(86)91607-0. [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K, Dourmap N, Jaffard R, Costentin J. Contextual fear conditioning is associated with an increase of acetylcholine release in the hippocampus of rat. Cognitive Brain Research. 2000;9(2):193–7. doi: 10.1016/s0926-6410(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Nakajima S, Leonard RJ, Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proceedings of the National Academy of Sciences USA. 1986;83(9):3022–6. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. Neural responses to threat and reward interact to predict stress-related problem drinking: a novel protective role of the amygdala. Biology of Mood and Anxiety Disorders. 2012;2(1):19. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Okamura M, Kaitsuka C, Haga T, Gurwitz D. Single nucleotide polymorphism of the human high affinity choline transporter alters transport rate. Journal of Biological Chemistry. 2002;277(47):45315–22. doi: 10.1074/jbc.M207742200. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, et al. A novel rat medial prefrontal cortical slice preparation to investigate synaptic transmission from amygdala to layer V prelimbic pyramidal neurons. Journal of Neuroscience Methods. 2006;151(2):148–58. doi: 10.1016/j.jneumeth.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, et al. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156(3):450–5. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Peters MS, Blakely RD, Sarter M. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. The Journal of Neuroscience. 2013;33(6):2326–37. doi: 10.1523/JNEUROSCI.4993-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, et al. Time courses of left and right amygdalar responses to fearful facial expressions. Human Brain Mapping. 2001;12(4):193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Bogdan R, Hariri AR. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosomatic Medicine. 2013;75(4):350–8. doi: 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. The Journal of Neuroscience. 1981;1(11):1242–59. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala–dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2012;60(1):523–9. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. The Journal of Comparative Neurology. 1985;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14(4):303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sansom A, Lind J, Cresswell W. Individual behavior and survival: the roles of predator avoidance, foraging success, and vigilance. Behavioral Ecology. 2009;20(6):1168–74. [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nature Reviews Neuroscience. 2005;6(1):48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M. Laminar distributions of neurons sensitive to acetylcholine, noradrenaline and dopamine in the dorsolateral prefrontal cortex of the monkey. Neuroscience Research. 1985;2(4):255–73. doi: 10.1016/0168-0102(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shinotoh H, Namba H, Fukushi K, et al. Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer’s disease: a positron emission tomography study. Annals of Neurology. 2000;48(2):194–200. [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. NeuroImage. 2000;12(4):425–33. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- Tung JY, Do CB, Hinds DA, et al. Efficient replication of over 180 genetic associations with self-reported medical data. PLoS One. 2011;6(8):e23473. doi: 10.1371/journal.pone.0023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory [published erratum appears in J Neurosci 1995 Mar;15(3): following table of contents] The Journal of Neuroscience. 1994;14(1):167–86. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Camp M, Jones VM, et al. Convergent effects of mouse Pet-1 deletion and human PET-1 variation on amygdala fear and threat processing. Experimental Neurology. 2013;250:260–9. doi: 10.1016/j.expneurol.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience. 1998;18(1):411–18. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yang M, Augustsson H, Markham CM, et al. The rat exposure test: a model of mouse defensive behaviors. Physiology and Behavior. 2004;81(3):465–73. doi: 10.1016/j.physbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42(3):1127–41. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


