Abstract
Social rejection elicits distress through the brain’s alarm system, the dorsal anterior cingulate cortex (dACC). The distress of rejection facilitates subsequent inclusion. As a result, traits that blunt this dACC response to social rejection might then threaten group membership, leading to further subsequent rejection. Alexithymia, the inability to identify and describe affective states, is associated with social impairment and reduced dACC activity under conditions of negative affect. Thus, we expected that alexithymia would relate to less dACC activation during rejection and that this blunted response would explain an association between alexithymia and greater rejection in everyday life. Using functional magnetic resonance imaging and daily diaries, we found that subclinical individual differences in the core feature of alexithymia, difficulty identifying affect, was associated with a blunted dACC response to social rejection. Deficits in affect identification were also associated with greater daily rejection and that this effect was mediated and suppressed by dACC activation to rejection. Our findings emphasize the crucial role of the dACC in response to social rejection and extend the literature on alexithymia’s ability to dampen neural responses and contribute to poor social functioning. The suppressing role of the dACC suggests future directions for clinical interventions on those with affective disorders.
Keywords: alexithymia, dACC, social rejection, fMRI
Human behavior is driven, in large part, by a quest for social acceptance (Baumeister and Leary, 1995). When this goal is thwarted by an instance of social rejection, individuals experience distress and negative affect that stems from the dorsal anterior cingulate cortex (dACC; Eisenberger et al., 2003). This signal from the social environment is useful in that it motivates us to adaptively respond to rejection in a manner that prevents future rejection (Eisenberger and Lieberman, 2004; MacDonald and Leary, 2005; Baumeister et al., 2007). Psychological dispositions that handicap the dACC response to rejection may then lead to increased rejection in everyday life. Alexithymia may play just such a crippling role.
ALEXITHYMIA: DEFICITS IN AFFECT IDENTIFICATION
Alexithymia, or ‘no words for feelings’, generally refers to a person’s dispositional inability to comprehend and regulate his/her own affective state (Nemiah et al., 1976). Attempts to quantify individual differences in this trait resulted in the construction of the 20-item Toronto Alexithymia Scale (TAS; Bagby et al., 1994a,b). Research using the TAS dissociated alexithymia into three features: deficits in identifying one’s feelings, deficits in describing one’s feelings and a larger syndrome of externally oriented thinking that was less specific to affect. Such alexithymic features have been implicated in various mental illnesses including eating disorders (Kessler et al., 2006), depression (Honkalampi et al., 2000) and anxiety disorders (Zeitlin and McNally, 1993). Beyond psychopathology, alexithymic features predict poor social functioning and blunted neural responses during social situations (Moriguchi et al., 2006, 2007, 2009; Bird et al., 2010; Bernhardt et al., submitted for publication; Cook et al., 2013). However, alexithymia’s influence on neural correlates of social rejection remains unknown.
THE dACC: A SOCIOMETRIC ALARM SYSTEM
The dorsal region of the anterior cingulate cortex (dACC) is a neural center with broad functions. A wealth of evidence suggests that the dACC functions as the brain’s alarm system that uses cognitive and affective processes to detect discrepancies between current and goal states, which signals distress when there is a discrepancy (Eisenberger and Lieberman, 2004). Supporting this notion, cognitive theory and research have shown that the dACC serves to detect conflict between desired and actual responses and the exertion of cognitive control to ameliorate the conflict (Bush et al., 2000; Luu and Posner, 2000; Botvinick et al., 2004; Fassbender et al., 2004; Mulert et al., 2005; Brown, 2013). Yet, dACC activation is also associated with the generation of negative affect, such as the painful distress of physical injury (Foltz and White, 1968), angry responses to provocation (Denson et al., 2008) and the expression of negative affect more generally (Etkin et al., 2011).
This ability to detect deviation from goal states and then elicit pain, distress and negative affect makes the dACC ideally suited to serve as the brain’s alarm system. The dACC’s alarm function is attuned to maintaining group membership. Social rejection, as compared with acceptance, is associated with robust increases in dACC activation, which in turn relates to greater self-reported distress (e.g. Eisenberger et al., 2003). Further, the dACC tracks state self-esteem, which functions as an indicator of social inclusion (Eisenberger et al., 2011).
ALEXITHYMIA AND THE dACC
The literature on alexithymia’s effect on dACC activation is incredibly mixed (Deng et al., 2013). Half of the studies report a blunted dACC response during emotional processing (see Aleman, 2005; e.g. Lane et al., 1997; Kano et al., 2003; Moriguchi et al., 2007; Karlsson et al., 2008), whereas the other half show a heightened dACC response (e.g. Berthoz et al., 2002; McRae et al., 2008). A recent meta-analysis ruled in favor of alexithymia’s ability to heighten dACC activity during emotional processing (van der Velde et al., 2013). Resolving this conflict, a recent study showed that valence determines the direction of the association, with reduced dACC activity among those with alexithymia under negative valence and greater activity for positively valenced stimuli (Deng et al., 2013).
As a negatively valenced emotional event (Williams, 2009), social rejection is an ideal situation to expect a negative association between alexithymia and dACC activation. Further, previous research showing blunted neural responses during other negatively valenced social situations (e.g. seeing others in pain; Moriguchi et al., 2007) suggest that socially focused neural regions, like the dACC, are dampened in their reactivity to appropriate social stimuli by alexithymia. These findings support the prediction that alexithymia will blunt the response of the brain’s social alarm system, the dACC.
A muted social alarm may magnify the likelihood of social rejection. Much as individuals who feel no physical pain often suffer horrific somatic injuries, a lack of a distress response to rejection would likely cause massive social injuries (e.g. expulsion from groups) for two key reasons. First, the dACC’s alarm function was likely co-opted by evolution to respond to exclusionary events because of the immense threat such rejection posed to our ancestors (Eisenberger, 2012). This alarm signal serves the function of orienting our attention to the threatening stimulus, inhibiting ongoing behavior, and motivating behaviors that might mitigate the threat and repair any harm (Eisenberger and Lieberman, 2004; MacDonald and Leary, 2005). Individuals who had their dACC surgically lesioned could detect and acknowledge a physically noxious stimulus but were not distressed by it (Foltz and White, 1968). Similarly, alexithymic individuals may be able to detect rejection in their environment, yet their blunted dACC response prevents them from finding it distressing.
A blunted dACC response to social rejection may prevent people from registering rejection as an aversive experience and subsequently learning from behaviors (or lack thereof) that caused social rejection. A leading notion is that affective states (e.g. alarm, distress, pain) influence behavior by providing feedback to an individual about the efficacy of that action (Baumeister et al., 2007). For instance, an individual who acted in a socially inappropriate manner (e.g. laughing at a funeral) and is shunned for it would benefit from the psychological pain and distress that the social rejection would typically elicit because this feedback would indicate that their behavioral response requires modification. Without such a dACC-generated signal, individuals may not revise their behavioral tendencies to achieve social inclusion. Thus, alexithymia’s potential handicapping of the dACC response to rejection should predict greater social rejection and suppress the effect of alexithymia on greater social rejection, with activity in this region reducing the ability of alexithymia to impair social functioning.
We did not predict that alexithymia’s three sub-factors—difficulty identifying affect, difficulty describing affect and externally oriented thinking—would equally relate to lower dACC activation and greater daily rejection (Bagby et al., 1994a,b). The few studies that assessed the unique contributions of each factor, as opposed to summing them into a single score, has indicated that the difficulty identifying affect subscale is uniquely effective at predicting blunted neural responses during socio-emotional tasks (e.g. Eichmann et al., 2008). Indeed, the external thinking and difficulty describing feelings subscales map more onto executive and intellectual abilities than affect identification (sample items: ‘It is difficult for me to find the right words for my feelings’; ‘I prefer to just let things happen rather than to understand why they turned out that way’). Thus, our hypotheses focused on the difficulty identifying feelings subscale of the TAS.
CURRENT STUDY
We hypothesized that sub-clinical individual differences in difficulty identifying affect would be associated with (i) less dACC activation during rejection, (ii) greater daily social rejection, and this blunted dACC response to rejection would (a) mediate and (b) suppress the relationship between alexithymia and social rejection. To test these hypotheses, participants reported their levels of alexithymia, recorded their daily levels of social rejection over 7 days and then were socially accepted and then rejected while undergoing functional magnetic resonance imaging (fMRI). The daily rejection reports were included in the middle of the experimental procedure for two reasons. First, daily reports of rejection were more likely to be made when a second laboratory visit was anticipated by participants. Second, our experimental induction of social rejection may have contaminated subsequent reports of social rejection.
METHOD
Participants
Participants were 27 healthy right-handed undergraduate students (14 females; Age: M = 18.78, s.d. = 1.01) who received course credit and money as compensation. Participants were screened for criteria relevant to safety and comfort in the MRI environment.
Procedure
Questionnaires. Participants arrived at the laboratory and completed a computerized battery of personality questionnaires, which included a demographics questionnaire and the 20-item TAS (Bagby et al., 1994a,b).
Daily reports of rejections. For the 7 days following the questionnaire session, participants received an Internet questionnaire in the evening, which contained an item that assessed daily rejection (i.e. How rejected did you feel today?). Participants responded using a 7-item Likert scale in which higher values represented greater daily levels of rejection. Greater scores across all days were considered to represent greater levels of social rejection.
MRI task. After the 7 days of reports were completed, participants arrived at our MRI facility. After entering the MRI scanner, they played three rounds of a computerized ball-tossing game (Cyberball) with two same-sex partners located in nearby scanners (as in Williams et al., 2000; Chester et al., 2014). In reality, participants played with a preset computer program that was designed to produce a within-participants experience of both social acceptance and rejection. Cyberball was implemented as a block-design with three rounds (60 s each). Before each round, participants were presented with instructions to rest for 10 s. This was followed by a 2 s screen instructing them to ‘get ready’ for the upcoming round. In rounds 1 and 2, participants were accepted for the entire duration of the task, receiving one-third of all ball-tosses. In round 3, participants received the ball three times, after which their partners only threw the ball to each other. Acceptance was operationalized as occurring throughout rounds 1 and 2, as well as throughout the first half of round 3. Rejection was operationalized as occurring during the second half of round 3 (i.e. 30 s), after participants had received the ball three times and then witnessed three more ball-tosses without receiving a toss themselves. This relatively short duration of the rejection block was chosen due to our desire to capture the initial aversive response to exclusion, not the appraisal and regulatory processes that come online as rejection unfolds, as outlined in the temporal need threat model of ostracism (Williams, 2009). After a series of anatomical scans, participants were then removed from the scanner and completed the 20-item Need Threat Scale, which measured participants’ level of social distress due to Cyberball (Williams, 2009).1
fMRI data
Functional images were acquired on a 3 tesla Siemens Magnetom TRIO scanner with a T2*-weighted gradient echo sequence with the following parameters: 2.5 s repetition time, 28 ms echo time, 64 × 64 matrix, 224 mm × 224 mm field of view, 40 axial slices of 3.5 mm acquired in interleaved order. A 3D shim was applied before functional data acquisition. These parameters allowed for whole brain coverage with 3.5 mm cubic voxels. A high-resolution, T1-weighted image was also acquired from each participant.
All preprocessing and statistical analyses were conducted using FSL [Oxford Center for Functional Magnetic Resonance Imaging (FMRIB); Smith et al., 2004; Woolrich et al., 2009]. Functional volumes were reconstructed from k-space using a linear time interpolation algorithm to double the effective sampling rate, the first of which was removed to allow for signal equilibration. Remaining functional volumes were corrected for head movement to the median volume, corrected for slice-timing skew using temporal sinc interpolation, pre-whitened and smoothed with a 5 mm FWHM Gaussian kernel. To remove drifts within sessions, a high-pass filter with a cutoff period of 120 s was applied. Non-brain structures were stripped from functional and anatomical volumes.
A fixed-effects analysis modeled event-related responses for each run of each participant. Acceptance and Rejection blocks were modeled as events using a canonical double-gamma hemodynamic response function (HRF) with a temporal derivative. Pre-block instructions and motion parameters were modeled as nuisance regressors, while rest blocks were left un-modeled to provide an implicit baseline. Functional volumes and first-level contrast images from this analysis were first registered to corresponding structural volumes, and then spatially normalized to an Montreal Neurological Institute (MNI) stereotaxic space template image. A top-level mixed-effects analysis was performed, which created group average maps for contrasts of interest. Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (family-wise error corrected) cluster significance threshold of P < 0.005 in our a priori region of interest (ROI; Worsley, 2001; Heller et al., 2006). An ROI mask was used to constrain fMRI analysis and multiple comparisons correction to dACC. This mask was created by Way et al. (2009) from the automated anatomical atlas (Tzourio-Mazoyer et al., 2002) using MNI coordinates established by Vogt et al. (2003), which used a rostral boundary of y = 33 and a caudal boundary of y = 0. Anatomically superior voxels within the mask were then trimmed from the original version to correspond to the border of the cingulate sulcus of subjects’ aggregated brain volume.
Analytic strategy. We predicted that difficulty identifying one’s feelings would be associated with increases in daily rejection through diminished dACC activity during rejection. This causal model is an example of statistical suppression, determined a priori, which occurs once the mediating variable is controlled for and the direct effect of the primary predictor becomes stronger (Davis, 1985; Mackinnon et al., 2000). Because our outcome of interest (i.e. daily rejection) violated the assumption of independence in ordinary least squares regression (i.e. daily reports nested within individual participants), we used multilevel modeling techniques to account for the data’s nested structure, using HLM Version 6.08 (Raudenbush et al., 2000; Nezlek, 2001; Raudenbush and Bryk, 2002). In addition to accounting for the data’s nested structure, the multilevel modeling algorithms within HLM use Bayes shrinkage, which weights observations by their reliabilities. Through this weighting, less reliable observations (e.g. outliers) are moved toward the mean (Nezlek, 2011). Methods that apply Bayes shrinkage are known to produce more accurate estimates (in terms of whether estimates correspond to population parameters) than procedures that do not use Bayes shrinkage (Littell et al., 1996; Raudenbush and Bryk, 2002). Participants’ 7 days of rejection reports yielded an intraclass correlation coefficient of 0.36, suggesting that 64% of the variability in feelings of rejection was within-person.
In these analyses, difficulty identifying feelings and dACC activity during exclusion were entered as Level 2 predictors and were grand-mean centered (Aiken and West, 1991). Given the significant gender differences we observed (see Results) in difficulty identifying feelings, we entered gender as a Level 2 covariate to control for this potential confound in a post hoc manner. Inspection of residual variances at each level of our model revealed that Level 1 residual variances were approximately normally distributed, whereas estimated Bayes residuals at Level 2 exhibited slight skew. Thus, robust standard errors were used to account for moderate normality violations. In analyses in which dACC activity was the outcome of interest (a non-nested outcome), ordinary least squares regression was used. Last, to provide an estimate of effect size that was consistent for each analysis (for the nested and non-nested outcomes), we present correlation coefficients that were derived from the t-tests and degrees of freedom obtained from the multilevel model fixed effects (Rosenthal, 1991).
RESULTS
Self-reports and demographics
Scores were calculated for each of the three subscales of the TAS by reverse-scoring and summing appropriate items (for descriptive and reliability information, see Table 1). Of the 27 participants, 25 of them completed all 7 days of the daily rejection item (for descriptive information, see Table 2). One participant completed 6 days and one participant completed 4 days of questionnaires. These missing data were accounted for in our multilevel model using maximum likelihood estimation.
Table 1.
Descriptive and reliability information for TAS subscales
| TAS subscale | Mean | s.d. | Response range | Cronbach α |
|---|---|---|---|---|
| Difficulty identifying feelings | 11.11 | 4.52 | 7–25 | 0.84 |
| Difficulty describing feelings | 11.67 | 4.84 | 5–25 | 0.82 |
| External thinking | 20.33 | 5.95 | 8–37 | 0.62 |
| Total | 43.11 | 10.81 | 20–66 | 0.77 |
Scores can range from 7 to 35 (Difficulty Identifying Feelings), 5 to 25 (Difficulty Describing Feelings), 8 to 40 (External Thinking) and 20 to 100 (total score).
Table 2.
Descriptive information for daily rejection scores. Scores can range from 1 to 7
| Mean | s.d. | Response range | |
|---|---|---|---|
| Day 1 | 1.92 | 1.38 | 1–6 |
| Day 2 | 2.31 | 1.52 | 1–5 |
| Day 3 | 1.96 | 1.34 | 1–5 |
| Day 4 | 1.89 | 1.31 | 1–5 |
| Day 5 | 1.85 | 1.17 | 1–6 |
| Day 6 | 1.69 | 1.12 | 1–5 |
| Day 7 | 1.85 | 1.35 | 1–7 |
| Average | 1.91 | 0.89 | 1.00–4.29 |
Gender and age were assessed as demographic variables that might impact components of alexithymia and daily rejection. Females reported more difficulty identifying feelings (M = 13.36, s.d. = 4.96) than males (M = 8.69, s.d. = 2.32), t(25) = 3.09, P = 0.005. However, gender did not impact the other two subscales of the TAS or rejection reports averaged across all 7 days, Ps > 0.09. Age was unassociated with difficulty identifying feelings or average rejection reports, Ps > 0.09. However, age showed negative associations with difficulty describing feelings, r(25) =−0.40, P = 0.039, and externally oriented thinking, r(25) = −0.43, P = 0.026.
NEUROIMAGING RESULTS
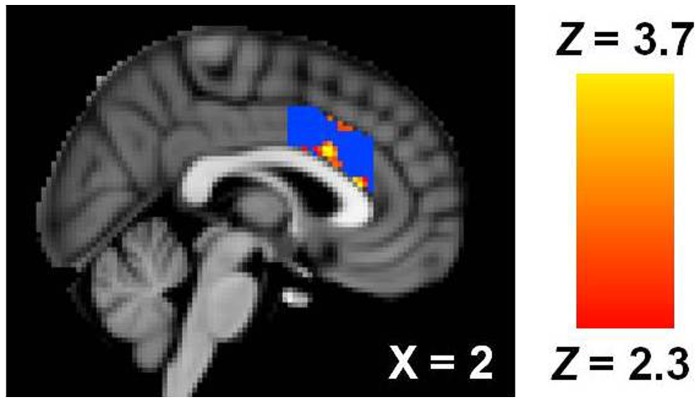
Validating the social rejection manipulation, participants reported average Need Threat Scale scores (NTS; Cronbach α = 0.92), an indicator of social distress, above the midpoint of the scale (i.e. 4), M = 4.41, s.d. = 0.99, t(26) = 2.13, P = 0.043, d = 0.59. Social rejection, compared with social acceptance, was associated with increased activity in the dACC (Figure 1; 289 voxels, peak Z = 4.01, peak MNI coordinates: x = 2, y = 22, z = 16; rejection > acceptance contrast). Functional data from this activated main effect cluster of the dACC were converted to units of percent signal change, averaged across each participant and extracted (as outlined by Mumford, J. http://mumford.bol.ucla.edu/perchange_guide.pdf). No association was observed between dACC activation from this contrast and social distress reports, r(25) = −0.26, P = 0.198. A null association as also observed between difficulty identifying feelings and social distress reports, r(25) = −0.27, P = 0.178.
Fig. 1.
dACC activation associated with rejection > acceptance in MNI space. Blue voxels indicate extent of ROI mask.
SUPPRESSION ANALYSES
We first examined the association between difficulty identifying feelings and dACC activity during rejection. As predicted, analyses revealed a significant negative association between difficulty identifying feelings and dACC activity, b = −0.01, t(25) = −3.06, P = 0.005, r = 0.53. Thus, people who have difficulty identifying their feelings exhibit diminished dACC activity during rejection. We then examined the direct effect of difficulty identifying feelings on daily rejection. As predicted, analyses revealed a significant positive association between difficulty identifying feelings and daily rejection, b = 0.11, t(25) = 2.83, P = 0.010, r = 0.50. Thus, people who have difficulty identifying their feelings exhibit greater daily rejection. Difficulty describing feelings was not associated with daily rejection, b = 0.01, t(25) = 0.20, P = 0.850, r = 0.09, though externally oriented thinking was, b = −0.07, t(25) = −2.95, P = 0.007, r = 0.52.
We next tested whether dACC activity during rejection predicted daily rejection, controlling for difficulty identifying feelings. As predicted, the association between dACC activity and daily rejection was significant, such that people who exhibited greater dACC activity during rejection also reported greater daily rejection on average, b = 7.31, t(24) = 2.59, P = 0.020, r = 0.48. As predicted, the positive association between difficulty identifying feelings and daily rejection became stronger after controlling for dACC activation, b = 0.16, t(24) = 3.89, P = 0.001, r = 0.63.
Last, we tested the statistical significance of the indirect effect (ab) for inconsistent mediation by estimating the 95% confidence interval of the indirect effect using the empirical-M test with the computer program PRODCLIN, which provided the confidence interval of the indirect effect (MacKinnon et al., 2007). As predicted, the indirect path through which difficulty identifying feelings predicts increased daily rejection via diminished dACC activity during rejection was statistically significant, as the 95% confidence interval did not include zero (−0.11 to −0.01; Figure 2). Thus, participants who tended to have difficulty identifying their feelings exhibited stronger daily rejection, in part because of diminished dACC activity during social rejection experiences.
Fig. 2.
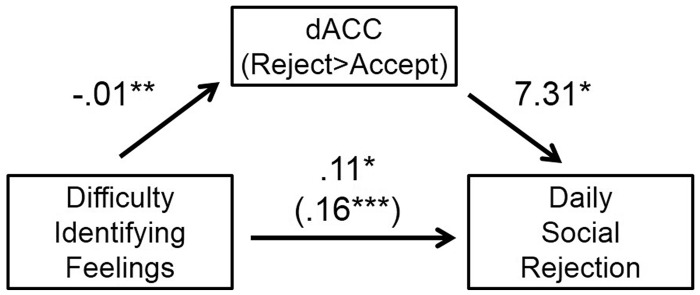
Statistical model whereby rejection-specific dACC activation mediates and suppresses the effect of self-reported difficulty with identifying feelings on daily rejection. Numerical values represent unstandardized regression coefficients (*P < 0.05; **P < 0.01; ***P < 0.001).
DISCUSSION
Rejection is a profound threat to human health and happiness (Cacioppo et al., 2003; Stillman et al., 2009; Dickerson, 2011; DeWall et al., 2012). The brain’s alarm system registers this threat, eliciting distress and negative affect, which serves to maintain group membership (Eisenberger et al., 2003; Eisenberger and Lieberman, 2004; MacDonald and Leary, 2004; Eisenberger, 2012). This study sought to test how alexithymia, a trait that alters individuals’ ability to decipher such affective signals (Bagby et al., 1994a,b; Nemiah et al., 1976) and blunts the responding of the dACC to negative emotional situations (e.g. Deng et al., 2013) might impact the typical dACC response to rejection and its implications for group membership in everyday life.
Using functional neuroimaging, we replicated the typical dACC response to social rejection (Eisenberger et al., 2003). This finding was extended by showing that a key feature of alexithymia, difficulty identifying one’s feelings, was negatively associated with dACC activation during rejection. Alexithymia’s blunting effect on the dACC response meshes well with other research that shows negative associations between alexithymia and dACC activation during socio-emotional events of a negative valence (e.g. Moriguchi et al., 2007; Deng et al., 2013). Indeed, meta-analytic findings that alexithymia is generally associated with greater dACC activation during emotional processing (van der Velde et al., 2013) may obscure the dynamic nature of this relationship.
Using a longitudinal daily diary design, we then showed that difficulty identifying one’s feelings predicted greater social rejection over 7 days. This finding extends previous research which implicated alexithymia is a uniquely robust contributor to social impairment (e.g. Bird et al., 2010; Cook et al., 2013), by showing that this trait promotes social exclusion as well. The heightened rejection that is associated with alexithymia poses a serious risk for those high in this trait because those without social bonds are far more at risk for physical illness and mortality (Cacioppo et al., 2003; Dickerson, 2011).
It may seem counterintuitive that a trait that diminishes the impact of rejection would lead to greater, and not lesser, reports of experiences of rejection. However, it is likely that individuals high in alexithymia still detect and understand that they are being rejected, as rejection is registered in multiple brain regions (e.g. ventrolateral prefrontal cortex, anterior insula; Eisenberger et al., 2003). However, a blunted dACC response to rejection would render this realization of exclusion un-colored by typical sensations of aversive distress. This social distress response serves a crucial function in preventing exclusion (MacDonald and Leary, 2005; Eisenberger, 2012). By disentangling the distress response to rejection from simple detection of the event, it is (somewhat paradoxically) possible to reduce the impact of rejection while increasing the experience of it on a daily basis.
This study implicated the dACC as a mechanism through which alexithymia is associated with relatively greater social rejection. Specifically, the effect of difficulty identifying one’s affective state on greater social rejection was mediated by a blunted dACC response to social rejection. This suggests that alexithymia may lead to social rejection because it reduces the ‘volume’ of the brain’s alarm system during instances of rejection, failing to alert the individual to the gravity of the situation and the outcomes it may have for their belongingness needs. Crucially, the dACC exerted a suppression effect whereby the effect of alexithymia on daily rejection grew stronger once dACC activation was statistically controlled for in the model. Such a finding suggests that greater dACC activation could serve to repair alexithymia’s role in heightened social rejection. If true, the deleterious effects of alexithymia on inclusion may be combated by interventions aimed at increasing the alarm response to cues of social rejection, though this remains speculative until further research is conducted. However, alexithymia also relates to other interpersonal deficits (e.g. impaired theory-of-mind; Moriguchi et al., 2006) that are likely to increase social rejection. Thus, any interventions that aim to increase the distress of rejection must weigh the potential costs of increasing the aversive experience of rejection experiences not due to a blunted neural alarm.
Limitations and Future Directions
These findings were limited in several ways. First, our dependent measure of rejection was based on self-report, which is biased by a lack of objective introspective accuracy (Nisbett and Wilson, 1977) and the extent to which the participants felt rejected and not a more objective measure of social rejection. As such, these perceptions of rejection may not reflect actual levels of social rejection in real life. It may seem perplexing that individuals who struggle with experiencing and identifying feelings would report more of any given feeling. These findings speak to the strength of social rejection, that even though alexithymia blunts the sting of rejection, it still registers to some extent in the minds of the rejected. Second, our model was only predictive of daily social rejection when using the difficulty identifying feelings subscale of the TAS and not the other two. As such, it appears that social rejection is most associated with deficits in identifying feelings, not communicating them, or a general external orientation. This is likely given theoretical conceptualizations of emotion as a feedback mechanism that guides behavior toward adaptive ends (Baumeister et al., 2007). If one cannot identify this signal, then one cannot benefit from it.
Third, because rejection always occurred later in time than acceptance, our fMRI contrast between acceptance and rejection conditions was confounded with the inevitable changes in the MRI signal that occur over the length of a scan. To reduce the impact of this potential confound, our data were highpass filtered to remove low-frequency shifts in the data over time, pre-whitened to remove temporal autocorrelation and a temporal derivative was included in the statistical model to account for time-based shifts in the hemodynamic response function (Poldrack et al., 2011). Such limitations of fMRI are counterbalanced against the ability of this technique to assess signatures of psychological processes that are likely difficult to measure through self-report, such as the alarming nature of rejection. Fourth, our sample fell into the bottom half of the possible distribution of alexithymia. Thus, it remains unclear whether our findings generalize to higher clinical levels of alexithymia. Future research should assess whether these effects hold across a greater range and among clinically alexithymic populations.
Fifth, participants generally reported very little felt rejection over the 7 day period we assessed. Restriction of range is a serious analytic issue and our findings should be interpreted in light of this issue. This lack of variability likely served as a conservative test of our hypothesis though future research should ensure that our findings hold among individuals experiencing a greater and more variable degree of rejection. Sixth, our relatively small sample size introduced the possibility of several inferential issues that should be corrected in the future by assuring that our findings replicate in larger samples. However, statistical simulations indicate that an even smaller sample size of 20 would still have a small chance of yielding a false-positive result or artificially inflated correlations (Lieberman et al., 2009). Seventh, we relied on reverse-inference in our interpretation of our findings, assuming that dACC activation during social rejection represents the subjective experience of social distress. Although this assumption is based on a large literature (for a review see Eisenberger, 2012), we cannot be certain that dACC activation truly represented social distress. Finally, both dACC activation during rejection and difficulty identifying feelings were unassociated with self-reported social distress. This is likely because administration of the NTS was delayed by 1 h after the rejection manipulation, and a reduction in self-reported social distress tends to appear ∼45 min after an instance of social rejection (Zadro et al., 2006). Our finding that participants reported a level of social distress above ambivalence (i.e. the midpoint of the NTS response scale) was likely obtained in spite of this tendency to underreport rejection and speaks to the strength of our manipulation. However, these null associations may reflect a true state of these constructs, and future research should measure self-reports of social distress immediately after rejection to see if these associations are observed as we expect they would.
CONCLUSION
Rejection is a threatening experience and evolution has bestowed us with neural systems to combat this threat (Eisenberger, 2012). Our research shows that alexithymia, a deficit in the ability to identify and understand affective responses, blunts the brain’s alarm response to rejection, which then explains greater rejection on an everyday basis. This blunted neural response to social rejection may prevent alexithymics from adaptively responding to social rejection and learning how to prevent further rejection, thereby setting in motion a vicious cycle in which they continue to experience greater rejection because they do not experience a strong neural response that signals distress. It is our hope that the current research may translate into the development of effective interventions to reduce the relationship between alexithymia and rejection.
Footnotes
1Some of these neural data, combined with other participants, are reported in another paper (Chester et al., 2014).
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. London: Sage; 1991. [Google Scholar]
- Aleman A. Feelings you can’t imagine: Towards a cognitive neuroscience of Alexithymia. Trends in Cognitive Sciences. 2005;9(12):553–5. doi: 10.1016/j.tics.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia scale—I: Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994a;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JDA. The twenty-item Toronto Alexithymia scale—II: Convergent, discriminant, and concurrent validity. Journal of Psychosomatic Research. 1994b;38(1):33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, DeWall CN, Zhang L. How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Personality and Social Psychology Review. 2007;11(2):167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Berthoz S. Effect of impaired recognition and expression of emotions on frontocingulate cortices: An fMRI study of men with alexithymia. American Journal of Psychiatry. 2002;159(6):961–7. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of Alexithymia but not autism. Brain. 2010;133(5):1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown JW. Beyond conflict monitoring: Cognitive control and the neural basis of thinking before you act. Current Directions in Psychological Science. 2013;22(3):179–85. doi: 10.1177/0963721412470685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG. The anatomy of loneliness. Current Directions in Psychological Science. 2003;12(3):71–4. [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Richman SB, Bushman BJ, DeWall CN. The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Social Cognitive and Affective Neuroscience. 2014;9:699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Brewer R, Shah P, Bird G. Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychological Science. 2013;24(5):723–32. doi: 10.1177/0956797612463582. [DOI] [PubMed] [Google Scholar]
- Davis MD. The logic of causal order. In: Sullivan JL, Niemi RG, editors. Sage University Paper Series on Quantitative Applications in the Social Sciences. Beverly Hills: Sage; 1985. [Google Scholar]
- Deng Y, Ma X, Tang Q. Brain response during visual emotional processing: An fMRI study of alexithymia. Psychiatry Research: Neuroimaging. 2013;213(3):225–9. doi: 10.1016/j.pscychresns.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Denson TF, Pedersen WC, Ronquillo J, Nandy AS. The angry brain: Neural correlates of anger, angry rumination, and aggressive personality. Journal of Cognitive Neuroscience. 2008;21(4):734–44. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Gilman R, Sharif V, Carboni I, Rice KG. Left out, sluggardly, and blue: Low self-control mediates the relationship between ostracism and depression. Personality and Individual Differences. 2012;53(7):832–7. [Google Scholar]
- DeWall CN, MacDonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychological Science. 2010;21(7):931–7. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Dickerson SS. Physiological responses to experiences of social pain. In: MacDonald G, Jensen-Campbell LA, editors. Social Pain: Neuropsychological and Health Implications of Loss and Rejection. Washington: American Psychological Association; 2011. pp. 79–94. [Google Scholar]
- Eichmann M, Kugel H, Suslow T. Difficulty identifying feelings and automatic activation in the fusiform gyrus in response to facial emotion. Perceptual and Motor Skills. 2008;107(3):915–22. doi: 10.2466/pms.107.3.915-922. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012;13(6):421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Byrne Haltom KE, Leary MR. The neural sociometer: Brain mechanisms underlying state self-esteem. Journal of Cognitive Neuroscience. 2011;23(11):3448–55. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–92. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cognitive Brain Research. 2004;20(2):132–43. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE. The role of rostral cingulumotomy in “pain” relief. International Journal of Neurology. 1968;6(3):353–4. [PubMed] [Google Scholar]
- Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of fMRI data. NeuroImage. 2006;33(2):599–608. doi: 10.1016/j.neuroimage.2006.04.233. [DOI] [PubMed] [Google Scholar]
- Honkalampi K, Hintikka J, Tanskanen A, Lehtonen J, Viinamäki H. Depression is strongly associated with Alexithymia in the general population. Journal of Psychosomatic Research. 2000;48(1):99–104. doi: 10.1016/s0022-3999(99)00083-5. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukudo S, Gyoba J, Kamachi M, Tagawa M, Mochizuki H, Itoh M, Hongo M, Yanai K. Specific brain processing of facial expressions in people with alexithymia: an H215O-PET study. Brain. 2003;126(6):1474–84. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. The British Journal of Psychiatry. 2008;192(1):32–8. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Kessler H, Schwarze M, Filipic S, Traue HC, von Wietersheim J. Alexithymia and facial emotion recognition in patients with eating disorders. International Journal of Eating Disorders. 2006;39(3):245–51. doi: 10.1002/eat.20228. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PML, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–72. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Berkman ET, Wager TD. Correlations in social neuroscience aren’t voodoo: Commentary on Vul et al. (2009). Perspectives on Psychological Science. 2009;4(3):299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131(2):202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39(3):384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding, and suppression effect. Prevention Science. 2000;1(4):173–81. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage. 2008;41(2):648–55. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Impaired self-awareness and theory of mind: An fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32(3):1472–82. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Empathy and judging other’s pain: An fMRI study of Alexithymia. Cerebral Cortex. 2007;17(9):2223–34. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Decety J, Hirakata M, Maeda M, Matsuda H, Komaki G. The human mirror neuron system in a population with deficient self-awareness: An fMRI study in alexithymia. Human Brain Mapping. 2009;30(7):2063–76. doi: 10.1002/hbm.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, Hegerl U. Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. International Journal of Psychophysiology. 2005;56(1):65–80. doi: 10.1016/j.ijpsycho.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Nemiah JC, Freyberger H, Sifneos PE. Alexithymia: a view of the psychosomatic process. Modern Trends in Psychosomatic Medicine. 1976;3:430–9. [Google Scholar]
- Nezlek JB. Multilevel random coefficient analyses of event and interval contingent data in social and personality psychology research. Personality and Social Psychology Bulletin. 2001;27(7):771–85. [Google Scholar]
- Nezlek JB. Multilevel Modeling for Social and Personality Psychology. Thousand Oaks, CA: Sage; 2011. [Google Scholar]
- Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychological Review. 1977;84(3):231–59. [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE. Handbook of Functional MRI Data Analysis. New York: Cambridge University Press; 2011. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. Thousand Oaks: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM. Lincolnwood: Scientific Software International; 2000. [Google Scholar]
- Rosenthal R. Meta-analytic Procedures for Social Research. Newbury Park: Sage; 1991. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, Supplement 1(0) 2004:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stillman TF, Baumeister RF, Lambert NM, Crescioni AW, DeWall CN, Fincham FD. Alone and without purpose: Life loses meaning following social exclusion. Journal of Experimental Social Psychology. 2009;45(4):686–94. doi: 10.1016/j.jesp.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van der Velde J, Servaas MN, Goerlich KS, Bruggeman R, Horton P, Costafreda SG, Aleman A. Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neuroscience and Biobehavioral Reviews. 2013;37(8):1774–85. doi: 10.1016/j.neubiorev.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18(11):3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the μ-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences. 2009;106(35):15079–84. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Ostracism: A temporal need-threat model. In: Zanna MP, editor. Advances in Experimental Social Psychology. Vol. 41. Philadelphia: Academic Press; 2009. pp. 275–314. [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79(5):748–62. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1):S173–86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Functional MRI: An Introduction to Methods. 2001;14:251–70. [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42(5):692–97. [Google Scholar]
- Zeitlin SB, McNally RJ. Alexithymia and anxiety sensitivity in panic disorder and obsessive-compulsive disorder. The American Journal of Psychiatry. 1993;150(4):658–60. doi: 10.1176/ajp.150.4.658. [DOI] [PubMed] [Google Scholar]