Abstract
The opinion of mind–body interaction has been increasingly acknowledged in recent years, as exemplified by accumulating evidence indicating that physical health (body) is associated with emotion and emotion regulation (mind). Yet, the neural basis linking emotion regulation with physical health remains largely uninvestigated. Here we used magnetic resonance imaging to investigate the neural basis of this pathway in a large population of healthy young adults. With a systematic study revealing the association of self-reported physical health and emotion traits of personality and general affective experiences, we further demonstrated that, for better physical health, individuals needed to regulate their emotion more effectively. Importantly, individuals who had larger gray matter (GM) volume in the amygdala reported not only a higher ability of emotion regulation but also better physical health. Further, GM volume in the amygdala mediated the correlation between emotion regulation ability and physical health. Our findings suggest that the amygdala plays a critical role in the neural circuit through which emotion regulation may influence physical health. Therefore, our study takes the first step toward exploring the neuroanatomical basis for body–mind interaction and may inform interventions aimed at promoting physical health by augmenting skills of emotion regulation.
Keywords: physical health, emotion regulation ability, the amygdala, voxel-based morphometry, individual difference
INTRODUCTION
Historically, the most popular opinion on the body–mind problem is the dualism that the body and mind are distinct substances, and the body is a machine-like entity separate from the mind (Descartes, 1985 [1641]). A specific example of the mind–body problem in medical practice and health psychology is the relationship between mental constructs (mind) and physical health (body) (e.g., Pelletier, 1992; Bates et al., 1997; Ryff and Singer, 1998; Astin et al., 2003). Owing to the dominance of this tradition of dualism in modern medical practices, physical health and mental health are viewed as separate entities, leading to the avoidance of considering mental constructs as a critical factor in physical health. In recent years, both theoretical and technical advances have illustrated the intrinsic link between physical health and mental characteristics such as emotion and personality in research of health psychology (e.g., Adler and Matthews, 1994; Smith et al., 2012). Yet, a critical issue that remains largely uninvestigated is the neural basis (brain) through which physical health (body) is linked with mental constructs (mind).
There is accumulating evidence indicating the association between physical health and emotion. For example, both negative and positive emotions have been shown to have an impact on physical health, as indexed by variables such as mortality, morbidity, disease severity, functioning of the immune and cardiovascular systems and self-reports of physical symptoms (Herbert and Cohen, 1993; Kiecolt-Glaser et al., 2002; Krantz and McCeney, 2002; Pressman and Cohen, 2005; Suls and Bunde, 2005; Chida and Hamer, 2008). In addition, neuroticism (i.e., the tendency to experience negative emotions such as depression and anxiety) and Type A behavioral pattern (i.e., hostility, anger and aggressiveness) have been acknowledged as well-established personality risks for physical health outcomes as diverse as heart disease, asthma, hypertension, longevity and symptom reporting (Goodwin and Friedman, 2006; Smith, 2006; Hampson and Friedman, 2008; Chida and Steptoe, 2009; Smith et al., 2012). Based on the association between physical health and emotion, it can be deduced that higher ability of emotion regulation, which promotes adaptive coping with environmental demands, as encompassed in the construct of emotional intelligence (Salovey and Mayer, 1990; Bar-On, 1997), contributes to better physical health (Schutte et al., 2007; Martins et al., 2010). A variety of cognitive and behavioral mechanisms have been proposed in the research of emotion intelligence (EI) to account for the association between emotion regulation ability and physical health (Woolery and Salovey, 2004; Zeidner et al., 2006; van Heck and den Oudsten, 2008; Keefer et al., 2009). However, the underlying neural basis through which emotion regulation ability may exert influences on physical health has not been examined. Here, we used magnetic resonance imaging (MRI) to identify the neural basis that bridges emotion regulation ability and physical health.
To do this, we first systematically examined the association between self-reported physical health and emotion traits of personality and general affective experiences in a large population of healthy young adults (N = 253). With a firmly established association between physical health and emotion at hand, we further tested the hypothesis that higher emotion regulation ability was associated with better physical health through the amygdala because (i) both functional and structural MRI studies indicate the amygdala as a core component in the neural circuit of emotion processing (Davis and Whalen, 2001; Phelps and LeDoux, 2005; Holmes et al., 2012) and emotion regulation (Tottenham et al., 2010; Ochsner et al., 2012), and (ii) the amygdala is critical in regulating physiological stress responses of the hypothalamic–pituitary–adrenal (HPA) axis (Herman and Cullinan, 1997; Tsigos and Chrousos, 2002), and individual differences in both activation and gray matter (GM) volume of the amygdala were associated with stressor-related physiological parameters, which linked to physical health (Urry et al., 2006; Gianaros et al., 2008, 2009; Taylor et al., 2008; Gianaros and Sheu, 2009).
MATERIALS AND METHODS
Participants
Two hundred and fifty three college students (mean age: 22.6 years; s.d.: 1.01; 135 females) from Beijing, China, participated in this study. Participants had no history of cognitive or neurological disorders (e.g., mental retardation, traumatic brain injury or psychiatric illnesses). The study was approved by the Institutional Review Board of Beijing Normal University. Written informed consent was obtained from all participants before the experiment.
Assessment of physical health
Participants’ physical health was assessed with Chinese Constitution Questionnaire (CCQ), which is a national standard inventory on health in China (Zhu et al., 2007). The questionnaire consists of 60 questions on a variety of physical symptoms and health problems, such as, ‘I catch cold more easily than others’. Participants were instructed to answer each question, based on their general experiences, on a 5-point Likert scale ranging from ‘Never’ to ‘All the time’. Nine items in the questionnaire are related to psychological problems, such as, ‘I get anxious and worried easily’. Given that we focused on examining the association between emotional regulation ability and physical health in this study, and that emotion regulation ability is tightly linked with mental health outcomes such as anxiety and depression (Schutte et al., 2007; Martins et al., 2010), the nine mental health items were excluded in the analyses to obtain a pure measure of physical health not contaminated by mental health. Scores of the remaining 51 items were summed up to yield an overall index of participants’ physical health, with higher scores indicating better physical health. Previous studies have shown that the questionnaire has high reliability and validity (Zhu et al., 2007, 2008). To further validate the CCQ in this study, we asked participants to evaluate their physical health status by responding to one statement: ‘In general, would you say your health is’, with response options ranging from 1 (very poor) to 6 (excellent).
Assessment of personality
Participants’ personality was assessed with the NEO Personality Inventory-Revised (NEO PI-R) (Costa and McCrae, 1992). The NEO PI-R is a self-report inventory consisting of 120 items adhering to the five-factor model of personality. Participants were instructed to rate their agreement to each item on a 5-point Likert scale ranging from ‘strongly disagree’ to ‘strongly agree’. This hierarchically structured inventory allows measurement of personality at both the dimensional and facet level. The five dimensions include neuroticism, extraversion, openness, agreeableness and conscientiousness. Each of the five dimensions has six interrelated but independent subscales measuring personality facets. For example, the neuroticism dimension consists of the facets of anxiety, angry hostility, depression, self-consciousness, impulsiveness and vulnerability. Dimension scores are computed by summing up the corresponding facet scores.
Assessment of general affective experiences
Affective experiences can be measured either as transient fluctuations in mood or as stable individual differences in general affective experience. In our study, participants’ general experiences of positive affect (PA) and negative affect (NA) were measured using the Positive and Negative Affect Schedule (PANAS) in the ‘general’ format (Watson et al., 1988). The PANAS consists of 20 items, half of which represent characteristics of PA, and the remaining items represent that of NA. PA items include ‘determined’, ‘proud’ and ‘inspire’; NA items include ‘jittery’, ‘guilty’ and ‘shamed’. Participants indicated to what extent they experienced each affect ‘in general’, that is, how they feel ‘on average’ on a 5-point Likert scale ranging from ‘very slightly’ to ‘extremely’.
Assessment of emotion regulation ability
EI is conceptualized as an array of abilities to perceive, use, understand and regulate emotions that leads to adaptive functioning, and the ability to regulate emotions is one core component in all EI models (e.g., Bar-On, 1997; Mayer and Salovey, 1997). In our study, emotion regulation ability was assessed by the Stress Management Scale of the Emotional Quotient Inventory (EQ-i) (Bar-On, 1997), which is a standardized self-report measure of various aspects of EI. The Stress Management Scale consists of two subscales, each of which composed of nine items. The subscale of stress tolerance assesses the competency to effectively and constructively manage emotions (e.g., ‘I know how to deal with upsetting problems’), and the subscale of impulse control assesses the competency to effectively and constructively control emotions (e.g., ‘It is a problem controlling my anger’) (Bar-On, 1997; Bar-On et al., 2003). Participants were asked to indicate the extent to which each statement accurately described them on a 5-point Likert-type scale, ranging from ‘very seldom or not true of me’ to ‘very often true of me or true of me’.
MRI data acquisition and analysis
Scanning was performed on a Siemens 3T Trio scanner (MAGENTOM Trio with a Tim system) with a 12-channel phased-array head coil at the BNU Imaging Center for Brain Research, Beijing, China. MPRAGE, an inversion-prepared gradient echo sequence (bandwidth = 190 Hz/pixel, flip angle = 7°, TR/TE/TI =2.53 s/3.39 ms/1.1 s), was used to acquire 3D T1-weighted whole brain structural images (voxel size 1 mm × 1 mm×1.33 mm, 128 slices).
Voxel-based morphometry (VBM) was used to explore the neural correlates of the behaviorally observed association. VBM provides a quantitative measure of tissue volume per unit volume of spatially normalized image (Ashburner and Friston, 2000). VBM preprocessing was performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Specifically, the images were first segmented into GM, white matter and cerebral spinal fluid, using the unified segmentation approach (Ashburner and Friston, 2005). The segmented GM images were aligned and warped to an iteratively improved study-specific template using nonlinear registration in the DARTEL (Ashburner, 2007). Then, we used the normalization function in the DARTEL to normalize GM images to the Montreal Neurological Institute (MNI) space (2 mm isotropic voxel). The normalized GM images were modulated by multiplying the Jacobian determinants derived from the normalization to preserve the local tissue volumes. The modulated GM images were then smoothed with a Gaussian kernel of 8 mm full width at half maximum. The resulted smoothed modulated normalized GM images were used for further analyses.
We used the Harvard–Oxford subcortical probabilistic structural atlas (Smith et al., 2004) with a probability threshold of 50% to define the left and right amygdalae as regions of interest (ROI). We calculated averaged GM volume across all voxels in each ROI. To determine the neural correlates of behavioral measures, we evaluated Pearson correlations between average GM volume of the left and right amygdalae and the behavioral measures of physical health and emotion regulation. Another way of calculating volumes of the amygdala is based on the automated segmentation procedure (Fischl et al., 2002) provided in Freesurfer 5.1 (http://surfer.nmr.mgh.harvard.edu). The procedure assigns a neuroanatomical label to each voxel in an MR volume based on probabilistic information automatically estimated from a manually labeled training set. The automated segmentations have been found to be statistically indistinguishable from manual labeling (Fischl et al., 2002). Pearson correlations were also calculated between the volumes of bilateral amygdala and behavioral measures. Total brain volume (TBV) was calculated based on all gray and white matter volumes and ventricular volumes to correct for individual differences in whole brain size.
RESULTS
Association between physical health and emotion
The mean, standard deviation and internal reliability of all behavioral measures are shown in Table 1. As indicated in Table 1, the self-report measures of physical health, personality, PA, NA and emotion regulation ability had good internal consistency. In addition, participants’ physical health assessed by CCQ (Zhu et al., 2007, 2008) was positively correlated with their self-report on their general physical health status, which they rated on a 6-point Likert scale ranging from ‘very poor’ to ‘excellent’ (r = 0.34, P < 0.001). This confirmed that the CCQ had good validity.
Table 1.
Descriptive statistics of all behavioral measures used in the study
| Mean | s.d. | Reliability | |
|---|---|---|---|
| Physical health | 187.94 | 21.89 | 0.922 |
| Personality | |||
| Neuroticism | 45.56 | 11.55 | 0.871 |
| Extraversion | 50.43 | 8.84 | 0.753 |
| Openness | 56.62 | 8.25 | 0.714 |
| Conscientiousness | 59.75 | 10.60 | 0.730 |
| Agreeableness | 61.74 | 7.46 | 0.883 |
| PANAS | |||
| PA | 34.28 | 4.68 | 0.811 |
| NA | 24.19 | 4.57 | 0.783 |
| Emotion regulation | 62.41 | 7.75 | 0.845 |
The reliability of all behavioral measures was Cronbach’s alpha for items in the questionnaires.
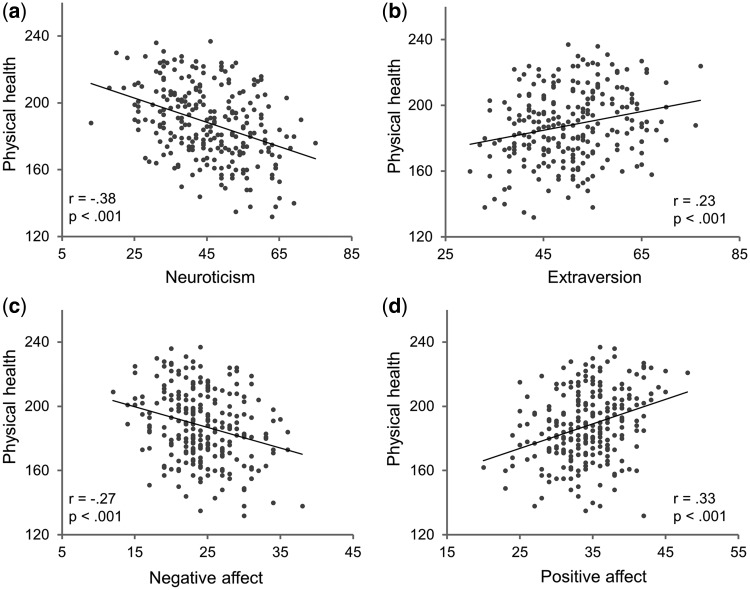
To explore the link between physical health and personality traits, we used a stepwise linear regression, with self-reported physical health as a dependent variable, and the five dimensions of personality assessed by the NEO PI-R (Costa and McCrae, 1992) and gender as predictor variables. We found that the personality dimensions were able to significantly predict self-reported physical health, with 16.1% of the variance of physical health accounted for by all the personality dimensions together (F = 24.07, P < 0.001, R2 = 0.161) (Table 2). Interestingly, the two dimensions that are most relevant to emotion, neuroticism (β = −0.346, P < 0.001) and extraversion (β = 0.123, P = 0.04), uniquely contributed to the prediction of physical health. None of the remaining three dimensions or gender contributed significantly to physical health (P > 0.05 in all cases). Post hoc correlation analyses further revealed that individuals who scored lower on neuroticism (r = −0.38, P < 0.001; with gender regressed out: r = −0.37, P < 0.001) or higher on extraversion (r = 0.23, P < 0.001; with gender regressed out: r = −0.24, P < 0.001) were likely to have better physical health (Figure 1, top).
Table 2.
Results of stepwise linear regression predicting physical health with the five dimensions of personality
| Beta | t-value | P-value | |
|---|---|---|---|
| Dependent variable: physical health (N = 253) | |||
| Neuroticism | −0.346 | −5.69 | <0.001 |
| Extraversion | 0.123 | 2.02 | 0.044 |
| Openness | 0.025 | 0.43 | 0.669 |
| Conscientiousness | 0.103 | 1.59 | 0.112 |
| Agreeableness | 0.071 | 1.20 | 0.233 |
| Gender | −0.097 | −1.66 | 0.099 |
| R2 | F | P | |
| 0.161 | 24.07 | <0.001 | |
Fig. 1.
Relationship between physical health and mental constructs. Scatter plots for correlation between physical health indexed by CCQ score and (a) neuroticism, (b) extroversion, (c) NA and (d) PA. Neuroticism and extraversion were measured by NEO PI-R; PA and NA were measured by PANAS.
To gain a more specific picture of the health–personality relationship, we performed two additional stepwise linear regression analyses, using self-reported physical health as the dependent variable and the six facets comprising neuroticism and those comprising extraversion as predictor variables, respectively. We found that in neuroticism, only the facet of anxiety (β = −0.39, P < 0.001) uniquely contributed to the prediction of physical health (F = 45.88, P < 0.001, R2 = 0.155) (Table 3), whereas in extraversion, only the facet of positive emotions (β = 0.30, P < 0.001) contributed significantly in this context (F = 24.74, P < 0.001, R2 = 0.09) (Table 4). Taken together, participants’ self-reported physical health seemed to be largely affected by the emotional characteristics of their personality traits. As personality traits of neuroticism and extraversion are defined as dispositions influencing how much positive and negation emotions one generally experiences in daily life (Costa and McCrae, 1992), we then explored whether the association between personality and physical health could be confirmed by and extended to the association between participants’ physical health and their general experiences of a broad range of PA and NA.
Table 3.
Results of stepwise linear regression predicting physical health with the six facets of neuroticism
| Beta | t-value | P-value | |
|---|---|---|---|
| Dependent variable: physical health (N = 253) | |||
| Anxiety | −0.393 | −6.77 | <0.001 |
| Angry hostility | −0.083 | −1.33 | 0.185 |
| Depression | −0.133 | −1.64 | 0.103 |
| Self-consciousness | −0.073 | −1.04 | 0.301 |
| Impulsiveness | −0.073 | −1.17 | 0.244 |
| Vulnerability | −0.013 | −0.18 | 0.859 |
| R2 | F | P | |
| 0.155 | 45.88 | <0.001 | |
Table 4.
Results of stepwise linear regression predicting physical health with the six facets of extraversion
| Beta | t-value | P-value | |
|---|---|---|---|
| Dependent variable: physical health (N = 253) | |||
| Warmth | 0.008 | 0.12 | 0.905 |
| Gregariousness | 0.024 | 0.38 | 0.705 |
| Assertiveness | 0.044 | 0.72 | 0.475 |
| Activity | 0.114 | 1.83 | 0.069 |
| Excitement-seeking | −0.006 | −0.11 | 0.917 |
| Positive emotions | 0.300 | 4.97 | <0.001 |
| R2 | F | P | |
| 0.090 | 24.74 | <0.001 | |
To do this, we correlated participants’ self-reported physical health with the PA and NA they generally experienced during their daily lives, measured by the PANAS in the ‘general’ format (Watson et al., 1988). We found that individuals who generally experienced more NA were likely to have worse physical health (r = −0.27, P < 0.001; with gender regressed out, r = −0.27, P < 0.001), whereas those who experienced more PA had a tendency to have better physical health (r = 0.33, P < 0.001; with gender regressed out, r = 0.31, P < 0.001) (Figure 1, bottom). Because individuals who experienced more PA were likely to have less NA (r = −0.16, P = 0.009), we further examined the relationship between physical health and PA with NA regressed out (and vice versa) to test whether PA (and NA) contributed to physical health independently. We found that participants’ physical health was significantly predicted by PA alone (with NA regressed out: r = 0.30, P < 0.001) or NA alone (with PA regressed out, r = −0.23, P < 0.001), consistent with the aforementioned finding that neuroticism and extraversion of personality traits contributed to physical health independently.
Association between physical health and emotion regulation ability
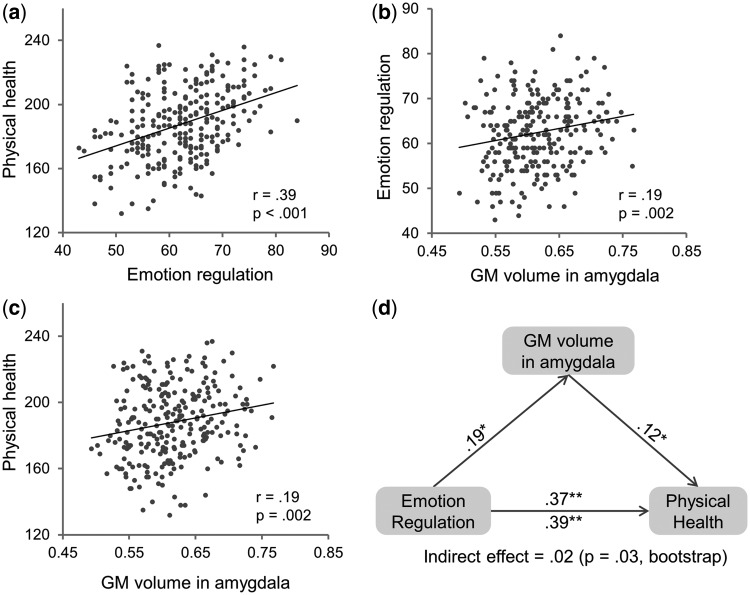
The close link between physical health and emotion leads to a logical implication that we can improve our physical health by regulating our emotions. To test this hypothesis, we correlated participants’ self-reported physical health with their ability to regulate their emotions, measured by the Stress Management Scale of the EQ-i (Bar-On, 1997). Conceptually, the Stress Management Scale covers an array of emotion regulation abilities to effectively and constructively manage and control emotions that are involved in coping with stressful situations (Bar-On et al., 2003; Wood et al., 2009). We found that individuals who reported to be better at regulating their emotions under stressful situations possessed better physical health (r = 0.39, P < 0.001; with gender regressed out, r = 0.37, P < 0.001) (Figure 2a). Emotion regulation involves reducing, strengthening or maintaining the experience of both positive and negative emotions depending on the current goals of an individual (Gross, 1998; Gross and John, 2003). Consistently, in our study, individuals who were more skilled in regulating emotions had a tendency to experience more PA (r = 0.30, P < 0.001) and less NA (r = −0.50, P < 0.001). Thus, it is possible that the association between physical health and emotion regulation ability was fully accounted for by the association between physical health and emotion. To rule out this possibility, we reexamined the correlation between self-reported physical health and emotion regulation ability after regressing out individual differences in affective experiences. We found that the correlation between physical health and emotion regulation ability remained significant after PA, NA and gender were regressed out (r = 0.24, P < 0.001), suggesting that the emotion regulation ability itself could affect physical health, where individuals better at regulating their emotions would exhibit better physical health, regardless of their emotional predisposition and general mood states.
Fig. 2.
Relationship between GM volume in the amygdala, emotion regulation ability and physical health. Scatter plots for correlation between (a) physical health and emotion regulation ability indexed by score on Stress Management Scale in the EQ-i, (b) emotion regulation ability and average GM volume in the amygdala and (c) physical health and average GM volume in the amygdala. (d) GM volume in the amygdala as a mediator in the correlation between emotion regulation ability and physical health in the mediation analysis. Path coefficients are shown next to arrows indicating each link in the analysis. For the association between emotion regulation ability and physical health, the value below the arrow indicates the zero-order correlation, and the value above the arrow represents the correlation after controlling the mediator of GM volume in the amygdala. All values represent standardized betas. *P < 0.01; **P < 0.001.
GM volume of the amygdala as a mediator
We then explored the neural basis through which emotion regulation ability linked with physical health. To do this, we focused on the amygdala, based on the converging evidence from neuropsychological and neuroimaging studies, indicating that the amygdala is a core component in neural circuits involved in EI (Bar-On et al., 2003; Killgore and Yurgelun-Todd, 2007) and emotion regulation (Ochsner et al., 2012). In addition, the amygdala has been found to be critical in modulating biological responses of the HPA axis and the sympathetic–adrenomedullary (SAM) system to stress, with profound health consequences (Tsigos and Chrousos, 2002). We used VBM to correlate average GM volume of the amygdala with behavioral measures of physical health and emotion regulation ability, respectively. The VBM analysis showed that individuals with higher self-reported ability of emotion regulation were likely to have greater GM volume in both the left (r = 0.17, P = 0.007; with TBV regressed out: r = 0.14, P = 0.03) and the right amygdala (r = 0.20, P = 0.001; with TBV regressed out: r = 0.18, P = 0.004). Although previous studies have consistently shown that adaptive regulation of negative emotions leads to decreased activation in the amygdala (Killgore and Yurgelun-Todd, 2007; Ochsner et al., 2012), the relationship between emotion regulation ability and amygdala GM volume was largely unclear. One recent study showed that greater amygdala GM volume predicts decreased stress-induced amygdala activation (Gianaros et al., 2008), which was in accordance with our results. More importantly, we found that individuals with better self-reported physical health also had a tendency to have larger GM volume in both the left (r = 0.19, P = 0.003; with TBV regressed out: r = 0.15, P = 0.02) and the right amygdala (r = 0.19, P = 0.002; with TBV regressed out: r = 0.15, P = 0.02). The result is in line with the recent study showing negative correlation between GM volume of the amygdala and stressor-evoked blood pressure reactions (Gianaros et al., 2008). Because similar results were observed in both hemispheres, the average GM volume across left and right amygdalae were collapsed (average GM volume of the amygdala and emotion regulation ability: r = 0.19, P = 0.002; average GM volume of the amygdala and physical health: r = 0.19, P = 0.002) (Figure 2b and c). Another way of calculating the volume of the amygdala is based on the automated segmentation procedure (Fischl et al., 2002), provided in Freesurfer 5.1 (http://surfer.nmr.mgh.harvard.edu). We observed a similar pattern in the association between volume of the amygdala and emotion regulation ability (r = 0.14, P = 0.03) and between volume of the amygdala and physical health (r = 0.13, P = 0.04).
We have shown that individuals who had larger GM volume in the amygdala not only possessed a higher ability of emotion regulation but also exhibited better physical health. This is indicative of the likelihood that the amygdala serves as the neural substrate through which emotion regulation is associated with physical health. To test this hypothesis, we conducted a mediation analysis with self-reported emotion regulation ability as the predictor, the GM volume of the amygdala as the mediator and self-reported physical health as the outcome. The mediation analysis showed that the correlation between emotion regulation ability and physical health decreased (r = 0.37, P < 0.001) after their correlation (r = 0.39, P < 0.001) was adjusted by the GM volume of the amygdala (Figure 2d). Bootstrap simulation (n = 5000) further confirmed that the indirect effect through the amygdala was significant (P = 0.03) with 95% confidence interval of (0.005, 0.057). In addition, the indirect effect through the amygdala remained largely unchanged with PA and NA regressed out from all three variables in the mediation model (P = 0.03, bootstrapping simulation with n = 5000 iterations), suggesting that emotional regulation may affect physical health through the amygdala independent of the general emotion experiences. In short, the mediation analysis suggests that individuals capable of regulating their emotions more effectively are healthier, in part, because they have greater GM volume in the amygdala.
DISCUSSION
Biomedical advances in the past century have led to a dramatic change on the patterns of health problems, that is, the leading causes of health problems are no longer infectious diseases, but rather those related to chronic stress and unhealthy lifestyles. Our study sheds new light on such modern health problems and implies promising solutions accordingly. With a systematic study revealing the association between self-reported physical health and emotion, we further demonstrated that, for better physical health, individuals needed to regulate their emotion more efficiently. Importantly, the amygdala was found to serve as a mediator in the link between self-reported emotion regulation ability and physical health, suggesting its critical role in the neural circuit through which emotion regulation influences physical health.
Why do individuals more capable of emotion regulation have better physical health? Research in health psychology has developed several models under the framework of stress coping to delineate the cognitive and behavioral mechanisms that link emotion regulation with physical health (Woolery and Salovey, 2004; Zeidner et al., 2006; van Heck and den Oudsten, 2008; Keefer et al., 2009). For example, the transactional/interactional stress-illness model suggests that individuals with different abilities of emotion regulation differ in the selection, manipulation and appraisal of stressful circumstances and coping responses, which in turn modulate physiological responses to stressors, subsequently contributing to illness and physical health (van Heck and den Oudsten, 2008). Alternatively, the health behavior model suggests that emotion regulation influences the degree to which a person engages in various health-promoting or health-degrading habits and coping behaviors (e.g., smoking, exercise), which mediates the association between emotion regulation and physical health (van Heck and den Oudsten, 2008). In contrast to the models hypothesizing causal relationships between emotion regulation and physical health, the constitutional predisposition model suggests that these two are not causally related; rather, they are co-effects of an underlying third set of variables (e.g., genetic or other psychobiologic factors) (Smith, 2006).
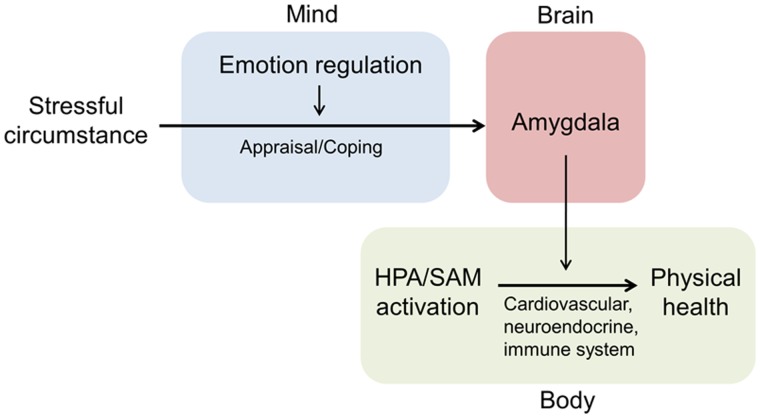
Our study provides neural constraints for these models by showing that the amygdala plays a pivotal role in the process through which emotion regulation improves physical health. On one hand, individuals’ emotion regulation ability was correlated with GM volume of the amygdala, consistent with previous studies in which the amygdala was found to be the core node of the neural network for regulating emotion (Bar-On et al., 2003; Killgore and Yurgelun-Todd, 2007; Ochsner et al., 2012). On the other hand, the GM volume of the amygdala was correlated with physical health, in accordance with previous findings that individual differences in GM volume and activation of the amygdala were associated with stressor-evoked cortisol release (Taylor et al., 2008), diurnal rhythm of cortisol secretion (Urry et al., 2006) and physiological responses to stress (e.g., blood pressure, preclinical atherosclerosis), which, in turn, contributed to physical health (Gianaros et al., 2008, 2009; Gianaros and Sheu, 2009). More importantly, our study indicated that the amygdala is a key node in the neural circuit underlying the link between the central processing of emotion regulation and peripheral expression of individual differences in physical health, thus bridging mental processes and bodily activities. Based on these results, it is likely that the transactional/interactional stress-illness model, among the several available models linking emotion regulation and physical health, more naturally accommodates the neural constraints placed by the amygdala. That is, when facing chronic stress, individuals with better ability to regulate emotion produce more positive appraisal and proactive coping responses (Keefer et al., 2009); consequently, they are less susceptible to stress-induced plasticity of neuronal activity in the amygdala, which cumulates in the alteration of the neuronal morphology (Roozendaal et al., 2009). Then, better preserved amygdala may diminish the prolonged excessive activity of the HPA axis and the SAM system through the projection from the amygdala to the hypothalamus, which exert adverse effects on neuroendocrine, cardiovascular and immune systems and ultimately on physical health (Tsigos and Chrousos, 2002) (Figure 3).
Fig. 3.
Model of the mind-brain-body pathways that links emotion regulation ability, the amygdala and physical health. HPA: the hypothalamic–pituitary–adrenal axis; SAM: the sympathetic–adrenomedullary system.
Note that physical health was measured by self-report questionnaire in our study. Self-report scales have been widely used in health psychology research because they clearly contain a valid component reflecting actual physical health, as evidenced by their extensive associations with objective health outcomes (e.g., blood pressure levels, disease incidence, mortality rates) (e.g., Watson and Pennebaker, 1989), though they also partly reflect individuals’ recognition or interpretation of their actual physical health (Watson and Pennebaker, 1989; Pressman and Cohen, 2005). Moreover, as for the association between emotion regulation and physical health, previous studies have shown that emotions and emotion regulation exert direct physiological consequences on physical health (Cohen and Rodriquez, 1995; Salovey et al., 2000), in addition to influencing how individuals perceive and interpret their actual physical health (Cohen and Rodriquez, 1995; Cohen et al., 2003). Given the critical role of the amygdala in regulating stress-related physiological responses through the HAP axis (Herman and Cullinan, 1997), it seems more likely to mediate the association between emotion regulation and actual physical health. Future studies are needed to further address this issue with objective measures of physical health.
In sum, our study takes the first step toward exploring the neural basis for the mind–body interaction and identifies the amygdala as a critical component in one of the candidate mind-brain-body pathways through which the brain may link emotion regulation with physical health. Accordingly, our study has considerable practical and theoretical implications. In practice, our study implies that by simply learning skills of emotion regulation, one can improve physical health, regardless of the extent of the NA or PA the person generally experiences. The ability to regulate emotion can be effectively developed by training in adults as well as children (Parker et al., 2005); therefore, our study may spur new means of promoting physical health by augmenting skills of emotion regulation. Future studies are needed to reveal the effectiveness of the different aspects of emotion regulation, such as regulation of subjective experience or behavioral responses, in interventions to improve physical health.
Theoretically, although it is well recognized that the brain plays a central role in the body–mind interaction through its coordination of emotion, cognition and physiological responses, surprisingly little is known about the putative neural pathways that link emotion regulation with physical health. Thus, our study serves as a starting point for future studies on delineation of mind-brain-body pathways to elucidate the neural mechanisms underlying the interaction of emotion regulation and physical health, in addition to the cognitive and behavioral mechanisms proposed in the existing models. Future studies are invited to reveal more brain regions possibly implicated in the complex interaction of emotion regulation and physical health, such as the hippocampus, prefrontal cortex and cingulate regions, to elucidate more neural pathways underlying the body–mind interaction. By doing this, our study advocates the emergence of a new research field of health cognitive neuroscience that relies on the combination of theoretical hypotheses from health psychology (Adler and Matthews, 1994; Baum and Posluszny, 1999; Suls and Rothman, 2004) with empirical evidence from health neuroscience (Gianaros and Sheu, 2009; McEwen and Gianaros, 2010).
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31230031, 91132703 and 31100808) and the National Basic Research Program of China (2010CB833903).
REFERENCES
- Adler N, Matthews K. Health psychology: why do some people get sick and some stay well? Annual Review of Psychology. 1994;45:229–59. doi: 10.1146/annurev.ps.45.020194.001305. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind-body medicine: state of the science, implications for practice. The Journal of the American Board of Family Practice. 2003;16(2):131–47. doi: 10.3122/jabfm.16.2.131. [DOI] [PubMed] [Google Scholar]
- Bar-On R. The Bar-On Emotional Quotient Inventory (EQ-i): A Test of Emotional Intelligence. Toronto, Canada: Multi-Health Systems; 1997. [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126(8):1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bates MS, Rankin-Hill L, Sanchez-Ayendez M. The effects of the cultural context of health care on treatment of and response to chronic pain and illness. Social Science and Medicine. 1997;45(9):1433–47. doi: 10.1016/s0277-9536(97)00068-3. [DOI] [PubMed] [Google Scholar]
- Baum A, Posluszny DM. Health psychology: mapping biobehavioral contributions to health and illness. Annual Review of Psychology. 1999;50:137–63. doi: 10.1146/annurev.psych.50.1.137. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134(6):829–85. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. Journal of the American College of Cardiology. 2009;53(11):936–46. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosomatic Medicine. 2003;65(4):652–7. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- Cohen S, Rodriquez MS. Pathways linking affective disturbances and physical disorders. Health Psychology. 1995;14(5):374–80. doi: 10.1037//0278-6133.14.5.374. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Descartes R. Meditations on first philosophy. In: Cottingham J, Stoothoff R, Murdoch D, editors. The Philosophical Writings of Descartes. Cambridge: Cambridge University Press; 1985 [1641]. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biological Psychiatry. 2009;65(11):943–50. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. 2009;47(3):922–36. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. The Journal of Neuroscience. 2008;28(4):990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Friedman HS. Health status and the five-factor personality traits in a nationally representative sample. Journal of Health Psychology. 2006;11(5):643–54. doi: 10.1177/1359105306066610. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–99. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hampson SE, Friedman HS. Personality and health: A lifespan perspective. In: John OP, Robins R, Pervin L, editors. The handbook of personality: Theory and research. 3rd edn. New York: Guilford Press; 2008. [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychological Bulletin. 1993;113(3):472–86. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. The Journal of Neuroscience. 2012;32(50):18087–100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer KV, Parker JDA, Saklofske DH. Emotional intelligence and physical health. In: Stough C, Saklofske DH, Parker JDA, editors. Assessing Emotional Intelligence. New York: Springer; 2009. [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. Journal of Consulting and Clinical Psychology. 2002;70(3):537–47. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Neural correlates of emotional intelligence in adolescent children. Cognitive, Affective & Behavioral Neuroscience. 2007;7(2):140–51. doi: 10.3758/cabn.7.2.140. [DOI] [PubMed] [Google Scholar]
- Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Annual Review of Psychology. 2002;53:341–69. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- Martins A, Ramalho N, Morin E. A comprehensive meta-analysis of the relationship between emotional Intelligence and health. Personality and Individual Differences. 2010;49(6):554–64. [Google Scholar]
- Mayer JD, Salovey P. What is emotional intelligence? In: Salovey P, Sluyter D, editors. Emotional Development and Emotional Intelligence: Implications for Educators. New York: Basic Books; 1997. [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JDA, Saklofske DH, Wood LM, Eastabrook JM, Taylor RN. Stability and change in emotional intelligence: Exploring the transition to young adulthood. Journal of Individual Differences. 2005;26:100–6. [Google Scholar]
- Pelletier KR. Mind-body health: research, clinical, and policy applications. American Journal of Health Promotion: AJHP. 1992;6(5):345–58. doi: 10.4278/0890-1171-6.5.345. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131(6):925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9(1):1–28. [Google Scholar]
- Salovey P, Mayer JD. Emotional intelligence. Imagination, Cognition and Personality. 1990;9:185–211. [Google Scholar]
- Salovey P, Rothman AJ, Detweiler JB, Steward WT. Emotional states and physical health. The American Psychologist. 2000;55(1):110–21. doi: 10.1037//0003-066x.55.1.110. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, Thorsteinsson EB, Bhullar N, Rooke SE. A meta-analytic investigation of the relationship between emotional intelligence and health. Personality and Individual Differences. 2007;42(6):921–33. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith TW. Personality as Risk and Resilience in Physical Health. Current Directions in Psychological Science. 2006;15(5):227–31. [Google Scholar]
- Smith TW, Gallo LC, Shivpuri S, Brewer AL. Personality and health: Current issues and emerging perspectives. In: Baum A, Revenson T, Singer J, editors. Handbook of Health Psychology. 2nd edn. New York: Taylor & Francis; 2012. [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Suls J, Rothman A. Evolution of the biopsychosocial model: prospects and challenges for health psychology. Health Psychology. 2004;23(2):119–25. doi: 10.1037/0278-6133.23.2.119. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53(4):865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heck GL, den Oudsten BL. Emotional Intelligence: Relationships to Stress, Health, and Well-being. In: Vingerhoets A, Nyklicek I, Denollet J, editors. Emotion Regulation: Conceptual and Clinical Issues. New York: Springer; 2008. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychological Review. 1989;96(2):234–54. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Wood LM, Parker JDA, Keefer KV. Assessing Emotional Intelligence Using the Emotional Quotient Inventory (EQ-i) and Related Instruments. In: Parker JDA, Saklofske DH, Stough C, editors. Assessing Emotional Intelligence: Theory, Research, and Applications. New York: Springer; 2009. [Google Scholar]
- Woolery A, Salovey P. Emotional intelligence and physical health. In: Nyklicek I, Temoshok L, Vingerhoets A, editors. Emotional Expression and Health: Advances in Theory, Assessment, and Clinical Applications. Hove and New York: Brunner-Routledge; 2004. [Google Scholar]
- Zeidner M, Matthews G, Roberts RD. Emotional intelligence, coping, and adaptation. In: Ciarrochi J, Forgas J, Mayer JD, editors. Emotional Intelligence in Everyday Life. 2nd edn. New York: Psychology Press; 2006. [Google Scholar]
- Zhu Y, Origasa H, Uebaba K, Xu F, Wang Q. Development and validation of the Japanese version of the constitution in Chinese Medicine Questionnaire. Kampo Medcine. 2008;59(6):783–92. [Google Scholar]
- Zhu Y, Wang Q, Origasa H. Evaluation on reliability and validity of the constitution in chinese medicine questionnaire [in Chinese] Chinese Journal of Behavioral Medical Science. 2007;16(7):651–4. [Google Scholar]