Abstract
Assessing neural commonalities and differences among depression, anxiety and their comorbidity is critical in developing a more integrative clinical neuroscience and in evaluating currently debated categorical vs dimensional approaches to psychiatric classification. Therefore, in this study, we sought to identify patterns of anomalous neural responding to criticism and praise that are specific to and common among major depressive disorder (MDD), social anxiety disorder (SAD) and comorbid MDD-SAD. Adult females who met formal diagnostic criteria for MDD, SAD or MDD-SAD and psychiatrically healthy participants underwent functional magnetic resonance imaging as they listened to statements directing praise or criticism at them or at another person. MDD groups showed reduced responding to praise across a distributed cortical network, an effect potentially mediated by thalamic nuclei undergirding arousal-mediated attention. SAD groups showed heightened anterior insula and decreased default-mode network response to criticism. The MDD-SAD group uniquely showed reduced responding to praise in the dorsal anterior cingulate cortex. Finally, all groups with psychopathology showed heightened response to criticism in a region of the superior frontal gyrus implicated in attentional gating. The present results suggest novel neural models of anhedonia in MDD, vigilance-withdrawal behaviors in SAD, and poorer outcome in MDD-SAD. Importantly, in identifying unique and common neural substrates of MDD and SAD, these results support a formulation in which common neural components represent general risk factors for psychopathology that, due to factors that are present at illness onset, lead to distinct forms of psychopathology with unique neural signatures.
Keywords: major depressive disorder, social anxiety disorder, comorbidity, functional neuroimaging, anhedonia, default mode network
INTRODUCTION
Major depressive disorder (MDD) and social anxiety disorder (SAD) are among the most prevalent and debilitating of all mental disorders (Kessler et al., 2005). More than 20% of the general population will experience a clinically significant episode of a mood disorder (Kessler and Wang, 2009), and almost 15% will experience SAD, making it the most common of the anxiety disorders (Kessler et al., 2005). It is becoming increasingly apparent that depression and anxiety co-occur at both the symptom and syndrome levels; one-quarter of individuals diagnosed with primary MDD also meet criteria for SAD (Kessler et al., 1999), and one-fifth of individuals with primary SAD also meet diagnostic criteria for MDD (Ohayon and Schatzberg, 2010). Compared with non-comorbid individuals, persons with comorbid SAD and MDD experience higher levels of suffering, greater impairment in social and occupational functioning, increased resistance to treatment and higher risk of suicide (Souery et al., 2007; Fava et al., 2008; Rush et al., 2008). Thus, there is a pressing need to develop comprehensive neural models of comorbid MDD and SAD that can inform effective treatment approaches for this debilitating condition.
While developing a neuroaffective conceptualization of comorbid MDD-SAD is crucial, understanding similarities and differences between non-comorbid MDD and SAD is also critical for identifying both specific and general neural characteristics of these two disorders. Indeed, citing high levels of overlap in the neural systems associated with MDD and with anxiety disorders reported in studies examining these disorders separately (Etkin and Wager, 2007; Hamilton et al., 2012), researchers have begun to consider the merits of dimensional, as opposed to categorical, approaches to the nosology of psychopathology (Insel et al., 2010). It is important to note, however, that a more critical assessment of the feasibility of dimensional approaches that involves direct neural comparisons of MDD and SAD is necessary to identify directly neural differences among these disorders. In this context, conducting a direct comparison of the neural underpinnings of MDD and SAD will allow us both to elucidate neural processes specific to these disorders and to inform the framework through which we understand psychopathology more generally.
One recent functional magnetic resonance imaging (fMRI) study examined individuals diagnosed with MDD, with Generalized Anxiety Disorder (GAD), and with comorbid MDD-GAD, as they completed an affective conflict resolution task (Etkin and Schatzberg, 2011). These investigators found common anomalies in responses of all clinical participants in the amygdala and ventral anterior cingulate cortex (ACC) under conditions of affective conflict, and depression-specific compensatory activity in dorsolateral prefrontal cortex (DLPFC). In another study, individuals with MDD, SAD and comorbid MDD-SAD underwent a social evaluative threat task in which they anticipated giving a speech during FMRI scanning. In this study, participants with non-comorbid SAD exhibited increased occipital cortex activity as they prepared for the speech and decreased insula activation following the speech stressor, whereas participants with non-comorbid MDD exhibited sustained medial frontal cortex activation throughout the stressor. Importantly, comorbid MDD-SAD participants exhibited both of these patterns of activation, indicating that, at a neural level, comorbid MDD-SAD may be understood as the additive combination of MDD and SAD (Waugh et al., 2012). Although these studies have made important preliminary contributions to neural models of depression, anxiety and their comorbidity, fundamental questions remain unaddressed. Specifically, MDD and SAD are associated with unique biases and patterns of behavior in both negative and positive emotional domains that are central in clinical manifestations of these disorders (Beck et al., 1979; Wells et al., 1995; Loas, 1996; Amir et al., 1998a). Thus, exploring the neural substrates of both negative and positive affective biases in MDD, SAD and their comorbidity has the potential to contribute significantly to the development of integrative and comprehensive neural models of these disorders.
This study was designed to examine the unique and common patterns of neural responding to negative and positive self-relational stimuli in non-comorbid and comorbid MDD and SAD. We utilized a two- (MDD: present, absent) by-two (SAD: present, absent) between-subjects factorial design to elucidate neural response anomalies to praise and criticism that were (i) related to MDD; (ii) related to SAD; (iii) associated with comorbid MDD-SAD or (iv) associated generally with the presence of psychopathology. There are distinct advantages of assessing neural responses to praise and criticism in MDD and SAD, as we did in this study, as opposed to other positive and negative affective stimuli such as static emotional faces or images. Given the prominent interpersonal difficulties that characterize both MDD and SAD, aberrant neural responses to praise and criticism are more likely to reflect core endophenotypes in these disorders. Consistent with this position, neural investigations of remitted MDD have found diminished response of affect regulatory structures to criticism (Hooley et al., 2005), while examinations of neural response in SAD have identified increased recruitment of salience network (Seeley et al., 2007) nodes in this disorder (Blair et al., 2008). Thus, using phrases containing praise and criticism directed at participants increases the relevance of the stimuli and, therefore, the ecological validity of the obtained results. Finally, data showing that increased criticism predicts relapse in MDD (Okasha et al., 1994) further underscores the clinical utility of using self-relational stimuli in experimental paradigms.
Given data from Hooley and colleagues (2005) showing decreased responding to criticism in the DLPFC in MDD, we predict that individuals with MDD and MDD-SAD will similarly show attenuated DLPFC response to criticism directed at them during scanning. Further, given findings from Blair et al. (2008) of increased amygdala response to criticism in SAD, we predict heightened responding of the amygdala and other salience network nodes, such as the anterior insula and dorsal anterior cingulate cortex (dACC), in both SAD and MDD-SAD. Finally, as implied in the above hypotheses, we predict that persons with MDD-SAD will show neural effects associated with both depression and with social phobia.
METHODS
Participants
Participants were recruited from local psychiatric outpatient clinics and through Web site postings. Sixty-one women participated in this study: 16 participants diagnosed with pure SAD; 15 participants diagnosed with pure MDD; 16 participants diagnosed with comorbid MDD-SAD; and 14 never-disordered control participants (CTL). We included only women in this study both given findings that depressive phenotypes differ between men and women (Nolen-Hoeksema et al., 1999) and to reduce error variance and improve the statistical sensitivity of our design. Diagnostic evaluations were based on DSM-IV-TR criteria using the Structured Clinical Interview for DSM-IV-TR Axis I (First et al., 2001) administered by interviewers who received extensive training, typically post-baccalaureate research assistants and advanced graduate students. Diagnoses were confirmed by PhD-level clinical psychologists. Further, we used the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) and the Social Phobia and Anxiety Inventory (SPAI; Beidel et al., 1989) to assess levels of depression and social phobia, respectively. Participants in the MDD-SAD group met diagnostic criteria for current SAD and current MDD, and did not meet current or lifetime criteria for any another Axis-I disorder. Participants in the two non-comorbid clinical groups (SAD, MDD) met diagnostic criteria for current SAD or current MDD, but did not meet criteria for any Axis-I disorder currently or across their lifetimes. Finally, CTL participants did not meet criteria for any current or past Axis-I psychopathology. Interrater reliability kappas ranged from 0.92 to 1.0 for diagnoses of MDD, SAD and MDD-SAD. All participants were between 18 and 55 years of age, had no lifetime history of psychotic ideation, no reported substance abuse within the past 6 months, no indication of impaired mental status and no physical limitations that prohibited them from undergoing fMRI.
Self-Other Affective Processing task
Participants completed the Self-Other Affective Processing (SOAP) task—modeled after a similar task developed and used by Blair and colleagues (Blair et al., 2008)—during scanning. In each 20 s block of the SOAP task, participants passively listened to four affective statements spoken by a recorded female voice. Each of the 5 s long (3 s of speech followed by 2 s of silence) affective statements was directed either at the participant (‘You are …’) or at a male other (‘He is …’). Given that responding in neural regions that subserve affect has been found to persist beyond the duration of an affective challenge (Siegle et al., 2002), we used a simple but active, directed button-pressing task to distract participants following the affective stimuli to make analysis of blood-oxygen-level-dependent (BOLD) time series data more tractable. Participants listened to eight blocks of each of six different kinds of statements: self- or other-related crossed with three valence categories: positive (POS), threat (THR) and negative (non-threat; NEG). These 48 blocks of affective statements were presented in pseudorandomized counter-balanced order over eight 240 s scanning runs. The affective adjectives used for the SOAP task were taken from the Affective Norms for English Words list (Bradley and Lang, 1999). Selected words were matched for both normed frequency and intensity ratings across the three valence categories. We included two categories of negative stimuli (THR and non-threat NEG) for possible examination of the specificity of neural responding to different types of negative stimuli in subsequent work, specifically the examination of semantically general (non-threat NEG) relative to semantically focused (THR) negative stimuli. In meeting the objective of this study to examine neural responding at different poles of the valence dimension, we focus here only on the POS (praise) and NEG (criticism) statements. We present in Supplementary Table S1 examples of POS and NEG self- and other-directed statements.
FMRI data acquisition and preprocessing
Data acquisition and preprocessing for this study followed standard protocols that we present in an online supplement to this article.
FMRI data analysis
Estimating maximum height of neural response
Conventional regression-based approaches for analyzing BOLD data, while elegant, can fail to identify meaningful aspects of these data, particularly in polysensory and limbic regions with response profiles that can be influenced by affective factors that vary among individuals. For example, in the context of block designs, the effects of brief and potentially meaningful increases in the hemodynamic response can go undetected due to ‘averaging out’ by conventional fitting procedures that aim to assign a single parameter to characterize several points in FMRI time series data. Given this concern, in the present analysis we estimated a single height parameter (described below) of the BOLD response for each participant for each condition. Similar approaches have been shown in previous work to identify unique and psychologically relevant aspects of the BOLD response in the amygdala and medial prefrontal cortex (Siegle et al., 2002; Waugh et al., 2010). To estimate this parameter, we first used the Analysis of Functional NeuroImages (AFNI) program 3dDeconvolve to obtain average stimulus response functions (SRFs), voxel-wise, for each participant for each condition. We used multiple regression to remove nuisance effects from SRFs; these nuisance effects included three translational and three rotational head movement estimates, zeroth through third order drift effects and heart rate and respiration artifacts (Chang and Glover, 2009). Next, for each participant for each condition, we estimated the maximum height from the corrected SRFs over a span of 13 BOLD acquisitions: 10 acquisitions over the 20 s block duration and 3 acquisitions following the end of blocks to account for potentially sustained processing of stimuli post-offset that can occur (e.g. Siegle et al., 2002) despite our use of an active control task. We estimated height as the change in BOLD signal from the average of the first two 2 s acquisitions—the ‘ramp up’ phase of the canonical SRF—to the maximum value of the 3rd through 13th acquisitions—the ‘plateau’ phase of the canonical SRF during a 20 s period of stimulation.
Voxel-wise analyses to identify interactions among MDD, SAD and valence
For our primary analysis, we conducted a two-by-two-by-two—[MDD: present (MDD and MDD-SAD) vs absent (SAD and CTL)] by [SAD: present (SAD and MDD-SAD) vs absent (MDD, CTL)] repeated over [valence: POS vs NEG stimuli]—analysis of variance (ANOVA), implemented in AFNI, on participants’ contrast maps for self- minus other-related statements. We added to our model as regressors of non-interest an age covariate, given a non-significant but trend-level difference in the average age of the MDD group relative to the other groups, and two dose-dependent medication covariates, one reflecting antidepressant load and another reflecting anxiolytic/sedative load; see Supplementary Table S2 for medications and dosages. The medication load covariates were calculated according to standards outlined by Sackheim in the context of defining treatment resistance to pharmacotherapy (Sackeim, 2001). These covariates are important in the context of this study given that we allowed individuals who were stabilized on psychotropic medications to participate and needed to account for medication effects on our group-wise analyses. We conducted this age- and medication-corrected factorial analysis to identify regions showing (i) effects of MDD that interact with valence; (ii) effects of SAD that interact with valence; and (iii) interactions of the MDD and SAD factors that, themselves, depended on the level of the valence factor. We present these specific results and not the full array of results rendered by the three-way ANOVA because they follow most directly from the aims of the study. We set the family-wise error-corrected α for these omnibus tests at 0.05 (per voxel α = 0.05, cluster threshold, κ = 50 voxels as determined by Monte Carlo simulation using the ANFI program AlphaSim). Given that we observed a high degree of consistency in response across several regions identified with individual omnibus analyses, neural response data from similarly responding regions identified in each omnibus analysis (see Table 2) were averaged before being analyzed further with t-tests.
Table 2.
Regions showing MDD-by-valence, SAD-by-valence and MDD-by-SAD-by-valence interactions
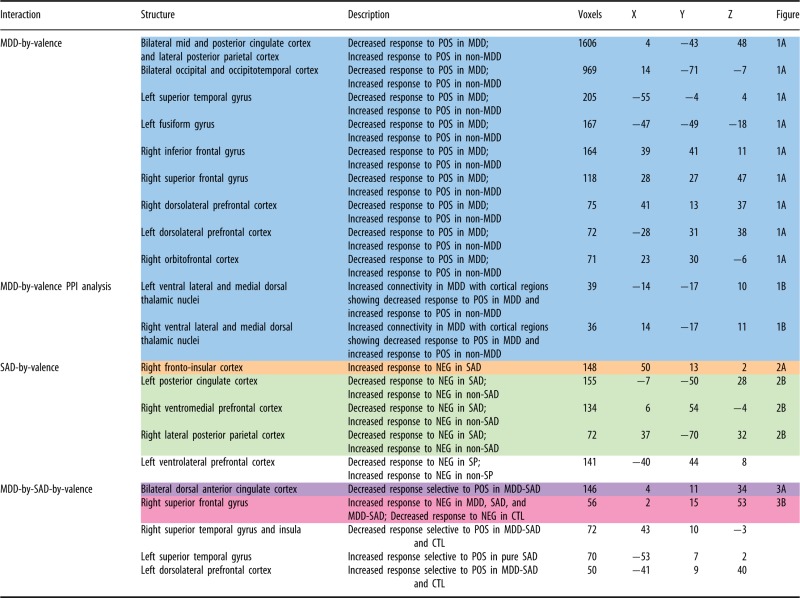 |
Data from similarly highlighted regions were averaged together prior to subsequent pairwise contrasts presented in figures.
Psychophysical interaction analysis
As we note in greater detail below, from the MDD-by-valence interaction analysis, we identified decreased response to praise in a diffuse cortical network in MDD. To examine whether this broad cortical effect was mediated by one or more subcortical structures, we conducted a psychophysical interaction (PPI) analysis using, as a seed, averaged BOLD signal from cortical regions showing a between-group (MDD vs non-MDD) difference in height of response to positive but not to negative stimuli. We examined condition-specific relations between this seed and several subcortical regions: Talairach defined amygdala, caudate, putamen, pallidum, hypothalamus, substantia nigra and thalamus. We conducted this PPI analysis—implementation described elsewhere (Hamilton et al., 2011)—in each participant for self- vs other-related POS and NEG statement conditions. We then submitted individual PPI coefficient maps to a two-by-two (MDD: present vs absent; repeated over valence: POS vs NEG) ANOVA that we examined for a significant interaction of MDD and valence (family-wise corrected α = 0.05; per voxel α = 0.05, cluster threshold, κ = 30 voxels as determined by Monte Carlo simulation using the ANFI program AlphaSim).
Neurobehavioral correlation analysis
For networks for which we found significant diagnosis-by-valence interactions in neural response, we extracted effect-related contrast coefficients for these regions and calculated the linear correlation between these coefficients and indices of severity of MDD (BDI-II) or SAD (SPAI), depending on the nature of the neural effect (e.g. for a network in which we found an effect of the MDD factor, we calculated the correlation between contrast coefficients for this network and the BDI-II). We used a Bonferroni correction to control for family-wise Type-I error associated with multiple statistical tests.
RESULTS
Participant demographic and clinical characteristics
Demographic, clinical and medication data for the four groups of participants are presented in Table 1. We present a description of these data in an online supplement to this article.
Table 1.
Demographic, clinical and pharmacological data for participant groups
| Age (mean ± SE) | BDI-II (mean ± SE)* | SPAI (mean ± SE)a | % antidepressant medicated | % anxiolytic/sedative medicated | |
|---|---|---|---|---|---|
| CTL | 33.21 ± 2.45 | 1.77 ± 0.83 | 258.92 ± 25.82 | 0 | 0 |
| MDD | 40.47 ± 2.99 | 25.86 ± 3.41 | 410.79 ± 30.00 | 67 | 33 |
| SAD | 28.69 ± 2.47 | 12.25 ± 2.18 | 511.66 ± 20.35 | 12.5 | 12.5 |
| MDD-SAD | 33.13 ± 3.17 | 29.69 ± 2.70 | 563.81 ± 17.75 | 31 | 19 |
SE = standard error; BDI-II = Beck Depression Inventory-II; SPAI = Social Phobia and Anxiety Inventory.
aBDI-II and SPAI did not correlate significantly in the CTL or psychopathology groups.
Neural response1
Interaction of MDD and valence
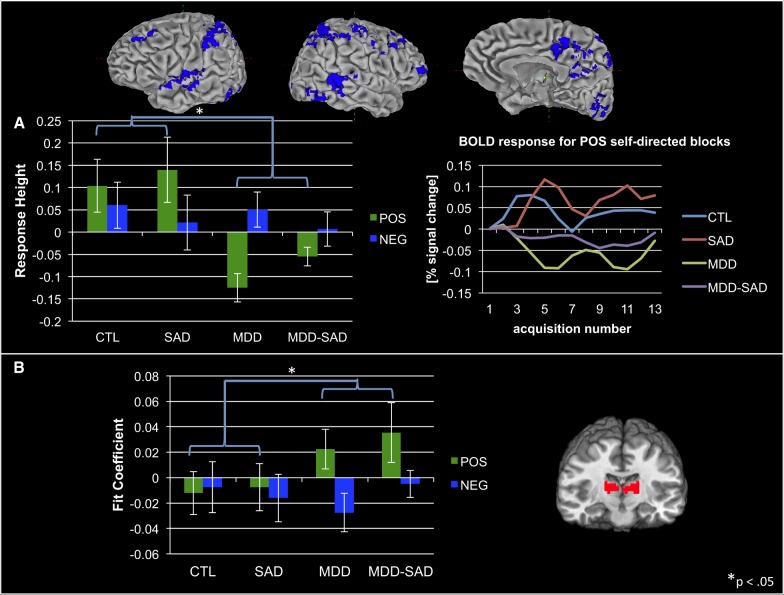
Participants with MDD (MDD and MDD-SAD), relative to participants without MDD (CTL and SAD), showed decreased responding to self- vs other-related POS statements in a large extent of auditory and visual as well as frontal and parietal association cortices. This pattern was not observed for NEG statements (see Figure 1A for group means and for regional BOLD time series data for the POS, self-related condition; see also Table 2, blue highlighted section, top, for the coordinates of regions driving these effects). Our post hoc PPI analysis identified significantly increased connectivity between this cortical network and ventral lateral and medial dorsal thalamic connectivity in groups with MDD relative to groups without MDD for POS but not for NEG self- vs other-related statements (see Figure 1B and Table 2, blue highlighted section, bottom).
Fig. 1.
(A) Decreased cortical response to positive self- vs other-related statements in MDD relative to non-MDD groups; averaged BOLD time series data from these same cortical regions for the positive self-related stimulus condition. (B) Increased connectivity in MDD relative to non-MDD groups between cortical regions from A, above, and ventral lateral and medial dorsal thalamic nuclei during processing of positive self- vs other-related statements. Note that we present neural response data from all groups, even though these results were obtained from a main-effects analysis of MDD vs non-MDD groups, to make clear to readers that main effects were driven by both MDD and MDD-SAD groups.
As we noted in Methods, we analyzed height of response (or, for the PPI analysis, differences in connectivity) for self- vs other-related statements. Given that such ‘multiple subtraction’ comparisons can hide the true nature of effects, we present in Supplementary Figures S1–S6 group means for response height (or connectivity) for self- and other-related statements for the valence conditions relevant to the effects reported here. These data, in addition to the time series data presented in the main figures for self-directed stimulus conditions, confirm that the reported effects are driven completely or in significant part by group differences in activity during self-relational processing.
Interaction of SAD and valence
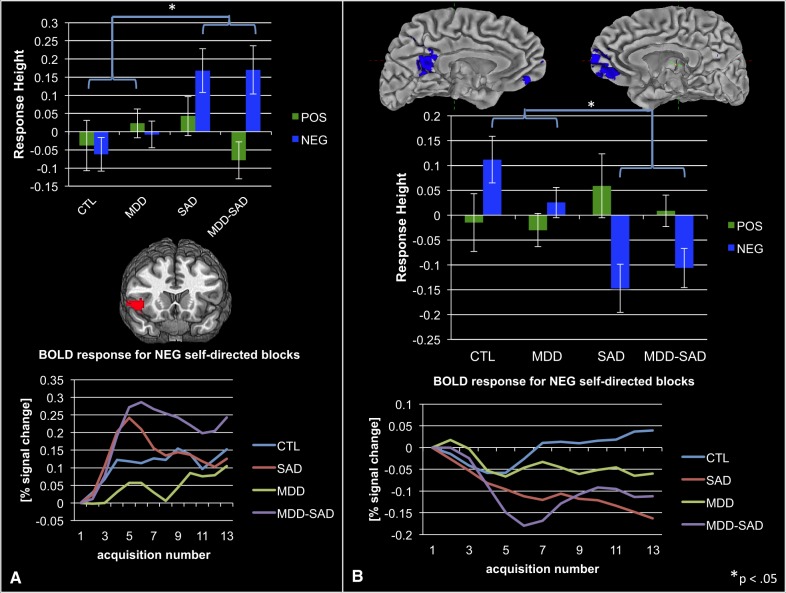
As predicted, participants with SAD (SAD, MDD-SAD) compared with those without SAD showed consistently increased response in right fronto-insular cortex to self-related NEG, but not POS, statements (see Figure 2A and Table 2, orange highlighted section). Conversely, in participants with SAD, relative to participants without SAD, we found decreased cortical response to NEG, but not to POS, self-related statements in three primary nodes of the default mode network (DMN)—ventromedial prefrontal, posterior cingulate and lateral posterior parietal cortices—as determined from maps from Greicius and colleagues (Greicius et al., 2003); see Figure 2B and Table 2, green highlighted section.
Fig. 2.
(A) Increased anterior insula response to negative self- vs other-related statements in groups with SAD, relative to groups without SAD; averaged anterior insula BOLD time series data recorded during processing of negative, self-related stimuli. (B) Decreased default-mode network responding in groups with SAD during processing of self- vs other-related negative statements; BOLD time series data from the default-mode network acquired as participants heard negative self-related statements. Note that we present neural response data from all groups, even though these results were obtained from a main-effects analysis of SAD vs non-SAD groups, to make clear to readers that main effects were driven by both SAD and MDD-SAD groups.
Interaction of MDD, SAD and valence
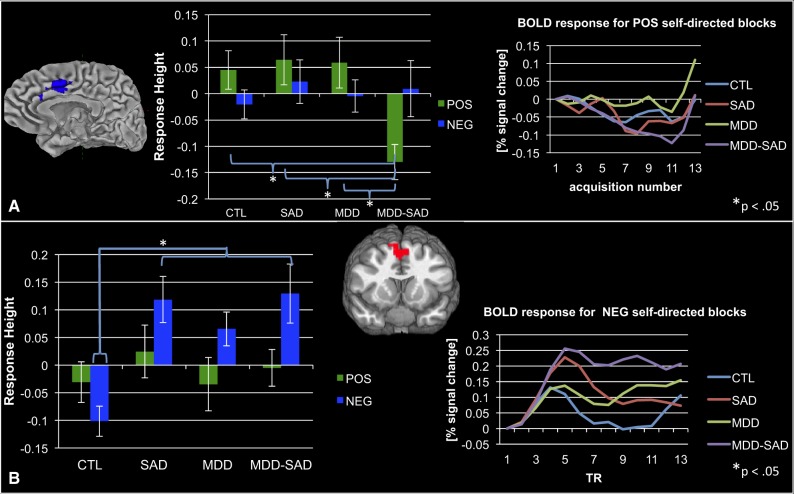
A number of regions showed a significant interaction of MDD and SAD that was moderated by the valence of information that participants heard (see Table 2, bottom). Two effects of greatest interest in the context of the aims of the present study were a decreased response in the dACC, bilaterally, to POS, but not to NEG, self- vs other-related statements in the comorbid MDD-SAD group relative to the MDD, SAD and CTL groups (see Figure 3A and Table 2, purple highlighted section) and increased response to NEG, but not to POS, self- vs other-related statements in groups with psychopathology (MDD, SAD and MDD-SAD) relative to the CTL group in the aspect of the superior frontal gyrus (SFG) along the cortical midline (see Figure 3B and Table 2, pink highlighted section).
Fig. 3.
(A). Decreased responding in the dorsal anterior cingulate cortex during processing of positive self- vs other-related stimuli in the comorbid MDD-SAD group relative to the CTL, SAD and MDD groups; dorsal anterior cingulate cortex BOLD time series data recorded while participants heard positive self-related stimuli. (B) Increased activation during processing of negative self- vs other-related statements in the superior frontal gyrus in the psychopathology groups (i.e. MDD, SAD and MDD-SAD) relative to the CTL group; superior frontal gyrus BOLD time series data acquired during processing of negative self-related stimuli.
Neurobehavioral correlation analysis
We found in participants with SAD (SAD, MDD-SAD) that more DMN deactivation was associated with increased severity of SAD as measured with the SPAI [r(30) = −0.44; P <0.05]; we did not observe this same effect in non-SAD participants [r(27) = −0.19; P >0.20]. All other neurobehavioral correlations computed did not reach the family-wise-error adjusted criterion for statistical significance (all uncorrected P >0.05).
DISCUSSION
This study is the first to examine anomalies in neural response to positive and negative self- and other-related stimuli in non-comorbid and comorbid MDD and SAD. In participants diagnosed with MDD, we documented a cortically diffuse decrease in the height of neural response to self-related positive, but not negative, stimuli, a pattern of activation that was potentially mediated by activity in ventral lateral and medial dorsal thalamic nuclei. In participants with SAD, we found increased insula response but decreased DMN response to negative, but not to positive, self-related stimuli. Further, in individuals with comorbid MDD-SAD, we found decreased response in the dACC to positive, but not to negative, self-related stimuli. Finally, in the MDD, SAD and MDD-SAD groups, relative to CTL participants, we found increased response to self- vs other-related negative stimuli in the SFG at cortical midline.
Cortical hypo-response to positive stimuli in MDD
In groups in which participants were diagnosed with MDD (i.e. MDD and MDD-SAD), we found a reduction in neural response in a broad array of secondary auditory and visual as well as associative cortical structures to praise relative to criticism. Reduced behavioral responding to positive stimuli in MDD is well documented (Loas et al., 1992), with neural investigations of this phenomenon focusing on structures of the basal ganglia postulated to subserve encoding of reward value (Pizzagalli et al., 2009; Hamilton et al., 2012). Recent neural accounts of reward processing that are based on statistically sensitive multi-voxel pattern analyses of fMRI data, however, have postulated that encoding for reward occurs on a cortex-wide scale (Vickery et al., 2011), particularly in the posterior cingulate and posterior parietal cortices in which we found depressed individuals to show reduced responding to praise. Given that reward value can be represented in large extents of cortex, the question remains of how cortical encoding of rewarding stimuli can be decreased so diffusely in MDD. One intriguing answer to this question emerged from the results of our PPI analysis showing that, in MDD, the ventral lateral and medial dorsal nuclei of the thalamus are more functionally connected to the cortical regions that are characterized by decreased activation during the receipt of praise. These nuclei receive input from a broad array of cortical structures via the cortico-striatal-pallido-thalamic circuit (Cummings, 1993); importantly, lesions of these nuclei resulting from thalamic infarction have been shown to result in increases in irritability and sad mood (Gentilini et al., 1987), both of which are central symptoms of MDD. Of greatest relevance to understanding our finding of diffuse cortical deactivation to praise in MDD are data reported by Portas and colleagues (Portas et al., 1998), who found that activity in the ventral lateral nucleus of the thalamus changed during performance of an attentional task as a function of arousal, indicating that this structure may play a critical role in mediating the interaction between arousal and attention. Therefore, in the context of MDD, functional anomalies in the ventral lateral nucleus of the thalamus may influence depressotypic attentional biases away from positive stimuli (Gotlib et al., 2011) and suggest a novel neural-level hypothesis to explain the presence of anhedonia in this disorder. Until additional data are acquired to test such a hypothesis more explicitly, however, this formulation remains speculative.
Neural underpinnings of vigilance-avoidance in SAD
We found that individuals with SAD (i.e. SAD and MDD-SAD groups), relative to participants without SAD, showed greater response to criticism in the right fronto-insular cortex. In contrast, we found reduced activation in response to criticism in the three primary loci of the DMN, as defined by Greicius and colleagues (Greicius et al., 2003): ventromedial prefrontal, posterior cingulate and lateral posterior parietal cortices. Recent neural findings suggest that the fronto-insular cortex is a component of the salience network of the brain, which, with the amygdala and dACC, is involved in processing the personal relevance of stimuli (Seeley et al., 2007), especially stimuli that signal threat (Dalton et al., 2005; van Wingen et al., 2011). Investigators have posited that the specific role of fronto-insular cortex within this network is to promote rapid awareness of and response to relevant stimuli (Craig, 2009; Allman et al., 2010). Thus, in this study, increased response of the fronto-insular cortex—which has been implicated reliably in anxiety disorders (Etkin and Wager, 2007)—to criticism in SAD could represent and undergird vigilance for and awareness of negative self-related stimuli. Importantly, this finding parallels results of previous work showing heightened response of another node of the salience network, the amygdala, to criticism in SAD (Blair et al., 2008). It is noteworthy, however, that we did not find between-groups differences in activation of subcortical structures, such as the amygdala and nucleus accumbens, typically involved in determining the personal significance of stimuli. Given the well-established role of the DMN in mediating self-relational thinking (Fox et al., 2005), and in light of findings that increased coherence among DMN nodes is associated with heightened pathology in SAD (Liao et al., 2010), deactivation of all primary nodes of this network in response to negative self-relevant stimuli in SAD may be a neural substrate of the avoidance of threatening information that characterizes this disorder.
Together, the patterns of response in salience and default-mode networks in SAD comprise a comprehensive and intuitive neural substrate of vigilance-avoidance models of this disorder (Amir et al., 1998b). Indeed, consistent with this formulation, follow-up analyses of onset times of neural responses show that, in SAD, during processing of criticism, fronto-insular cortex responds significantly in advance of the DMN.
Neural substrates of comorbid SAD and MDD
Participants with comorbid MDD-SAD exhibited patterns of neural activation that provide intriguing clues about how depression and social anxiety interact neurally. First, we found neural-level influences of both MDD and SAD in the comorbid group: MDD-SAD participants exhibited both depressotypic decreases in cortical responding to praise and a socially anxious cortical response to criticism. In addition to these effects, the comorbid group showed, uniquely, decreased dACC response to praise. The dACC is a hub of the salience network (Seeley et al., 2007) that plays an important role in volitional control of the autonomic nervous system (Critchley et al., 2003). Importantly, meta-analyses of neural investigations of anxiety (Etkin and Wager, 2007) and of major depression (Hamilton et al., 2012) have found that aberrant dACC response to affective challenge is a consistent aspect of these disorders. Further, recent work has shown that the same region of the dACC that was identified in the present analysis has abnormally increased functional connectivity in MDD to three primary neural networks: the default-mode, executive and salience networks (Sheline et al., 2010). Given these data, combined with our finding of reduced dACC response to praise in the comorbid MDD-SAD group, we posit that in comorbid depression and anxiety, this region acts as a convergence zone for the neural effects of negative self-schemas that are associated with MDD and the anxiety concerning social interactions that characterizes SAD.
A common neural substrate of heightened negative affective reactivity in MDD, SAD and their comorbidity
We found increased response to criticism in the SFG at cortical midline in all three groups with psychopathology. Examinations of performance of a broad range of tasks following damage to the SFG reveal deficits in cognitive control operations, particularly those involving initiation of novel responses and set switching (Peraud et al., 2002). Further refining conceptions of SFG function, functional neuroimaging studies have found this structure to activate during shifts of attention away from one feature of an object toward another (Nagahama et al., 1999). Thus, in the context of the current findings of increased SFG response to criticism in MDD, SAD and their comorbidity, it is possible that this structure plays a causal role in higher level, volitional engagement of attention toward self-relevant negative information, consistent with negative self schemas in MDD (Bradley and Mathews, 1983) and increased vigilance to threat in SAD (Mogg et al., 2004).
LIMITATIONS
There are three noteworthy limitations of this study. First, degrees of freedom lost in incorporating a factorial design with three factors and 15 participants per cell, on average, may have rendered this study insensitive to important neural effects in understanding MDD, SAD and their comorbidity. Second, given both that abstract neutral adjectives are relatively uncommon and that neutral stimuli are often misinterpreted as negative in SAD (Amir et al., 1998b), we did not include a neutral stimulus condition in this study. Future studies with larger samples and different categories of stimuli will be important in replicating and extending the present results. Finally, given the potential transdiagnostic implications of the present work, the lack of a transdiagnostic dimensional measure (such as neuroticism) in the study limits our capacity to determine the broader clinical significance of the neural data presented.
CONCLUSIONS AND FUTURE DIRECTIONS
The present findings concerning shared and unique aspects of the neural functioning of individuals diagnosed with non-comorbid and comorbid MDD and SAD come at a critical and exciting time in the development of clinical neuroscience, as we consider new nosologic systems in psychiatry—particularly dimensional approaches to classification—that may more accurately reflect findings from genetic and imaging data (Insel et al., 2010). A primary motivation for considering dimensional approaches to psychiatric classification is the substantial overlap in neural system dysfunction reported in studies of different DSM-based diagnostic categories. For example, our recent meta-analysis of functional neuroimaging studies of MDD revealed a pattern of reliable over-response in the salience network to negative affective challenge in depression (Hamilton et al., 2012) that echoed findings from a similar meta-analysis of functional neuroimaging studies of anxiety disorders (Etkin and Wager, 2007). Although it is important to acknowledge this overlap, it is equally important to recognize that critical tests of dimensional formulations of psychopathology require that researchers conduct studies that directly compare specific diagnostic groups, with an eye toward challenging assumptions on which dimensional formulations are based. Although such investigations are rare, this study, along with recent work examining comorbid MDD and GAD (Etkin and Schatzberg, 2011), identifies both common and distinct neural signatures in MDD and SAD. Collectively, this work suggests a testable formulation in which common neural factors, representing a pluripotent risk for psychopathology, interact with factors that play a critical role in differentiating forms of psychopathology at the onset of a disorder. Identifying these latter factors in high-risk individuals is critical for understanding the development of different forms of psychopathology that have distinct neural signatures.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
Preparation of this manuscript was supported by Grant MH80683 from the National Institute of Mental Health awarded to Ian H. Gotlib. The authors thank Emily Dennis, Juliana Gonzales, Kalpa Bhattacharjee, Arkadiy Maksimovskiy, and Maria Lemus for their help in data collection and preparation. Preparation of this manuscript was supported by Grant MH80683 from the National Institute of Mental Health awarded to Ian H. Gotlib.
Footnotes
1 Given that onset times of neural responding can affect the height of neural responses, in Supplementary Table S3 we present data on group differences in neural response onset times for POS and NEG stimuli.
REFERENCE
- Allman JM, Tetreault NA, Hakeem AY, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Structure and Function. 2010;214(5–6):495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Amir N, Foa EB, Coles ME. Automatic activation and strategic avoidance of threat-relevant information in social phobia. Journal of Abnormal Psychology. 1998a;107(2):285–90. doi: 10.1037//0021-843x.107.2.285. [DOI] [PubMed] [Google Scholar]
- Amir N, Foa EB, Coles ME. Negative interpretation bias in social phobia. Behaviour Research and Therapy. 1998b;36(10):945–57. doi: 10.1016/s0005-7967(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of Depression. New York: The Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beidel DC, Turner SM, Stanley MA, Dancu CV. The social phobia and anxiety inventory—concurrent and external validity. Behavior Therapy. 1989;20(3):417–27. doi: 10.1016/0005-7967(89)90093-4. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008;65(10):1176–84. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Mathews A. Negative self-schemata in clinical depression. British Journal of Clinical Psychology. 1983;22:173–81. doi: 10.1111/j.2044-8260.1983.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Bradley, M.M., Lang, P.J. (1999). Affective Norms for English Words (ANEW): instruction manual and affective ratings. Technical Report C-1. The Center for Research in Psychophysiology, University of Florida.
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47(4):1448–59. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human-behavior. Archives of Neurology. 1993;50(8):873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17(6):969–80. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. American Journal of Psychiatry. 2011;168(9):968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. American Journal of Psychiatry. 2008;165(3):342–51. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM–IV–TR Axis I Disorders. New York: State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilini M, Derenzi E, Crisi G. Bilateral paramedian thalamic artery infarcts—Report of 8 cases. Journal of Neurology Neurosurgery and Psychiatry. 1987;50(7):900–9. doi: 10.1136/jnnp.50.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Jonides J, Buschkuehl M, Joormann J. Memory for affectively valenced and neutral stimuli in depression: evidence from a novel matching task. Cognition and Emotion. 2011;25(7):1246–54. doi: 10.1080/02699931.2010.538374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Scott LA, Hiller JB, Yurgelun-Todd DA. Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biological Psychiatry. 2005;57(7):809–12. doi: 10.1016/j.biopsych.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions' of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Stang P, Wittchen HU, Stein M, Walters EE. Lifetime co-morbidities between social phobia and mood disorders in the US National Comorbidity Survey. Psychological Medicine. 1999;29(3):555–67. doi: 10.1017/s0033291799008375. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. The epidemiology of depression. In: Gotlib IH, Hammen CL, editors. The Handbook of Depression. New York: Guildord Press; 2009. [Google Scholar]
- Liao W, Chen HF, Feng YA, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52(4):1549–58. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: a model centered on anhedonia. Journal of Affective Disorders. 1996;41(1):39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Loas G, Salinas E, Guelfi JD, Samuellajeunesse B. Physical anhedonia in major depressive disorder. Journal of Affective Disorders. 1992;25(2):139–46. doi: 10.1016/0165-0327(92)90076-i. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113(1):160–5. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10(2):193–9. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. Journal of Personality and Social Psychology. 1999;77(5):1061–72. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Social phobia and depression: prevalence and comorbidity. Journal of Psychosomatic Research. 2010;68(3):235–43. doi: 10.1016/j.jpsychores.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Okasha A, Elakabawi AS, Snyder KS, Wilson AK, Youssef I, Eldawla A. Expressed emotion, perceived criticism, and relapse in depression—a replication in an Egyptian community. American Journal of Psychiatry. 1994;151(7):1001–5. doi: 10.1176/ajp.151.7.1001. [DOI] [PubMed] [Google Scholar]
- Peraud A, Meschede M, Eisner W, Ilmberger J, Reulen HJ. Surgical resection of grade II astrocytomas in the superior frontal gyrus. Neurosurgery. 2002;50(5):966–75. doi: 10.1097/00006123-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166(6):702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. Journal of Neuroscience. 1998;18(21):8979–89. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Wisniewski SR, Warden D, et al. Selecting among second-step antidepressant medication monotherapies. Archives of General Psychiatry. 2008;65(8):870–81. doi: 10.1001/archpsyc.65.8.870. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry. 2001;62:10–17. [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan ZZ, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: Assessment of sustained event-related fMRI amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Massat I, et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. Journal of Clinical Psychiatry. 2007;68(7):1062–70. doi: 10.4088/jcp.v68n0713. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011;16(6):664–71. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery TJ, Chun MM, Lee D. Ubiquity and specificity of reinforcement signals throughout the human brain. Neuron. 2011;72(1):166–77. doi: 10.1016/j.neuron.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Hamilton JP, Chen MC, Joormann J, Gotlib IH. Neural temporal dynamics of stress in comorbid major depressive disorder and social anxiety disorder. Biology of Mood and Anxiety Disorders. 2012;2:11. doi: 10.1186/2045-5380-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Hamilton JP, Gotlib IH. The neural temporal dynamics of the intensity of emotional experience. Neuroimage. 2010;49(2):1699–707. doi: 10.1016/j.neuroimage.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Clark DM, Salkovskis P, Ludgate J, Hackmann A, Gelder M. Social phobia - the role of in-situation safety behaviors in maintaining anxiety and negative beliefs. Behavior Therapy. 1995;26(1):153–61. doi: 10.1016/j.beth.2016.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.