Abstract
Brain reward systems mediate liking and wanting for food reward. Here, we explore the differential involvement of the following structures for these two components: the ventral and dorsal striatopallidal area, orbitofrontal cortex (OFC), anterior insula and anterior cingulate. Twelve healthy female participants were asked to rate pleasantness (liking of food and non-food odors) and the desire to eat (wanting of odor-evoked food) during event-related functional magnetic resonance imaging (fMRI). The subjective ratings and fMRI were performed in hunger and satiety states. Activations of regions of interest were compared as a function of task (liking vs wanting), odor category (food vs non-food) and metabolic state (hunger vs satiety). We found that the nucleus accumbens and ventral pallidum were differentially involved in liking or wanting during the hunger state, which suggests a reciprocal inhibitory influence between these structures. Neural activation of OFC subregions was correlated with either liking or wanting ratings, suggesting an OFC role in reward processing magnitude. Finally, during the hunger state, participants with a high body mass index exhibited less activation in neural structures underlying food reward processing. Our results suggest that food liking and wanting are two separable psychological constructs and may be functionally segregated within the cortico-striatopallidal circuit.
Keywords: brain reward systems, liking and wanting, food odors, metabolic state, body mass index
INTRODUCTION
Brain reward processing and pleasurable experiences are ubiquitous in human behaviors such as tasting and eating food (Small et al., 2001), smelling odorants (Royet et al., 2000), listening to music (Menon and Levitin, 2005), reacting to sexual stimulation (Demos et al., 2012), winning games or money (Rademacher et al., 2010) and engaging in affiliative interactions (Krach et al., 2010). Moreover, abnormalities or alterations in reward processing are related to a range of disorders, including obesity (Stice et al., 2013) and psychiatric disorders (e.g., affective and eating disorders, schizophrenia) (Der-Avakian and Markou, 2012; Dichter et al., 2012; DiLeone et al., 2012). For example, anhedonia (i.e., the decreased response to pleasurable stimuli) and avolition (the lack of motivation) are considered primary features of major depression and schizophrenia (Der-Avakian and Markou, 2012). Previous studies on reward-related brain activation in healthy adults have identified the neural structures [e.g., nucleus accumbens (NAc), orbitofrontal cortex (OFC), anterior cingulate] that are either common or distinct to the various phases (anticipatory vs consummatory), stages (evaluation, decision making and outcome) or properties (magnitude, probability, uncertainty and valence) of reward processing (Liu et al., 2011; Diekhof et al., 2012; Kühn and Gallinat, 2012). However, few studies have focused on the psychological components of these reward processes. The incentive salience theory on taste and ingestive behavior (Berridge, 1996, 2009) suggests that food reward can be operationalized in a motivational component called ‘wanting’ (subsuming the attribution of incentive salience to a reward) and an affective component named ‘liking’ (subsuming the hedonic impact of reward). Although these two components are underlain by common neural correlates localized in the mesocorticolimbic regions, such as the NAc and the ventral pallidum (VP), they also involve distinct neural mechanisms. Liking is primarily driven by restricted sites of the striatopallidal circuit (opioid hotspots in the NAc and VP), whereas wanting is more widely distributed within the mesolimbic circuit (ventral tegmental area, NAc, dorsal striatum and VP) (Berridge, 1996, 2009; Smith et al., 2009; Richard et al., 2012). This dual-process model of brain reward has been fruitful in demonstrating that drug addiction involves the neural sensitization of the dopaminergic system that assigns incentive salience to stimuli without necessarily affecting hedonic responsiveness (Robinson and Berridge, 2003). However, because these findings were based on neurochemical manipulations in rats, they may not be generalizable to primates in which neocortical integration is maximized. In humans, neuroimaging studies have primarily focused on the neural correlates of pleasantness judgment for a variety of stimuli (taste, odor, face, music, painting and financial reward), revealing regions that are associated with reward (medial OFC and ventral striatum) (Kringelbach, 2005; Liu et al., 2011; Kühn and Gallinat, 2012). Because liking and wanting typically occur quasi-simultaneously, they are highly correlated and difficult to disentangle in humans (Havermans, 2011; Finlayson and Dalton, 2012). In the present study, subjective measures of liking and wanting were used to compare the respective neural correlates of affective and motivational components of reward-related behaviors.
To date, only one study has focused on the hemodynamic correlates of both wanting and liking. Exposing healthy participants to food pictures during functional magnetic resonance imaging (fMRI), Born et al. (2011) found that the posterior insula and cingulate were preferentially activated during liking and the striatum during wanting when they compared pre- and post-meal conditions. However, regions presumed to be specifically related to food wanting (hypothalamus) and to food liking (OFC) were not differentially activated by these components. Furthermore, it remains to be determined whether these results are vision specific.
Here, we used event-related fMRI in healthy participants who were required to rate liking or wanting of food (Food) and nonfood (NFood) odors before and after a meal. Food odors are potent stimuli that induce affective responses throughout the lifespan (Soussignan et al., 1999; 2005; Armstrong et al., 2007) and provide visceral conditioned cues and anticipatory signals for ingestion (Jansen et al., 2003; Soussignan et al., 2012). Based on previous findings (O'Doherty et al., 2003; Bragulat et al., 2010; Born et al., 2011), we hypothesized that the brain regions underlying these two psychological components would be partially overlapping (NAc and VP) and partially distinct for wanting (the hypothalamus and dorsal striatum) and liking (OFC). We further examined their differential involvement as a function of the subject’s metabolic state and body mass index (BMI).
MATERIALS AND METHODS
Participants
Twelve healthy right-handed women [mean age ± SD: 24.14 ± 3.06 years; range: 21.41–34.66 years] participated in the study. Their BMI was 21.45 ± 2.66 (range: 17.63–27.48). Participants were further screened for the absence of eating disorders using the Bulimic Inventory Test, Edinburgh (BITE) (Henderson and Freeman, 1987). The mean BITE score was 4.00 ± 3.22 (range: 0–9). The subjects tested here were part of a wider study including female patients with eating disorders. The study was conducted in accordance with the Declaration of Helsinki. See Supplementary Data for additional details.
Stimuli and materials
Fifty-six odorants were used: 28 for training purposes and 28 for the fMRI scanning session. For fMRI, odorants were composed of 14 Food and 14 NFood odorants (see Supplementary Data) and were presented to the participants using an airflow olfactometer, which allows the stimuli to be synchronized with breathing (Vigouroux et al., 2005). Details regarding the stimulating and recording systems of behavioral responses and physiological signals (breathing) are provided in Supplementary Material (sections ‘Stimuli’ and ‘Stimulating and recording materials’).
Experimental procedure
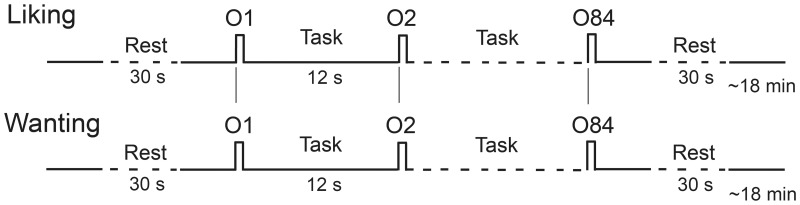
Two sessions were planned for each participant on two consecutive days; participants were alternatively scanned in hunger and satiety states (Figure 1). In each session, two functional runs were performed during which subjects successively reported their odor liking and wanting. A structural image acquisition sequence was performed between two functional runs during the first or the second day. During each run, 28 odorants were delivered three times each, such that 84 stimuli were presented. They were delivered according to an event-related fMRI design with a jittered interstimulus interval of ∼12 s, depending on the participant’s respiration. The orders of the two sessions and of the runs were counterbalanced across participants; the order of the presentation of odorants was randomized for each run.
Fig. 1.
Timeline of the procedure for a session. Each session performed in the morning (hunger state) or the afternoon (satiety state) included two runs during which liking and wanting tasks were performed, respectively. O, odor.
During the liking run, the participants were asked to press one of five buttons with the corresponding finger, depending on their liking judgment (thumb: very unpleasant; forefinger: unpleasant; middle finger: neutral; ring finger: pleasant; pinkie: very pleasant). During the wanting run, if the odor evoked food, the participants were asked to press one of five buttons, depending on their desire to eat the food evoked by the odor (not at all, not desired, just a little, much desired, urge). If the odor did not evoke food, they did not press a button (see Supplementary Data for details concerning instructions, training of subjects and experimental conditions for the fasted and satiated states).
Behavioral data analysis
Because liking and wanting scores are correlated (Jiang et al., 2008, 2013), we performed a multivariate analysis of variance (MANOVA) using Task (liking vs wanting) and State (hunger vs satiety) factors with repeated measurements on the Odorant factor (Winer et al., 1991). Analyses of variance (ANOVA) with repeated measurements were then used to separately analyze the scores derived from the judgment tasks. The differences between pairs or groups of means were assessed using multiple orthogonal contrasts. Regression analyses were performed between BMI and liking/wanting ratings.
Functional and structural data acquisition and pre-processing
Images were acquired using a 1.5-Tesla MAGNETOM Sonata whole-body imager (Siemens Medical®, Erlangen, Germany) equipped with a 4-channel circularly polarized head coil. For functional imaging, we obtained 26 interleaved, 4-mm-thick axial slices using a T2*-weighted echo-planar sequence with the following parameters: repetition time (TR) = 2500 ms, echo time (TE) = 50 ms, flip angle (FA) = 80°, field-of-view (FOV) = 240 × 240 mm2 and imaging matrix = 64 × 64 (voxel size: 3.75 × 3.75 × 4 mm3). In total, 460 scans were collected for each functional run. A high-resolution structural T1-weighted anatomical image (inversion-recovery 3D Gradient-Echo sequence, 1 × 1 × 1 mm3) parallel to the bicommissural plane and covering the entire brain was acquired over ∼10 min. Foam wedges were used to restrict head motion. An oil-filled capsule was fixed on the right temple to subsequently locate the right side of the images.
We processed all functional images using Statistical Parametric Mapping software (SPM5, Wellcome Department of Cognitive Neurology, London, UK) (Friston et al., 1995). Additional details considering pre-processing are given in Supplementary Methods (Functional data analysis).
Functional data analysis
For each subject, activation associated with six conditions of interest [Category (Food, NFood), State (Hunger, Satiety) and Task (Liking, Wanting)] was modeled using boxcar predictors convolved with both the canonical hrf and its time derivative (Friston et al., 1998; Hopfinger et al., 2000). A high-pass filter (cutoff frequency of 1/120 Hz) was used to eliminate instrumental and physiological signal fluctuations at very low frequencies. Stimulus onset asynchronies were fixed at the time of odor delivery. Confounding factors (head motion) were included in the model. No participant moved more than 3 mm in any direction within or across runs. Thus, no data were eliminated due to motion artifacts. Random-effects analyses were performed to extrapolate statistical inferences at the population level, as described in the SPM5 software. Whole-brain analyses were performed on functional images for the different experimental conditions. Activation common to six conditions (Liking, Wanting, Hunger, Satiety, Food and NFood) was determined by a conjunction (intersection) of the simple contrasts. Because only liking was rated for both Food and NFood odors, we performed two types of contrast analyses. First, we contrasted activation functional images between liking and wanting in the hunger and satiety states for Food odors only (liking vs wanting). Second, we contrasted activation images between Food and NFood conditions in the hunger and satiety states for the liking task only (Food vs NFood). For those analyses, the level of significance was set at P < 0.005, uncorrected at the cluster level for multiple comparisons across the much larger volume of the whole brain. We used an extent threshold (k) superior or equal to 5 adjacent activated voxels.
Analyses were then performed on brain regions of interest known to be involved in olfactory and food reward processing (Mawlawi et al., 2001; Ongur et al., 2003; Craig, 2005; Kringelbach, 2005; Vogt, 2005). These volumes of interest (VOIs), which were subdivided into subregions, were the OFC (four areas), anterior insula (two areas), anterior cingulate gyrus (two areas), hypothalamus and ventral and dorsal striatopallidum (VDSP, six areas). They were drawn from the MNI template (Ch2better.nii) using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) and human brain atlases (Duvernoy, 1999; Mai et al., 2008). Additional details are provided in Supplementary Data (‘VOI definition’).
The mean activation signals were extracted in the different experimental conditions (Task, Category and State) for each subregion of VOIs and for each of the 12 participants using the MarsBar toolbox (http://marsbar.sourceforge.net; Brett, Anton, Valabregue, Poline, 2002). Repeated measures ANOVA and mean comparisons were then performed to compare the levels of activation as a function of different conditions (Category, State, Task and Side). Because liking was rated for both Food and NFood odors, but wanting for food odors only, two series of statistical analyses for each VOI were performed. To compare liking–wanting activation, a three-way (Task × State × Subregion) ANOVA with repeated measures was performed for Food odors alone. To compare Food–NFood activation, a three-way (Category × State × Subregion) ANOVA with repeated measures was performed for data recorded in the liking task alone. Brain linear regression analyses were further performed to evaluate whether VOI-activation data were dependent on self-rated liking/wanting data and on anthropometric data (BMI). According to our hypotheses, this assessment was performed for certain areas of the VDSP and the OFC only.
RESULTS
Behavioral and physiological data
Metabolic state. The hunger scores that were collected at the beginning and the end (Time factor) of each fMRI scan session were 5.63 ± 2.18 and 7.05 ± 2.33, respectively, when subjects were tested in the hunger state, and 1.58 ± 0.79 and 2.05 ± 1.29, respectively, when they were tested in the satiety state. The hunger scores were significantly higher in the pre-meal than post-meal period [F(1, 11) = 74.44, P < 0.001], ascertaining that the participants were in the hunger and satiated states, respectively (see Supplementary Results). No significant Time effect and no significant Time × State interaction were noted.
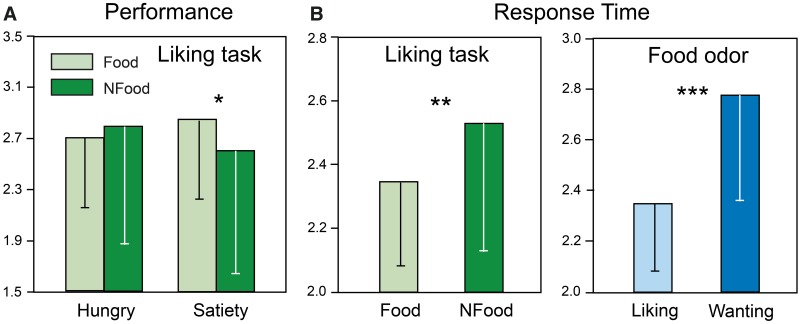
Liking and wanting scores as a function of metabolic state. The mean liking scores were determined in the hunger and satiety states for Food and NFood odors. ANOVAs revealed a significant State × Category interaction [F(1, 11) = 8.31, P = 0.015] due to a lower liking score for Food than for NFood odors in the satiety state (Figure 2A). A significant State × Category × Odor interaction [F(13, 143) = 2.17, P = 0.014] indicated that liking scores were higher in the hunger than in the satiety state for several Food odors [beef (P = 0.029), Gruyère (P = 0.049), potato (P = 0.037), pizza (P = 0.029) and shellfish (P = 0.049)] and were lower in the hunger than in the satiety state for smoked bacon (P = 0.016). For NFood odors, the liking scores indicated less pleasantness for camphor and lavender in the hunger than in the satiety state (P = 0.002 and 0.029, respectively). Further analyses concerning Food odors are provided in Supplementary Results.
Fig. 2.
A) Mean scores of liking ratings for Food and NFood odors as a function of the metabolic state; and (B) mean response times for Food and NFood odors in the liking task and for liking and wanting ratings for Food odors. Vertical bars: standard deviations; *P < 0.05; **P < 0.01; ***P < 0.001.
Liking response times as a function of metabolic state. Response time (RT) was defined as the interval between odorant delivery and the subject’s response. First, they were determined in the hunger and satiety states for Food and NFood odors during the liking task. A two-way ANOVA (State × Category) with repeated measures on the two factors indicated a significant effect of Category due to lower RTs for Food than for NFood odors (Figure 2B; F(1, 11) = 9.69, P = 0.010]. Second, we compared RTs between liking and wanting tasks for Food odors only (Figure 2B). A MANOVA indicated significant main effects for Task [Roy’s GR(13, 32) = 1.99, P = 0.0565] and Odor [Roy’s GR(13, 32) = 4.81, P < 0.001] factors but not for State [Roy’s GR(13, 32) = 0.78, P = 0.67], and no significant Task × State interaction [Roy’s GR(13, 32) = 0.24, P = 0.99]. A two-way ANOVA (Task × Odor) with repeated measures on the two factors indicated significant main effects for Task [F(1, 11) = 36.10, P < 0.001] and Odor [F(13, 143) = 3.81, P < 0.001] factors and a significant Task × Odor interaction [F(13, 143) = 2.13, P = 0.016]. Mean comparisons further indicated that RTs were significantly higher in wanting than liking tasks for bitter almond, banana, peanut, strawberry, blue cheese, Gruyère, smoked bacon, shellfish and Pâté (at least P < 0.05).
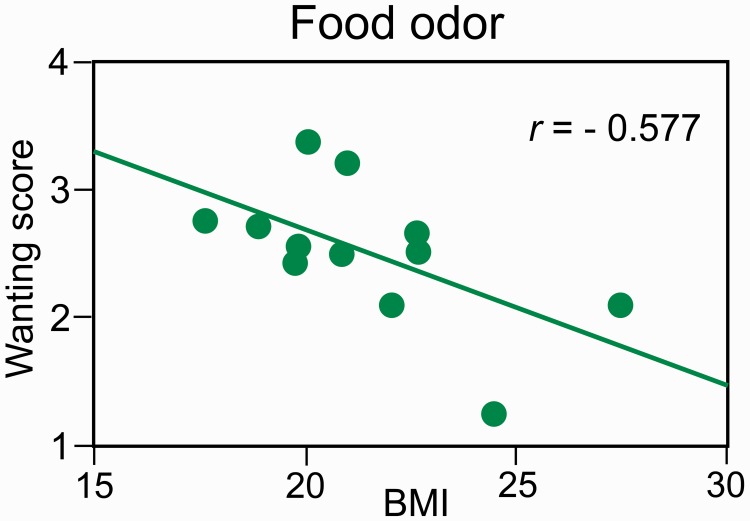
Liking/wanting scores as a function of BMI and BITE scores. Only the wanting scores for Food odors in the hunger state were correlated with BMI [Figure 3; r = −0.577, F(1, 10) = 5.00, P = 0.049]. The liking scores were correlated with the wanting scores in the hunger state for Food odors [r = 0.748, F(1, 10) = 12.70, P = 0.005]. BMI and BITE scores were not significantly correlated [r = 0.376, F(1, 10) = 1.64, P = 0.22].
Fig. 3.
Negative relationship between wanting scores elicited by Food odors and BMI values.
Impact of sniffing. Details are provided in Supplementary Data.
Cerebral imaging data
All results concerning whole-brain analyses are presented in Supplementary Results (fMRI data section). The following results concern analyses applied to VOIs.
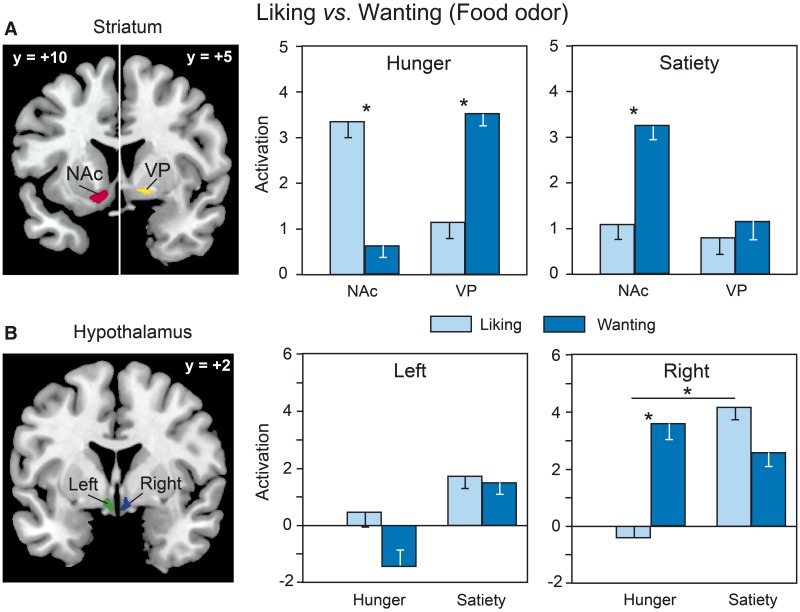
Liking and wanting activation for Food odors. When we compared the activation signals between liking and wanting tasks for Food odors, we found a significant Area × Task × State interaction [F(5, 55) = 2.35, P = 0.05] for the VDSP (Figure 4). This interaction is primarily due to a higher activation in the NAc for liking than for wanting during the hunger state (P = 0.01) and for wanting than for liking during the satiety state (P = 0.05); it further reflects a higher activation in the VP for wanting than for liking during the hunger state (P = 0.03). We also found a marginally significant Side × Task × State interaction in the hypothalamus [F(1, 11) = 4.03, P = 0.07], indicating a greater right-sided activation during the wanting than the liking task in the hunger state (P = 0.049).
Fig. 4.
Differential activation for Food odors as a function of the reward task (liking vs wanting) and the metabolic state (hunger vs satiety) in (A) the NAc and VP; and (B) the left and right hypothalamus. VOI mappings are superimposed on coronal sections of the standard MNI brain.
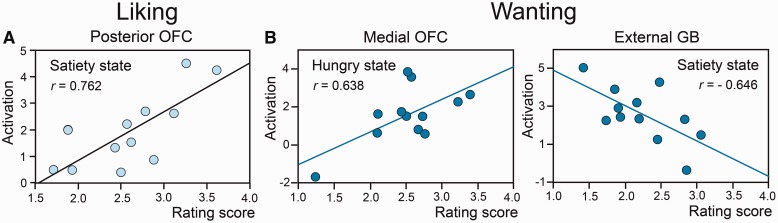
For the liking task, the brain activation was positively and bilaterally correlated with the ratings of subjective pleasantness in the posterior OFC in the satiated state [r = 0.762, F(1, 10) = 13.86, P = 0.004] (Figure 5A). For the wanting task, the wanting scores were positively correlated with activation in the hunger state in the left medial OFC [r = 0.638, F(1, 10) = 6.85, P = 0.026] (Figure 5B). Furthermore, they were negatively correlated with activation in the external globus pallidus (GP) in the satiety state [r = −0.646, F(1, 10) = 7.17, P = 0.023; Figure 5B].
Fig. 5.
Relationships observed for Food odors between (A) the liking scores and the level of activation recorded in the bilateral posterior OFC in the satiated state; and (B) the wanting scores and the level of activation recorded in the left OFC in the hunger state and the bilateral external GP in the satiety state.
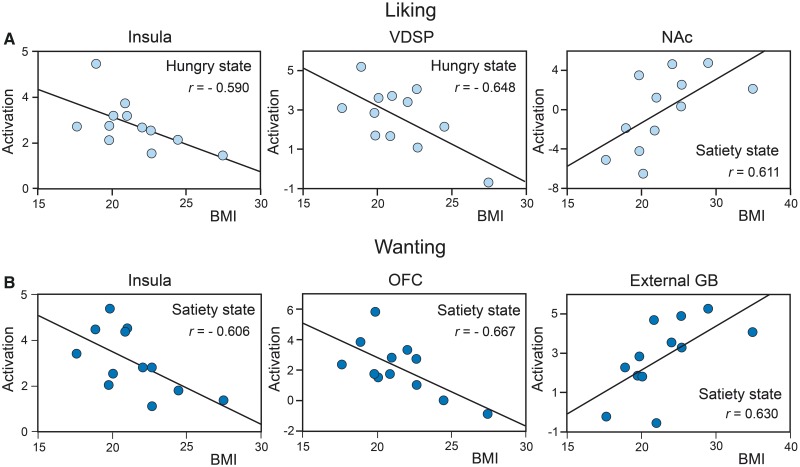
Liking and wanting activations as a function of BMI. In the hunger state, the liking task-related brain activation for Food odors was negatively correlated with BMI in the left insula [r = −0.590, F(1, 10) = 5.35, P = 0.043] and in the VDSP [r = −0.648, F(1, 10) = 7.23, P = 0.023; Figure 6A]. In the satiated state, it was positively correlated with BMI in the left NAc [r = 0.611, F(1, 10) = 5.95, P = 0.035; Figure 6A].
Fig. 6.
Significant correlations between the BMI and the level of activation recorded for Food odors in (A) the liking task in the left insula and the VDSP in the hunger state and in the left NAc in the satiety state; and (B) the wanting task in the left insula, the left OFC and the right external GP in the satiety state.
Brain activation during the wanting task for Food odors in the satiety state (Figure 6B) was negatively correlated with BMI in the left insula [r = −0.606, F(1, 10) = 5.80, P = 0.037] and in the left OFC [r = −0.667, F(1, 10) = 8.02, P = 0.018] and was positively correlated with BMI in the right external GP (r = 0.630, F(1, 10) = 6.58, P = 0.028].
Liking activation as a function of odor category. In the liking task, a significant main effect of odor Category was detected in the anterior cingulate [F(1, 11) = 8.29, P = 0.015] reflecting a greater activation for Food than NFood odors. For the VDSP, a significant Area × Side × Category interaction [F(5, 55) = 3.13, P = 0.015] was found due to a higher activation in the right VP (P = 0.018) and the right internal GP (P = 0.025) for NFood than Food odors and in the NAc (P = 0.048) for Food than NFood odors.
DISCUSSION
We investigated whether the explicit evaluation of hedonic (liking) and motivational (wanting) components of food reward was functionally segregated within the cortico-striatopallidal network in participants exposed to odorants during pre- and post-prandial states. We provide evidence that the liking and wanting of various foodstuffs that are evoked by olfactory cues are underlain by the activation of brain regions that were previously associated with the reward system (NAc, VP and OFC). Furthermore, the contrast in the activation signal between liking and wanting tasks and its correlates with subjective evaluation indicated a partial dissociation within the cortico-striatopallidal circuit that was dependent on the participants’ metabolic state (hunger vs satiety) and BMI.
Liking and wanting for food odors
Liking and wanting, which were rated during separate tasks, were discriminated by the participants because they reacted more rapidly to evaluate odor-evoked pleasantness than the desire to eat and because some odorants scored higher in the liking than wanting task. Thus, our procedure was sufficiently valid to differentiate the two psychological constructs.
The analysis of activation signals highlights that core regions of the ventral striatopallidum (NAc and VP) were differentially recruited in the liking and wanting tasks, whereas we did not observe such differences in its dorsal counterparts (putamen, caudate nucleus and GP). Furthermore, the pattern of activation within the VDSP was task dependent in the hunger state: it was significantly higher in the NAc for liking than for wanting but higher in the VP for wanting than for liking. Additionally, satiety induced a greater activity in the NAc for wanting than for liking. These results differ, in part, from those obtained by Born et al. (2011). These authors, using food pictures as stimuli, found a higher activation in the posterior insula and cingulate for liking and in the striatum for wanting; they also found an increase in NAc activity for both liking and wanting, but only as a function of dietary restraint. Whether this disparity between studies reflects modality-specific differences (olfaction vs vision) or other procedural discrepancies remains to be clarified. However, regarding hedonic processing, our findings concur with numerous neuroimaging studies demonstrating that pleasantness ratings of various sensory rewards (odors, tastes, food images, music, faces and paintings) increase activity in the NAc/ventral striatum (Kühn and Gallinat, 2012; Simmons et al., 2014). From regression analyses of subjective ratings on brain activity, we further observed that participants who reported higher pleasure for food odors in the satiety state exhibited stronger activation in the posterior OFC, whereas those who reported more desire to eat odor-evoked food in the hunger state exhibited higher activation in the medial OFC. Collectively, our findings emphasize the critical involvement of the cortico-striatopallidal pathway in the valuation of liking and wanting and of their magnitude. How can this pattern of findings that indicate both an overlap and dissociation for liking and wanting be explained?
Striatopallidal dissociation. The NAc and VP reactivities to liking and wanting valuation were dependent on the metabolic state. First, in the hunger state when the reward value of food odors is high, the higher NAc activation during liking than wanting may be due to the direct connection between the primary olfactory cortex and the NAc (Newman and Winans, 1980; Price, 2004; van Hartevelt and Kringelbach, 2012), and the close anatomical coupling between olfactory and emotional/hedonic processes (Royet et al., 2000; 2003). Regarding the preferential involvement of the VP in wanting, we note that this structure represents a central point for both the NAc output and input from other reward-related structures (e.g., OFC, lateral hypothalamus, ventral tegmental area) (Smith et al., 2009). Additionally, the VP is considered essential for integrating motor, affective, motivational and cognitive signaling pathways to gain external rewards (i.e., wanting) (Mogenson et al., 1989; Mogenson and Yang, 1991; Smith et al., 2009). Thus, when participants were requested to report their desire to eat the odor-evoked food, their rating was potentially first influenced, at least implicitly, by the odor-evoked palatability of the foods and the consequences (pleasant vs unpleasant) of their intake. However, as the rating task focused on wanting rather than liking, we may speculate that the VP, as a ‘limbic final common pathway’ in food reward (Smith et al., 2009), was activated to a greater extent because it is involved in the conscious motivation to eat food. Our results also suggest that the differential striatopallidal activation between the NAc and VP during the liking and wanting tasks may reflect an inhibitory reciprocal influence, such as that found in animal studies (Zahm, 2000; Smith et al., 2009; 2011).
Second, in the satiety state, we observed a higher activity in the NAc during food wanting than food liking. Although there is currently no clear explanation for this finding, animal studies indicate that NAc neurons may be activated to assign incentive value to palatable food in the hunger state (Ahn and Phillips, 1999) but may also reveal elevated levels of dopamine metabolites in satiety (Chance et al., 1987). A tentative interpretation would be that the NAc is involved in a devaluation process of food wanting during satiety. In the rat, the NAc is necessary to reinforce devaluation effects in Pavlovian conditioned tasks (Lex and Hauber, 2010; Singh et al., 2010), and kappa, but not mu, opioid agonists in the NAc were shown to reverse the devaluation (satiety) effect of pre-feeding for a given flavor (Woolley et al., 2007). Thus, depending on the metabolic state, opposite effects can be observed in the NAc through the mobilization of different receptors.
Orbitofrontal dissociation. The coding of liking or wanting for food odors appeared heterogeneous in the OFC. Liking scores during satiety were positively correlated with the posterior OFC activity, whereas the wanting scores during the hunger state were positively correlated with the medial OFC activity alone. First, this distinct activation pattern between liking and wanting as a function of the internal state may be related to the fact that food wanting was more state dependent than food liking, as has been previously found (Jiang et al., 2008; Born et al., 2011). Second, the human OFC is a key structure of reward processing. On the one hand, the posterior OFC, which is the major target of the primary olfactory cortex (Gottfried and Zald, 2005), is activated by hedonically contrasted odors (Gottfried et al., 2002). On the other hand, reward processing in the medial OFC has been related to not only liking (positive valence or pleasantness rating; Rolls et al., 2003; Kringelbach, 2005; Liu et al., 2011; Kühn and Gallinat, 2012) but also wanting (O'Doherty et al., 2003; Piech et al., 2009). For example, Piech et al. (2009) reported stronger responses in the medial OFC to high vs low levels of attractiveness of food menu items in the hunger state. This suggests that the medial OFC may preferentially represent the subjective incentive value (wanting) of food reward by integrating not only the motivational salience of the reward (attractiveness) but also the motivational state of the subject.
Hypothalamic activation. It is well established that the hypothalamus is critical in the control of food intake (wanting) and energy balance (Berridge and Valenstein, 1991; Berthoud, 2007). We found greater activation in the hypothalamus for wanting than for liking during the hunger state, which is consistent with the results of Born et al. (2011), showing that the hypothalamus may be involved in reward processes. Interestingly, a growing body of evidence has highlighted the interplay between the hypothalamus and mesolimbic reward pathways. For example, leptin, which acts to signal satiety at the hypothalamic level, may also inhibit dopamine signaling in the NAc by binding to receptors on dopaminergic neurons in the ventral tegmental area (Hommel et al., 2006; Grosshans et al., 2012). Further, ghrelin, which acts as a hunger signal in the hypothalamus, may increase fMRI responses in reward-related brain regions (Malik et al., 2008).
Liking and wanting as a function of BMI
Our findings suggest a complex relationship among brain reward processing, motivational state (hunger vs satiety) and BMI. When hungry, higher-BMI subjects exhibited lower activation during liking in brain regions processing reward (VDSP and insula). This result supports the reward deficiency hypothesis that individuals who experience lower activation of reward circuitry through food cues may overeat to compensate for this deficit (Comings and Blum, 2000; Blum et al., 2012). Thus, the reward value and the pleasantness of food cues may be reduced in the striatopallidal pathways in overweight/obese individuals, and overeating would be one way to obtain reward. This view is consistent with fMRI studies in overweight/obese women exposed to food (Stice et al., 2009, 2010) or in obese adolescents exposed to food advertisements on television (Gearhardt et al., 2014). Furthermore, it is consistent with neuroimaging studies that demonstrated decreased basal D2 receptor availability in the dorsal striatum of obese individuals (Wang et al., 2001; Volkow et al., 2008).
During satiety, subjects with a higher BMI exhibited either lower activation in the left insula and OFC during wanting or higher activation in the left NAc and the right external GP during liking and wanting, respectively. These results were not consistent with those observed by Born et al. (2011) who found that normal-weight subjects with a higher BMI exhibited a lower wanting-related change of activation in the striatum (the putamen and GP) and anterior insula during wanting alone. The origin of these divergent results between studies is unclear but may, again, reflect procedural differences. In contrast, we observed that higher-BMI participants exhibited stronger striatal activation (the NAc, external GP) during liking and wanting tasks, suggesting that the reward value of food odors in these structures is higher in the satiety state in women who are at higher risk of being overweight/obese. This finding is consistent with recent evidence indicating that, after eating, the dorsal striatum activation in overweight/obese individuals in response to low-energy food is elevated and positively correlated with impaired satiety scores (Ho et al., 2012). Thus, this result suggests that an alteration of interoceptive perception may influence target regions of the reward circuit.
Food vs NFood odors during liking
A greater activation in the NAc and the anterior cingulate for Food than NFood odors in the liking task is consistent with previous studies in olfaction (Bragulat et al., 2010) and confirms that the NAc plays a critical role in hedonic coding of appetitively conditioned olfactory cues. Specifically, the ventral part of the anterior cingulate receives projections from the NAc/ventral striatum and is primarily involved in assessing the salience of emotional/motivational information and in coding the pursuit of expected future hedonic rewards for reward decision (Bush et al., 2000; Allman et al., 2001).
Limitations of the study
A number of issues are addressed in Supplementary Discussion, as they may constitute limitations of the present study. They concern the measurement of incentive salience, the method used to control for anatomical variability between subjects, the impact of pleasantness on differential OFC activity between Food and NFood odors and the possible effect of sensory-specific satiety.
Conclusion
The present study demonstrates that odors are relevant sensory probes to gain insight into the functional heterogeneity of food reward processing within the cortico-striatopallidal circuitry. The explicit valuation of liking and wanting distinctly recruits core regions involving the NAc, VP and OFC. This result sheds new light on the current debate on the difficulty of disentangling the brain circuit underlying food reward processes in humans (Finlayson and Dalton, 2012; Havermans, 2012). Although our finding of an inverse relationship between BMI and brain reward processing cannot specify the nature of the causal mechanisms, it is consistent with studies suggesting that overeating is a risk factor through its impact on the functional properties of the reward circuit.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank all the members of CERMEP (particularly D. Ibarrola, C. Vighi and F. Vey) for their invaluable assistance. We are also greatly indebted to S. Garcia for designing the software (with Matlab) to analyze the behavioral and breathing data. The authors gratefully thank all participants for their availability and patience in addition to the companies Mane, René Laurent, Arômes & Parfums, International Flavor & Fragrances, Givaudan-Roure and Lenoir who provided the odorants that were used as stimuli. This study was funded by the Regional Council of Burgundy (grant No. 04B-9); www.region-bourgogne.fr. Centre National de la Recherche Scientifique, www.cnrs.fr.
REFERENCES
- Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. Journal of Neuroscience. 1999;19:RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–17. [PubMed] [Google Scholar]
- Armstrong JE, Hutchinson I, Laing DG, Jinks AL. Facial electromyography: responses of children to odor and taste stimuli. Chemical Senses. 2007;32:611–21. doi: 10.1093/chemse/bjm029. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and Biobehavioral Reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiology and Behavior. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Valenstein ES. What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behavioral Neuroscience. 1991;105:3–14. doi: 10.1037//0735-7044.105.1.3. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiology and Behavior. 2007;91:486–98. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Current Pharmaceutical Design. 2012;18:113–18. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born JM, Lemmens SG, Martens MJ, Formisano E, Goebel R, Westerterp-Plantenga MS. Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. American Journal of Clinical Nutrition. 2011;94:392–403. doi: 10.3945/ajcn.111.012161. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Bruno C, et al. Food-related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity. 2010;18:1566–71. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chance WT, Foley-Nelson T, Nelson JL, Fischer JE. Neurotransmitter alterations associated with feeding and satiety. Brain Research. 1987;416:228–34. doi: 10.1016/0006-8993(87)90901-2. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–41. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends in Cognitive Science. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. Journal of Neuroscience. 2012;32:5549–52. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. Journal of Neurodevelopmental Disorders. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–66. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nature Neuroscience. 2012;15:1330–35. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain Surface. Three Dimensional Sectional Anatomy and MRI. 2nd edn. New York: Springer; 1999. [Google Scholar]
- Finlayson G, Dalton M. Current progress in the assessment of ‘liking’ vs. ‘wanting’ food in human appetite. Comment on ‘“You say it’s liking, i say it’s wanting …”. On the difficulty of disentangling food reward in man’. Appetite. 2012;58:373–78. doi: 10.1016/j.appet.2011.10.011. discussion 252–55. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith C, Poline JB, Healther JD, Frackowiak RS. Spatial Registration and Normalization of Images. Human Brain Mapping. 1995;2:165–89. [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magnetic Resonance in Medecine. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience. 2014;9(7):932–38. doi: 10.1093/scan/nst059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 2002;22:10819–28. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Research Reviews. 2005;50:287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Grosshans M, Vollmert C, Vollstadt-Klein S, et al. Association of leptin with food cue-induced activation in human reward pathways. Archives of General Psychiatry. 2012;69:529–37. doi: 10.1001/archgenpsychiatry.2011.1586. [DOI] [PubMed] [Google Scholar]
- Havermans RC. “You Say it's Liking, I Say it's Wanting …”. On the difficulty of disentangling food reward in man. Appetite. 2011;57:286–94. doi: 10.1016/j.appet.2011.05.310. [DOI] [PubMed] [Google Scholar]
- Havermans RC. How to tell where ‘liking’ ends and ‘wanting’ begins. Appetite. 2012;58:252–55. [Google Scholar]
- Henderson M, Freeman CP. A self-rating scale for bulimia. The ‘BITE’. British Journal of Psychiatry. 1987;150:18–24. doi: 10.1192/bjp.150.1.18. [DOI] [PubMed] [Google Scholar]
- Ho A, Kennedy J, Dimitropoulos A. Neural correlates to food-related behavior in normal-weight and overweight/obese participants. PLoS One. 2012;7:e45403. doi: 10.1371/journal.pone.0045403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buchel C, Holmes AP, Friston KJ. A study of analysis parameters that influence the sensitivity of event-related fMRI analyses. Neuroimage. 2000;11:326–33. doi: 10.1006/nimg.2000.0549. [DOI] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, et al. Overweight children overeat after exposure to food cues. Eating Behaviors. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Jiang T, Schaal B, Boulanger V, Kontar F, Soussignan R. Children's reward responses to picture- and odor-cued food stimuli: a developmental analysis between 6 and 11years. Appetite. 2013;67:88–98. doi: 10.1016/j.appet.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Jiang T, Soussignan R, Rigaud D, et al. Alliesthesia to food cues: heterogeneity across stimuli and sensory modalities. Physiology and Behavior. 2008;95:464–70. doi: 10.1016/j.physbeh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Krach S, Paulus FM, Bodden M, Kircher T. The rewarding nature of social interactions. Frontiers in Behavioral Neuroscience. 2010;4:22. doi: 10.3389/fnbeh.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews: Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. The neural correlates of subjective pleasantness. Neuroimage. 2012;61:289–94. doi: 10.1016/j.neuroimage.2012.02.065. [DOI] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of nucleus accumbens dopamine in outcome encoding in instrumental and Pavlovian conditioning. Neurobiology of Learning and Memory. 2010;93:283–90. doi: 10.1016/j.nlm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edn. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metabolism. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–84. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M, Tsai CT. Subpallidal-pedunculopontine projections but not subpallidal-mediodorsal thalamus projections contribute to spontaneous exploratory locomotor activity. Brain Research. 1989;485:396–98. doi: 10.1016/0006-8993(89)90584-2. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Advances in Experimental Medicine and Biology. 1991;295:267–90. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Newman R, Winans SS. An experimental study of the ventral striatum of the golden hamster. II. Neuronal connections of the olfactory tubercle. Journal of Comparative Neurology. 1980;191:193–212. doi: 10.1002/cne.901910204. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23:7931–39. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Piech RM, Lewis J, Parkinson CH, et al. Neural correlates of appetite and hunger-related evaluative judgments. PLoS One. 2009;4:e6581. doi: 10.1371/journal.pone.0006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. Olfaction in The Human Nervous System. UK: Elsevier Academic Press; 2004. [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews. 2012;37:1919–31. doi: 10.1016/j.neubiorev.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, De Araujo IET. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20:713–28. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. Journal of Neuroscience. 2000;20:7752–9. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Ingeholm JE, et al. The ventral pallidum and orbitofrontal cortex support food pleasantness inferences. Brain Structure and Function. 2014;219:473–83. doi: 10.1007/s00429-013-0511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, McDannald MA, Haney RZ, Cerri DH, Schoenbaum G. Nucleus accumbens core and shell are necessary for reinforcer devaluation effects on pavlovian conditioned responding. Frontiers in Integrative Neuroscience. 2010;4:126. doi: 10.3389/fnint.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–33. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E255–64. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural Brain Research. 2009;196:155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussignan R, Ehrle N, Henry A, Schaal B, Bakchine S. Dissociation of emotional processes in response to visual and olfactory stimuli following frontotemporal damage. Neurocase. 2005;11:114–28. doi: 10.1080/13554790590922513. [DOI] [PubMed] [Google Scholar]
- Soussignan R, Schaal B, Boulanger V, Gaillet M, Jiang T. Orofacial reactivity to the sight and smell of food stimuli. Evidence for anticipatory liking related to food reward cues in overweight children. Appetite. 2012;58:508–16. doi: 10.1016/j.appet.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Soussignan R, Schaal B, Marlier L. Olfactory alliesthesia in human neonates: prandial state and stimulus familiarity modulate facial and autonomic responses to milk odors. Developmental Psychobiology. 1999;35:3–14. doi: 10.1002/(sici)1098-2302(199907)35:1<3::aid-dev2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neuroscience and Biobehavioral Reviews. 2013;37:2047–58. doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiology and Behavior. 2009;97:551–60. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. Journal of Neuroscience. 2010;30:13105–9. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hartevelt TJ, Kringelbach ML. The olfactory system. In: Mai JK, Paxinos G, editors. The Human Nervous System. 3rd edn. Amsterdam: Academic Press; 2012. pp. 1219–38. [Google Scholar]
- Vigouroux M, Bertrand B, Farget V, Plailly J, Royet JP. A stimulation method using odors suitable for PET and fMRI studies with recording of physiological and behavioral signals. Journal of Neurocience Methods. 2005;142:35–44. doi: 10.1016/j.jneumeth.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews: Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1991. [Google Scholar]
- Woolley JD, Lee BS, Kim B, Fields HL. Opposing effects of intra-nucleus accumbens mu and kappa opioid agonists on sensory specific satiety. Neuroscience. 2007;146:1445–52. doi: 10.1016/j.neuroscience.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neuroscience and Biobehavioral Reviews. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.