Abstract
Functional magnetic resonance imaging (fMRI) of thoracic spinal cord neurons was used to examine the neural correlates of visceral emotional responses. Participants completed four spinal fMRI runs involving passive viewing (i.e. no movement) and motoric responses to negative or neutral images. Negative images, particularly in the movement condition, elicited robust activity in motoric nuclei, indicating ‘action preparedness’. These images also enhanced activity in autonomic and sensory nuclei, thus providing a clear neural representation of visceral responses to emotional stimuli.
Keywords: magnetic resonance imaging, functional MRI, spinal cord, thoracic spinal cord, emotion
A core feature of emotional experience is a subjective visceral response to a stimulus or event. These responses imbue events with a vividness that enhances perception (Phelps et al., 2006) and memory (Sharot et al., 2004), and that may influence decision making (Damasio, 1996; Ohira, 2010). However, bodily responses to emotions are not a unitary phenomenon; instead, they involve three components: (i) a motoric response involving the contraction of muscles; (ii) an autonomic nervous system response to release stored energy reserves; and (iii) a somatosensory response that leads to interoception, the interpretation of internal body states (Wiens, 2005).
Quantifying neural activity related to such phenomenologically complex experiences poses significant methodological challenges. Paramount among these is the difficulty in separating neural activity related to the bodily responses from activity associated with the cognitive interpretation of these responses. Previous research has successfully identified key structures related to the latter. Activation of the right insula has been consistently linked with interoception (Critchley et al., 2004). Additionally, the right dorsal anterior cingulate gyrus has been linked to the detection of autonomic nervous system responses such as changes in heart rate and blood pressure (Critchley et al., 2003). However, these brain regions are also involved with numerous cognitive functions, thus complicating the interpretation of such activations. In order to isolate neural activity related to bodily responses, it is necessary to acquire measures of central nervous system activity in regions that are not involved with attentional or cognitive processes.
One such region is the spinal cord, which has different sensory, motoric and autonomic functions but is not directly related to any cognitive process. The spinal cord is divided into four different regions—cervical, thoracic, lumbar and sacral—each of which is composed of a number of individual segments projecting to and receiving information from different regions of the body. Each of these segments consists of dorsal and ventral aspects, which correspond to sensory and motoric activity and, in the thoracic segments, a lateral aspect that corresponds to autonomic activity. Although often thought to be a simple structure, the patterns of activity in the spinal cord reflect a number of potentially interacting systems. Numerous separate descending pathways from the brain modulate motoric regions of the spinal cord, and similarly, multiple separate ascending pathways bring different types of somatosensory information back to the brain. In addition to these brain–spinal cord interactions, the spinal cord houses interneurons that project both intra-segmentally and inter-segmentally and that are involved in a number of reflexive responses that occur independent of the brain. Therefore, studies examining the excitability of a single descending pathway from the brain (e.g. the corticospinal tract) are measuring only a fraction of the activity occurring in the spinal cord. Examining activity at the level of the spinal cord itself will provide researchers with unique information about nervous system activity that cannot be identified using traditional brain fMRI (functional magnetic resonance imaging).
In previous studies, we have used fMRI of the spinal cord (‘spinal fMRI’; Stroman, 2005) to examine whether the spinal cord activity can be modulated by emotional stimuli. In the first study to examine spinal cord responses to emotions, we measured activity in the cervical spinal cord, which innervates the upper limbs including the hands (Smith and Kornelsen, 2011). We found that negative emotional stimuli elicited greater levels of activity in ventral (motoric) regions of several spinal cord segments, particularly those related to hand responses. This activity was more pronounced when participants made a movement (pressed a button on a response pad) than when they passively viewed the emotional images. Because of this very specific pattern of activity, we suggested that spinal cord neurons show evidence of emotion-dependent ‘action preparedness’.
However, emotional responses are obviously not limited to rapid gestural responses, as shown by measurements of the cervical spinal cord. Instead, emotional responses generally involve muscle tension in the chest and abdomen as well as autonomic nervous system activity. Neuroanatomical studies suggest that these responses should be linked with activity in a different region of the spinal cord, the thoracic cord. Spinal nerves emanating from the thoracic spinal cord innervate muscles in the trunk and abdomen, stimulating muscle contraction of these regions. Afferent somatosensory projections from the peripheral nervous system synapse with this region as well. These responses are likely linked to the subjective awareness of our emotionally aroused state. Additionally, the thoracic spinal cord is critical for a number of autonomic nervous system responses ranging from pupil dilation and cardiopulmonary responses (segments T1–T4) to the secretion of norepinephrine and epinephrine by the adrenal medulla (T10–T12; see Shields, 1993, for a detailed review). Therefore, measuring activity in the thoracic spinal cord would help delineate the neural architecture involved in these visceral emotional responses.
Until recently, measuring activity in the thoracic spinal cord has not been possible, as the challenges associated with spinal fMRI are exacerbated in these thinnest segments of the spinal cord. However, methodological advances now allow us to reliably detect functional activity in this region of the spinal cord (Kornelsen et al., 2013). In the current study, we used spinal fMRI to measure neuronal activity in thoracic spinal cord segments T1–T12 during the perception of emotionally arousing photographs. Participants completed four separate neuroimaging runs: passive (non-motoric) viewing of negative images, passive viewing of neutral images, motoric responses (button presses) to negative images and motoric responses to neutral images. These stimuli and experimental tasks are identical to our earlier study of the cervical spinal cord (Smith and Kornelsen, 2011), thus facilitating comparisons across studies.
METHODS
Participants
Thirteen healthy right-handed undergraduate university students participated in this study (eight female, age range = 18–26 years, mean age = 21.2 years). All participants underwent MR screening to ensure that they could be safely scanned. Ethical approval was obtained from our institutions’ Human Research Ethics Boards. All participants provided written informed consent prior to their participation in these studies. All participants received a $25 honorarium in exchange for their participation.
Stimulus materials and paradigm
Participants completed four separate neuroimaging runs. Participants viewed images of neutral and negative emotion-evoking photographs taken from the International Affective Picture System (IAPS), a standardized image database commonly used in emotion studies (Lang et al., 2008). This set of images was supplemented with additional photographs taken from publicly available sources, as the IAPS images did not have enough high-arousal, negative pictures for our design (Smith and Kornelsen, 2011). Negative images included photographs of fear-related scenes (e.g. attacking animals, people being threatened) and disgust-related scenes (e.g. images of skin diseases). Neutral images included photographs of the interiors of houses (e.g. kitchens) as well as landscape and cityscape images. The negative and neutral image sets were equated for visual complexity. Pilot testing was performed to ensure that negative and neutral images differed in terms of valence and arousal. On a 9-point scale, negative images were rated as being highly arousing (mean = 7.23, s.d. = 1.98) and quite negative (mean = 2.01, s.d. = 1.84, with a low score indicating strong negative emotional responses). Neutral images were rated as being non-arousing (mean = 2.17, s.d. = 1.31) and emotionally neutral (mean 5.20, s.d. = 2.10).
In the current experiment, two neuroimaging runs depicted images of neutral scenes, and two neuroimaging runs depicted images of negative emotion-evoking scenes. In two of the runs, the participants viewed the images while making a motoric response in the form of a button press. Participants were asked to make a button press response with the index finger if the image depicted an indoor scene and with the middle finger if the image depicted an outdoor scene. In the other two runs, the participants passively viewed the images. Thus, the four separate neuroimaging runs were: passive (non-motoric) viewing of negative images (Passive Negative), passive viewing of neutral images (Passive Neutral), motoric responses (button presses) to negative images (Active Negative) and motoric responses to neutral images (Active Neutral). The order of the four fMRI runs was counterbalanced across participants. Within each run, the images were presented, after an initial 40 s rest block, in 60 s stimulation blocks followed by 40 s rest blocks; these were repeated three times for a total functional scan time of 340 s. Spinal cord imaging generally uses blocked, rather than event-related, designs; this is because the imaging parameters used in spinal fMRI typically require 8–10 s in order to acquire a single volume. Each of the three stimulation blocks consisted of 15 images presented individually for 4 s. The individual images were not repeated across blocks or fMRI runs. This procedure helped to prevent participants from habituating to the emotional images (Stark et al., 2004).
MR methodology
Functional imaging of the thoracic spinal cord was conducted using previously established imaging parameters (Kornelsen et al., 2013). Experiments were conducted with a 3.0 Tesla Siemens Magnetom Trio system. A standard Siemens radio frequency (RF) body coil was used for excitation, and a Siemens Spine Matrix was used for reception. Spinal cord fMRI was performed using a single-shot fast spin-echo sequence with partial Fourier sampling (HASTE) with an echo time (TE) = 38 ms and a repetition time (TR) = 1000 ms per slice. Seven 2 mm thick contiguous sagittal slices were acquired with the following acquisition parameters: flip angle = 125°, 195 Hz/pixel bandwidth, 90 × 128 matrix size, echo spacing = 9.68 ms, field of view (FOV) = 200 mm × 100 mm, 50% FOV phase encode. Slices were centered rostro-caudally on the T5 vertebra and spanned the thoracic spinal cord segments. Spatial saturation pulses were applied to eliminate signal from surrounding areas to avoid aliasing and to reduce motion artifacts arising from regions anterior to the spine.
Data analysis
Data were preprocessed and analyzed with Matlab custom-written software (Mathworks, Inc., Natick, MA). Data from two participants were removed because of movement of the spinal cord out of the field of view. Each spinal fMRI data series was realigned using a raw data image to define the location and curvature of the spinal cord, according to established spinal fMRI data analysis methodology (Stroman, 2006). The C7/T1 and T8/T9 intervertebral disks were marked in the sagittal plane, and a reference line was manually drawn along the anterior, posterior, left and right edges of the spinal cord. The data at each time point were combined into a three-dimensional volume and were linearly interpolated to 0.5 mm cubic voxels. The volume was resliced transverse to the manually drawn reference lines. The reference lines were used to realign the slice data to correct for motion between time points and to apply spatial smoothing in the rostro-caudal direction. Each series was analyzed using the general linear model as described in previous research (Stroman, 2006) with the basis set generated for the stimulation paradigm described above, consisting of a block paradigm of 40 s rest before and after each 60 s block of stimulus presentation. The analyzed individual results were then spatially normalized by reslicing the spinal cord every 1 mm along the length of the reference lines between the C7/T1 and T8/T9 discs and by scaling to a 140 mm length and fine-tuning the alignment in each transverse slice. A random effects analysis was conducted, and activity maps of the significantly activated voxels (t-threshold = 3.5, df = 10, P = 0.0029) were created for each condition. The average percent signal change from baseline was calculated for each data series with baseline calculated as mean intensity during rest and then averaged per condition. The number of active voxels was tabulated for each run for all participants in order to perform comparisons across conditions.
RESULTS
Spinal cord activity was elicited during both conditions with both stimulus types. The Active Negative condition elicited the greatest amount of spinal cord activity in terms of the spatial extent as quantified by the number of active voxels. The Active Negative condition had more active voxels than each of the other three conditions. The total number of active voxels for the condition Active Negative was 464 with an average of 42 active voxels per series. These comparisons were shown to be significant using one-tailed, paired sample Student’s t-tests (P< 0.05 Bonferroni corrected for multiple comparisons) for the Passive Neutral (194 active voxels total, average of 18 per series; P = 0.0000), Active Neutral (241 active voxels total, average of 22 per series; P = 0.0000) and Passive Negative (299 active voxels total, average of 27 per series; 0.0009) conditions.
A random effects analysis was used to create activity maps of significantly active voxels for each condition. The Active Negative condition elicited widespread activity along the thoracic spinal cord (Figure 1). Bilateral dorsal activations were found in somatosensory nuclei related to the pectoral muscles (T1–T2) and lower abdomen and hips (T11–T12). Right ventral responses were found in T2, T6–T7 and T12, regions related to the control of musculature in the upper limbs, chest and abdomen. Activation occurred in the right lateral horn in upper, middle and lower thoracic segments corresponding to projections of the autonomic nervous system preganglionic fibers to the cervical ganglia (T2, T4), celiac ganglion (T6–T7, T9) and to the adrenal medulla (T10–T11). These projections are responsible for sympathetic activation of the eyes (i.e. pupil dilation), head and cardiopulmonary system (T1–T4), the liver and digestive systems (T5–T9) and the secretion of norepinephrine and epinephrine (T10–T12). In contrast, responses made during the Active Neutral condition elicited activity in left dorsal sensory nuclei in T1 (Figure 2A). Importantly, the number of active voxels detected during Active Neutral runs was significantly smaller than in Active Negative runs despite the fact that an identical motoric response was performed in both conditions. Consistent with previous studies of the cervical spinal cord (Smith and Kornelsen, 2011), these results demonstrate that the presence of emotional images significantly enhanced activity in the thoracic spinal cord.
Fig. 1.
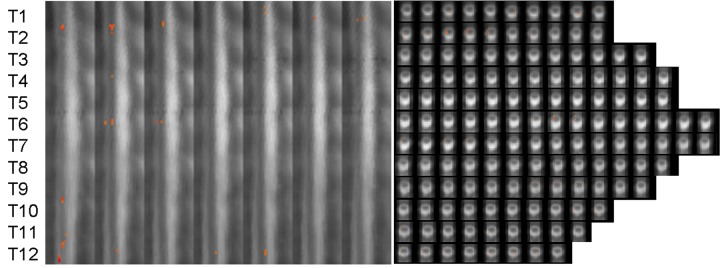
Distribution of activity elicited during the Active Negative condition. The approximate location of the thoracic spinal cord segments T1–T12 are shown on the left of the image for both the sagittal and axial orientations. In sagittal view, the slices are shown from right to left with the dorsal side of the spinal cord to the right of each frame. Axial slices are shown with the dorsal side of the spinal cord at the bottom of each frame and with the right side of the spinal cord to the left of the frame.
Fig. 2.
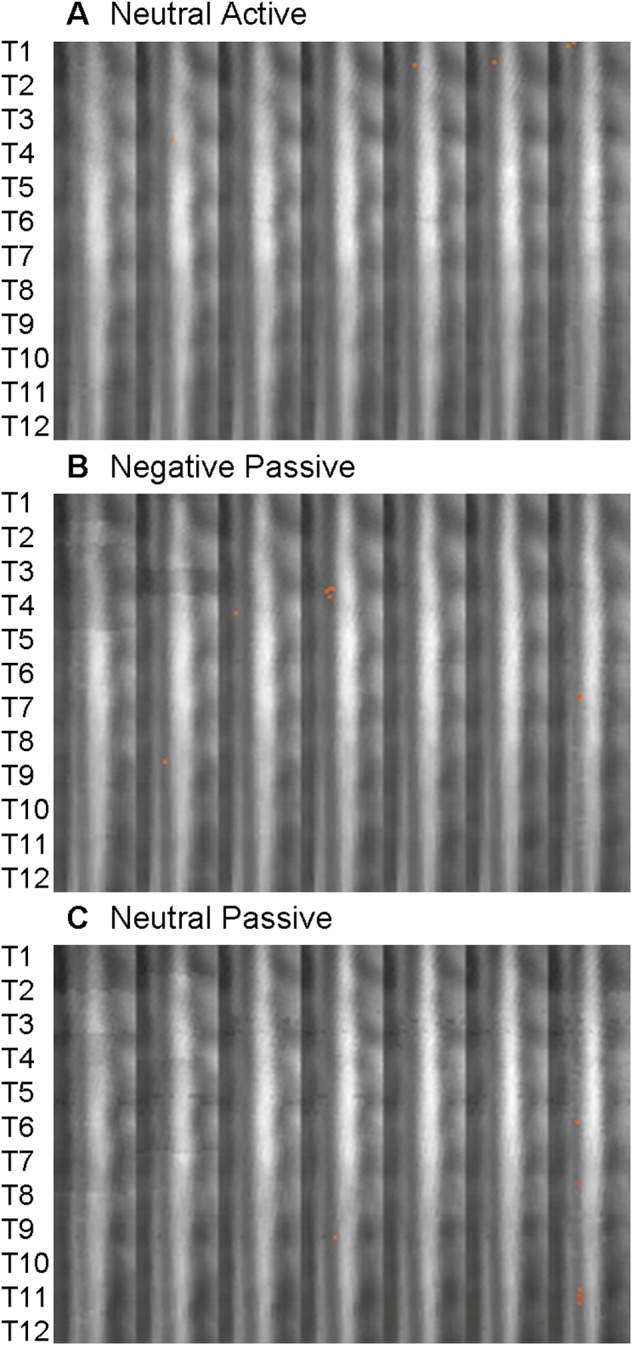
Distribution of activity elicited during the (A) Neutral Active, (B) Negative Passive and (C) Neutral Passive conditions. The approximate location of the thoracic spinal cord segments are shown on the left. The slices are shown from right to left with the dorsal side of the spinal cord to the right of each frame.
Emotional stimuli also modulated spinal cord activity in the passive-viewing conditions (i.e. where no movement was performed). Passively viewing negative images elicited activity in midline dorsal and ventral T4, left dorsal T7 and right lateral T8 (Figure 2B). These responses reflect muscular contractions in the pectoral areas, and importantly, somatosensory responses to the visual images. Autonomic nuclei in the right lateral T8 segment project to the celiac ganglia, which contributes to sympathetic activity of the liver and digestive systems. Although the Passive Neutral condition produced activity in left-lateralized T6, T7, T9 and T11, the number of active voxels was significantly less than in the emotional condition (P = 0.0025; Figure 2C). Thus, simply viewing negative emotional images—without making any motoric response—enhances activity in the thoracic spinal cord.
The average percent signal change did not differ significantly between conditions. The elicited average signal changes from baseline were as follows: Active Negative 4.15%, Active Neutral 3.92%, Passive Negative 3.56% and Passive Neutral 4.48%. These values are in agreement with previously reported spinal fMRI signal change values (Lawrence et al., 2011) and are in accordance with the expected average percent signal change for spinal fMRI (Stroman, 2005).
DISCUSSION
The pattern of results suggests that two—potentially interacting—mechanisms are underlying the emotional modulation of thoracic spinal cord activity. One mechanism is an arousal response to the emotional photographs. In the passive viewing (i.e. no movement) conditions, negative photographs elicited significantly more activity than did neutral photographs. The extensive somatosensory activity in the dorsal regions of the thoracic spinal cord likely reflects bodily responses to the emotional stimuli, which were previously rated as being quite emotionally arousing (Smith and Kornelsen, 2011). The dorsal neurons synapse with second-order neurons that project to the anterolateral tract of the spinal cord, the same tract that receives pain, temperature and mechanosensory input. This information travels rostrally to the ventral posterior nucleus of the thalamus, which then projects to numerous cortical sites (Brown, 1982). It is likely, therefore, that the activity we detected in the spinal cord represents the initial central nervous system representation of visceral responses to emotional stimuli.
Although arousal undoubtedly influenced these results, the precise pattern of activity—significantly larger levels of activity elicited in the Active Negative condition—suggests that a second, perhaps complementary, mechanism is at work. The pronounced emotion-dependent activity in ventral regions related to movement is consistent with an action preparedness theory of emotion (Pereira et al., 2010; van Loon et al., 2010; Smith and Kornelsen, 2011). We found an emotion-dependent enhancement of activity in thoracic spinal cord segment T2, which projects to the brachial plexus and is involved in movements of the upper extremity (Loukas et al., 2010). The fact that this area produced the greatest concentration of active voxels, complements previous findings that revealed activity in the cervical spinal cord is enhanced when viewing negative images depicting hands as compared with neutral images of hands or negative images of feet (McIver et al., 2013). The second highest concentration of active voxels in our Active Negative condition was at segment T12. This can be accounted for by the projection of the lateral horn directly to the adrenal medulla to elicit a fast sympathetic response to negative stimuli, and the proximity to the lumbar spinal cord segments, which are involved in the movement of the lower limbs. The fact that these specific spinal cord segments showed larger responses than other thoracic segments makes neuroanatomical sense. The middle thoracic region, T6–T9, the area of lowest concentration of active voxels in our study, stimulates the stomach, pancreas and intestines; these regions are less likely to be involved with rapid emotional movements than are the limbs and should therefore produce smaller responses in the current paradigm.
The current results demonstrate that the emotional modulation of spinal cord activity detected in our previous studies extends beyond the cervical spinal cord to thoracic segments involved in the control of the torso and viscera. In previous research, we found that emotional stimuli—particularly, highly arousing negative images—enhanced activity in ventral regions of the cervical spinal cord related to movement of the hands (Smith and Kornelsen, 2011). A subsequent study demonstrated that this modulation was quite task-specific (McIver et al., 2013). Emotional images in which the actor would respond using his or her hands (e.g. an image of someone with a spider on her hand) elicited more activity in cervical spinal cord segments related to hand responses than did emotional images in which the actor would respond using his or her feet (e.g. an image of someone about to step on a nail). These data suggest that the input from the brain is specifically targeting spinal cord segments that would allow for a situation-appropriate response to be made. The current research demonstrates that in addition to these limb-specific motoric plans, emotional responses involve a general contraction of muscles in the chest, suggesting an experience of tension. More importantly, spinal fMRI allows us to measure somatosensory and autonomic activity in the central nervous system before it has been interpreted by the insula and dorsal anterior cingulate gyrus in the brain. Therefore, our results delineate the neural architecture of visceral emotional responses at an earlier stage of processing than has been observed in any previous work.
Although these results are novel, several questions remain unanswered. As with our initial study of emotion-dependent activity in the cervical spinal cord, our ‘negative’ stimuli included highly arousing images from a number of different withdrawal-related emotions (e.g. threat/fear, disgust). Doing so allowed us to generate a stimulus set with varied content, thus reducing the likelihood of participants habituating to the emotionality of the images; however, this does prevent us from examining the effects of specific emotions (e.g. fear). As we have now demonstrated that high-arousal emotional images affect spinal cord activity, we will examine potential valence-specific responses in subsequent studies. Future studies will also examine whether the emotional responses in ventral regions of the spinal cord are selective for images in which movement is depicted or implied. It is possible that a highly arousing negative image implying movement (e.g. an image of someone pointing a gun at the viewer) will elicit greater activity than a more static image (e.g. an image of a crashed plane). This manipulation would help clarify the degree to which the observed activity is related to ‘action preparedness’.
A final limitation of the current study is that it is limited to one region of the spinal cord. In an ideal world, we would be able to measure multiple regions of the spinal cord at the same time. Unfortunately, the imaging field of view is a limiting factor. By acquiring our images in the sagittal orientation, we have maximized the volume of spinal cord that can be acquired within a reasonable amount of time (Stroman, 2005). Therefore, spinal fMRI is limited to scanning different regions of the cord in different scanning sessions (e.g. cervical or thoracic). In future studies, it will be informative to scan the brain and different spinal cord regions in the same individual; such multi-session studies will allow us to link together activity in all regions of the central nervous system. Doing so will help us delineate the neural architecture underlying the interactions between emotion, arousal and movement.
Conflict of Interest
None declared.
Acknowledgments
This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Manitoba Medical Services Foundation (MMSF).
REFERENCES
- Brown AG. The dorsal horn of the spinal cord. Quarterly Journal of Physiology. 1982;67:193–212. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London B. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Kornelsen J, Smith SD, McIver TA, Sboto-Frankenstein U, Latta P, Tomanek B. Functional MRI of the thoracic spinal cord during vibration sensation. Journal of Magnetic Resonance Imaging. 2013;37:981–5. doi: 10.1002/jmri.23819. [DOI] [PubMed] [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N. (2008). International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Technical report A-8. University of Florida, Gainesville, FL.
- Lawrence J, Kornelsen J, Stroman PW. Noninvasive observation of cervical spinal cord activity in children by functional MRI during cold thermal stimulation. Magnetic Resonance Imaging. 2011;29:813–8. doi: 10.1016/j.mri.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Loukas M, Tubbs RS, Apaydin N, Louis RG, Jr., Wartman C, Shoja MM. A review of the T2 segment of the brachial plexus. Singapore Medical Journal. 2010;51:464–7. [PubMed] [Google Scholar]
- McIver TA, Kornelsen J, Smith SD. Limb-specific emotional modulation of cervical spinal cord neurons. Cognitive, Affective, and Behavioral Neuroscience. 2013;13:464–72. doi: 10.3758/s13415-013-0154-x. [DOI] [PubMed] [Google Scholar]
- Ohira H. The somatic marker revisited: brain and body in emotional decision making. Emotion Review. 2010;2:245–9. [Google Scholar]
- Pereira MG, de Oliveira L, Erthal FS, et al. Emotion affects action: midcingulate cortex as a pivotal node of interaction between negative emotion and motor signals. Cognitive, Affective, and Behavioral Neuroscience. 2010;10:94–106. doi: 10.3758/CABN.10.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–9. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7:1376–80. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Shields RWJ. Functional anatomy of the autonomic nervous system. Clinical Neurophysiology. 1993;10:2–13. doi: 10.1097/00004691-199301000-00002. [DOI] [PubMed] [Google Scholar]
- Smith SD, Kornelsen J. Emotion-dependent responses in spinal cord neurons: a spinal fMRI study. Neuroimage. 2011;58:269–74. doi: 10.1016/j.neuroimage.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Walter B, et al. Hemodynamic effects of negative emotional pictures—a test-retest analysis. Neuropsychobiology. 2004;50:108–18. doi: 10.1159/000077948. [DOI] [PubMed] [Google Scholar]
- Stroman PW. Magnetic resonance imaging of neuronal function in the spinal cord: Spinal fMRI. Clinical Medicine and Research. 2005;3:146–56. doi: 10.3121/cmr.3.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroman PW. Discrimination of errors from neuronal activity in functional MRI of the human spinal cord by means of general linear model analysis. Magnetic Resonance in Medicine. 2006;56:452–6. doi: 10.1002/mrm.20966. [DOI] [PubMed] [Google Scholar]
- van Loon AM, van den Wildenberg WPM, van Stegeren AH, Hajcak G, Ridderinkhof RJ. Emotional stimuli modulate readiness for action: a transcranial magnetic stimulation study. Cognitive, Affective, and Behavioral Neuroscience. 2010;10:174–81. doi: 10.3758/CABN.10.2.174. [DOI] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinion in Neurology. 2005;18:442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]


