Abstract
Field studies on HIV risk perception suggest that people rely on impressions they have about the safety of their partner. The present fMRI study investigated the neural correlates of the intuitive perception of risk. First, during an implicit condition, participants viewed a series of unacquainted persons and performed a task unrelated to HIV risk. In the following explicit condition, participants evaluated the HIV risk for each presented person. Contrasting responses for high and low HIV risk revealed that risky stimuli evoked enhanced activity in the anterior insula and medial prefrontal regions, which are involved in salience processing and frequently activated by threatening and negative affect-related stimuli. Importantly, neural regions responding to explicit HIV risk judgments were also enhanced in the implicit condition, suggesting a neural mechanism for intuitive impressions of riskiness. Overall, these findings suggest the saliency network as neural correlate for the intuitive sensing of risk.
Keywords: risk, fMRI, feelings, intuition, implicit
The perception of a health risk is a prerequisite for protective behavior change. However, the mechanisms of risk perception remain insufficiently understood. Two fundamentally different modes of risk processing have been proposed: The ‘risk-as-analysis’ view emphasizes the role of deliberative reasoning and probability calculus, whereas the ‘risk-as-feelings’ view suggests that sensing risk encompasses intuitive and affective processes (Loewenstein et al., 2001; Slovic et al., 2006). In real-life situations, these two modes may clash, as when feelings prevail over analysis (e.g. feeling frightened during the takeoff in a passenger plane, even though one knows that the probability of crashing is vanishingly small) or when people engage in risky health behaviors despite better knowledge (e.g. smokers). This issue is also relevant in the field of sexual behavior and the perception of HIV risk (Thompson et al., 1996). Studies show that the rates of condom use remain low even among well-informed individuals (e.g. Keller, 1993), and the two-mode view of risk perception might explain this phenomenon: In particular, people who engaged in unsafe sex often report that their partner appeared as safe and that they relied on this feeling without further deliberation (Maticka-Tyndale, 1991; Gold et al., 1992; Gold, 1993). These striking examples suggest an intuitive ‘risk-as-feelings’ mode, which, however, has been difficult to examine. Here, we use functional imaging to determine the neural correlates associated with the intuitive perception of HIV risk.
To be functional, a system for risk perception needs to detect sources of risk and trigger an alarm signal in order to cope with risk and danger. Recent evidence from neuroimaging suggests that these operations involve the saliency network (Seeley et al., 2007). This network, which includes the anterior insular cortex (aINS), mediofrontal cortex (MFC) and dorsal anterior cingulate cortex (dACC), responds to salient stimuli, plays a role in the experience of feelings and regulates attention and working memory processes (Medford and Critchley, 2010; Menon and Uddin, 2010). Accordingly, it is proposed that risky stimuli may command increased activity in regions of the saliency network. A key characteristic of the salience network is that it operates implicitly. Thus, salient stimuli are detected based on their intrinsic relevance even without instruction (Menon and Uddin, 2010). This suggests—in line with the ‘risk-as-feelings’ model and the implicit–explicit distinction in social cognition (Lieberman et al., 2002; Frith and Frith, 2008)—that neural responses associated with sensing (HIV) risk would also occur spontaneously. Specifically, differential reactions to risky stimuli may not depend on explicit processing goals (i.e. ‘is it risky to have unprotected sex with this partner?’), but may also be observed in implicit conditions (Schmälzle et al., 2012).
The main goal of the present study was to investigate the neural correlates of the intuitive perception of risk. Toward this end, fMRI data were collected during an implicit and an explicit HIV risk perception task. In both conditions, participants viewed the same opposite sex portraits of unknown individuals. The implicit condition consisted of a person recognition task that did not make any reference to HIV risk. In the subsequent explicit condition, participants provided ratings of HIV risk. We predicted that regions comprising the saliency network are responsive to HIV risk in both the explicit and implicit condition.
MATERIALS AND METHODS
Participants
Twenty-six healthy volunteers (13 female, mean age = 21.75 years, SD = 3.1) were recruited on the campus of the University of Konstanz. Participants received either monetary reward or course credits for participation and provided written consent to the study protocol, which was approved by the Ethic Review Board of the University of Konstanz. All participants had normal or corrected to normal vision. Three participants were excluded from the analyses because of excessive head movement or insufficient numbers of trials in one of the individually categorized risk conditions.
Stimulus materials
The stimulus set consisted of colored photographs depicting 160 males and 160 females, which had already been used in previous research (Schmälzle et al., 2011; Renner et al., 2012). The photographs were retrieved with permission from a popular online photo-sharing community (www.flickr.com). To assure high ecological validity, stimuli showed a colored photo of a single person located in the foreground, with his or her face clearly visible. To be representative of the study’s target population in terms of age and race, only photographs of Caucasians between 18 and 35 years old were included. To resemble naturalistic viewing conditions and to facilitate impression formation, self-portraits exhibiting attire, socioeconomic status cues and situational context features were included. To increase ecological validity, each participant viewed pictures showing opposite sex persons. Ten additional pictures (5 females) served as memory probes for the person recognition task in the implicit condition.
Implicit condition: person recognition task
During the first functional run, assessment of implicit risk perception was conducted while participants performed a memory task unrelated to HIV risk (cf. Engell et al., 2007; Schmälzle et al., 2011). The condition was composed of 10 memory blocks. Within one block, participants were presented with 15 images from the 160 picture set and one picture from the task stimulus set followed by a test stimulus from the task stimulus set. The task was to decide whether the test stimulus had been presented in the preceding stream of pictures. This was the case for half of the blocks, resulting in a probability of 50% across blocks. The order of picture presentation was fully randomized for each participant. Each stimulus was presented for 750 ms, followed by a black screen during the interstimulus interval (ISI). The duration of the ISIs was exponentially jittered around a mean duration of 2000 ms, ranging from 500 ms up to 8000 ms. ISI durations were distributed semi-randomly across the blocks, ensuring an approximately equal length of each block.
Explicit condition: HIV risk perception task
The memory task was followed by an explicit risk perception task. Participants were instructed to provide their first impression regarding the HIV risk of the depicted person. It was stressed that they should react spontaneously and that they had only limited time. The 160 pictures of opposite sex persons were presented in randomized order. As in the implicit condition, each stimulus was presented for 750 ms. This was followed by a black screen presented for 500 ms, after which participants were asked to evaluate how likely it is that the presented person is infected with HIV (rather likely/rather unlikely). After participants gave their answer, the next trial was initiated, with an average intertrial interval (ITI) of 2.5 s, exponentially distributed between 1000 and 7000 ms. Responses were collected using an MR-compatible serial response box, and response assignment was counterbalanced across participants. Low and high risk categories comprised 116.1 and 43.9 trials (SD = 16.1), respectively. Extensive control analyses confirmed that the same effects as reported below are also obtained when the frequency of low- vs high-risk stimuli entering fMRI model estimation were balanced by subsampling randomly from the low risk category.
MRI acquisition and analysis
Scanning was conducted using a 1.5 Tesla Philips Intera MR-System equipped with Power Gradients. For functional imaging, a T2*-weighted fast field echo-echo planar imaging (FFE-EPI) sequence utilizing parallel scanning technique was acquired. In-plane resolution was 3 × 3 mm2, and the slice thickness was 3.5 mm (32 axial slices; no gap; field of view = 240 mm; acquisition matrix: 80 × 80 voxels; echo time = 40 ms; flip angle = 90°; repetition time = 2.5 s). In addition, a high-resolution (200 sagittal slices; isotropic voxels of 1 mm) T1-weighted structural scan was obtained for each participant.
Preprocessing and statistical analysis of the functional data were performed using SPM5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London). Using default settings, functional images were slice-time corrected, realigned, normalized and smoothed using a Gaussian kernel with an Full Width at Half Maximum of 9 mm. Furthermore, a high-pass filter with a cutoff period of 128 s as well as global scaling was applied to the data.
Whole-head fMRI analysis served to identify neural regions that showed an increased activation to risky as compared with safe persons based on each participant’s idiosyncratic HIV ratings from the explicit condition. The data were analyzed in an event-related design incorporating two covariates of interest (risky vs safe) and, to improve model-fit, additional covariates of no interest (time and dispersion derivatives and realignment parameters). Control of multiple comparisons was achieved using False Dicovery Rate (FDR) with the criterion set to q = 0.05, and the resulting clusters were used to derive regions of interest (ROIs) for data from the implicit condition. Using MarsBaR, data were extracted from ROIs, and the mean beta of peak voxel was calculated. MRICroN was used for visualizing results.
RESULTS
fMRI results: explicit condition
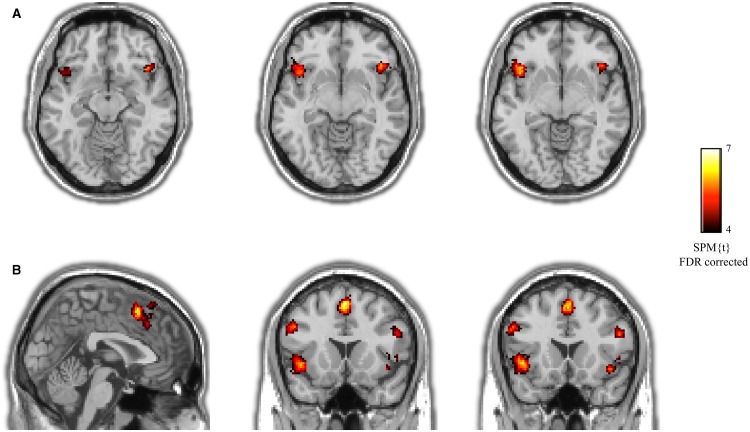
Whole-head fMRI analysis revealed a significant increase in activation to high- as compared with low-risk stimuli in regions comprising the saliency network (see Figure 1). Specifically, bilateral anterior insular cortices (left: MNI coordinates x = −46, y = 20, z = −2, t22 = 6.39, p = 9.9 × 10−7, cluster size = 1360 mm3; right: MNI 44, 20, −10, t22 = 5.94, p = 2.8 × 10−6, cluster size = 464 mm3) showed increased blood oxygenation level dependent (BOLD) signals during the processing of risky as compared with safe persons. Furthermore, risky stimuli elicited increased activity in the MFC (MNI 0, 16, 54, t22 = 6.89, p = 3.2 × 10−7, cluster size = 123 mm3) and right supplementary motor area (SMA; MNI 14, 2, 68, t22 = 5.08, p = 2.1 × 10−5, cluster size = 232 mm3), which closely correspond to brain regions comprising the saliency network (Seeley et al., 2007). Of note, the dACC, a key node of the saliency network that is located just inferior to the MFC cluster, did not survive correction for multiple comparisons, but showed the same pattern of results and immediately emerged upon lowering the threshold. Furthermore, results for a dACC search volume survived small volume correction (p = 5 × 10−3, 5 mm radius around MNI coordinates 0, 22, 36; cf. Raichle, 2011). Finally, risky stimuli elicited increased activity in the left and right dorsolateral prefrontal cortex (dlPFC; left: −48, 16, 32, t22 = 5.58, p = 6.5 × 10−6, cluster size = 320 mm3; right: 52, 20, 26, t22 = 6.07, p = 2.1 × 10−6, cluster size = 280 mm3), possibly related to the engagement of working memory and executive control processes.
Fig. 1.
(A) Activations of aINS toward risky persons in the explicit condition. (B) Illustration of MFC and dlPFC, which in addition to the insula showed an increased activation toward risky persons.
There were no additional brain regions beyond the saliency network showing increased activation to high-risk stimuli, and testing for effects in the opposite direction, i.e. increased activity to low as compared with high stimuli, did not reveal any significant effects.
fMRI results: implicit condition
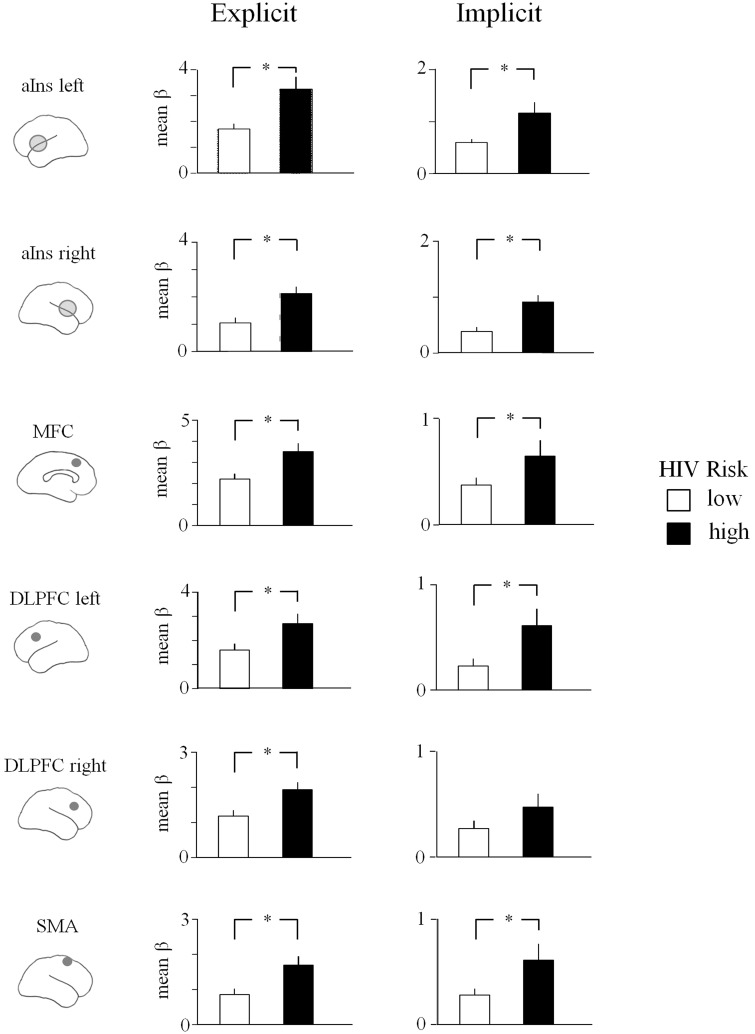
ROI analysis determined whether neural regions sensitive to risk in the explicit risk condition were already differentially engaged during the preceding implicit condition. Similar to the explicit condition, aINS activity during the implicit condition exhibited neural differences associated with HIV risk status. Specifically, as shown in Figure 2, increased activity for risky as compared with safe persons was observed in the left (t22 = 2.6, p = 8 × 10−3) and right (t22 = 3.6, p = 1 × 10−3) aINS. In addition, risk status affected BOLD activation also in the MFC (t22 = 2.9, p = 4 × 10−3) and right SMA (t22 = 2.8, p = 5 × 10−3). Furthermore, increased activity toward risky as compared with safe persons was observed in the left dlPFC (t22 = 2.9, p = 4.5 × 10−3) and approached significance for the right dlPFC (t22 = 1.6, p = 6.5 × 10−2). The dACC, which was identified at lower threshold in the explicit condition, was not significant in the implicit condition (t22 = 0.3, ns). Overall, a similar pattern of results as observed for the explicit risk rating task was also evident during the implicit condition, although during this condition no mention of HIV risk had been made.
Fig. 2.
Mean beta activations in ROIs for risky and safe persons in the explicit and the implicit condition. ROIs were derived based on the activated clusters observed in the explicit condition (see Figure 1).
To compare implicit and explicit conditions, repeated measures analyses of variance were conducted including factors of Condition (implicit vs explicit) and Risk (low vs high). All ROIs showed a significant increase in activation, Fs(1, 21) > 22.5, ps < 0.0001. Of interest, a significant interaction of Condition × Risk was observed in left and right insular cortex, Fs(1, 21) > 7.9, ps < 0.01, left and right dlPFC, Fs(1, 21) > 8.6, ps < 0.01, and MFC F(1, 21) = 10.7, p < 0.01, indicating an increased differentiation of high- and low-risk stimuli in the explicit condition (see Figure 2).
Exploratory whole-head analysis revealed no activated brain regions in the implicit condition when testing for the contrasts ‘high’ > ‘low risk’ and ‘low’ > ‘high risk’.
DISCUSSION
The present study examined the neural mechanisms of impressions about a potential partner’s HIV risk. The results revealed that HIV risk perception is associated with activity in the anterior insula and medial frontal regions. Furthermore, the dACC, which is often coactivated with aINS (Craig, 2010; Medford and Critchley, 2010), exhibited risk-related differences in the explicit condition when probed using ROI analysis. These structures are key regions of a large-scale network devoted to the processing of salient stimuli (Seeley et al., 2007; Shirer et al., 2012). Overall, the present study provides evidence that ‘gut feelings’ of risk are, at least in part, represented by the saliency network, which seems to underlie the presumed ‘risk-as-feelings’ mode of risk perception.
Interestingly, enhanced activations toward high-risk stimuli in key regions of the saliency network were observed in both experimental conditions. While the explicit condition resembles an active screening mode of risk perception, the implicit condition engages spontaneous routines of person perception during which the concept of HIV risk was not yet activated. Although the differentiation between high- and low-risk stimuli was stronger during the explicit condition, regions of the saliency network already exhibit differences during the implicit condition. These findings suggest that the active evaluation of HIV risk during the explicit condition amplified a pattern of differentiation already seen during the implicit condition. These findings are discussed with respect to current theories of the functions of the saliency network (Craig, 2009; Medford and Critchley, 2010; Menon and Uddin, 2010).
Previous research shows that the observed risk-sensitive regions of the saliency network respond to a broad range of stimuli and tasks. The anterior insula is consistently observed in studies of emotional stimulus processing (Kober et al., 2008), empathy for and direct experience of pain (Lamm et al., 2007; Kober et al., 2008) and a broad variety of social emotions (Lamm and Singer, 2010). Similarly, mediofrontal regions have been shown to be involved in negative affect processing (Shackman et al., 2011), decision making in social situations as well as under uncertainty (Nakao et al., 2012) and studies of action monitoring (Amodio and Frith, 2006). Furthermore, risky decision making was also associated with structures of the saliency network in a variety of economic and gambling tasks in which the outcome was uncertain and participants knew the probability of each outcome (see meta-analysis by Mohr et al., 2010). These findings strongly suggest that risk perception is not represented in dedicated ‘risk’ brain regions. Rather, intuitive risk perception seems to involve a network that is engaged by diverse stimuli with the common denominator of assessing and responding to salience and personal relevance. Overall, acknowledging the limits of reverse inferences (Poldrack 2006), the current findings align well with the larger literature on risk as well as studies on emotional and interoceptive processes.
The anterior insular cortex may be particularly relevant for the experience of feelings of riskiness and safety. Studies of various emotional states and interoceptive body awareness show activations of the anterior insula, which lead to the hypothesis that this region engenders subjective awareness and self-related visceral experiencing (Craig, 2009; Medford and Critchley, 2010). On that view, the anterior insular cortex enables a momentary feeling state based on the integration of salient (interoceptive and exteroceptive) stimuli prevailing at the current moment (Craig, 2010). The functional properties associated with the saliency network seem thus well suited for detecting and experiencing risks, and ultimately initiating appropriate behavioral actions. Specifically, an alarm signal may be generated, which shifts processing priorities in other large scale networks implicated in the working memory and attention processes (Menon and Uddin, 2010). Consistent with this notion, the processing of risky stimuli was associated with increased activity in the dlPFC, which is one of the key regions of the central executive network (Seeley et al. 2007).
A further main finding of the present study is that the saliency network is already engaged by high-risk stimuli in an implicit condition. This suggests that everyday risk perceptions seem to occur spontaneously and independent from context-specific processing goals related to health or sexual diseases. In general, it may be speculated that the detection of risky stimuli and their tagging for processing facilitates deliberative processes and thus promotes protection motivation. In the absence of risk-related feelings acting as warning signals, however, a sense of security would prevail and the systems for coping with risks would not be activated. Such a mechanism seems particularly important in situations of sexual arousal and diminished control (Kruse and Fromme, 2005; Ariely and Loewenstein, 2006), which may help to explain why even informed people ‘skip’ using condoms when a partner does not appear to be risky. However, dangerous consequences may arise when the intuitive sensing of risk lacks validity, as in the case of HIV.
The present findings indicate that the differentiation between high and low risk is considerably more pronounced in the explicit as compared with the implicit task condition. This was also corroborated in an additional full-factorial analysis (Factors Task: implicit vs explicit, Risk: low vs high; family-wise error small volume corrected) among ROIs in the anterior insula, MFC, dlPFC (bilateral) and SMA, defined according to the AAL atlas. Highly significant interaction effects were obtained in the left anterior insula (MNI −42, 18, −4, p = 5 × 10−3), MFC (MNI −2, 24, 38, p = 1 × 10−3), dlPFC (left: MNI −46, 20, 0, p = 0.024, right: MNI 54, 22, 22, p = 3.4 × 10−3) and SMA (MNI 4, 18, 48, p = 4.6 × 10−3). There were no effects in which the differentiation of riskiness was more pronounced in the implicit as compared with the explicit condition, not even at lenient thresholds. While the pattern of findings is consistent with the hypothesis that explicit processing of risk amplifies implicit processes, it has to be noted that this reasoning is preliminary. The current study employed a variant of the ‘subsequent memory paradigm’ (Wagner et al., 1998), which we adapted to study risk-related processing. This paradigm, however, required a fixed order of conditions, thus introducing potential confounds related to habituation or familiarity1. Future research balancing order of presentation would resolve methodological issues, and, importantly, provide insight into the effects of explicitly activating the HIV risk stereotype on subsequent stimulus processing in which participants have a task focus unrelated to HIV.
An essential feature of intuitive risk perception via a ‘risk-as-feelings’ mode concerns its perceived veridicality. Specifically, people who engaged in unprotected sex often report that they were convinced that their partners were safe and college students claim to ‘just know’ whether somebody is safe or not (Maticka-Tyndale, 1991; Gold et al., 1992; Gold, 1993; Keller, 1993). Such feelings of knowing and the self-evident validity of experiences are typical of intuitive processing in many domains. Interestingly, the absence of risk (low > high risk) did not reveal a brain signature of safety. One may accordingly speculate that the saliency network codes risk on a continuum from safety to low risk, providing a further instance of the greater potency of negative rather than positive events (Baumeister et al., 2001). Furthermore, the trust in one’s intuitions may reflect a generalization process because the saliency network is invoked by all kinds of social and physically salient stimuli, presumably engaged many times every day, and, for the most part successful in navigating social life and avoiding dangers. Furthermore, implicit processes in person processing are assumed to develop gradually by experience (Lewicki et al., 1992). A learning and experience-based perspective on the intuitive judgment of HIV risk seems to be hampered by the low base rate and the lack of corrective feedback, which may over time lead to a false sense of control for HIV risk. Overall, people relying on their intuitions may assume that they prevent their risk for infection by listening to their intuitions about riskiness or safety (Thompson et al., 1996).
Though problematic in the case of HIV risk, a spontaneous and feeling-based system for intuitive risk perception is highly adaptive in many cases. With regard to the evolutionarily very relevant health risk domain of infectious diseases, Schaller and colleagues advanced the idea of a ‘behavioral immune system’ for protection against pathogens (Schaller et al., 2010). Briefly, this system includes sensory processes for infection detection, which then set off disease-avoiding affective, cognitive and behavioral reactions. A wealth of evidence suggests that such mechanisms, which are also present in other species, act as ‘a crude first line of defense against diseases’ (Schaller and Duncan, 2007). In the context of HIV risk behaviors, however, reliance on assumed or inferred HIV risk status provides a dangerous, unreliable and illusory strategy against sexually transmitted diseases (Thompson et al., 2002). In particular, the physical appearance of a person does not provide reliable information about HIV risk status (Thompson et al., 2002), and even if there were a ‘kernel of truth’ in one’s risk inferences, the level of protection would be far from perfect. We recently speculated that a high-risk stereotype of HIV based on interrelated person characteristics provides the basis for intuitive judgments of HIV risk (Renner et al., 2012). Specifically, person characteristics such as a lack of responsibility and low trustworthiness have been identified as cardinal features of the high-risk stereotype in young adults (Renner and Schwarzer, 2003), and perceived HIV risk consistently shows strong negative correlations with ratings of responsibility and trustworthiness (Schmälzle et al., 2011; Renner et al., 2012). Furthermore, social psychological research on person perception revealed that these characteristics can be extracted easily and with short exposure times, making people prone to form first impressions. While previous fMRI studies of trustworthiness impressions revealed activations in structures of the saliency network, i.e. amygdala and insular cortex (Winston et al., 2002; Castle et al., 2012), the present study did not observe effects in the amygdala. This finding is consistent with previous meta-analytic fMRI studies of risk processing, which also did not report effects in the amygdala (Mohr et al., 2010). However, studies with improved fMRI methodology, i.e. scanner field strength and optimized scanning sequences (e.g. Sabatinelli et al., 2005), are needed to determine the role of the amygdala in risk processing.
Results from factor analysis of the same stimuli as used in the present research show that ratings of HIV risk, trustworthiness, responsibility and arousal represent a common factor of person perception distinct from a valence/approach factor with loadings of attractiveness, pleasantness and willingness to interact (Renner et al., 2012). Furthermore, in a series of studies, temporally sensitive event-related brain potentials (ERP) differentiated between risky and safe stimuli very early in the processing stream (<300 ms) and carried a similar signature as is observed during the processing of salient emotional stimuli (Schmälzle et al., 2011, 2012). Importantly, while trustworthiness and responsibility dimensions showed a similar ERP signature to HIV risk, there were no corresponding effects for attractiveness (Schmälzle et al., 2011, 2012). In addition, a recent study demonstrated that the ERP signature of HIV risk was distinct from risk for leukemia, a life-threatening, but non-contagious, disease (Barth et al., 2014). Overall, accumulating evidence points to the specificity of intuitive HIV risk judgments and sexually transmitted diseases, which appear to be distinct from general judgments of valence/attractiveness and health/disease. It seems promising to close in future research the gap between sensory and perceptual features displayed by the stimulus and ratings of person characteristics related to the HIV risk stereotype on the one hand, and their association with the activation of regions of the saliency network and feelings of knowing and the experience of self-evident validity on the other hand. Furthermore, in the case of HIV, the risk of infection depends on one’s potential partner and is thus contextualized to social inferences. However, other sources of risk are inherently non-social, such as large-scale technological, economic or private health risks. Future research should thus examine to what extent different domains of risk are represented in the same brain regions.
SUMMARY
Research at the intersection of health and neuroscience is growing rapidly (e.g. Falk et al., 2010; Schmälzle et al., 2013). In this vein, functional imaging was used here to determine the neural correlates underlying feelings of HIV risk. The present study revealed the saliency network, particularly the anterior insula and mediofrontal cortex, as possible neural correlates of the intuitive and experiential mode of risk perception. These findings help to better understand why impressions about HIV risk are formed with little effort and are perceived as veridical. In order to effectively promote precautionary behaviors, people will not only need to know the facts about HIV transmission and safe sexual practices but should also be aware of the ‘risks’ associated with their intuitions about risky and safe sexual partners.
Supplementary Material
Acknowledgments
This work was supported in part by the German Research Foundation [DFG, RE 3430, Schu 1074/10-3 and Schu 1074/11-2].
Footnotes
1 Both task conditions were compared in an additional exploratory analysis. The explicit compared with the implicit condition prompted enhanced activation in widespread regions of the visual system as well as regions commonly associated with executive functions (i.e. left and right lateral frontal as well as left parietal cortex, pre-SMA), and motor regions (presumably related to the higher motor demands of the explicit task). Conversely, the implicit condition (i.e. person recognition task) showed higher activations compared with the explicit condition especially in the posterior cingulate/precuneus, medial prefrontal and bilateral parietal cortex, i.e. regions encompassing the ‘default mode network’ (Raichle, 2011). Furthermore, the implicit condition yielded higher activations in auditory cortex. Overall, differences between the tasks were regionally confined rather than showing general increases/decreases.
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ariely D, Loewenstein G. The heat of the moment: the effect of sexual arousal on sexual decision making. Journal of Behavioral Decision Making. 2006;19(2):87–98. [Google Scholar]
- Barth A, Schmälzle R, Renner B, Schupp HT. Neural correlates of serious and life-threatening risks: contagiousness makes a difference. Frontiers in Behavioral Neuroscience. 2013;7:166. doi: 10.3389/fnbeh.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister, R.F., Bratslavsky, E., Finkenauer, C., Vohs, K.D. (2001). Bad is stronger than good. Review of General Psychology, 5(4), 323–70.
- Castle E, Eisenberger NI, Seeman TE, et al. Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(51):20848–52. doi: 10.1073/pnas.1218518109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Structure and Function. 2010;214(5–6):563–77. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19(9):1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. Journal of Neuroscience. 2010;30(25):8421–4. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60(3):503–10. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Gold RS. On the need to mind the gap: on-line versus off-line cognitions underlying sexual risk-taking. In: Terry D, Gallois C, McCamish M, editors. The Theory of Reasoned Action: Its Application to AIDS Preventive Behavior. Oxford: Pergamon Press; 1993. pp. 227–52. [Google Scholar]
- Gold RS, Karmiloff-Smith A, Skinner MJ, Morton J. Situational factors and thought processes associated with unprotected intercourse in heterosexual students. AIDS Care. 1992;4(3):305–23. doi: 10.1080/09540129208253101. [DOI] [PubMed] [Google Scholar]
- Keller ML. Why don’t young adults protect themselves against sexual transmission of HIV? Possible answers to a complex question. AIDS Education and Prevention. 1993;5(3):220–33. [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MI, Fromme K. Influence of physical attractiveness and alcohol on men’s perceptions of potential sexual partners and sexual behavior. Experimental and Clinical Psychopharmacology. 2005;13(2):146–56. doi: 10.1037/1064-1297.13.2.146. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2(12):e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214(5–6):579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Lewicki P, Hill T, Czyzewska M. Nonconscious acquisition of information. American Psychologist. 1992;47(6):796–801. doi: 10.1037//0003-066x.47.6.796. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Gaunt R, Gilbert DT, Trope Y. Reflection and reflexion: a social cognitive neuroscience approach to attributional inference. Advances in Experimental Social Psychology. 2002;34:199–249. [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127(2):267–86. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Maticka-Tyndale E. Sexual scripts and AIDS prevention: variations in adherence to safer-sex guidelines by heterosexual adolescents. Journal of Sex Research. 1991;28(1):45–66. [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 2010;214(5–6):535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PN, Biele G, Heekeren HR. Neural processing of risk. Journal of Neuroscience. 2010;30(19):6613–19. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Bai Y, Nashiwa H, Northoff G. Resting-state EEG power predicts conflict-related brain activity in internally guided but not in externally guided decision-making. Neuroimage. 2012;66:9–21. doi: 10.1016/j.neuroimage.2012.10.034. [DOI] [PubMed] [Google Scholar]
- Poldrack, R.A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences, 10, 59–63. [DOI] [PubMed]
- Raichle ME. The restless brain. Brain Connectivity. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner B, Schmälzle R, Schupp HT. First impressions of HIV risk: it takes only milliseconds to scan a stranger. PLoS One. 2012;7(1):e30460. doi: 10.1371/journal.pone.0030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner B, Schwarzer R. Risikostereotype, Risikowahrnehmung und Risikoverhalten im Zusammenhang mit HIV. Zeitschrift für Gesundheitspsychologie. 2003;11(3):112–21. [Google Scholar]
- Sabatinelli, D., Bradley, M.M., Fitzsimmons, J.R., Lang, P.J. (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage, 24(4), 1265–70. [DOI] [PubMed]
- Schaller M, Duncan LA. The behavioral immune system: Its evolution and social psychological implications. In: Forgas JP, Haselton MG, von Hippel W, editors. Evolution and the Social Mind: Evolutionary Psychology and Social Cognition. New York: Psychology Press; 2007. pp. 293–307. [Google Scholar]
- Schaller M, Miller GE, Gervais WM, Yager S, Chen E. Mere visual perception of other people's' disease symptoms facilitates a more aggressive immune response. Psychological Science. 2010;21(5):649–52. doi: 10.1177/0956797610368064. [DOI] [PubMed] [Google Scholar]
- Schmälzle R, Barth. A, Renner B, Schupp HT. Implicit and explicit processes in risk perception: neural antecedents of perceived HIV risk. Frontiers in Human Neuroscience. 2011;5(43):1–10. doi: 10.3389/fnhum.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R, Häcker F, Renner B, Honey CJ, Schupp HT. Neural correlates of risk perception during real-life risk communication. Journal of Neuroscience. 2013;33(25):10340–7. doi: 10.1523/JNEUROSCI.5323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R, Renner B, Schupp HT. Neural correlates of perceived risk: the case of HIV. Social Cognitive and Affective Neuroscience. 2012;7(6):667–76. doi: 10.1093/scan/nsr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex. 2012;22(1):158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovic P, Peters E. Risk perception and affect. Current Directions in Psychological Science. 2006;15(6):322–5. [Google Scholar]
- Thompson SC, Anderson K, Freedman D, Swan J. Illusions of safety in a risky world: a study of college students’ condom use. Journal of Applied Social Psychology. 1996;26(3):189–10. [Google Scholar]
- Thompson SC, Kyle D, Swan J, Thomas C, Vrungos S. Increasing condom use by undermining perceived invulnerability to HIV. AIDS Education and Prevention. 2002;14(6):505–14. doi: 10.1521/aeap.14.8.505.24115. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.