Abstract
This study investigated the attention modulation of disgust in comparison with anger in a dot-probe task. Results indicated a two-stage processing of attention modulation by threats. When participants viewed the cues that were represented by Chinese faces (i.e. the in-group condition), it was found at the early processing stage that an angry face elicited a larger occipital P1 component whereas a disgusted face elicited a smaller P1 for validly than for invalidly cued targets. However, the result pattern was reversed at the later processing stage: the P3 amplitudes were larger for valid disgust cues but were smaller for valid angry cues, when both were compared with invalid cue conditions. In addition, when participants viewed the cues that were represented by foreign faces (i.e. the out-group condition), the attention modulation of disgust/anger diminished at the early stage, whereas enhanced P3 amplitudes were observed in response to validly cued targets in both disgusting and angry conditions at the later stage. The current result implied that although people can perceptually differentiate the emotional categories of out-group faces as accurately as in-group faces, they may still be not able to psychologically understand the subtle differences behind different categories of out-group facial expressions.
Keywords: disgust, anger, attention, threat-related emotion
INTRODUCTION
Disgust, as a basic emotion like fear, anger, sadness and happiness, is found across all cultures. Like other threat-related emotions (anger and fear), disgust represents a certain set of stimuli that signify potential danger in our environment, e.g. rotting food or dirty animals, which would contaminate individuals both physically and psychologically (Oaten et al., 2009). The experience of disgust would typically result in a distinctive facial expression, characterized as wrinkled nose, mouth agape and raised lips (Ekman et al., 1975). Functional magnetic resonance imaging (fMRI) studies have revealed the enhanced activation of the anterior insular cortex for feeling disgust or perceiving facial expressions of disgust (Phillips et al., 1997; Wicker et al., 2003). Intracerebral event-related potentials (ERPs) revealed specific potentials in response to disgust beginning 300 ms after the stimulus onset and lasting 200 ms in the ventral anterior insula (Krolak-Salmon et al., 2003).
The adaptive function of disgust is to facilitate avoidance, preventing people from biological/psychological contamination as soon as possible (Jones, 2007). As one of the threat-related emotions, disgust is associated with the evolutionary-precoded responses of reduced blood pressure, decreased heart rate and skin conductance (Oaten et al., 2009). Although disgust is assumed to appropriately interact with cognition, so far as we know, little effort has been devoted to investigating the neural substrates/correlates of how disgust affects specific cognitive processes (Curtis et al., 2011). Given the indispensability of attention to cognitive processing and its sensitivity to emotional modulation (Kastner and Ungerleider, 2000), the current study mainly focused on the attention modulation of disgust. To date, this direction of research topics has been empirically studied at behavioral level, and some inconsistent results have been reached (Charash and McKay, 2002; Charash et al., 2006). For instance, previous behavioral studies have found an attention bias toward disgust words: participants had more interference when responding to disgust words in the Stroop color-naming task (Charash and McKay, 2002); they also responded faster to disgust words in the backward masking task, when compared with the neutral condition (Charash et al., 2006). In contrast, some researchers have observed the attention suppression effects of disgust, as evidenced by reduced attentional blinks when compared with the neutral condition in an attentional blink task, where facial expression pictures were employed as primes (Vermeulen et al., 2009). Such inconsistency may be partially due to the limitation of behavioral measurements: behavioral data alone could not reveal whether the attention modulation of disgust happens at the early sensory perceptual stage or at the later post-perceptual stage. The ERP technique has a high time resolution and therefore it will be an ideal method for use in tracking dynamic changes of neural activities in distinct processing stages.
Threat-related emotions are traditionally associated with enhanced attentional resources (Vuilleumier et al., 2001; Pessoa et al., 2002; Vuilleumier, 2005). For instance, when a person manipulates attention and emotion independently, unattended fearful faces elicited a larger P1 automatically, representing neural indexes of bottom-up processing (Oya et al., 2002; Schupp et al., 2007; Zhang et al., 2014). A research conducted by Pourtois et al. (2005) has demonstated that such quick response mainly depended on a visual pathway preferentially tuned to coarse-magnocellular inputs (i.e. low spatial-frequency information), and that the response could persist unchanged even when the recognition of the object was disrupted. Furthermore, when participants explicitly attended to fearful rather than neutral stimuli, ERP studies have revealed an enlarged central P3, serving as neural correlates of emotion modulation (Schupp et al., 2004a,b; Conroy and Polich, 2007). Taken together, these results suggest that visual attention can be involuntarily attracted by threat-related emotions or voluntarily directed toward them. However, some studies that focused on the influence of different threat-related emotions have found that even with similar valence ratings, fear and anger can influence judgment and choice in different ways (Lerner and Keltner, 2000). More recently,Krusemark and Li (2013) asked participants to search the horizontal bar among seven vertical bars with fearful, disgusting or neutral affective pictures as visual backgrounds, and a rapid discrimination between the two threat-related emotions was shown as early as 96 ms after the stimulus onset, represented by larger occipital P1 amplitudes in the fearful condition and smaller P1 amplitudes in the disgusting condition. The result indicated that whereas fear enhanced attention, disgust suppressed it (Krusemark and Li, 2013). Therefore, many researchers have suggested that the interaction between attention and certain emotions may be determined by its evolutionary purpose per se, rather than by the valence (Brosch et al., 2008). Disgusting images usually suppress visual attention to minimize the exposure to threats, resulting in inhibited behavioral/ERP responses. In the current study, we compared disgust with another threat-related emotion and made the two emotions have similar valence and arousal. Anger was selected mainly because of two reasons. First, anger and disgust share common facial action pattern which serves to diminish sensory exposure (i.e. a facial pattern of closure) whereas the facial action pattern of surprise and fear relates to sensory exposure (Susskind and Anderson, 2008; Susskind et al., 2008). Second, anger is the only type of threat-related emotions that tends to promote approach rather than avoidance behavior (Hutcherson and Gross, 2011), so anger and disgust have relatively opposite approach–avoidance responses.
The present study mainly aimed to compare the effect of attention modulation between disgust and anger and to demonstrate how disgust affects attention in order to facilitate its evolutionary-precoded responses. We recorded ERP and behavioral responses when participants performed a standard dot-probe task (MacLeod et al., 1986; Brosch et al., 2008). This task is an often-used paradigm to investigate selective attention to threat, with a facilitated response to the target that appears at the same location of threat (Yiend, 2010). In this study, two facial expressions, i.e. one angry/disgusted face and one neutral face, were used as cues in the experiment. Previous ERP results have indicated that automatic attention attraction by emotionally significant stimuli may reliably enhance occipital P1 in the dot-probe task, representing an increased perception in the visual cortex (Pollak and Tolley-Schell, 2003; Pourtois et al., 2004; Brosch et al., 2008). Furthermore, participants may also explicitly pay more attention to emotionally significant stimuli in the dot-probe task, as evidenced by a larger P3 in ERPs (Pollak and Tolley-Schell, 2003). Therefore, we hypothesized that the attention modulation of disgust is opposite to that of anger. For instance, we predicted that an angry face may attract attention rapidly, resulting in an early attention orienting as evidenced by a larger P1, paralleled by a facilitated behavior response; conversely, a disgusted face may generate an opposite pattern of effects, reflected by a reduced P1 and a slowed reaction time.
In addition, it would be also interesting to investigate whether the effects of emotional faces on attention differ when the face is expressed by members of another culture group. Some previous studies have demonstrated that face recognition is generally more accurate for perceivers from the same cultural group as emotional expresser, termed as ‘in-group advantage’ (Elfenbein and Ambady, 2002a,b, 2003a; Nelson and Russell, 2013). From an evolutionary view, such in-group advantage prevents people from wasting too much energy to empathy stranger, but instead it facilitates emotion commutation between people in the same-culture group (Elfenbein and Ambady, 2003b). In addition to emotion recognition, the in-group advantage also exists in other cognitive processes (Sporer et al., 2007; Ambady and Bharucha, 2009). For example, an eye-tracking study found that people are robustly less sensitive to the changes made in other-race faces (Hirose and Hancock, 2007). Furthermore, one fMRI study showed greater recruitment of bilateral posterior superior temporal sulci in the same-culture mental state decoding, compared with that in other-culture condition (Adams et al., 2010). Based on these results, we hypothesized that the attention modulation of threat-related emotions may be more significant in the condition with facial stimuli expressed by the same cultural group.
METHODS
Participants
Sixty healthy subjects (30 females; age range = 21–26 years) were recruited from Beijing Normal University in China as paid participants. They were randomly assigned into two groups: behavioral experiment (n = 30) and ERP experiment (n = 30). All subjects were right-handed and had normal or corrected-to-normal vision. They gave their written informed consent prior to the experiment.
Stimuli
Chinese faces were selected from the native Chinese Facial Affective Picture System (Gong et al., 2011), with an equal number of face pictures of males and females. A total of 80 faces (20 disgusted, 20 angry and 40 neutral faces) were used. Meanwhile, 80 foreign faces were selected from the NimStim Set of Facial Expressions (http://www.macbrain.org/resources.htm) (20 disgusted, 20 angry and 40 neutral faces). Among the two sets of 80 facial pictures, 120 pictures (40 disgusted, 40 angry and 40 neutral faces) were collected from 40 actors/actresses. The other 40 neutral facial pictures were collected from another 40 actors/actresses. Notably, to prevent our results from being contaminated by different familiarities between Chinese and foreign faces (Jack et al., 2009), we carefully matched the recognition rates of these two stimulus sets, so that any significant differences due to culture belonging cannot be accounted for by varied recognition difficulties between facial expressions.
Each picture had been assessed for its valence and arousal on a 9-point scale, as well as recognition rates with a large sample of Chinese participants in a previous survey. The univariate ANOVA performed on the average valence scores showed that the two categories of threat-related faces did not differ significantly in emotional valence [F(2,154) = 173, p < 0.001, = 0.692; mean ± standard error: disgust = 3.00 ± 0.085, anger = 2.83 ± 0.085, neutral = 4.49 ± 0.060; disgust vs anger: p = 0.509] while their valence ratings significantly differed from neutral faces (ps < 0.001). The univariate ANOVA performed on the average arousal scores showed that the two categories of threat-related faces did not differ significantly in arousal [F(2,154) = 129, p < 0.001, = 0.627; disgust = 6.03 ± 0.147, anger = 6.17 ± 0.147, neutral = 3.75 ± 0.104; disgust vs. anger: p = 1.000] while their arousal ratings significantly differed from the neutral faces (ps < 0.001). In addition, the main effect of culture belonging was significant in arousal [F(1,154) = 8.91, p = 0.003, = 0.055]; the arousal scores were higher in response to Chinese faces (5.55 ± 0.109) than foreign faces (5.086 ± 0.109). No significant interaction effect was found in arousal between emotion and culture belonging [F(2,154) < 1]. The univariate ANOVA performed on the average recognition rates showed that neither the main effect of culture belonging [F(1,154) < 1, Chinese faces = 0.88 ± 0.04, foreign faces = 0.86 ± 0.05] and emotion [F(2,154) < 1, disgust = 0.87 ± 0.02, anger = 0.86 ± 0.03, neutral = 0.89 ± 0.01] nor the interaction between them [F(2,154) < 1] was significant.
All faces were gray-scale photographs. They were presented with the same contrast and brightness on the black background (3.0° × 3.5° visual angle). The upper and lower triangles that were used as targets were white (1.2° × 1.2° visual angle).
Procedure
Stimuli were presented on a LCD monitor at a viewing distance of ∼100 cm. The experiment consisted of four blocks (Chinese disgust, Chinese anger, foreign disgust and foreign anger), each containing 160 trials. The order of the four blocks was pseudorandomized across subjects. Blocks were separated by self-terminated breaks.
The design of the dot-probe task was very similar to that used in previous studies (MacLeod et al., 1986; Brosch et al., 2008). As shown in Figure 1, each trial started with a 300-to-600-ms fixation, followed by a 100-ms cue that consisted of two faces. In the two blocks of foreign faces, the cue was represented by two foreign faces; in the two blocks of Chinese faces, the cue was represented by two Chinese faces. In the two disgusted blocks, the cue was represented by a disgusted and a neutral face; in the two angry blocks, the cue was represented by an angry and a neutral face. Each face was presented eight times in a random order in corresponding blocks. The location of the neutral face in each trial was equally likely to be left or right. After the cue and a short interval (100–300 ms), a target (one upper or lower triangle) was presented with the duration of 150 ms. In valid trials, the target appeared at the location previously occupied by the emotional face; in invalid trials, the target appeared at the location previously occupied by the neutral face. Valid and invalid trials were presented in random order with equal probability (50% each). The location of the target in each trial was equally likely left or right. After the presentation of the target, the subjects were required to respond as quickly and as accurately as possible regarding the location of the triangle, pressing the ‘F’ key for the left location and the ‘J’ key for the right location on the computer keyboard with their left and right index fingers. The response screen would not disappear until a button press or until 1000 ms elapsed. Responses with latencies of <1000 ms were considered valid.
Fig. 1.
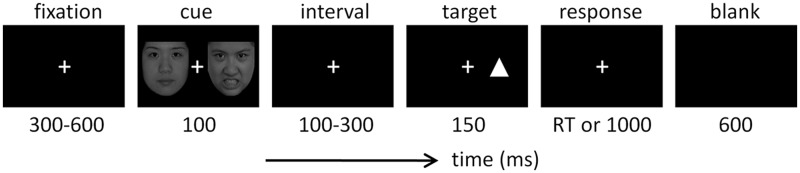
Illustration of one experimental trial in this study.
Participants were required to respond only to one kind of triangle (the upper or the lower) during the experiment. The assignment of the target as an upper or lower triangle was counterbalanced between participants. The only difference between the electroencephalography (EEG) and the behavioral tasks was that 10 and 50% trials required a motor response during the two experiments, respectively (Brosch et al., 2008).
Behavioral measures
This study analyzed the accuracy rate (ACC) and reaction time (RT) recorded in the ERP experiment (n = 30, 10% target design, 16 trials in each condition) and in the behavioral experiment (n = 30, 50% target design, 80 trials in each condition).
EEG recording and analysis
Brain electrical activity was recorded referentially against left mastoid and offline re-referenced to the average of the left and right mastoids, by a 64-channel amplifier with a sampling frequency of 250 Hz (NeuroScan Inc., Herndon, USA). Besides electrooculogram electrodes, 62-channel EEG data were collected with electrode impedances kept below 5 kΩ. Ocular artifacts were removed from EEGs by using a regression procedure implemented in NeuroScan software (Scan 4.3).
The recorded EEG data were filtered with a 0.01–30 Hz finite impulse response filter with a zero phase distortion. Filtered data were segmented beginning 100 ms prior to the onset of targets (i.e. triangles) and lasting for 1000 ms. All epochs were baseline-corrected with respect to the mean voltage over the 100 ms preceding the onset of targets, followed by averaging in association with experimental conditions. To prevent the ERP results from being contaminated by movement-related potentials, the average ERPs of the 30 subjects were computed based on non-response trials (160 × 90% = 144 trials per condition).
We analyzed the amplitudes of occipital P1 and parietal P3 components across different sets of electrodes in accordance with grand-mean ERP topographies and relevant literatures (Luck, 2005; Zhang et al., 2013a). The mean amplitude of P1 was calculated at the electrode sites of O1, O2, PO3 and PO4 (time window = 110–140 ms). The mean amplitude of P3 was calculated at CP1, CPz, CP2, P1, Pz and P2 (time window = 450–650 ms).
Statistics
Descriptive data were presented as mean ± standard deviation (SD) unless otherwise noted. The significance level was set at 0.05. In order to directly investigate the attention modulation of emotions in the dot-probe experiment, this study employed a differential measurement, namely, the attentional bias score for further analyses (see also Lubman et al., 2000; Townshend and Duka, 2001). The attentional bias score was calculated by subtracting the data of the validly cued condition from the associated data of the invalidly cued condition. Then, two-way repeated-measures ANOVAs were performed on the attentional bias scores of ACC, RT, the P1 amplitude and the P3 amplitude, with culture belonging of the faces (Chinese vs foreign) and emotion of the faces (disgust vs anger) as within-subject factors. Partial eta-squared () was reported to demonstrate the effect size in the ANOVA tests.
RESULTS
ACC
The ACC in the behavioral experiment was 98.8 ± 1.71%. The attentional bias score of ACC in 2 (culture belonging) × 2 (emotion) conditions was below 5% (0.05 ± 1.8%). No significant difference was found between conditions.
The ACC in the ERP experiment was 96.7 ± 2.01%. The attentional bias score of ACC in 2 × 2 conditions was below 7% (2.01 ± 2.4%). No significant difference was found between conditions.
RT
In the behavioral experiment, the interaction effect of culture belonging by emotion on the attentional bias score of RT was significant [F(1,29) = 9.93; p = .004; = 0.255]. Simple effect analysis indicated that the effect of culture belonging significantly influenced the attentional bias score of RT. When the cue was presented using Chinese faces [F(1,29) = 15.0; p = 0.001], the attentional bias score of RT following the disgusted-face cue (−5.57 ± 11.3 ms) was significantly smaller than that following the angry-face cue (4.85 ± 9.20 ms). However, the foreign faces did not significantly influence the attentional bias score of RT between emotions [F(1,29) = 1.44; p = 0.241; disgust = 2.92 ± 9.22 ms; anger = − 0.44 ±11.8 ms].
In the ERP experiment, the main effect of culture belonging on the attentional bias score of RT was significant [F(1,29) = 4.63; p = 0.040; = 0.138]. The attentional bias score of RT following the Chinese-face cue (−0.66 ± 6.48 ms) was smaller than that following the foreign-face cue (7.05 ± 5.86 ms).
P1
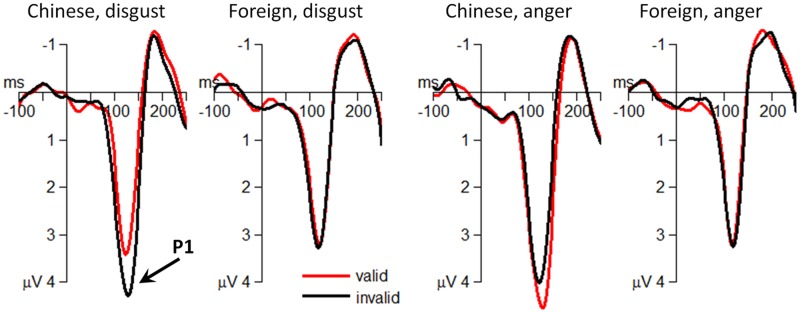
The interaction effect of culture belonging by emotion on the attentional bias score of P1 amplitudes was significant [F(1,29) = 12.3; p = 0.002; = 0.298] (Figure 2). Simple effect analysis indicated that the effect of culture belonging significantly influenced the attentional bias score of P1 amplitudes. When the cue was presented using Chinese faces [F(1,29) = 33.5; p < 0.001], the attentional bias score of P1 amplitudes following the disgusted-face cue (0.74 ± 0.93 μV) was significantly larger than that following the angry-face cue (−0.71 ± 0.85 μV). However, the foreign faces did not significantly influence the attentional bias score of P1 amplitudes between emotions [F(1,29) < 0.01; disgust = 0.16 ± 0.75 μV; anger = 0.15 ± 1.61 μV].
Fig. 2.
The grand-mean ERP waveforms at the occipital electrode site of O2.
P3
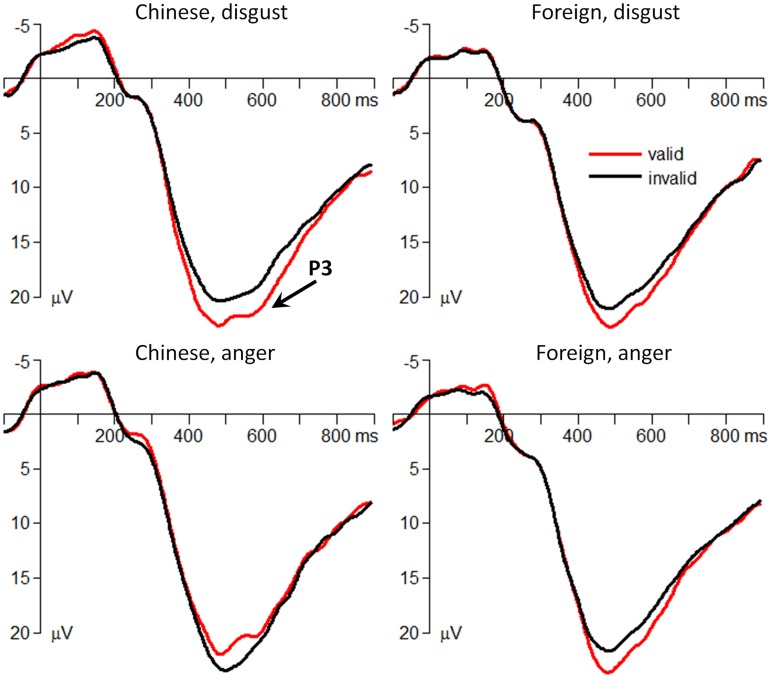
The interaction effect of culture belonging by emotion on the attentional bias score of P3 amplitudes was significant [F(1,29) = 15.5; p < 0.001; = 0.348] (Figure 3). Simple effect analysis indicated that the effect of culture belonging significantly influenced the attentional bias score of P3 amplitudes. When the cue was presented using Chinese faces [F(1,29) = 21.1; p < 0.001], the attentional bias score of P3 amplitudes following the disgusted-face cue (−2.64 ± 4.01 μV) was significantly smaller than that following the angry-face cue (1.65 ± 2.61 μV). However, the foreign faces did not significantly influence the attentional bias score of P3 amplitudes between emotions [F(1,29) < 1; disgust = −1.43 ± 3.71 μV; anger = −1.95 ± 2.47 μV]. In addition, one-sample t-test showed that the two (disgust and anger) attentional bias scores in the foreign-face condition were significantly smaller than zero [disgust: t(29)= −2.12, p =0.042; anger: t(29)= −4.32, p < 0.001].
Fig. 3.
The grand-mean ERP waveforms at the parietal electrode site of Pz.
DISCUSSION
The present study aimed to test the hypothesis that in order to facilitate the evolutionary goal of disgust, this emotion suppresses rather than enhances attention. Our results revealed that when participants viewed the angry faces of the host members, the P1 component showed higher amplitudes for validly than for invalidly cued targets. Conversely, the P1 amplitudes were significantly lower for validly than for invalidly cued targets in the disgusted condition. This P1 result was well in line with behavior data—angry cues facilitated while disgusted cues inhibited behavior responses in the valid cue condition. Interestingly, the ERP pattern was reversed at a later processing stage as evidenced by the parietal P3—the P3 amplitudes were larger for valid disgusted cues and smaller for valid angry cues, when both were compared with corresponding invalid cue conditions. In addition, consistent with our hypothesis on culture belonging, the attention modulation of disgust and anger was diminished at an early stage when participants viewed emotional faces of the non-host members.
At the early processing stage in the Chinese-face condition (the in-group condition), the finding of suppressed attention caused by disgust was remarkable; this was because traditional effects of threat-related stimuli were assumed to attract rather than to suppress attention (Rozin and Royzman, 2001; Vuilleumier, 2005; Vaish et al., 2008). The occipital P1 has been proved to be sensitive to early emotional modulation in visual perception (Schupp et al., 2007; Eldar and Bar-Haim, 2010; Zhang et al., 2013b). In the current study, whereas Chinese angry faces attracted attention, Chinese disgusted faces generated an opposite pattern of effects, reflected by a reduced P1 in valid condition, paralleled by inhibited behavior responses. Previous studies also supported the finding that angry faces attracted while disgusted faces suppressed attention. For instance, researchers found divergent effects of fear and disgust on attention, as represented by a larger occipital P1 in fearful but a smaller P1 in disgusting condition (Krusemark and Li, 2013). In our opinion, the present findings are in accordance with the evolutionary purposes of anger and disgust. Angry faces typically signal a threat-related consequence of social interaction or an attempt to control or change the behavior of others (Neuberg et al., 2011). When facing angry faces, people are required to respond quickly and correctly, because if they do not they might get hurt (De Quervain et al., 2004; O’Gorman et al., 2005). Therefore, angry faces may attract attention to facilitate self-protective behavior or to ward off challengers in competition for resources, status or territory (Ewbank et al., 2009). Heightened attention and facilitated task performance conform to the well-established role of anger to promote approach tendencies (Hutcherson and Gross, 2011). On the contrary, a disgusted facial expression is a form of threat related to physical or psychological contamination, typically associated with cognitive avoidance (Curtis et al., 2011). The clear departure of disgust-induced P1 between valid and invalid cue conditions implies an involuntary attention suppression to maintain the goal of avoidance to threats (Curtis et al., 2011). Taking the inhibited behavior results together, we can infer that disgust may firstly initiate a distinct downstream operation of attention suppression, which is the preparation for later disgust-specific actions of avoidance.
At the later processing stage in the in-group condition, the attention of participants was not always attracted to the position of the pre-target emotional cue. In fact, the result pattern at this stage was reversed as compared with that at the early stage: while Chinese disgusted faces generated a higher P3 for validly than for invalidly cued targets, Chinese angry faces generated a lower P3 for validly than for invalidly cued targets instead. One possible explanation for the P3 phenomenon is that this component may reflect top-down modulation and is associated with the voluntary orienting of attention (Friedman et al., 2001; Polich, 2007; Zhang et al., 2014). According to the principles of least effort (Zipf, 1949; i Cancho and Solé, 2003), when the target replaced the location of a disgusted face, due to the early attention suppression of disgust, participants needed to exert a top-down modulation to voluntarily orient more attention toward the target so as to ensure a quick behavioral response, therefore resulting in a larger P3 for validly than for invalidly cued targets. Conversely, participants did not need to allocate extra attention to the location of an angry face at the valid condition because they have already paid attention to this location as soon as the cues were presented, thus resulting in a smaller P3 for validly than for invalidly cued targets. The current result suggested that the attention modulation of emotions at the later stage is voluntarily controlled; thus, attention could be flexibly tuned to fit cognitive goals with least effort. This result is consistent with a previous study, which trained anxious participants to voluntarily avoid threat and found a smaller P3 in attention-related tasks after training (indicating a more eased attention control) (Eldar and Bar-Haim, 2010). Another possible explanation for the P3 phenomenon may be due to the interaction effect of endogenous attention and emotional categories of the cues. On the one hand, the angry faces intensively attracted attention (Fox et al., 2000), which was evidenced by the P1 activity in the current study. When the target did not appear at the location of the angry cue, participants needed more attention resources to disengage their attention from the location of anger and attend to the target (Koster et al., 2004; Belopolsky et al., 2011), therefore resulting in a larger P3 for the invalid- than for the valid-cued condition. On the other hand, disgust faces first diverted participants’ attention, so at the later stage participants needed to devote more attention to the location of the disgusted cue, resulting in a larger P3 for the valid- than for the invalid-cued condition. However, these two potential P3 mechanisms need more supporting data to be confirmed.
In addition, the finding of clear departure of the attention modulation effects between in-group and out-group conditions was interesting. We found in the out-group condition that the attention modulation of disgust/anger diminished at the early stage, whereas it was relatively unaffected at the later stage (enhanced P3 amplitudes in response to validly cued targets in both disgusting and angry conditions). This result suggested that the culture belonging mainly affects the early stage of attention modulation by threat-related emotions. It is noteworthy that the diminished attention modulation at an early stage cannot be accounted for by the higher difficulty in recognizing facial expressions of the non-host members (Jack et al., 2009). Instead, the result may be due to the higher arousal scores of Chinese faces compared with foreign faces, as high arousal is usually assumed to represent high motivation-driven attention (Schupp et al., 2004a). The similar ERP pattern at the later stage between foreign angry and disgusted cues suggested that Chinese participants treated anger and disgust as general threats, both resulting in more attention allocation compared with the neutral condition. Our result implied that although people can perceptually differentiate the emotional categories of out-group faces as accurately as in-group faces, they still cannot psychologically understand the subtle differences behind different categories of out-group facial expressions.
The culture difference is a universal phenomenon, existing across fundamental domains of cognitive and social psychology. In the domain of perception, several studies suggested that Westerners tend to focus on objects whereas East Asians tend to focus on contextual and background information (e.g. Gutchess et al., 2006). In the domain of social cognition, individuals from Western cultures tend to value uniqueness and freedom and view the self as independent of others, whereas individuals from cultures like China tend to value social harmony and adherence to group norms and view the self as interconnected and interdependent with others (e.g. Zhu et al., 2007). However, despite the widespread culture difference in cognition, few studies directly examined the reasons behind it. Recently, by reviewing links between culture and brain, Ambady and Bharucha (2009) suggested there may be at least three reasons for cultural differences, i.e. genetic difference, cultural learning mediated by brain plasticity and the degree of similarity between cultural environments. We think the three reasons all seem to be the plausible sources of the current findings. Further studies on this issue may shed novel insight on emotion processing and help to solve the misunderstandings of social interactions between different culture groups.
Finally, it should be pointed out that this study took a basic emotion framework for granted and investigated the attention modulation of two discrete emotions. However, the brain basis of emotion are still in debate: the locationist account of emotion assumes that discrete emotion categories consistently and specifically correspond to distinct brain regions; the psychological constructionist account of emotion assumes that emotions are constructed of more general brain networks which are not specific to discrete categories (Lindquist et al., 2012). The current study did not aim to support either of the theories. Instead, our results implied that anger and disgust can be separated to some degree as regard to their distinct ERP responses in this experiment. This opinion is supported by Ekman and Cordaro (2011), who argued that the discrete view of emotion does not necessarily equal to locationist approach, and that each discrete emotion may be triggered by an inherited mechanism that corresponds to a specific pattern of autonomic nervous system activity.
CONCLUSION
To sum up, this study examined how disgust facilitates avoidance by investigating the attention modulation of disgust/anger in a dot-probe task. It was found that at the early processing stage in the in-group condition, an angry face elicited a larger occipital P1 component whereas a disgusted face elicited a smaller P1 for validly than for invalidly cued targets. However, the result pattern was reversed at the later processing stage: the P3 amplitudes were larger for valid disgust cues but were smaller for valid angry cues, when both were compared with invalid cue conditions. However, the attention modulation of disgust/anger diminished at the early stage in the out-group condition, and enhanced P3 amplitudes were observed in response to validly cued targets in both disgusting and angry conditions at the later stage. Considering that the diminished attention modulation at an early stage cannot be accounted for by the higher difficulty in recognizing facial expressions of the out-group members, the current result implied that although people can perceptually differentiate the emotional categories of out-group faces as accurately as in-group faces, they may still be not able to psychologically understand the subtle differences behind different categories of out-group facial expressions.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (31300867) and the National Key Basic Research Program of China (973 Program, 2014CB744600).
REFERENCES
- Adams RB, Jr, Rule NO, Franklin RG, Jr, et al. Cross-cultural reading the mind in the eyes: an fMRI investigation. Journal of Cognitive Neuroscience. 2010;22:97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Ambady N, Bharucha J. Culture and the brain. Current Directions in Psychological Science. 2009;18:342–5. [Google Scholar]
- Belopolsky AV, Devue C, Theeuwes J. Angry faces hold the eyes. Visual Cognition. 2011;19:27–36. [Google Scholar]
- Brosch T, Sander D, Pourtois G, Scherer KR. Beyond fear rapid spatial orienting toward positive emotional stimuli. Psychological Science. 2008;19:362–70. doi: 10.1111/j.1467-9280.2008.02094.x. [DOI] [PubMed] [Google Scholar]
- Charash M, McKay D. Attention bias for disgust. Journal of Anxiety Disorders. 2002;16:529–41. doi: 10.1016/s0887-6185(02)00171-8. [DOI] [PubMed] [Google Scholar]
- Charash M, McKay D, Dipaolo N. Implicit attention bias for disgust. Anxiety, Stress, and Coping. 2006;19:353–64. [Google Scholar]
- Conroy MA, Polich J. Affective valence and P300 when stimulus arousal level is controlled. Cognition and Emotion. 2007;21:891–901. [Google Scholar]
- Curtis V, de Barra M, Aunger R. Disgust as an adaptive system for disease avoidance behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:389–401. doi: 10.1098/rstb.2010.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quervain DJF, Fischbacher U, Treyer V, et al. The neural basis of altruistic punishment. Science. 2004;305:1254–8. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Ekman P, Cordaro D. What is meant by calling emotions basic. Emotion Review. 2011;3:364–70. [Google Scholar]
- Ekman P, Friesen WV, Press CP. Pictures of Facial Affect. New York: Consulting Psychologists Press; 1975. [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40:667–77. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA, Ambady N. Is there an in-group advantage in emotion recognition? Psychological Bulletin. 2002a;128:243–9. doi: 10.1037/0033-2909.128.2.243. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA, Ambady N. On the universality and cultural specificity of emotion recognition: a meta-analysis. Psychological Bulletin. 2002b;128:203–35. doi: 10.1037/0033-2909.128.2.203. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA, Ambady N. When familiarity breeds accuracy: cultural exposure and facial emotion recognition. Journal of Personality and Social Psychology. 2003a;85:276–86. doi: 10.1037/0022-3514.85.2.276. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA, Ambady N. Universals and cultural differences in recognizing emotions. Current Directions in Psychological Science. 2003b;12:159–64. [Google Scholar]
- Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, Calder AJ. Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage. 2009;44:1144–51. doi: 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: are angry faces detected more efficiently? Cognition and Emotion. 2000;14:61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gong X, Huang Y, Wang Y, Luo Y. Revision of the Chinese facial affective picture system. Chinese Mental Health Journal. 2011;25:40–6. [Google Scholar]
- Gutchess AH, Welsh RC, Boduroĝlu A, Park DC. Cultural differences in neural function associated with object processing. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:102–9. doi: 10.3758/cabn.6.2.102. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Hancock PJB. Equally attending but still not seeing: an eye-tracking study of change detection in own-and other-race faces. Visual Cognition. 2007;15:647–660. [Google Scholar]
- Hutcherson CA, Gross JJ. The moral emotions: a social–functionalist account of anger, disgust, and contempt. Journal of Personality and Social Psychology. 2011;100:719. doi: 10.1037/a0022408. [DOI] [PubMed] [Google Scholar]
- i Cancho RF, Solé RV. Least effort and the origins of scaling in human language. Proceedings of the National Academy of Sciences. 2003;100:788–91. doi: 10.1073/pnas.0335980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack RE, Blais C, Scheepers C, Schyns PG, Caldara R. Cultural confusions show that facial expressions are not universal. Current Biology. 2009;19:1543–8. doi: 10.1016/j.cub.2009.07.051. [DOI] [PubMed] [Google Scholar]
- Jones D. Moral psychology: the depths of disgust. Nature. 2007;447:768–71. doi: 10.1038/447768a. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy. 2004;42:1183–92. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Hénaff MA, Isnard J, et al. An attention modulated response to disgust in human ventral anterior insula. Annals of Neurology. 2003;53:446–53. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Krusemark EA, Li W. From early sensory specialization to later perceptual generalization: dynamic temporal progression in perceiving individual threats. The Journal of Neuroscience. 2013;33:587–94. doi: 10.1523/JNEUROSCI.1379-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Beyond valence: toward a model of emotion-specific influences on judgement and choice. Cognition and Emotion. 2000;14:473–93. [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences. 2012;35:121–43. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychological Medicine. 2000;30:169–75. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-related Potential Technique, London. UK: The MIT Press; 2005. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Nelson NL, Russell JA. Universality revisited. Emotion Review. 2013;5:8–15. [Google Scholar]
- Neuberg SL, Kenrick DT, Schaller M. Human threat management systems: self-protection and disease avoidance. Neuroscience & Biobehavioral Reviews. 2011;35:1042–51. doi: 10.1016/j.neubiorev.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman R, Wilson DS, Miller RR. Altruistic punishing and helping differ in sensitivity to relatedness, friendship, and future interactions. Evolution and Human Behavior. 2005;26:375–87. [Google Scholar]
- Oaten M, Stevenson RJ, Case TI. Disgust as a disease-avoidance mechanism. Psychological Bulletin. 2009;135:303–22. doi: 10.1037/a0014823. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. The Journal of Neuroscience. 2002;22:9502–12. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider L. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences. 2002;99:11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112:323–35. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: time course and topographic evoked-potentials mapping. Human Brain Mapping. 2005;26:65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex. 2004;14:619–33. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5:296–320. [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004a;41:441–9. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Öhman A, Junghöfer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004b;4:189–201. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Selective visual attention to emotion. The Journal of Neuroscience. 2007;27:1082–9. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporer SL, Trinkl B, Guberova E. Matching faces differences in processing speed of out-group faces by different ethnic groups. Journal of Cross-Cultural Psychology. 2007;38:398–412. [Google Scholar]
- Susskind JM, Anderson AK. Facial expression form and function. Communicative and Integrative Biology. 2008;1(2):148–9. doi: 10.4161/cib.1.2.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind JM, Lee DH, Cusi A, Feiman R, Grabski W, Anderson AK. Expressing fear enhances sensory acquisition. Nature Neuroscience. 2008;11:843–50. doi: 10.1038/nn.2138. [DOI] [PubMed] [Google Scholar]
- Townshend J, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology. 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Vaish A, Grossmann T, Woodward A. Not all emotions are created equal: the negativity bias in social-emotional development. Psychological Bulletin. 2008;134:383–401. doi: 10.1037/0033-2909.134.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen N, Godefroid J, Mermillod M. Emotional modulation of attention: fear increases but disgust reduces the attentional blink. PLoS One. 2009;4:e7924. doi: 10.1371/journal.pone.0007924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: a review of attentional processing of emotional information. Cognition and Emotion. 2010;24:3–47. [Google Scholar]
- Zhang D, Gu R, Wu T, et al. An electrophysiological index of changes in risk decision-making strategies. Neuropsychologia. 2013a;51:1397–407. doi: 10.1016/j.neuropsychologia.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, He W, Wang T, et al. Three stages of emotional word processing: an ERP study with rapid serial visual presentation. Social Cognitive and Affective Neuroscience. 2014;9:1897–903. doi: 10.1093/scan/nst188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Luo W, Luo Y. Single-trial ERP analysis reveals facial expression category in a three-stage scheme. Brain Research. 2013b;1512:78–88. doi: 10.1016/j.brainres.2013.03.044. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. Neuroimage. 2007;34:1310–16. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Zipf GK. Human Behavior and the Principle of Least Effort. London, UK: Addison-Wesley Press; 1949. [Google Scholar]