Abstract
Genetic moderation of interpersonal psychotherapy (IPT) efficacy for economically disadvantaged women with major depressive disorder was examined. Specifically, we investigated whether genotypic variation in corticotropin releasing hormone receptor 1 (CRHR1) and the serotonin transporter gene (5-HTT) moderated effects of IPT on depressive symptoms over time. We also tested genotype moderation of IPT mechanisms social adjustment and perceived stress. Non-treatment seeking urban women at or below the poverty level with infants were recruited from the community (N = 126; M age = 25.33; SD = 4.99; 54.0% African-American, 22.2% Caucasian, and 23.8% Hispanic/biracial) and randomized to individual IPT or enhanced community standard (ECS). Results revealed that changes in depressive symptoms over time depended on both intervention group and genotypes (5-HTTLPR and CRHR1). Moreover, multiple-group path analysis indicated that IPT improved depressive symptoms, increased social adjustment and decreased perceived stress at post-treatment among women with the 0 copies of the CRHR1 TAT haplotype only. Finally, improved social adjustment at post-intervention significantly mediated the effect of IPT on reduced depressive symptoms at 8 months post-intervention for women 0 copies of the TAT haplotype only. Post-hoc analyses of 5-HTTLPR were indicative of differential susceptibility, albeit among African-American women only.
Over 26% of Americans ages 18 and older suffer from a diagnosable mental disorder in a given year (Kessler, Chiu, Demler, & Walters, 2005), including enduring conditions such as depression, bipolar disorder, and schizophrenia. Major depressive disorder (MDD) is a significant public health problem that is particularly prevalent in women during their childbearing years (Kessler et al., 1993; Kessler et al., 2003; Regier et al., 1988). Twenty percent of women will experience an episode of MDD at some point during their lives and women residing in poverty are at even greater risk for MDD (Segre, O'Hara, Arndt, & Stuart, 2007; Williams & Colling, 1995). Despite the magnitude of the problem, many economically disadvantaged women do not seek treatment or receive sub-standard treatments that are ineffective (Wang et al., 2005). Of particular concern are findings that racial and ethnic minority groups, many of whom reside in poverty, receive poorer quality healthcare and have worse outcomes when care is received (Smedley, Stith, & Nelson, 2002). Herein we evaluate whether individuals vary in response to the provision of an evidence-based psychosocial treatment for MDD as a function of genetic moderation. In secondary analyses, we explore differential susceptibility in relation to self-reported ethnoracial group status.
Early Life Stress and Depression
A plethora of studies have elucidated the role of early life stress in the etiology of depression (Caspi et al., 2003; Caspi, Hariri, Holmes, Uher & Moffit, 2010; Danese, 2008; Heim & Binder, 2012). Disadvantaged women are frequently exposed to traumatic events, including child maltreatment and community and domestic violence (Browne & Bassuk, 1997; Cicchetti & Lynch, 1993; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995), which further increase the likelihood that they will experience MDD (Chapman, Whitfield, Felitti, Dube, Edwards, & Anda, 2004). In fact, lifetime rates of MDD have been as high as 64% in women with histories of abuse (Longhurst, & Mazure, 1999). Statistics such as these underscore the criticality of providing and evaluating the efficacy of treatments for MDD in socioeconomically disadvantaged women. Because the stressors associated with parenting further exacerbate the likelihood that depression will emerge (Cicchetti & Toth, 1995, 1998), the conduct of investigations with low-income mothers is of paramount importance.
Interactive Effects of Trauma and Depression
In accord with a developmental psychopathology perspective, multi-level investigations of pathways to mental disorder are increasingly being called for (Cicchetti & Dawson, 2002; Cicchetti & Toth, 2009). Such multi-level investigations have highlighted the heterogeneity of outcomes among individuals with similar risk factors, including those at heightened genetic risk. For example, despite moderate to high heritability estimates for depression, including rates as high as 48%-75% for individuals with recurrent depression (McGuffin, Katz, Watkins, & Rutherford, 1996; Sullivan, Neale, & Kendler, 2000), five genome-wide case-control association studies of over 7,000 individuals with depression failed to identify any genetic variant reliably associated with depression (Uher, 2011). Moreover, significant variability also exists with regard to the role of trauma in contributing to the etiology of depression as not all individuals who experience early trauma, including child maltreatment, develop depression in adulthood (Cicchetti & Toth, in press).
In efforts to better understand pathways to psychopathology, in recent years efforts have been directed toward examining the interactive effects of early life stress and depression (Caspi et al., 2003; Heim & Binder, 2012). Specifically, a significant corpus of research has sought to identify candidate genetic variations that interact with early trauma in contributing to the emergence of MDD (Hornung & Heim, 2014). Candidate genes of particular interest in understanding the moderating effects of genes in relation to early life stress have included the CRHR1 and the 5-HTTLPR genes. The rationale for choosing these genes as potential moderators in the current investigation is delineated below.
Corticotropin Releasing Hormone Receptor 1 (CRHR1)
Genes involved in biological stress systems are important candidates for investigations of genetic moderation of the effects of major stressors such as depression. Corticotropin releasing hormone (CRH) is a key activator of the hypothalamic-pituitary-adrenal (HPA) axis, binding to receptors that initiate the stress response, culminating with release of cortisol from the adrenal cortex (Gunnar & Vasquez, 2006). CRH receptors occupy widespread regions of the primate brain. (Chrousos, 1998; Lupien, McEwen, Gunnar, & Heim, 2009; Sanchez, Young, Plotsky, & Insel, 1999). CRH receptors may function as transcription factors and thereby serve as regulators of gene transcription (Lupien et al, 2009). Genotypic variation in CRHR1 has been linked to HPA-axis dysregulation in adults who reported having been maltreated in childhood (Tyrka et al., 2009) and, in interaction with childhood adversity, to risk for, and protection against, depression (Bradley et al., 2008; Polanczyk et al., 2009) and neuroticism (DeYoung, Cicchetti, & Rogosch, 2011). Furthermore, a three-way GxGxE interaction (5-HTTLPR x CRHR1 x Child Maltreatment) has been found for high internalizing symptoms (Cicchetti, Rogosch, & Oshri, 2011).
Serotonin Transporter Gene (5-HTT)
The serotonin transporter gene (5-HTT) is one of the major genes involved in serotonergic neurotransmission. The 5-HTT gene has a polymorphism in the linked polymorphic region (5-HTTLPR) in the 5’- regulatory region due to a 44-base pair deletion that eventuates in either the short (S) or long (L) allele (Lesch et al., 1996). Because the short variation of 5-HTTLPR appears to be dominant, heterozygous (SL) individuals can be functionally categorized with individuals who possess the homozygous (SS) genotype. In vitro research has revealed that the long allele variant has two to three times the transcriptional activity of the short variant (Lesch et al., 1996).
The 5-HTT gene has been shown to play a pivotal role in brain development and in the emergence of individual differences in mood and emotion regulation (Caspi, et al., 2013). Although there is some disagreement in the literature (compare Risch et al., 2009, to Caspi et al., 2010 and Karg, Burmeister, Shedden, & Sen, 2011), variation in the promoter region of the 5-HTT gene has been linked to stress sensitivity in humans. The majority of GxE research on 5-HTTLPR in humans conducted to date has primarily focused on depression. 5-HTTLPR short allele carriers are characterized by negative activity that develops into depression only under particular stress sensitive conditions. This stress sensitivity may result in the development of anxiety and fear neural circuitry (Hariri & Holmes, 2006), and an attentional bias toward negative emotions and sensitivity to potential threat (Caspi et al., 2010; Watson & Clark, 1984). The short allele of 5-HTTLPR is often considered to be the risk allele for depression, whereas the long allele is thought to be a protective factor against the occurrence of mood dysregulation.
Beyond Genetic Vulnerability
Interestingly, the view that a “risk” genotype makes an individual vulnerable to the effects of environmental adversity, whereas a “protective” genotype inoculates one to adversity, has fallen into disfavor (Uher, 2011). According to the differential susceptibility to environmental influence hypothesis proffered by Belsky (1997, 2005; Belsky & Pluess, 2009), genes that confer risk in harsh environments may confer benefits in normal or nurturing environments. In other words, the characteristics of individuals (including their genotypes) that render them disproportionately more vulnerable to experiencing adversity also may make them more likely to benefit from supportive contexts (Belsky, Bakersman-Kranenburg, & van IJzendoorn, 2007; Ellis, Boyce, Belsky, Makersman-Branenburg, & van IJzendoorn, 2011). A number of empirical studies have demonstrated that the same genetic variant that increases susceptibility to the effects of adversity may also result in an increased likelihood of benefitting from positive environmental experiences (Cicchetti & Rogosch, 2012; Cicchetti, Rogosch, Hecht, Crick & Hetzel, 2014; Davies & Cicchetti, 2014). These results also have been found in a meta-analysis of studies that primarily included Caucasian children and adolescents (van IJzendoorn, Belsky, & Bakersman-Kranenburg, 2012). Thus, the differential susceptibility framework does not view susceptible individuals as more vulnerable to adversity; rather, susceptible individuals are viewed as more malleable or plastic (Pluess & Belsky, 2013).
Psychosocial Interventions for MDD
Cognitive behavioral therapy (CBT) and interpersonal psychotherapy (IPT) are two of the most widely investigated psychosocial treatments for MDD and both have been found to be efficacious (Elkin, Shea, Watkins, et al., 1989; Hollon & Ponniah, 2010). Because impoverished women with young children experience a multitude of interpersonal stressors, the provision of IPT might be particularly effective in treating MDD in this population. In fact, investigations of the efficacy of IPT with low-income and minority populations have yielded promising results in reducing depressive symptoms as well as alleviating posttraumatic stress disorder (Grote, Swartz, Geibel, Zuckhoff, Houck, & Frank, 2009; Krupnick, Green, Stockton, Miranda, Krause, & Mete, 2008; Mufson, Moreau, Weissman, Wickramaratne, Martin, & Samilov, 1994; Mufson, Weissman, Moreau, & Garfinkle, 1999; Rosello & Bernal, 1999; Spinelli & Endicott, 2003).
Most recently, Toth and colleagues conducted a randomized clinical trial of IPT with economically disadvantaged mothers with MDD (Toth, Rogosch, Oshri, Gravener-Davis, Sturm, & Morgan-Lopez, 2013). Notably, women in this sample also had extensive histories of trauma, with over 86% of women receiving IPT having histories of child maltreatment and with over 90% experiencing at least one lifetime traumatic event. Depressive symptoms at the end of treatment and at eight-months post-intervention were significantly lower among women who received IPT than in those who received treatment generally available in the community. Social adjustment and perceived stress also were identified as mediators of sustained positive treatment effects (Toth et al., 2013).
Genetic Moderation of Intervention Outcome
The importance of incorporating multi-level measurement strategies into intervention outcome studies has been increasingly highlighted (Cicchetti & Gunnar, 2008). However, to date little research has been directed specifically toward identifying genetic predictors of response to psychosocial interventions. In a notable exception involving a study of a family intervention directed toward reducing externalizing behavior in toddlers, the dopamine D4 receptor polymorphism (DRD4) was found to moderate intervention effects. Specifically, parental insensitivity was related to externalizing behaviors in preschoolers, but only in the presence of the DRD4 7-repeat polymorphism (Bakermans-Kranenburg & Van IJzendoorn, 2006). Differential susceptibility to intervention effects based on the presence of the DRD4 7-repeat allele in children also have been reported (Bakermans-Kranenburg, Van IJzendoorn, Femke, Pijlman, Mesman, & Juffer, 2008). Brody and colleagues (Brody, Beach, Philibert, Chen, & Murry, 2009) also found 5-HTTLPR to moderate intervention effects such that youths with the risk allele (SS or SL) benefited more from the preventive parenting program than did youth with the LL genotype.
With respect to the examination of the potential moderating effects of genetic factors on stressful life events and response to interventions directed specifically toward depression, investigations also have focused on pharmacological treatments and have identified individuals with 5-HTTLPR short alleles (SS or SL) as having poorer responses to medication treatment (Keers, Uher, Huezo-Diaz, et al., 2011; Mandelli, Marino, Pirovano et al., 2009). However, not all investigations of genetic moderation of psychotropic medications have yielded positive results (Bukh, Bock, Vinberg, Werge, Gether, & Kessing, 2010), underscoring the importance of continued research on this issue. To our knowledge, the only investigation to examine possible genetic moderation of a psychosocial intervention on depression involved the parents of children who participated in a parent training program for depressed African American parents. The intervention was associated with a greater impact on reducing parental depressive symptoms when children were at increased genetic risk (i.e., one or two copies of the short allele) for negative affect and poor self-control (Beach, Brody, Kogan, Philbert, Chen, & Lei, 2009).
Current Study
In the current investigation, genetic moderators of intervention efficacy were examined in the Toth et al. (2013) RCT of interpersonal psychotherapy in socially disadvantaged racially and ethnically diverse mothers with MDD. Specifically, we investigated the potential moderating effects of CRHR1 and of 5-HTTLPR on depressive symptoms. Moreover, we examined CRHR1 and 5-HTTLPR as possible moderators of the previously identified IPT mediators – perceived stress and social adjustment. Based on prior research revealing genetic moderation of early life stress and depression (Caspi et al., 2003; Karg et al., 2011), we expected to identify similar moderating effects on intervention response.
Given that the extant literature to date on genetic moderation of intervention has identified those with risk alleles (e.g., SS/SL) as being more likely to benefit from treatment, similar findings might be hypothesized for the current investigation. However, it is important to note that at least with respect to the 5-HTTLPR meta-analysis, these results on genetic moderation for individuals with risk allelles (SS/SL) held only for Caucasians (van IJzendoorn, Belsky, & Bakersman-Kranenburg, 2012). For instance, a study that did not provide evidence for SS/SL as the risk allele was conducted among a racially and ethnically heterogeneous sample of youths. Specifically, Sadeh and colleagues (2010) showed that lower SES was associated with higher levels of callous-unemotional and narcissistic traits only among youth with the LL genotype, thus demonstrating that SS/SL allele may not represent the risk allele with heterogeneous, non-Caucasian samples. Furthermore, in recent years, investigations of ancestrally heterogeneous samples of African American and mixed ethnicities have begun to demonstrate that the LL genotype may confer vulnerability to depression (see, e.g., Banny, Cicchetti, Rogosch, Oshri, & Crick, 2013; Cicchetti, Rogosch, & Oshri, 2011; Davies & Cicchetti, 2014; Laucht, Treutlein, Blomeyer, Buchmann, Schmid, Becker...Banaschweski, 2009). Thus, race and ethnicity may play an extremely important role in the nature of GxE interactions (Cicchetti et al., 2014; van IJzendoorn et al., 2012).
Given the large percentage of non-Caucasian participants included in the current investigation, in conjunction with findings that long alleles are more common in African-American individuals (Odgerel, Talati, Hamilton, Levinson, & Weissmann, 2013), we intended to utilize ancestral proportion scores to investigate whether the results on differential susceptibility in relation to intervention would operate similarly in a more diverse sample.
Method
Participants
The sample for this investigation included 126 racially and ethnically diverse low-income urban women (aged 18-40; mean age=25.33; 54.0% African-American, 22.2% Caucasian, and 23.8% Hispanic/biracial) with a 12 month-old infant. Informed consent for participation was obtained from mothers prior to the initiation of data collection, and the research was conducted in accord with the Institutional Review Board approval. All women met criteria for Major Depressive Disorder. We recruited a community sample of non-treatment-seeking women from primary care clinics serving low-income women and from Women, Infant and Children (WIC) clinics. Seventy-eight percent of the sample was at or below the U.S. Department of Health and Human Services definition of poverty level, and 96% met WIC criteria (185% of the poverty level). A project recruitment coordinator initially screened women with the Center for Epidemiologic Studies-Depression Scale (CES-D; Radloff, 1977), and those scoring above 16 were targeted for further assessments to determine eligibility for inclusion. Women who subsequently scored 19 or higher on the Beck Depression Inventory II (BDI-II; Beck, Steer, & Brown, 1996), and who met MDD diagnostic criteria based on the operational criteria on the Diagnostic Interview Schedule (DIS-IV; Robins, Cottler, Bucholz, & Compton, 1995) were eligible to participate. Following diagnostic confirmation, women were randomized to Interpersonal Psychotherapy (IPT) or Enhanced Community Standard (ECS) (see Intervention Groups section below for details on interventions).
For all but 6.3% of the women, the onset of their first major depressive episode preceded the infant's birth. Accordingly, the current sample was not comprised of women with depression restricted to the post-partum period, but rather was of longer standing duration. Regarding comorbid DSM-IV diagnoses, 50% of women met criteria for an anxiety disorder (non-PTSD), 33.6% met criteria for posttraumatic stress disorder, and16.4% met criteria for antisocial personality disorder. No statistically significant differences were found in rate of comorbid disorders between the IPT and ECS groups
Although scores on the Hamilton Rating Scale for Depression (HRSD-R) of 14 or higher are generally considered indicative of MDD, utilization of this cut-off criteria for study admission has been criticized because individuals may be erroneously excluded (Bagby, Ryder, Schuller & Marshall, 2004; Morris et al., 2007). Therefore, the HRSD-R was not used to exclude participants in the current investigation. Women meeting diagnostic criteria for lifetime bipolar disorder or for any lifetime psychotic spectrum disorder were excluded. Women with Mood Disorder Due to a General Medical Condition and Substance-Induced Mood Disorder also were excluded, as were women with any current alcohol or substance abuse disorder, as defined by DSM-IV criteria. Women with other co-morbid disorders were not excluded.
Procedures
Assessments were conducted at baseline, post-intervention, and at an eight-month post-intervention follow-up. All assessments were conducted by trained interviewers who were unaware of group condition or study hypotheses. Due to possible variations in literacy and reading ability, all self-report measures were read to participants while they followed along and marked their answers. Following confirmation of diagnostic status, women were randomized to the IPT or to the ECS group, using a progressive block randomization procedure over the extended period of participant recruitment. Demographic variables including age, race, ethnicity, education, and number of children were used as blocking variables. Because the clinical trial involved women who were not seeking treatment, we expected that there would be a number of participants who would not be interested in the active IPT arm when offered, and thus, would decline treatment, thereby not complying with their random assignment to receive the intervention (Little & Yau, 1998). In this “treatment-as-received” investigation, women who were not interested in the active IPT intervention were considered in the ECS group (n=39). This decision was made to maximize the sample in each intervention group (IPT and ECS), thus making gene x intervention analyses more feasible. Therefore, 58 women participated in IPT with 84% completing all 14 sessions and the mean number of sessions attended was 13.68. Group assignment was not revealed until completion of the baseline research assessments, at which time participants were informed of their group assignment by the recruitment coordinator.
Intervention Groups
Interpersonal Psychotherapy (IPT)
IPT was delivered in accord with the treatment manual (Weissman et al., 2000) and included the provision of 14 one-hour sessions on a weekly basis. Although traditionally provided in clinic settings, flexibility of delivery site (home vs. clinic) was offered to reduce the possible stigma associated with receiving mental health services for low income racially and ethnically diverse participants and to increase receptivity to services. Depression was explained to participants as common feelings that can be associated with the many challenges parents face with childrearing. At times, language focused more on “feeling overwhelmed, stressed and down” because it was difficult for some clients to acknowledge feeling “depressed.” Therefore, psychoeducation around depression that therapists typically provide in the initial phase sometimes was provided later in treatment once therapeutic rapport was stronger. Therapists included Master's or Doctoral level practitioners who were trained in IPT in accord with credentialing recommendations. Therapists had a minimum of 10 years of experience with the provision of psychotherapy to low-income populations and at least two years of supervised experience in the provision of IPT. Weekly individual and group supervision was provided by supervisors who also met credentialing requirements for the supervisory level. Fidelity was monitored through the completion of therapist questionnaires at the Initial, Intermediate, and Termination Phases of IPT. The questionnaires, which were reviewed by supervisors, included information on sessions held, as well as therapists’ evaluations of the extent of progress on client goals. One audiotape from each of the Initial (sessions 1-3), Intermediate (sessions 4-11), and Termination (sessions 12-14) Phases for each client were randomly selected to be reviewed by an individual who had been trained to meet credentialing criteria established for IPT supervisors and who was not providing treatment to participants in order to ensure treatment fidelity. A standard rating scale was developed and utilized to rate tapes for adherence to the treatment protocol.
Enhanced Community Standard (ECS)
Because it is not ethical to withhold treatment from women who have been identified as depressed, all women in the ECS arm were actively offered referral to services typically available in the community (n=68). However, these women were not required to be in treatment unless they chose to do so. Overall, 66.2% elected to be involved in treatment for depression, and all of these women received individual counseling or psychotherapy. In this subgroup participating in treatment, additional interventions also were received, including medication (40.4%), support groups (21.1%), family/marital counseling (12.3%), and day treatment (12.3%). All women in the ECS group also had access to a project staff member who provided periodic informational newsletters, basic education about MDD, support, and referrals to community mental health centers to assist them with accessing treatment, as requested. The staff member was very active in referring ECS participants to treatment and if needed, would assist them in attending their initial intake appointments for support or follow-up with phone calls to ascertain how treatment was proceeding. Thus, treatment received in the ECS group varied from no active intervention to psychotherapy plus additional services.
Measures
The Center for Epidemiologic Studies-Depression (CES-D; Radloff, 1977)
The CESD is a frequently used, well-validated 20-item scale to screen for depression. Scores > 16 predict a high likelihood of MDD.
Diagnostic Interview Schedule-IV (DIS-IV; Robins et al, 1995)
The DIS-IV is a structured interview designed to assess diagnostic criteria for Axis I disorders, as well as for antisocial personality disorder, as outlined in the Diagnostic and Statistical Manual of Mental Disorders (4th edition; American Psychiatric Association, 1994). The DIS-IV ascertains diagnoses present in the past year, the past six months, and those that are current or remitted. The DIS has been shown to be reliable and valid for use in psychiatric epidemiological field studies (Robins, Helzer, Croughan, & Ratcliff, 1981; Robins, Helzer, Ratcliff, & Seyfried, 1982). Robins et al. (1981) compared DSM diagnoses made using the DIS to those made by psychiatrists and reported mean κ = .69, sensitivity of 75%, and specificity of 94%. Given the forced choice structured format of the DIS, interviewers do not need to be trained clinicians. All interviewers were trained to criterion reliability in the administration of the DIS and computer-generated diagnoses were utilized.
Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996)
The BDI-II is the most widely used self-report instrument for measuring the severity of depression. It includes 21-questions in a multiple-choice format and scores of 19 or above indicate levels of depression with clinical significance. Previous studies report that the BDI-II demonstrates good internal consistency (coefficient alpha of .91) and validity (Dozois, Dobson & Ahnberg 1998; Storch, Roberti, & Roth, 2004). In the current study, the average internal consistency of the BDI-II based on the three assessments was α = .94.
The Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983)
The PSS is a self-report measure of perceived stress. The PSS is a psychometrically sound 14-item questionnaire that measures the degree to which respondents feel their lives are unpredictable, uncontrollable, and overwhelming. Previous research with this measure has reported high internal consistency (Cronbach's alpha of .91), concurrent validity with a measure of mental health, and convergent validity with the Posttraumatic Stress-Arousal Symptoms Scale (Mitchell, Crane, & Kim, 2008). Test-retest reliability has been reported to range from .85 to .55 for a 2-day and a 6-week period, respectively (Cohen et al., 1983). The PSS has also been found to be correlated with depression and with physical symptoms (e.g., Cohen et al., 1983; Whiffen & Gotlib, 1993). The reliability score of the PSS based on the three assessments was α = .84.
Social Adjustment Scale – Self Report (SAS-SR; Weissman, 1999)
The SAS-SR is a 54-item measure which evaluates functioning in six role areas, including work, social and leisure activities, relationships with extended family, role as a marital partner, parental role, and role within the family unit. Instrumental and expressive features of functioning within these roles are assessed. The overall social adjustment scale was used in the present study. The measure has been used extensively in studies of treatments for mental disorders (Bateman & Fonagy, 1999; Grote et al., 2009; Gunlicks-Stoessel, Mufson, Jekal, & Turner, 2010; Lenze et al., 2002), and research has demonstrated a high correlation (.72) between interview ratings of overall adjustment and the SAS-SR (Weissman & Bothwell, 1976). The average SAS-R internal consistency in the current study based on the three time assessments was α = .80.
DNA collection, extraction and genotyping
Using an established protocol, trained research assistants obtained DNA samples from women by collecting buccal cells with the Epicentre Catch-All Collection Swabs. Subsequently, using the conventional method, DNA was extracted with the Epicentre BuccalAmp DNA Extraction Kit, in order to prepare DNA for PCR amplification. Genotyping was conducted following previously published protocols. DNA was whole-genome amplified using the Repli-g kit (Qiagen, Chatsworth, CA, Catalog No. 150043) per the kit instructions to ensure the availability of data over the long term for this valuable sample. Amplified samples were then diluted to a working concentration.
CRHR1 was genotyped using assays for SNPs rs110402, rs242924, and rs7209436 purchased from Applied Biosystems, Inc. (ABI, Bedford, MA) as C2544843 10, C2257689 10, and C1570087 10, respectively. Individual allele discriminations were made using Taq Man Genotyping Master Mix (ABI Catalog No. 4371357) with amplification in an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200. 5-HTTLPR samples were genotyped for fragment length polymorphisms of 5-HTTLPR with Hot Star Taq PCR Mix (Qiagen, Catalog No. 203205) and previously described primers (Gelernter, Kranzler, & Cubells, 1997), followed by fragment analysis using a CEQ8000 (Beckman-Coulter, Inc., Fullerton, CA).
If a genotype for either gene or SNP could not be determined after the first run, then it was repeated up to four times. The call rates for the three CRHR1 SNPs were all 1.00. Genotype distributions were in Hardy-Weinberg equilibrium (HWE; all p>.05). Haplotypes for the three CRHR1 SNPs were determined using Arlequin v3.5.1.3, which employs a pseudo-Bayesian approach to estimate phase (Excoffier & Lischer, 2010). Arelquin was able to estimate haplotypes for every participant with a posterior probability higher than 0.94, which allowed us to assign a score of zero, one, or two copies of the TAT haplotype to participants with a high degree of certainty. The TAT haplotype accounted for 34.9% of all haplotypes in the sample, with its complement, CGG accounting for 61.2%. Table 1 presented the allele and haplotype frequencies for the CRHR1 SNPs, TAT haplotype, and 5-HTTLPR genotype for both intervention groups (IPT and ECS). As displayed in Table 1, the groups did not differ by CRHR1 SNPs, TAT haplotype, or 5-HTTLPR.
Table 1.
Baseline demographic, genotype, and depression variables for IPT and ECS groups
| IPT (n=58) | ECS (n=68) | Test | |
|---|---|---|---|
| Married | 10 (16.7%) | 6 (8.8%) | χ2(1)=1.79, p=.18 |
| HS degree | 30 (50.0%) | 44 (64.7%) | χ2(1)=2.83, p=.09 |
| Ancestral Proportion Scores | |||
| African | .62 (SD=.43) | .60 (SD=.42) | t(120)=−.30, p=.76 |
| Native American | .13 (SD=.24) | .11 (SD=.21) | t(120)=−.63, p=.53 |
| European | .25 (SD=.37) | .30 (SD=.39) | t(120)=.70, p=.48 |
| Age | 25.98 (SD=5.10) | 24.76 (SD=4.87) | t(124)=−1.37, p=.17 |
| BDI-II | 29.29 (SD=876) | 30.81 (SD=8.95) | t(124)=.96, p=.34 |
| CRHR1 SNP | |||
| rs7209436 | χ2(2)=5.80, p=.06 | ||
| CC | 29 (50.0%) | 21 (30.9%) | |
| CT | 24 (41.4%) | 34 (50.0%) | |
| TT | 5 (8.6%) | 13 (19.1%) | |
| rs110402 | χ2(2)=4.13, p=.13 | ||
| GG | 27 (46.6%) | 22 (32.4%) | |
| AG | 26 (44.8%) | 33 (48.5%) | |
| AA | 5 (8.6%) | 13 (19.1%) | |
| rs242924 | χ2(2)=4.01, p=.13 | ||
| GG | 24 (41.4%) | 24 (35.3%) | |
| TG | 30 (51.7%) | 31 (45.3%) | |
| TT | 4 (6.9%) | 13 (19.1%) | |
| TAT haplotype | χ2(1)=1.76, p=.21 | ||
| 0 copies | 29 (50.0%) | 26 (38.2%) | |
| 1-2 copies | 29 (50.0%) | 42 (62.0%) | |
| 5-HTTLPR | χ2(1)=0.00, p=1.00 | ||
| LL | 29 (50.0%) | 34 (50.0%) | |
| LS/SS | 29 (50.0%) | 34 (50.0%) |
Notes: For ancestral proportion scores n=66 in the ECS group.
All DNA samples were genotyped in duplicate for quality control. In addition, human DNA from cell lines was purchased from Coriell Cell Respositories for all representative genotypes in duplicate and genotypes confirmed by sequencing using DTCS chemistry on an ABI 3130x1. These and a no template control were run alongside study samples representing 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same conditions.
Ancestral proportion scores
For ancestral proportion testing, DNA from study participants were subjected to SNP genotyping of the Burchard et al panel of 106 SNPs (Lai et al., 2009; Yaeger et al., 2008), known to be informative for ancestry from Africa, Europe, and Native America. The SNPs were genotyped using the iPLEX platform from Sequenom Bioscience, Inc which uses the Sequenom MassArray. Samples are subjected to single base primer extension (SBE) with fluorophore labeled nucleotides from primers designed for SNPs of interest. The samples including the SBE products were placed on the iPLEX platform and MALDI-TOF was used to identify the allele based on the fluorophore passing the detector at the expected time associated with the mass of the SBE primer. The SNP genotyping results were then recoded and uploaded into STRUCTURE v2.3.4 which uses algorithms developed by Pritchard and colleagues (Falush Stephens, & Pritchard, 2003, 2007; Hubisz, Falush, Stephens, & Pritchard, 2009). Three SNP tests were excluded based on high allele call rates of the non-DNA containing wells. The data from remaining 103 loci were uploaded into the software and set to analyze with an Admixture model of ancestry and initialization of the simulation on the GALA cohort (initialize of POPINFO). The simulation was set to run with a Burn-in of 10,000, MCMC Reps of 1,000 and assuming 3 populations within the group. The results of the simulations were subsequently identified as percent association (continuous variable ranging from 0.00-1.00) to each ancestry group based on the known ancestry of the GALA cohort1.
Results
The data analytic strategy for this investigation involved repeated measures analysis of covariances (ANCOVAs) and multiple group path analyses. Repeated measures ANCOVAs were utilized to examine whether IPT effects on depressive symptoms over time (baseline, post-intervention, follow-up) were moderated by genotype. Two sets of analyses were conducted. The first set investigated whether CRHR1 (0 vs 1-2 copies of the TAT haplotype) moderated the effect of IPT on depressive symptoms over time and the second set tested whether 5-HTTLPR genotype (LL vs. SL/SS) moderated IPT effects. The Greenhouse–Geisser correction was used when the assumption of sphericity was violated and corrected degrees of freedom are reported where appropriate.
To address Keller's (2014) concerns regarding covariate interaction inclusion in gene x environment studies, we repeated the above analyses with the inclusion of the following interaction terms: proportion African ancestry x intervention and proportion African ancestry x gene, proportion European ancestry x intervention and proportion European ancestry x gene, and proportion Native American ancestry x intervention and proportion Native American ancestry x gene.
Finally, multiple group path analyses were employed to examine whether mechanisms of IPT effects on depressive symptoms (2 mediators: perceived stress and social adjustment) depended on genotype. Again, two sets of models were tested; the first set explored moderation by number of copies of the CRHR1 TAT haplotype and the second set tested moderation by 5-HTTLPR allelic group.
Baseline demographic, genotype, and depression variables for IPT and ECS groups are presented in Table 1. As expected and consistent with randomization, there were no statistically significant differences between intervention groups on any baseline demographic characteristics or baseline depressive symptoms. As illustrated in Table 2, self-identified African-American and Caucasian women varied in their 5-HTTLPR LL allelic frequency, with LL being more common among African-American women and SS/SL being more common among Caucasian women. Regarding CRHR1, African-American and Hispanic/biracial women differed in their allelic frequencies for SNPs rs110402 and rs24292. Moreover, African-American women varied from Caucasian and Hispanic/biracial women in the number of copies of the CRHR1 TAT haplotype such that 0 copies of the haplotype are more common among African-American women.
Table 2.
CRHR1 and 5-HTTLPR genotypes among self-identified racial/ethnic groups
| African-American (n=68) | Caucasian (n=28) | Hispanic/biracial (n=30) | Test | |
|---|---|---|---|---|
| CRHR1 SNP | ||||
| rs7209436 | χ2(4)=6.21, p=.18 | |||
| CC | 33 (48.5%) | 9 (32.1%) | 8 (26.7%) | |
| CT | 27 (39.7%) | 13 (46.4%) | 18 (60.0%) | |
| TT | 8 (11.8%) | 6 (21.4%) | 4 (13.3%) | |
| rs110402 | ||||
| GG | 35 (51.5%)a | 9 (32.1%) | 5 (16.7%)b | χ2(4)=13.14, p=.01 |
| AG | 25 (36.8%) | 13 (46.4%) | 21 (70.0%) | |
| AA | 8 (11.8%) | 6 (21.4%) | 4 (13.3%) | |
| rs242924 | ||||
| GG | 34 (50.0%)a | 9 (32.1%) | 5 (16.7%)b | χ2(4)=12.21, p=.02 |
| TG | 27 (39.7%) | 13 (46.4%) | 21 (70.0%) | |
| TT | 7 (10.3%) | 6 (21.4%) | 4 (13.3%) | |
| TAT haplotype | χ2(2)=9.16, p=.01 | |||
| 0 copies | 38 (55.9%)a | 9 (32.1%)b | 8 (26.7%)b | |
| 1-2 copies | 30 (44.1%) | 19 (67.9%) | 22 (73.3%) | |
| 5-HTTLPR | χ2(2)=6.75, p=.03 | |||
| LL | 39 (57.4%)a | 8 (28.6%)b | 16 (53.3%) | |
| LS/SS | 29 (42.6%) | 20 (71.4%) | 14 (46.7%) |
Note: Groups with different superscripts are statistically significantly different (p<.05).
CRHR1 as a moderator of IPT effects on depressive symptoms
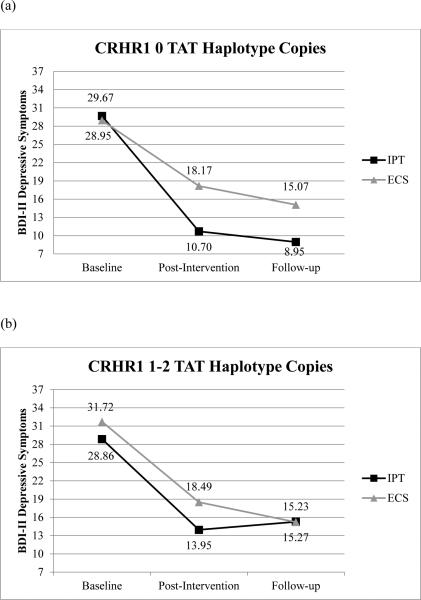
To examine whether CRHR1 moderated the effect of IPT on change in depressive symptoms over time a 3 (baseline, post-intervention, follow-up) x 2 (IPT vs. ECS) x 2 (CRHR1 TAT haplotype: 0 copies vs. 1-2 copies) repeated measures analysis of covariance (ANCOVA) was conducted with ancestry (3 proportion scores: African, European, and Native American) included as covariates. There was not a significant main effect of time, ancestry, or CRHR1 in this model and the interactions of time by ancestry were also non-significant. Although the time x intervention effect on depressive symptoms was statistically significant (F (1.78, 205.19) = 3.35, p=.04), this was qualified by a three-way interaction. Specifically, the significant interaction of time x CRHR1 x intervention group (F (1.78, 205.19) = 3.27, p=.046) indicated that depressive symptoms varied over time depending on intervention group and the number of copies of the CRHR1 TAT haplotype. Additional ANCOVAs indicated that among women with 0 copies of the TAT haplotype, those who participated in IPT reported significantly fewer depressive symptoms at post-intervention (F (1, 48) = 5.26, p =.03) and follow-up (F (1, 48) = 5.56, p =.02) compared to those who participated in ECS. Among women with 1 or 2 copies of the TAT haplotype, no differences in depressive symptoms between intervention groups (IPT vs ECS) were found at post-intervention (F (1, 64) = 2.43, p =.12) or follow-up (F (1, 64) = .001, p =.98). See Figure 1 for graphical representation of interaction.
Figure 1.
(a) Change in depressive symptoms over time among the CRHR1 0 TAT copies haplotype copy group (b) Change in depressive symptoms over time among the CRHR1 1-2 TAT copies haplotype group
To address Keller's (2014) argument concerning covariate inclusion in gene x environment studies, we re-analyzed the above repeated measures ANCOVA with the following interactions: ancestry (3 proportion scores: African, Native American, European) x CRHR1 and ancestry x intervention group. None of the interactions were statistically significant and the pattern of results was the same as above. Specifically, the three-way interaction of primary interest for this investigation (i.e., time x CRHR1 x intervention group) remained statistically significant in this model (F (1.78, 194.35) = 3.68, p=.03). Therefore, with the inclusion of covariate by environment and covariate by gene interactions, the results continued to demonstrate that change in depressive symptoms over time varied not only by intervention group, but also by the presence of the CRHR1 TAT haplotype.
5-HTTLPR as a moderator of IPT effects on depressive symptoms
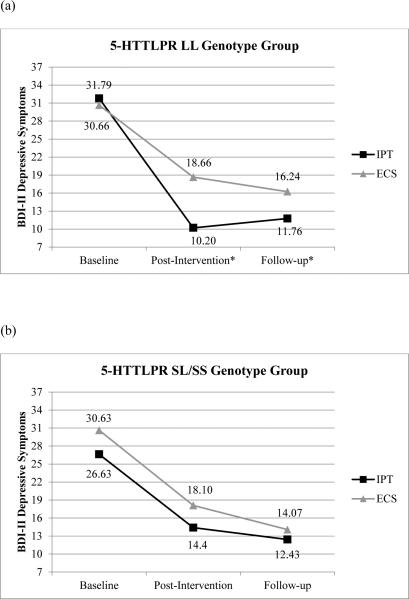
To examine whether 5-HTTLPR moderated the effect of IPT on change in depressive symptoms over time a 3 (baseline, post-intervention, follow-up) x 2 (IPT vs. ECS) x 2 (5-HTTLPR LL vs. SL/SS groups) repeated measures analysis of covariance (ANCOVA) was conducted with 3 ancestral proportion scores included as covariates. There was not a significant main effect of time or 5-HTTLPR in this model. The significant effect of intervention group (F (1, 115) = 4.58, p=.03) and significant time x intervention group interaction (F (1.82, 209.17) = 3.26, p=.045) were clarified by the significant three-way interaction of time x intervention group x 5-HTTLPR genotype group (F (1.82, 209.17) = 3.68, p=.03). Results demonstrated that changes in depressive symptoms over time depended on both intervention group (IPT vs. ECS) and 5-HTTLPR genotype (LL vs. SL/SS). Additional ANCOVAs indicated that among women with LL genotype, those who participated in IPT reported significantly fewer depressive symptoms at post-intervention (F (1, 57) = 8.62, p =.005) but not at follow-up (F (1, 57) = 2.79, p =.10) compared to those who participated in ECS. Among women with SL/SS genotypes, no differences in depressive symptoms between intervention groups (IPT vs ECS) were found at post-intervention (F (1, 55) = 1.19, p =.28) or follow-up (F (1, 55) = .26, p =.61). See Figure 2 for graphical representation of this interaction.
Figure 2.
(a) Change in depressive symptoms over time among the 5-HTTLPR LL genotype group (b) Change in depressive symptoms over time among the 5-HTTLPR SL/SS genotype group
To again address Keller's (2014) argument concerning covariate inclusion in gene x environment studies, we re-analyzed the above repeated measures ANCOVA with the following interactions: ancestry (3 variables: African, Native American, European) x 5-HTTLPR and ancestry x intervention group. None of the interactions were statistically significant and the pattern of results was the same as above with the following exceptions: the interaction of time and intervention was non-significant in this model and the three-way interaction of time x 5-HTTLPR x intervention group was marginally significant (F (1.83, 199.23) = 2.95, p=.06).2
Genetic moderation of mechanisms of IPT
We next tested whether perceived stress and social adjustment mediated IPT effects on depressive symptoms and whether CRHR1 and 5-HTTLPR moderated these intervention effects on the proposed mediators. Separate sets of longitudinal path models were investigated; one set for CRHR1 and another for 5-HTTLPR. Path models were tested using Mplus Version 7.0 (Muthén and Muthén, 1998–2012). Missing data were handled using full information maximum likelihood (FIML). Model fit was estimated with the chi-square statistic, comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR)3.
Baseline (pre-intervention) depressive symptoms, perceived stress, social adjustment, and intervention group (IPT vs. ECS) were entered as correlated exogenous variables. Depressive symptoms, stress, and social adjustment at post-intervention were entered as mediators and residual covariances were modeled. All exogenous variables and mediators were modeled to predict depressive symptoms at follow-up (endogenous variable). Ancestral proportion scores (3 variables: proportion African, European, and Native American) were included in preliminary models as covariates but did not uniquely predict mediators or the outcome and did not interact with genotype or intervention to predict mediators or the outcome. To maintain the most parsimonious model, ancestry was trimmed from the final models. The pattern of results did not vary with or without the inclusion of each ancestry variable and respective interactions.
CRHR1
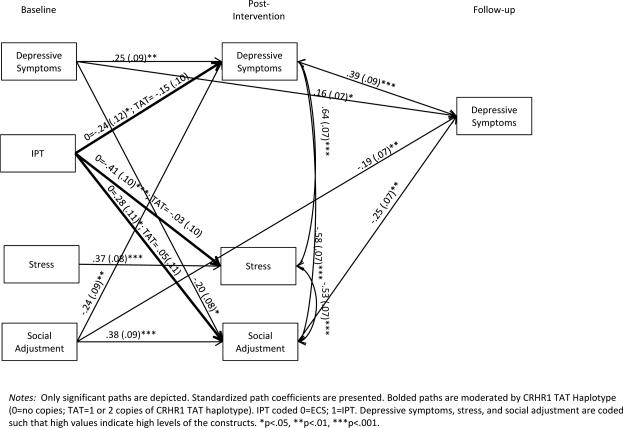
To test for moderated mediation with CRHR1, a model which fully constrained all paths to be equal across CRHR1 groups (0 copies of TAT haplotype n=53, 1-2 copies n=70) was tested. This model evidenced fair fit to the data (χ2 (22) = 34.92 p=.04, CFI = .96, RMSEA .10, SRMR=.09). A partially unconstrained model was tested next which relaxed the constraints between groups for the paths from IPT to the 3 mediators (depressive symptoms, stress, and social adjustment). This partially unconstrained model was a good fit to the data (χ2 (19) = 26.07, p=.13, CFI = .98, RMSEA .08, SRMR=.08) and was significantly better than the fully constrained model (Δχ2 (3) = 8.85, p<.05). This indicates that when paths from IPT to mediators were allowed to be different across CRHR1 genotype, the model fit was significantly improved and is therefore, indicative of significant moderation by genotype. Results of the partially unconstrained model are presented in Figure 4. Participation in IPT predicted less depressive symptoms (b=-.24, p<.05), less perceived stress (b=-.41, p<.001) and greater social adjustment (b=.28, p<.05) at post-intervention for individuals with 0 copies of the CRHR1 TAT haplotype only. IPT was unrelated to these mediators for individuals with 1 or 2 copies of the TAT haplotype. Thus, findings are indicative of genetic moderation of IPT intervention on 3 mechanisms (depressive symptoms, stress, and social adjustment). Moreover, higher levels of social adjustment at post-intervention uniquely predicted less depressive symptoms at follow-up (b=-.25, p<.01).
Figure 4.
Depressive symptoms at post-intervention among African-American women
To examine whether social adjustment mediated the effect of IPT on depressive symptoms at follow-up differently for individuals with and without copies of the CRHR1 TAT haplotype, 95% asymmetrical confidence intervals were utilized (Tofighi & MacKinnon, 2011). Confidence intervals (CIs) that do not include the value zero indicate significant mediation. Results indicated that improved social adjustment significantly mediated the effect of IPT on depressive symptoms for individuals with 0 copies of the CRHR1 TAT haplotype (95% CI [.18, 3.67]) only.
5-HTTLPR
A model fully constraining all paths to be equal across 5-HTTLPR allelic groups was tested first (LL allelic group n=62, SS/SL allelic group n=61) and evidenced good model fit (χ2 (22) = 22.26 p=.44, CFI = 1.00, RMSEA .01, SRMR=.09). A partially unconstrained model was tested next which relaxed the constraints between groups for the paths from IPT to the 3 mediators (depressive symptoms, stress, and social adjustment). This model was also a good fit to the data (χ2 (19) = 19.61, p=.42, CFI = 1.00, RMSEA .02, SRMR=1.0). The partially unconstrained model was not a significantly better fit to the data than the fully constrained model (Δχ2 (3) = 2.65, p=n.s.). Thus, results did not support moderation by 5-HTTLPR genotype.
Results of the fully constrained model indicated that participation in the IPT intervention predicted less depressive symptoms (b=-.24, p<.05), less perceived stress (b=.40, p<.001) and greater social adjustment (b=-.28, p<.05) at post-intervention. Higher levels of social adjustment at baseline and post-intervention uniquely predicted less depressive symptoms at follow-up (bbaseline=.20, p<.01; bpost=.24, p<.01). Perceived stress at post-intervention did not uniquely predict depressive symptoms at follow-up (b=.04, p=n.s.). Moreover, significant stability was found for stress (b=.37, p<.001) and social adjustment (b=.38, p<.001) from baseline to post-intervention, and depressive symptoms from baseline to post-intervention (b=.18, p<.01) and baseline to follow-up (b=.38, p<.001).
Post-hoc analyses
The following analyses were conducted post-hoc to further clarify our results regarding 5-HTTLPR. Specifically, we were interested in further understanding how the racial and ethnic heterogeneity of our sample may influence our findings. We present these results as tentative given the small cell sizes. Caution is warranted when interpreting the findings and replication is necessary with much larger samples more well-equipped to examine these issues thoroughly.
In order to explore racial/ethnic group differences in 5-HTTLPR moderation of IPT effects, we substituted the ancestral proportion scores from the previous models (ancestral proportion scores are continuous variables that do not easily allow for group comparisons) with a self-reported race/ethnicity variable consisting of 3 groups: African-American, Caucasian, and Hispanic/biracial. A 2 (intervention group: IPT vs. ECS) x 2 (5-HTTLPR: LL vs. SL/SS allele groups) x 3 (self-reported race/ethnicity: African-American, Caucasian, and Hispanic/biracial) ANOVA was conducted to examine differences in depressive symptoms at post-intervention among groups. Results supported a significant three-way interaction of intervention x 5-HTTLPR x self-reported race/ethnicity (F (2, 114) = 3.90, p=.02).
Follow-up ANOVAs and t-tests clarified the three-way interaction. Results showed a significant interaction among 5-HTTLPR and intervention group among African-American women only (F (1, 64) = 6.40, p=.01). Moreover, t-tests revealed that among African-American women, those with the LL genotype who participated in IPT evidenced significantly lower depressive symptoms at post-intervention compared to African-American women with the LL genotype who participated in ECS (t (37) = 3.11, p=.004). Among African-American women with the SS/SL genotype, there were no differences between intervention groups. See Figure 4 for a graphical representation of 5-HTTLPR x IPT interaction among African American women only. Among Caucasian women, we did not find a significant interaction between 5-HTTLPR and intervention group. Similarly, among Hispanic/biracial women, we also did not find a significant interaction between 5-HTTLPR and intervention group. However, given small cell sizes for Caucasian and Hispanic women when divided by genotype group and intervention group, we lacked sufficient power to test these interactions adequately and therefore do not present them graphically.
The above 2×2×3 ANOVA was repeated to examine group differences in depressive symptoms at the 8-month follow-up. The three-way interaction of intervention x 5-HTTLPR x race/ethnicity was marginally significant (F (2, 114) = 2.70, p=.07) at this time point. The pattern of results was the same as at post-intervention.
Discussion
Recent research has underscored the role of genotypes and epigenetic processes in the emergence of depression in individuals who have experienced childhood trauma (Hornung & Heim, 2014). Moreover, in conjunction with recent research on genetic moderation of interventions outcome, the current investigation provides a foundation on which to build future research on the efficacy of interventions for individuals with MDD. Given the prevalence of early trauma in the current sample of mothers with MDD, the demonstrated efficacy of IPT in reducing depressive symptoms is particularly compelling. (Toth et al., 2013). As such, an important next step involved the examination of potential genetic moderation of treatment effects. To our knowledge, this is the first investigation of genetic moderation of depression in response to the provision of interpersonal psychotherapy in low-income racially and ethnically diverse mothers with extensive trauma histories.
Two candidate genes, CRHR1 and 5-HTT, were chosen to elucidate individual variation among depressed mothers who were randomly assigned to either the IPT or ECS interventions. The inclusion of the molecular genetic level is consistent with one of the principles of a developmental psychopathology perspective – namely, that a multiple levels of analysis approach provides a more comprehensive understanding of developmental processes than does a single level of analysis (Cicchetti, 2006; Cicchetti & Dawson, 2002; Cicchetti & Toth, 2009).
Contrary to the extant literature where risk alleles have been found to moderate responsivity to intervention, depressed women with “protective” genotypes evinced the greatest reduction in depressive symptoms following IPT compared to women with “risk” genotypes. Specifically, for CRHR1, a 3-way-interaction was obtained between time, CRHR1 number of copies of TAT haplotype (0 vs 1, 2), and intervention of group (IPT, ECS). Change in depressive symptoms was found to be dependent upon intervention group and the number of CRHR1 TAT haplotypes, such that among women with 0 copies of the TAT haplotype, those who received IPT experienced a greater reduction in depressive symptoms at post-intervention and follow-up compared to those in the ECS condition. However, among women with 1 or 2 copies of the TAT haplotype, there were no differences between intervention groups at either time point. These findings remained significant with the inclusion of ancestry x gene and ancestry x intervention interactions, thus meeting Keller's (2014) recommendation for covariate interaction inclusion in GxE studies. Figures 1A and 1B denote that women with 0 copies of the CRHR1 TAT haplotype who received IPT had depression scores in the non-clinical range (adjusted mean = 8.95) at the 8 month follow-up assessment of the intervention, whereas women with 1-2 copies of the CRHR1 TAT haplotype who received IPT evinced higher depressions scores (adjusted mean = 15.27; BDI II clinical depression cut-off = 19).
Likewise, for 5-HTTLPR, a 3 (time: baseline, post-intervention, follow-up) x 2 (intervention group) x 2 (5-HTTLPR genotype) repeated measures ANCOVA with ancestral proportion scores as covariates revealed a statistically significant time x intervention group x 5-HTTLPR interaction. Specifically, depressed women with the LL genotype of 5-HTTLPR who received IPT experienced greater reductions in their depressive symptoms at post-intervention compared to those who received ECS. No differences between intervention conditions were found in depressive symptoms at either time point for women with SS/SL genotypes. These findings, contrary to those found in investigations conducted with Caucasian individuals, suggest that identification of “risk” versus “protective” genotypes may vary among diverse racial and ethnic groups (Cicchetti et al., 2014). That is, a genotype that operates as a risk allele in a Caucasian sample may operate as a protective allele in an African-American sample, and vice-versa.
Next we examined whether previously identified mechanisms of IPT effects (i.e. improved social adjustment and decreased perceived stress; Toth et al., 2013) also were moderated by genotype. In other words, in addition to exploring genetic moderation of IPT effects on depressive symptoms, we also investigated whether genotype moderated identified mediated mechanisms of this intervention. Again, contrary to findings obtained with more ancestrally homogeneous Caucasian samples, the depressed women in this ethnoracially diverse group who experienced the largest decrease in their depressive symptoms were those who had protective genotypes and who had experienced improvements in the mediating mechanisms of perceived stress and social adjustment.
Specifically, the efficacy of IPT at reducing depressive symptoms, decreasing stress and improving social adjustment varied depending on CRHR1 genotype such that improvements in these domains were found only for women in the IPT condition with 0 copies of the TAT haplotype. These results demonstrate a genetic moderation (i.e., CRHR1 TAT haplotype moderated IPT effects on three mechanisms – depressive symptoms, perceived stress, and social adjustment). Furthermore, improvements in social adjustment at post-intervention mediated the effects of IPT on depressive symptoms at the 8 month follow-up for those women with 0 copies of the TAT haplotype. Overall, women who received the IPT intervention and who possessed 0 copies of the CRHR1 TAT haplotype were significantly more likely to have fewer depressive symptoms, and to report less perceived stress and greater social adjustment at post-intervention than women with 1 or 2 copies of the TAT haplotype of CRHR1. In contrast, statistical analyses that examined whether the LL or SS/SL genotypes of 5-HTTLPR served as a moderator of the mediating mechanisms that demonstrated improvements following participation in IPT (i.e., reduction of depressive symptoms; less perceived stress; greater social adjustment) were not substantiated.
Differential susceptibility theory has focused on individual differences to both negative and positive experiences as a function of their interaction with risk genotypes and developmental outcome (Belsky et al., 2007; Belsky & Pluess, 2009). The findings of the current investigation are consistent with differential susceptibility theory, but only for African-American women. This finding is critically important as it raises the possibility that differential susceptibility in GxE studies may operate differently in racially and culturally diverse groups. Moreover, it highlights the necessity of conducting intervention efficacy and effectiveness studies with diverse groups in order to more cogently understand which interventions are most likely to be beneficial for particular individuals.
Although the current investigation makes an important contribution to the burgeoning body of research on the genetic moderation of intervention outcome studies, it is not without limitations. First, random assignment in this non-treatment seeking sample proved to be challenging as a not insignificant number of women who were assigned to the IPT intervention refused to participate in treatment. Therefore, in order to retain the largest number of participants possible, our analyses were conducted on treatment as received and did not meet the “gold standard” of more traditional RCTs. Secondly, the sample size limited our ability to thoroughly assess differential susceptibility by each ethnic and racial group. Given the cost and time commitment inherent in intervention efficacy studies, smaller than ideal samples are likely to remain a reality, thereby underscoring the criticality of conducting cross-sample and cross-site replications. Finally, although not necessarily a limitation, it is important to note that because considerable effort was expended to ensure that women in the enhanced community standard group were able to access services for their depression, the magnitude of the efficacy of IPT actually may have been reduced.
Despite promising insights, our understanding of the genetic moderation and moderated-mediation of intervention outcome remains in its nascent stages. Future research on GxE and on epigenetic moderation and mediation would do well to emphasize and incorporate a developmental perspective (GxExD). Genetic variation may affect the ways in which individuals vary in their responsiveness to positive and negative experiences. Importantly, these individual differences may operate differently at different developmental periods. Moreover, the effects of prior development may influence the effects of genes and experience during a particular developmental period. Additionally, environmental experiences may affect the timing of genetic effects and gene expression. For example, outcomes might vary as a function of factors such as when in the developmental period a depressive episode first occurred and the severity and chronicity of depression. Consistent with prior research (Heim & Binder, 2012), genetic moderation of outcome also might be expected to vary due to the presence or absence of trauma. Furthermore, experience exerts effects on the epigenome and these also would be likely to operate differently across the course of development.
There appear to be many ways whereby there can be genetic effects on intervention efficacy. For example, as demonstrated herein, some individuals may be more susceptible to the positive effects of intervention. Alternatively, different interventions may be more efficacious with different individuals as a function of their genetic make-up. This suggests that specific interventions may be able to be matched to an individual's genotype group. Intervention also may affect DNA methylation resulting in changes in gene expression that may differ across developmental periods. Perhaps DNA methylation changes in response to intervention could eventuate in the design of both prevention and intervention strategies that alter the expression of genes to optimize and promote healthy physical and mental health outcomes.
As this study is the first demonstration of a 3-way interaction involving genes, intervention group, and time, it will be important to replicate these findings in future research (Duncan & Keller, 2011). The inclusion of ancestry-informative markers (AIMs), also known as ancestral proportion scores, in the present study enables us to estimate the geographical origins of an individual's ancestors and to discern the proportion of ancestry that is derived from each geographical region. Because our sample is ancestrally heterogeneous, unlike many samples in the extant GxE literature, we were able to obtain a more accurate portrayal of race and racial admixture than that available via self-report of race alone. The utilization of these Ancestry Informative Markers represents an increase in methodological sophistication and addresses concerns raised by Duncan and Keller (2011) regarding the absence of these AIMs markers in GxE research with ancestrally heterogeneous samples.
Additionally, we controlled for potential covariate interactions in the models. Keller (2014) commented on the fact that few GxE investigations had entered relevant covariate interaction terms in the same model with the GxE term. Therefore, we computed ancestry x gene and ancestry x intervention interactions as Keller (2014) suggested. With the exception of the three-way interaction of time x 5-HTTLPR x intervention which was reduced to marginal significance with the inclusion of all covariate interactions (p=.06), our results held with the incorporation of these interaction terms, thus eliminating potential alternative explanations of our GxE findings.
Implications for Differential Susceptibility and Future Directions
The findings of the current investigation proffer some fascinating implications with respect to their relevance for addressing questions related to differential susceptibility theory and the provision of intervention for racially and ethnically diverse groups. Because we did not compare IPT with another evidence-based intervention, but rather with a non-evidence based model comprised of services generally available in the community, we cannot determine whether individuals with particular genotypes who benefitted from IPT would not benefit equally from another evidence-based intervention such as cognitive behavioral therapy. Answers to this question are particularly important with respect to determining whether individuals with certain genotypes are more likely to derive benefit from one versus another model of treatment. If so, then important strides can be made with respect to ascertaining a seminal question in the intervention literature, namely, what works for whom (Fonagy, Target, Cottrell, Phillips, & Kurtz, 2002)? The provision of RCTs that include competing models of evidence-based interventions (e.g., IPT vs CBT) would hold great promise for addressing this issue.
Relatedly, the fact that individuals with the same diagnosis often vary with respect to their responsivity to the same therapeutic intervention further highlights the role that genetic variation and different environmental stressors play in contributing to intervention efficacy. In accord with a developmental psychopathology perspective, we also maintain that a consideration of developmental factors will enhance the ascertainment of interventions that are differentially effective for individuals with differing genotypes and experiences of adversity (GxExD). Although the burgeoning research on the genetic moderation of intervention outcome might lead the overly-zealous to conclude that we are poised to begin to provide interventions based on different genetic profiles, we caution against this premature conclusion. Given the complexity of mental illness and the methodological challenges that accompany GxE investigations of intervention efficacy, extensive replications and carefully designed studies that clearly define the characteristics and risk environments of participants are needed. Even in the absence of genetic moderation, we know far too little about mediators of intervention outcome. We share the belief that the conduct of high-quality research that incorporates progress in genetic and epigenetic technology has the potential to inform a more person-specific approach to the provision of intervention, but that it is unlikely, nor even advisable, that this goal will be achieved in the short-term (Uher, 2011). Importantly, research that suggests that individuals with a particular genotype are less likely to respond positively to certain interventions should impel us to continue to develop and evaluate interventions that are more likely to help those who have not yet benefitted, ultimately contributing to reductions in the overall burden of mental illness for individuals, families, and society.
Figure 3.
Moderated mediation of IPT effects on depressive symptoms
Acknowledgements
We are grateful to the Jacobs Foundation and to the National Institute of Mental Health (MH091070: PI's Dante Cicchetti and Sheree L. Toth) for their support of this work.
Footnotes
There were 4 participants for whom samples were determined to be poor prior to sample submission for SNP testing for ancestral proportion scores. Therefore these samples were not tested for markers and are missing from analyses involving ancestry.
All repeated measures ANCOVAs also were tested with just proportion African ancestry (rather than all 3 proportion scores-African, European, and Native American) included in the model. Eliminating proportion European and proportion Native American variables did not change the results and the three-way interactions of interest (i.e., time x gene x intervention) remained significant in both the CRHR1 model and the 5-HTTLPR models. The only differences in results when only the African ancestry proportion score was included was that the three-way interaction of interest in the CRHR1 model with all covariate interactions was reduced to marginal significance.
Because 3 mothers were missing data on the exogenous variable baseline perceived stress, the total sample size for the longitudinal moderated mediation path models was 123.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, D.C.: 1994. [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: Has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental psychobiology. 2006;48(5):406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Pijlman FT, Mesman J, Juffer F. Experimental evidence for differential susceptibility: dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers' externalizing behavior in a randomized controlled trial. Developmental psychology. 2008;44(1):293. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Banny A, Cicchetti D, Rogosch FA, Crick NR, Oshri A. Vulnerability to depression: A moderated mediation model of the roles of child maltreatment, peer victimization, and 5-HTTLPR genetic variation among children from low-SES backgrounds. Development and Psychopathology. 2013;25(3):599–614. doi: 10.1017/S0954579413000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman B, Fonagy P. Effectiveness of partial hospitalization in the treatment of Borderline Personality Disorder: a randomized controlled trial. American Journal of Psychiatry. 1999;156:1563–1569. doi: 10.1176/ajp.156.10.1563. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Kogan SM, Philibert RA, Chen Y.-f., Lei M-K. Change in caregiver depression in response to parent training: Genetic moderation of intervention effects. Journal of Family Psychology. 2009;23:112–117. doi: 10.1037/a0013562. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Belsky J. Theory testing, effect-size evaluation, and differential susceptibility to rearing influence: The case of mothering and attachment. Child Development. 1997;68:598–600. [PubMed] [Google Scholar]
- Belsky J. Differential susceptibility to rearing influences: An evoluntionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evoluntionary pyschology and child development. Guilford Press; New York, NY: 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakersman-Kranenburg MJ, van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Secience. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: Moderation by the corticotrophin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Beach SR, Philibert RA, Chen YF, Murry VM. Prevention Effects Moderate the Association of 5-HTTLPR and Youth Risk Behavior Initiation: Gene× Environment Hypotheses Tested via a Randomized Prevention Design. Child development. 2009;80(3):645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Browne A, Bassuk S. Intimate Violence in the Lives of Homeless and Poor Housed Women: Prevalence and Patterns in an Ethnically Diverse Sample. American Journal of Orthopsychiatry. 1997;6:261–278. doi: 10.1037/h0080230. [DOI] [PubMed] [Google Scholar]
- Bukh JD, Bock C, Vinberg M, Werge T, Gether U, Kessing LV. No interactions between genetic polymorphisms and stressful life events on outcome of antidepressant treatment. European Neuropsychopharmacology. 2010;20(5):327–335. doi: 10.1016/j.euroneuro.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Usher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implicatons for studying complex disease and traits. The American Jouranl of Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, Poulton R. Influence of life stress on depression: Moderation by polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Annals of the New York Academy of Sciences. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Dawson G. Multiple levels of analysis. Development and Psychopathology. 2002;14:417–420. doi: 10.1017/s0954579402003012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Toward an ecological/transactional model of community violence and child maltreatment: Consequences for children's development. Psychiatry. 1993;56:96–118. doi: 10.1080/00332747.1993.11024624. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Gene by Environment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development and Psychopathology. 2012;24:411–427. doi: 10.1017/S0954579412000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Hecht KF, Crick NR, Hetzel S. Moderation of maltreatment effects on childhood borderline personality symptoms by gender and oxytocin receptor and FK506 binding protein 5 genes. Development and Psychopathology. 2014;26 doi: 10.1017/S095457941400042X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and Psychopathology. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Developmental psychopathology and disorders of affect. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder, and adaptation. Vol. 2. Wiley; New York: 1995. pp. 369–420. [Google Scholar]
- Cicchetti D, Toth SL. The development of depression in children and adolescents. American Psychologist. 1998;53:221–241. doi: 10.1037//0003-066x.53.2.221. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A multilevel perspective on child maltreatment. In: Lamb M, Garcia Coll C, editors. Handbook of child psychology and developmental science, 7th ed., Vol. 3: Socioemotional process. Wiley; New York: in press. [Google Scholar]
- Cohen S, Kamarck T, Marmelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Danese A. Genetic opportunites for psychiatric epidemiology: on life stress and depression. Epidemiologia e psichiatria sociale. 2008;17:201–210. doi: 10.1017/s1121189x00001299. [DOI] [PubMed] [Google Scholar]
- Davies PT, Cicchetti D. How and why does the 5-HTTLPR gene moderate associations between maternal unresponsiveness and children's problems? Child Development. 2014;85(2):484–500. doi: 10.1111/cdev.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung C, Cicchetti D, Rogosch FA. Moderation of the association between childhood maltreatment and neurotocism by the corticotropin-releasing hormomre receptor 1 gene. Journal of Child Psychology and Psychiatry. 2011;52:898–906. doi: 10.1111/j.1469-7610.2011.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10:83–89. [Google Scholar]
- Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Parloff MB. National Institute of Mental Health treatment of depression collaborative research program: General effectiveness of treatments. Archives of general psychiatry. 1989;46(11):971. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–87. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Molecular Ecology Notes. 2007;7:574–578. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Target M, Cottrell D, Phillips J, Kurtz Z. What works for whom? A critical review of treatments for children and adolescents. The Guilford Press; New York: 2002. [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Grote NK, Swartz HA, Geibel SL, Zuckoff A, Houck PR, Frank E. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatric Services. 2009;60:313–321. doi: 10.1176/appi.ps.60.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunlicks-Stoessel M, Mufson L, Jekal A, Turner B. The impact of perceived interpersonal functioning on treatment for adolescent depression: IPT-A versus treatment as usual in school-based health clinics. Journal of Consulting and Clinical Psychology. 2010;78:260–267. doi: 10.1037/a0018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2nd ed. Wiley; New York, NY: 2006. pp. 533–577. [Google Scholar]
- Hariri A, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends in Cognitive Science. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental neurology. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Ponniah K. A review of empirically supported psychological therapies for mood disorders in adults. Depression and anxiety. 2010;27(10):891–932. doi: 10.1002/da.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung O, Heim C. Gene-environment interactions and intermediate phenotypes: early trauma and depression. Frontiers in Endocrinology. 2014;5:14. doi: 10.3389/fendo.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources. 2009;9:1322–32. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Uher R, Huezo-Diaz P, Smith R, Jaffee S, Rietschel M, Aitchison KJ. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. The pharmacogenomics journal. 2011;11(2):138–145. doi: 10.1038/tpj.2010.14. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene× environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological psychiatry. 2014;75(1):18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz MS, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29(2-3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic Stress Disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Krupnick JL, Green BL, Stockton P, Miranda J, Krause E, Mete M. Group interpersonal psychotherapy for low-income women with posttraumatic stress disorder. Psychotherapy Research. 2008;18:497–507. doi: 10.1080/10503300802183678. [DOI] [PubMed] [Google Scholar]
- Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, García-Bailo B, Ordovás JM. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Human Genetics. 2009;125:199–209. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, Banaschweski T. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: evidence from a high-risk community sample of young adults. International Journal of Neuropsychopharmacology. 2009;12(6):737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Dew MA, Mazumdar S, Begley AE, Cornes C, Miller MD, Reynolds CF. Combined pharmacotherapy and psychotherapy as maintenance treatment for late-life depression: effects on social adjustment. American Journal of Psychiatry. 2002;159:466–468. doi: 10.1176/appi.ajp.159.3.466. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heiles A, Sabol SZ, Greenberg S, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1521. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Little RJA, Yau L. Statistical techniques for analyzing data from prevention trials: Treatment of no-shows using Rubin's Causal Model. Psychological Methods. 1998;3:147–159. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mandelli L, Marino E, Pirovano A, Calati R, Zanardi R, Colombo C, Serretti A. Interaction between SERTPR and stressful life events on response to antidepressant treatment. European Neuropsychopharmacology. 2009;19(1):64–67. doi: 10.1016/j.euroneuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Archives of general psychiatry. 1996;53(2):129. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- Mitchell AM, Crane PA, Kim Y. Perceived stress in survivors of suicide: psychometric properties of the perceived stress scale. Research in Nursing & Health. 2008;31:576–585. doi: 10.1002/nur.20284. [DOI] [PubMed] [Google Scholar]
- Morris DW, Rush AJ, Jain S, Fava M, Wisniewski SR, Balasubramani GK, Trivedi MH. Diurnal mood variation in outpatients with major depressive disorder: implications for DSM-V from an analysis of the Sequenced Treatment Alternatives to Relieve Depression Study data. Journal of Clinical Psychiatry. 2007;68:1339–1347. [PubMed] [Google Scholar]
- Mufson L, Moreau D, Weissman M, Wickramaratne P, Martin J, Samilov A. Modification of interpersonal psychotherapy with depressed adolescents (IPT-A): Phase I and II studies. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:695–705. doi: 10.1097/00004583-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Mufson L, Weissman M, Moreau D, Garfinkel R. Efficacy of interpersonal psychotherapy for depressed adolescents. Archives of General Psychiatry. 1999;56:573–579. doi: 10.1001/archpsyc.56.6.573. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Seventh Edition. Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Odegerel Z, Talati A, Hamilton SP, Levinson DF, Weissman MM. Genotyping serotonin transporter polymorphisms 5-HTTLPR and res25531 in European- and African American subjects from the National Institute of Mental Health's Collaborative Center for Genomic Studies. Translational Psychiatry. 2013;3(9):e307, 1–6. doi: 10.1038/tp.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: Replication and extension. Archives of General Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Regier DA, Herschfeld RM, Goodwin FK, Burke JD, et al. The NIMH depression awareness, recognition, and treatment program: structure, aims, and scientific basis. The American Journal of Psychiatry. 1988;145:1351–1357. doi: 10.1176/ajp.145.11.1351. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM–IV. Washington University Press; St. Louis, MO: 1995. [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Ratcliff KS, Seyfried W. Validity of the Diagnostic Interview Schedule, Version II: DSM-III diagnoses. Psychological Medicine. 1982;12:855–870. doi: 10.1017/s0033291700049151. [DOI] [PubMed] [Google Scholar]
- Rossello J, Bernal G. The efficacy of cognitive-behavioral and interpersonal treatments for depression in Puerto Rican adolescents. Journal of Consulting and Clinical Psychology. 1999;67:734–745. doi: 10.1037//0022-006x.67.5.734. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, Gelernter J, Verona E. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of Abnormal Psychology. 2010;119:604–609. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotrophin-releasing factor 1 and 2 receptors in nonhuman primate brain. Journal of Comparative Neurology. 1999;408:365–377. [PubMed] [Google Scholar]
- Segre LS, O'Hara MW, Arndt S, Stuart S. The prevalence of postpartum depression. Social Psychiatry and Psychiatric Epidemiology. 2007;42(4):316–321. doi: 10.1007/s00127-007-0168-1. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. Institute of Medicine Report; National Academy Press; Washington, D.C.: 2002. [PubMed] [Google Scholar]
- Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. American Journal of Psychiatry. 2003;160:555–562. doi: 10.1176/appi.ajp.160.3.555. [DOI] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory – Second Edition in a sample of college students. Depression and Anxiety. 2004;19:187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. R Mediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth SL, Rogosch FA, Oshri A, Gravener J, Sturm R, Morgan-Lopez A. The efficacy of interpersonal psychotherapy for economically disadvantaged mothers. Development and Psychopathology. 2013;4(1):1065–1078. doi: 10.1017/S0954579413000370. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects on hypothalamic-pituitary-adrenal axis reactivity. Biological Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R. Genes, environment, and individual differences in responding to treatment for depression. Harvard Review of Psychiatry. 2011;19(3):109–124. doi: 10.3109/10673229.2011.586551. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States. Archives of General Psychiatry. 2005;62:629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L. Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin. 1984;96:465–490. [PubMed] [Google Scholar]
- Weissman MM. Social Adjustment Scale–Self Report (SAS-SR): User's manual. Multi-Health Systems; North Tonawanda, NY: 1999. [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Markowitz JW, Klerman GL. Comprehensive Guide to Interpersonal Psychotherapy. Basic Books; New York: 2000. [Google Scholar]
- Whiffen VE, Gotlib IH. Comparison of postpartum and nonpostpartum depression: Clinical presentation, psychiatric history, and psychosocial functioning. Journal of Consulting and Clinical Psychology. 1993;61:485–494. doi: 10.1037//0022-006x.61.3.485. [DOI] [PubMed] [Google Scholar]
- Williams DR, Collins C. U.S. socioeconomic and racial differences in health. Annual Review of Sociology. 1995;21:349–386. [Google Scholar]
- Yaeger R, Avila-Bront A, Abdul K, Nolan PC, Grann VR, Birchette MG, Joe AK. Comparing genetic ancestry and self-described race in African Americans born in the United States and in African. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:1329–38. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]