Abstract
Recently, gel-based proteomics has been increasingly applied to investigate proteins involved in female reproductive tract in healthy and disease states. Gel-based proteomics coupled by mass spectrometry (MS) facilitate the identification of new proteins playing roles in cellular and molecular interactions underlying female reproductive biology and it is a useful method to identify novel biomarkers of diseases by studying thousands of proteins simultaneously involved in female reproductive tract in healthy state compared to disease state. This review will discuss the best studies areas contributed to female reproductive biology in which gel-based proteomics coupled by MS has been applied to generate proteome of female reproductive tract in a healthy state.
KEY WORDS: Corpus luteum, embryo implantation, endometrial receptivity, oocyte, ovary and two-dimensional gel electrophoresis
INTRODUCTION
Many investigations are being conducted to understand biological aspects of normal physiology of female reproductive tract, female reproductive diseases and infertility with female factor, in particular at the molecular and protein levels, although much still remains to be known. To find out biological complexities of female reproduction, each protein at the tissue level requires to be addressed. Significant advances in molecular biology research particularly in high-throughput proteomics technologies have provided unbelievable opportunities to investigate global and targeted protein expression and modification in different biological systems.
To date, many data in molecular biological research have been withdrawn from genomic analysis; however, genomic data alone presents nothing about protein expression, functions and isoforms. Then, integration of genomic data with results from the analysis of protein expression and function would be more valuable if we want to know that how cells or tissues act at the molecular level in physiological and pathological conditions. Therefore, today, many efforts turn to the investigation of proteins expressed by the genome of a tissue or an organism so-called “proteome” using application of proteomics technologies. The use of proteomics technologies enables us to study the expression of several hundreds or thousands of proteins in order to reveal physiological state of a tissue or an organ at the molecular level and to identify disease-specific biomarkers.
In the field of reproductive biology, proteomics tools have been exploited to address many molecular biological questions related to various reproductive tissues (including ovary, gametes, testis, endometrium, and placenta) and to know molecular interactions which occur during gametogenesis, fertilization, endometrial receptivity, and embryo implantation. Both gel-based proteomics including two-dimensional gel electrophoresis (2DE) gel and 2DE-fluorescence difference gel electrophoresis (DIGE) and gel-free proteomics such as liquid chromatography (LC) and capillary electrophoresis are useful methods to identify proteins playing role in reproductive system. Currently, proteomics has been dominated by 2DE gel coupled by mass spectrometry (MS) as it is still most reproducible and effective technology to separate overall proteins of the microorganisms, cells, and heterogeneous tissues.[1] However, 2DE gel is not still widely employed by reproductive biologists to address questions attributed to proteins, proteins functions, and interactions involved in the reproductive system. Therefore, this current review will provide an overview in application of 2DE gel coupled by MS about female reproductive system in both human and animal species to introduce proteomic technique as a useful method to investigate proteins and molecules involved in the biology of female reproductive system.
OVERVIEW ON GEL-BASED PROTEOMICS TECHNIQUE
Gel-based proteomics is the most popular and a well-established technique for global protein separation and quantification. Through this technique, overall protein expression of tissues could be analyzed at the large scale, and it is a cheaper approach in comparison with gel-free proteomics.[2]2DE gel, MS and bioinformatics tools are the key components of the gel-based proteomics.[3] Polyacrylamide gel-based proteomics consists of 2DE gel and 2DE-DIGE, a 2DE variant.[4] In 2DE, the complex protein samples are separated in two dimensions according to their net charge at different pH (isoelectric focusing) and their molecular weights sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE electrophoresis).[2] Protein migration in a perpendicular direction provides a spot map of the proteins distributed in 2DE gel. The technique has a great resolving power to make it possible to visualize >10,000 spots corresponding to >1000 proteins.[5] The next typical steps of 2DE gel technique are spot visualization, spot evaluation and expression analysis. The final step is protein identification by MS.[2] Application of 2DE-DIGE can reduce the protein ratio errors because of low gel-to-gel variations which could frequently occur in 2DE gel technique. 2DE-DIGE includes tagging three protein extracts with different fluorescent CyDyes (Cy2, Cy3, and Cy5) that are subsequently mixed and separated on a single gel.[1] Most important steps involved in 2DE gel technique are summarized in Figure 1.
Figure 1.
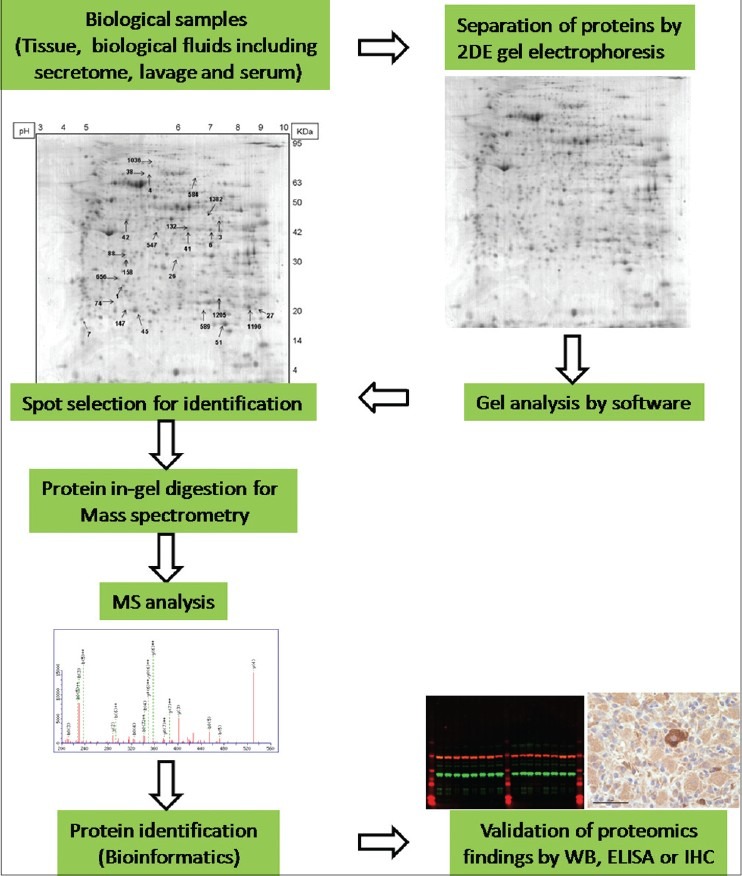
Schematic representation of various steps involved in two-dimensional gel electrophoresis coupled by mass spectrometry to identify proteins in various cells, tissues and organs. WB = Western blot; ELISA = Enzyme-linked immunosorbent assay; IHC = Immunohistochemistry
ENDOMETRIAL RECEPTIVITY AND INVESTIGATING EMBRYO IMPLANTATION BIOMARKERS
The endometrium is hormonally regulated organ that is receptive to blastocyst implantation only for a limited time (4–5 days of each menstrual cycle) called the “implantation window.”[6] Over 75% of the loss pregnancies are associated to implantation defects.[7] Therefore, characterizing implantation molecular events is crucial as failure of implantation is a main reason for loss of a normal pregnancy. Moreover, the high incidence of in vitro fertilization (IVF) failure is mainly due to disruption of implantation after embryo transfer in IVF cycles.[8] However, as a huge number of proteins and mediators are involved in the process of embryo implantation, and also many of these factors have multiple functions and high rates of redundancy, a single biomarker for embryo implantation has not yet been discovered.[9]
To date, the results of 2DE gel application on the endometrial tissue and secretions have shown different patterns of endometrial protein expression during various time points of the menstrual cycle. In most studies, endometrial proteome in the secretory phase has been compared with endometrial proteome in the proliferative phase within a normal menstrual cycle. Study of cycling human endometrium by 2DE-DIGE identified 76 proteins, production of 41 different genes, differentially regulated between mid-proliferative (days 8–10, nonreceptive) and mid-secretary (days 19–23, receptive) phases of the menstrual cycle. The proteome changes were confirmed by immunohistochemistry on 3 representative proteins including Rho GDP-dissociation inhibitor 1-α, chloride intracellular channel and membrane-associated progesterone receptor membrane component 1 (PGRMC1). To find crucial interactive networks in the cycling of human endometrium, they did biological pathway analysis using Ingenuity Pathway Analysis Software. They reported that the Jnk and epidermal growth factor signaling pathways play regulatory roles in protein expression during the mid-secretory phase.[10]
Parmar et al. employed 2DE gel to study endometrial proteome at the late proliferative phase in comparison with mid-secretory phase of the menstrual cycle of fertile women. 57 spots were up-regulated in the proliferative phase compared to 104 spots which were down-regulated in the mid-secretory phase. Of identified proteins, the level of calreticulin (CALR), fibrinogen, adenylate kinase isoenzyme 5 and transferrin was up-regulated in proliferative phase while annexin V, peroxiredoxin 6, α1-antitrypsin (AAT), and creatine kinase were down-regulated in mid-secretory phase. In this study also, heat-shock protein 27 (HSP 27), transferrin, and AAT precursor were detected in both proteome of endometrial and uterine fluid samples at mid-secretory phase of menstrual cycle, implying that endometrium is one of the sources of protein secretion in uterine fluid.[11] In similar study, 57 out of 215 spots were differentially altered at the proliferative phase compared to the secretory phase of the menstrual cycle. Identification of proteins in the 49 differentially altered spots revealed that proteins involved in cell proliferation and cytoskeleton were up-regulated in the proliferative phase compared to the secretory phase. Further, the level of HSPs including 96 kDa glucose-regulated protein (GRP96), GRP78, HSP 70 isoforms increased during the proliferative phase. It may be concluded that since the proliferation needs higher protein synthesis, chaperones such HSPs which are involved in correct folding of synthesized proteins are produced more during the proliferative phase.[12]
Two-dimensional gel electrophoresis-fluorescence difference gel electrophoresis study on endometrial samples of fertile women at the prereceptive phase (2 days after luteinizing hormone [LH] surge, LH + 2) and at the receptive phase (7 days after LH surge, LH + 7) within a normal menstrual cycle revealed only two consistently regulated proteins in both groups. Annexin A2 was up-regulated in the receptive endometrium, probably due to facilitating adhesion of the embryo to the apical surface of the endometrial epithelium.[13] Further, PGRMC1 decreased in the receptive endometrium[13] as reported by Chen et al.[10]
Uterine secretome contains nutrients, cytokines, enzymes, transport proteins, antiproteases, plasma-derived proteins and other biologically active factors. Molecular alterations in the glandular and surface epithelium could result in changes of protein contents in uterine fluid during different phases of the menstrual cycle, embryo implantation, and various stages of pregnancy.[14] Thus, using lavage of the uterine cavity (uterine fluid) could be useful to investigate endometrial proteome particularly secretory proteins. Using lavage samples in studies could be helpful as collection of endometrial lavage is less invasive than sample biopsy so it could be more acceptable and less annoying for patients or women participating in studies. Further, sample of uterine secretome has less complexity than the tissue, as it contains no abundant structural and housekeeping proteins such as actin and vimentin which are present in endometrium, also abundant plasma proteins like albumin can be easily removed from uterine fluid by provided various immunodepletion kits. Finally endometrial biopsy for implantation study could impact on the process of embryo implantation while using uterine fluid for endometrial study not only it does not affect the process of implantation also it could be helpful method to predict implantation outcome in women are under treatment of IVF.
A research on endometrial lavage at prereceptive (LH + 4) and receptive phases (LH + 9) of the menstrual cycle was performed by Scotchie et al. using 2DE-DIGE.[15]82 out of 152 identified proteins were differentially expressed. Surprisingly, some proteins which are crucial for implantation including glycodelin, leukemia inhibitory factor, and osteopontin,[16] were not identified in uterine secretion. It may be because these proteins are low abundance and highly glycosylated and phosphorylated in lavage samples which could impact ability of identification of these proteins in analyses.[15] Casado-Vela et al. identified 803 various proteins in endometrial secretion at mid-secretory phase using a combination of techniques including high-performance liquid chromatography (HPLC) tandem MS, SDS-PAGE followed by HPLC/MS/MS and 2DE gel followed by matrix-assisted laser desorption ionization time-of-flight MS.[17] Although this study could provide a list of proteins present in the uterine secretion in secretory phase, the level of identified proteins were not compared to different phase of the menstrual cycle or with the same phase of menstrual cycle in endometrium of infertile women. In an extensive study using 2DE-DIGE, the protein content of uterine lavage at receptive and nonreceptive phases of the menstrual cycle was compared in fertile and infertile women. The expression level of three isoforms of α2-macroglobulin, AAT, and activin receptor type-2B increased at mid-secretory phase (receptive) when compared to mid-proliferative phase (nonreceptive) across menstrual cycle of fertile women, suggesting they may have roles in embryo implantation. Comparison of mid-secretory phase between fertile and infertile women showed a reduction of 6 proteins and elevation of 12 proteins in uterine lavage of infertile women. This different pattern in proteins expressions in the uterine lavage of fertile and infertile women may impact on fertility of women.[18]
As reviewed earlier, most endometrial proteome studies have been conducted on the endometrial or uterine fluid samples at mid-proliferative and mid-secretory phases across the menstrual cycle in fertile or infertile women however, proteome analysis of human endometrium during various stages of pregnancy has not been achieved due to increasing risk of miscarriage and ethical considerations. A few animal studies have been carried out to identify differentially expressed proteins in pregnant endometrium compared to nonpregnant endometrium using 2DE gel.
We compared ovine endometrial (caruncular and intercaruncular) proteome of pregnancy on days 12 (conceptus preattachment), 16 (embryo implantation), and 20 (postimplantation) of pregnancy with ovine endometrial (caruncular and intercaruncular) proteome of oestrous cycle on days 12 and 16 using 2DE gel-based proteomics. In the caruncular endometrium, 58% (500 out of 867) and in the intercaruncular endometrium, 75% (998 out of 1324) of detected protein spots demonstrated some changes in expression during embryo implantation and early pregnancy. In caruncular endometrium, 45 protein spots (5% of total spots) changed between days 12 and 16 of pregnancy whereas 85 protein spots (10% of total spots) were differentially expressed between days 16 of pregnancy and oestrous cycle. In intercaruncular endometrium, 31 protein spots (2% of total spots) altered between days 12 and 16 of pregnancy while 44 protein spots (4% of total spots) were different between days 12 and 16 of oestrous cycle. Among the identified proteins, 11 proteins in the caruncular endometrium and 6 proteins in the intercaruncular endometrium were identified. The identified proteins play a role in proteins synthesis, protein degradation, antioxidant defense, cell structural integrity, cell adhesion, and signal transduction which may be important for embryo implantation and establishment of the pregnancy.[19] Chae et al.[20] studied proteome of pig endometrium on 7 days after menstruation (nonpregnant) and 40, 70, and 93 days of pregnancy. They reported 63 up-or down-regulated proteins between nonpregnant and pregnant endometrium. Several proteins involved in the development, cell differentiation, cell proliferation and cell death including transferrin, protein DJ-1, transgelin, and galectin 1 changed significantly in pregnant pig endometrium, indicating their importance in the maintenance of pregnancy. Findings of this study suggest that differentially expressed proteins in endometrium may regulate uterine development for the maintenance of pregnancy. In similar study, endometrial proteome of nonpregnant and pregnant pig (day 14) was compared together using 2DE-DIGE followed by LC-MS/MS along with iTRAQ labeling and nano-LC-MS/MS. Data of both proteomics techniques revealed 14 significantly changed proteins among endometrial proteome of pregnant and nonpregnant pigs. Expression of several proteins including signal transducer and activator of transcription 1, interferon and aldose reductase 1 (AKR1B1) altered between pregnant and nonpregnant endometrium. It was postulated that these proteins are possibly involved in embryo-maternal dialogue.[21]
Proteome studies relevant to endometrial tissue and secretions at the time of embryo implantation and during different phases of endometrial cycle have been resulted in identifying many proteins essential for embryo-maternal dialog, demonstrating that 2DE gel coupled by MS can apply to identify proteins playing crucial roles in endometrial function and development. Further, these findings reveal the complexity of the endometrial tissue in nature, in particular during receptivity, pregnancy, and infertility. With improvement of proteomic technology and better designing of in vivo and in vitro biological studies, it will likely provide better opportunity to identify novel biomarkers for embryo implantation, maintenance of pregnancy, and infertility in the near future.
CORPUS LUTEUM AND OOCYTE PROTEOME
The human corpus luteum (CL) is a principal source of steroid hormones particularly progesterone (P4) during the luteal phase that is important to the maintenance of early pregnancy. Application of gonadotropins for ovarian stimulation in IVF cycles results in changing endocrine environment and CL dysfunction.[22] Thus, research on proteome of CL would be helpful to reveal proteins and molecules involved in rescuing of the CL and maintenance of P4 production during early pregnancy. Further, it is valuable to know more about proteins which are involved in regression of the CL at the end of luteal phase within the normal menstrual cycle and also about molecular and proteome basis of the CL when there is a need to apply exogenous P4 or human chorionic gonadotrophin or estradiol to support luteal phase in patients undergoing IVF cycles. To date, however, a few proteomics studies have been carried out to investigate CL proteome.
We studied proteome of ovine CL on days 12, 16, and 20 of pregnancy compared with proteome of ovine CL on days 12 and 16 of the oestrous cycle using 2DE gel-based proteomics. At the time of embryo implantation on day 16, 77 protein spots were up-regulated, and 101 protein spots were down-regulated in CL of pregnancy compared to regressed CL. To identify proteins, 24 significantly changed protein spots were analyzed by tandem mass spectroscopy. We found several proteins, playing crucial roles in key biological pathways including oxidative stress, steroidogenesis, signal transduction, and apoptosis which have not previously been reported in association with alterations occurring in the CL during peri-implantation periods. The identified proteins are probably involved in rescuing the CL from regressing and allowing the CL to produce P4 during early pregnancy.[23]
A study on mouse CL proteome was carried out when the CL is functional during pregnancy (pregnancy CL), when the pregnancy CL is regressing after parturition (regressing CL) and finally the CL during lactation (lactation CL). The results demonstrated 24 proteins with a few differences between pregnancy CL and lactation CL probably because both pregnancy and lactation CLs are functional, secreting steroid hormones. 10 out of 24 identified proteins were enzymes defining a ketogenic metabolic landscape which can reveal the prevalence of de novo cholesterol synthesis in steroidogenic cells particularly in lactation CL. In addition, protein of 20 alpha-hydroxysteroid dehydrogenase, a well-known marker of CL regression, and 3 proteins of ferritin, cathepsin D, and gamma actin which are involved during CL regression were found higher in regressing CL when compared with functional CLs (pregnancy and lactation CLs).[13]
Gametes are good candidates to be subjected to proteomics technologies as their proteins create oocyte or sperm, having great importance in fertilization and pregnancy outcomes and it can enhance our knowledge about their biology to generate a healthier offspring in reproductive medicine. Further, the mature oocyte contains maternal proteins required for fertilization, oocyte-to-embryo transition and the early embryo development. For proteome analysis of gametes, a large number of them are required. As accessibility of human oocytes is difficult, and it is sometimes annoying for women, proteomics study on human oocytes has not been performed to date, however, due to easily accessibility of sperms, many proteomics studies have been carried out on human sperms.[24,25,26] Thus, the proteomics studies of oocyte have been carried out only in animals, particularly in mice.
Ma et al.[27] applied 2DE gel to analyze proteome and phosphoproteome of mouse oocyte at metaphase-II (MII) stage while the zona pellucida of oocytes had been removed. 380 proteins were identified from a total of 869 protein spots. Of these, 90 protein spots representing 53 unique proteins have been subjected to phosphopotein staining using Pro-Q Diamond dye, demonstrating they are present in the MII oocytes in phosphorylated forms. A comparative proteomics study on mouse oocyte at the germinal vesicle (GV) and MII stages (zona pellucida had been removed) was conducted by Cao et al.[28] using 2DE gel. The results revealed 56 up-regulated protein spots and 39 down-regulated ones at the MII oocytes compared with GV oocytes. Of 95 differentially expressed proteins spots between GV and MII oocytes, 63 proteins were identified.[28] However, in the similar study, only 12 differential proteins were identified using 2DE gel.[29] The reason may be because zona pellucida, which contains similar proteins, had not been removed from MII and GV oocytes and thus it might impact sensitivity of detection. Many of 63 identified proteins are known to be involved in oocyte meiosis and early embryonic development including adenylosuccinate synthetase, nucleoplasmin-2 and protein arginine deiminase type 6. They also identified for the 1st time, a highly expressed novel protein, E330034G19Rik, which might be an oocyte-specific protein.[28]
Jiang et al.[30] investigated proteins associated to oocyte aging and the effects of caffeine on oocyte aging in porcine using 2DE-DIGE. They reported 23 highly expressed proteins including citrate synthase, aldose reductase, and 3 lowly expressed proteins such as beta-actin and peptidyl arginine deiminase like protein during the oocyte aging process. In caffeine-treated oocytes, expression of 6 identified proteins such as heat shock 70 kDa protein 1B (HSPA1B) and protein glial fibrillary acidic increased while expression of 12 proteins such as 90-kDa heat shock protein (HS90A) and CALR decreased compared to oocytes in MII stage. They concluded that caffeine treatment could change expression levels of gamete proteins in favor of prolonging physiological aging. In conclusion, proteomic studies of oocyte may enhance our knowledge in connection with molecular and protein relationship underlying physiology, quality, and aging of oocyte.
STRENGTHS AND LIMITATIONS OF GEL-BASED PROTEOMICS APPLICATION IN REPRODUCTIVE SAMPLES
As reviewed, gel-based proteomics coupled with MS is a useful technique to investigate protein identification and protein expression alterations in female reproductive tissues. Nevertheless, obtained data from gel-based proteomics techniques should be cautiously interpreted particularly when the interested tissues are heterogeneous in nature. Heterogeneous tissues such as ovary, CL and endometrium contain various types of cells including epithelial cells, fibroblast cells, immune cells, blood cells, and endothelial cells. Further, these tissues undergo remodeling during the oestrous cycle, embryo implantation, and early pregnancy. In general, the issues that should be considered in analyzing and interpreting the data acquired from the heterogeneous tissues are: (1) The cell types which express the identified protein, (2) the amount of protein expressed in the different cell types, (3) changes in the ratios of different cell types in the tissues. These issues can be addressed by performing immunolocalization (immunohistochemistry [IHC]) and histomorphological analysis. In addition, no single approach can fully unravel the relationship between the amount of protein expressed (obtained data from gel-based proteomics) and gene expression or tissue remodeling. IHC, quantitative reverse transcription-polymerase chain reaction and cell culture followed by Western blot (WB) may be carried out to understand this relationship. For instance, in cell culture studies of endometrium, epithelial and stromal cells could be separately cultured and then protein levels, quantified by WB, could be correlated with the number of cells by counting cells recognized by the relevant antibodies.[23]
Data obtained from gel-based proteomics are required to be strongly validated to overcome technical errors performed during sample analysis or to reduce false positive or false negative results arising from individual biological variation. To validate the 2DE gel findings, 1DE/2DE WB and enzyme-linked immunosorbent assay (ELISA) were frequently utilized, however, in some cases, WB or ELISA findings did not show strong agreement with 2DE gel findings. It is possibly because the majority of antibodies are not isoform-specific. Therefore, where the 2DE gels yielded specific isoform separately, the WB reflected total immunoreactive quantities of the protein, and this may cause a dismaching between the 2DE gel and WB quantitations of proteins.[31]
Detection of low-abundance proteins in reproductive biological samples is one of the main limitations of current gel-based proteomics since large quantities of abundant soluble proteins obscure their detection.[32] The most low-abundance proteins including regulatory proteins, signal transduction proteins, and receptors are known to be important in cell function. To obviate this, cellular proteomics approach is increasingly used.[2] Moreover, to detect the low-abundance proteins, the abundant proteins could be removed from the reproductive samples by various methods such as affinity columns and sample sub-fractionation.[33] Thus, robust sample preparation techniques remain one of the most important steps in gel-based proteomics. Applying narrow range immobilized pH gradient/IPG (2–3 pH units and 1 pH unit) enables the most proteins to be resolved. As a consequence, more low-abundance proteins can be detected, and it also has markedly improved the reproducibility of 2DE gels.[2]
Most significant challenges in gel-based proteomics arise from working with samples of biological fluids including plasma, urine, secretome, and peritoneal fluid. The collection of these samples is easier and noninvasive when they are compared to tissue samples, however, there are some drawbacks to use samples of biological fluids in proteomics studies. For instance, the wide dynamic range of protein concentrations and presence of high-abundance proteins in plasma such as albumin and IgG makes difficult identification of low-abundance proteins in plasma and also other biological fluids.[34,35] To overcome this problem, high-abundance proteins can be removed by immunodepletion, affinity depletion, chromatography, and subfractionation of biological fluid samples.[36,37,38] Collectively, careful sample selection (tissue or biological fluid), high-throughput sample preparation, removal of abundance proteins, and sample fractionation strategies could significantly improve the data drawn from gel-based proteomics when a hypothesis is defined in female reproductive research.
CONCLUSION
This review provides an opportunity to deeper understanding of proteomics application, in particular, gel-based proteomics technology as a powerful technique to unravel molecular complexity underlying female reproductive biology and infertility. Despite good progress in SDS-PAGE electrophoresis, bioinformatics, and protein detection, there is still a long way to achieve highly standardized gel-based technique. Indeed, there are a few limitations to exploit the gel-based proteomics to investigate proteome profile of female reproductive tract. To challenge this, it is crucially required to robust sample preparation and to enhance the sensitivity of the technique. If the limitations of the gel-based proteomics are obviated, it will expectedly open new doors to research on the biology, physiology, and pathology of female reproductive tract including CL, oocyte, and endometrium.
ACKNOWLEDGEMENTS
We are grateful to thank Professor Paul A. Fowler (The University of Aberdeen) for his continued support. Study by Arianmanesh carried out in Paul A. Fowler laboratory was supported by NHS Grampian R and D (project number RG05/019).
Footnotes
Source of Support: Study by Arianmanesh carried out in Paul A. Fowler laboratory was supported by NHS Grampian R and D (project number RG05/019).
Conflict of Interest: None declared.
REFERENCES
- 1.Abdallah C, Dumas-Gaudot E, Renaut J, Sergeant K. Gel-based and gel-free quantitative proteomics approaches at a glance. Int J Plant Genomics. 2012:494572. doi: 10.1155/2012/494572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevalier F. Highlights on the capacities of “Gel-based” proteomics. Proteome Sci. 2010;8:23. doi: 10.1186/1477-5956-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert JP, Ethier M, Smith JC, Figeys D. Proteomics: From gel based to gel free. Anal Chem. 2005;77:3771–87. doi: 10.1021/ac050586d. [DOI] [PubMed] [Google Scholar]
- 4.Qin XJ, Ling BX. Proteomic studies in breast cancer (Review) Oncol Lett. 2012;3:735–743. doi: 10.3892/ol.2012.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulze WX, Usadel B. Quantitation in mass-spectrometry-based proteomics. Annu Rev Plant Biol. 2010;61:491–516. doi: 10.1146/annurev-arplant-042809-112132. [DOI] [PubMed] [Google Scholar]
- 6.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: Advances and challenges. Science. 2002;296:2185–8. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 8.Horcajadas JA, Pellicer A, Simón C. Wide genomic analysis of human endometrial receptivity: New times, new opportunities. Hum Reprod Update. 2007;13:77–86. doi: 10.1093/humupd/dml046. [DOI] [PubMed] [Google Scholar]
- 9.Diedrich K, Fauser BC, Devroey P, Griesinger G. Evian Annual Reproduction (EVAR) Workshop Group. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13:365–77. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- 10.Chen JI, Hannan NJ, Mak Y, Nicholls PK, Zhang J, Rainczuk A, et al. Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res. 2009;8:2032–44. doi: 10.1021/pr801024g. [DOI] [PubMed] [Google Scholar]
- 11.Parmar T, Gadkar-Sable S, Savardekar L, Katkam R, Dharma S, Meherji P, et al. Protein profiling of human endometrial tissues in the midsecretory and proliferative phases of the menstrual cycle. Fertil Steril. 2009;92:1091–103. doi: 10.1016/j.fertnstert.2008.07.1734. [DOI] [PubMed] [Google Scholar]
- 12.Rai P, Kota V, Sundaram CS, Deendayal M, Shivaji S. Proteome of human endometrium: Identification of differentially expressed proteins in proliferative and secretory phase endometrium. Proteomics Clin Appl. 2010;4:48–59. doi: 10.1002/prca.200900094. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez F, Garrido-Gómez T, López JA, Camafeita E, Quiñonero A, Pellicer A, et al. Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum Reprod. 2009;24:2607–17. doi: 10.1093/humrep/dep230. [DOI] [PubMed] [Google Scholar]
- 14.Leese HJ, Hugentobler SA, Gray SM, Morris DG, Sturmey RG, Whitear SL, et al. Female reproductive tract fluids: Composition, mechanism of formation and potential role in the developmental origins of health and disease. Reprod Fertil Dev. 2008;20:1–8. doi: 10.1071/rd07153. [DOI] [PubMed] [Google Scholar]
- 15.Scotchie JG, Fritz MA, Mocanu M, Lessey BA, Young SL. Proteomic analysis of the luteal endometrial secretome. Reprod Sci. 2009;16:883–93. doi: 10.1177/1933719109337165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seppälä M, Koistinen H, Koistinen R, Dell A, Morris HR, Oehninger S, et al. Glycodelins as regulators of early events of reproduction. Clin Endocrinol (Oxf) 1997;46:381–6. doi: 10.1046/j.1365-2265.1997.1510943.x. [DOI] [PubMed] [Google Scholar]
- 17.Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, García-Velasco JA, et al. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res. 2009;8:4622–32. doi: 10.1021/pr9004426. [DOI] [PubMed] [Google Scholar]
- 18.Hannan NJ, Stephens AN, Rainczuk A, Hincks C, Rombauts LJ, Salamonsen LA. 2D-DiGE analysis of the human endometrial secretome reveals differences between receptive and nonreceptive states in fertile and infertile women. J Proteome Res. 2010;9:6256–64. doi: 10.1021/pr1004828. [DOI] [PubMed] [Google Scholar]
- 19.Al-Gubory KH, Arianmanesh M, Garrel C, Bhattacharya S, Cash P, Fowler PA. Proteomic analysis of the sheep caruncular and intercaruncular endometrium reveals changes in functional proteins crucial for the establishment of pregnancy. Reproduction. 2014;147:599–614. doi: 10.1530/REP-13-0600. [DOI] [PubMed] [Google Scholar]
- 20.Chae JI, Kim J, Lee SG, Jeon YJ, Kim DW, Soh Y, et al. Proteomic analysis of pregnancy-related proteins from pig uterus endometrium during pregnancy. Proteome Sci. 2011;9:41. doi: 10.1186/1477-5956-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fröhlich T, Kösters M, Bauersachs S, Samborski A, Kessler B, Wolf E, et al. 119 quantitative proteome analysis of endometrium from pregnant and nonpregnant PIGS. Reprod Fertil Dev. 2012;25:206–7. [Google Scholar]
- 22.Devoto L, Kohen P, Muñoz A, Strauss JF., 3rd Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reprod Biomed Online. 2009;18(Suppl 2):19–24. doi: 10.1016/s1472-6483(10)60444-0. [DOI] [PubMed] [Google Scholar]
- 23.Arianmanesh M, McIntosh RH, Lea RG, Fowler PA, Al-Gubory KH. Ovine corpus luteum proteins, with functions including oxidative stress and lipid metabolism, show complex alterations during implantation. J Endocrinol. 2011;210:47–58. doi: 10.1530/JOE-10-0336. [DOI] [PubMed] [Google Scholar]
- 24.de Mateo S, Martínez-Heredia J, Estanyol JM, Domínguez-Fandos D, Vidal-Taboada JM, Ballescà JL, et al. Marked correlations in protein expression identified by proteomic analysis of human spermatozoa. Proteomics. 2007;7:4264–77. doi: 10.1002/pmic.200700521. [DOI] [PubMed] [Google Scholar]
- 25.Li LW, Fan LQ, Zhu WB, Nien HC, Sun BL, Luo KL, et al. Establishment of a high-resolution 2-D reference map of human spermatozoal proteins from 12 fertile sperm-bank donors. Asian J Androl. 2007;9:321–9. doi: 10.1111/j.1745-7262.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Heredia J, Estanyol JM, Ballescà JL, Oliva R. Proteomic identification of human sperm proteins. Proteomics. 2006;6:4356–69. doi: 10.1002/pmic.200600094. [DOI] [PubMed] [Google Scholar]
- 27.Ma M, Guo X, Wang F, Zhao C, Liu Z, Shi Z, et al. Protein expression profile of the mouse metaphase-II oocyte. J Proteome Res. 2008;7:4821–30. doi: 10.1021/pr800392s. [DOI] [PubMed] [Google Scholar]
- 28.Cao S, Guo X, Zhou Z, Sha J. Comparative proteomic analysis of proteins involved in oocyte meiotic maturation in mice. Mol Reprod Dev. 2012;79:413–22. doi: 10.1002/mrd.22044. [DOI] [PubMed] [Google Scholar]
- 29.Vitale AM, Calvert ME, Mallavarapu M, Yurttas P, Perlin J, Herr J, et al. Proteomic profiling of murine oocyte maturation. Mol Reprod Dev. 2007;74:608–16. doi: 10.1002/mrd.20648. [DOI] [PubMed] [Google Scholar]
- 30.Jiang GJ, Wang K, Miao DQ, Guo L, Hou Y, Schatten H, et al. Protein profile changes during porcine oocyte aging and effects of caffeine on protein expression patterns. PLoS One. 2011;6:e28996. doi: 10.1371/journal.pone.0028996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowler PA, Tattum J, Bhattacharya S, Klonisch T, Hombach-Klonisch S, Gazvani R, et al. An investigation of the effects of endometriosis on the proteome of human eutopic endometrium: A heterogeneous tissue with a complex disease. Proteomics. 2007;7:130–42. doi: 10.1002/pmic.200600469. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed N, Rice GE. Strategies for revealing lower abundance proteins in two-dimensional protein maps. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:39–50. doi: 10.1016/j.jchromb.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 33.Greenough C, Jenkins RE, Kitteringham NR, Pirmohamed M, Park BK, Pennington SR. A method for the rapid depletion of albumin and immunoglobulin from human plasma. Proteomics. 2004;4:3107–11. doi: 10.1002/pmic.200300815. [DOI] [PubMed] [Google Scholar]
- 34.Righetti PG, Boschetti E, Lomas L, Citterio A. Protein equalizer technology: The quest for a “democratic proteome”. Proteomics. 2006;6:3980–92. doi: 10.1002/pmic.200500904. [DOI] [PubMed] [Google Scholar]
- 35.Anderson NL. The clinical plasma proteome: A survey of clinical assays for proteins in plasma and serum. Clin Chem. 2010;56:177–85. doi: 10.1373/clinchem.2009.126706. [DOI] [PubMed] [Google Scholar]
- 36.Ametzazurra A, Matorras R, García-Velasco JA, Prieto B, Simón L, Martínez A, et al. Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum Reprod. 2009;24:954–65. doi: 10.1093/humrep/den450. [DOI] [PubMed] [Google Scholar]
- 37.Imre T, Kremmer T, Héberger K, Molnár-Szöllosi E, Ludányi K, Pócsfalvi G, et al. Mass spectrometric and linear discriminant analysis of N-glycans of human serum alpha-1-acid glycoprotein in cancer patients and healthy individuals. J Proteomics. 2008;71:186–97. doi: 10.1016/j.jprot.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Abbott KL, Lim JM, Wells L, Benigno BB, McDonald JF, Pierce M. Identification of candidate biomarkers with cancer-specific glycosylation in the tissue and serum of endometrioid ovarian cancer patients by glycoproteomic analysis. Proteomics. 2010;10:470–81. doi: 10.1002/pmic.200900537. [DOI] [PMC free article] [PubMed] [Google Scholar]


