Abstract
Fertility preservation is becoming increasingly important to improve the quality of life in cancer survivors. Despite guidelines suggesting that discussion of fertility preservation should be done prior to starting cancer therapies, there is a lack of implementation in this area. A number of techniques are available for fertility preservation, and they can be used individually or together in the same patient to maximize efficiency. Oocyte and embryo cryopreservation are now established techniques but have their limitations. Ovarian tissue cryopreservation though considered experimental at present, has a wider clinical application and the advantage of keeping the fertility window open for a longer time. Both chemotherapy and radiotherapy have a major impact on reproductive potential and fertility preservation procedures should be carried out prior to these treatments. The need for fertility preservation has to be weighed against morbidity and mortality associated with cancer. There is thus a need for a multidisciplinary collaboration between oncologists and reproductive specialists to improve awareness and availability.
KEY WORDS: Cancer, counseling, cryopreservation, fertility preservation
INTRODUCTION
The National Cancer Registry of India suggests that the annual number of patients who develop cancer in India is set to rise from about 9.79 lakhs in 2010 to 11.4 lakhs in 2020.[1] More than 140,000 cancer patients are diagnosed in their reproductive years that is, up to age of 45 years and childhood cancer too seems to be increasing. It is believed that in 2010, every 250th adult will be a survivor of childhood cancer.[2] Approximately, 40-80% of females face possible infertility as a result of their cancer treatments that is, chemotherapy, radiation, and surgery.
Fertility preservation in essence means preserving the ability of an individual or couple to start a family at a time of their choosing. Oncofertility is a term coined for fertility preservation in cancer patients. Improvement in cancer management and increasing survival rates has created a need for oncofertility. Current data suggest that for most tumors posttreatment pregnancy does not increase the risk of cancer progression or obstetric or neonatal outcome.[3] The emphasis therefore has moved from providing life to providing quality of life.
This article gives an overview of the various fertility preservation options available in women and the recommendations of the International Society for Fertility Preservation and the American Society for Reproductive Medicine (ASRM) with regard to fertility preservation in different malignancies and providing fertility preservation services respectively.
REPRODUCTIVE COUNSELING
Unfortunately, fertility preservation services are rarely offered or even discussed with the patient before starting cancer therapy. Studies have shown that infertility is a significant survival concern. Patients who received information regarding their sexual and reproductive health had lower levels of psychological distress than patients who did not receive this information. Informed decision reduces reproductive regret in these young men and women.[4]
In 2006, the American Society of Clinical Oncology first published recommendations on fertility preservation stating that “as part of education and informed consent before cancer therapy, oncologists should address the possibility of infertility with patients treated during their reproductive years and be prepared to discuss possible fertility preservation options or refer patients to reproductive specialists.”[5] The ASRM practice committee recommends that mental health professionals and genetic counselors be available to counsel the patient and aid them in decision-making. Genetic counselors are required to discuss any potential risks of transmission of the disease to the resulting offspring and available genetic testing in heritable diseases.[6]
CANCER THERAPY AND FERTILITY
Chemotherapy and radiotherapy remain the mainstay of cancer treatments. Both can be damaging to the ovary depending on the agent used, dose given, and age of the patient.
Chemotherapy
Chemotherapeutic drugs act by interrupting vital cell processes and arresting the normal cellular proliferation cycle, no wonder then that they have such a damaging effect on the germ cells. They cause DNA abnormalities as well as oxidative damage in somatic and germ cells. Persistent unrepaired DNA double-strand breaks activate apoptotic death in oocytes.[7] Genetic effects on the oocyte result in aneuploidy and early embryonic mortality.[8]
Ovarian effects
The clinical impact of chemotherapeutic drugs on the ovary is variable ranging from no effect to complete ovarian atrophy. The degree of damage is dependent upon the type of the chemotherapeutic agent used, dose given, age of the patient and her baseline ovarian reserve. The prepubertal ovary is less susceptible to damage by chemotherapeutic agents while older women have a lower ovarian reserve and hence are more susceptible to premature ovarian failure (POF).[9]
Reduction in ovarian reserve occurs because of apoptosis of the growing follicles and activation of the resting follicle with subsequent apoptosis, leading to a burn-out effect. Fibrosis of stromal blood vessels adds to the ovarian damage.[10,11] The clinical manifestation of this follicular loss ranges from a complete amenorrhea to premature menopause and varying degree of infertility.
Cytotoxic Drugs and their action on the ovary: Action of the various group of drugs is listed below[12] [Figure 1].
Figure 1.
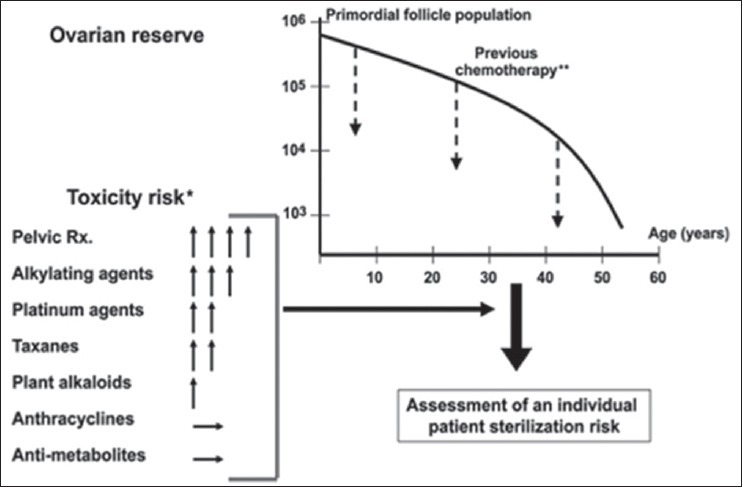
The risk of ovarian failure postchemotherapy is determined largely by the interaction of two factors: The type and amount of drug received and the age of the patient at treatment. Assessment of individual risk can be made using these factors; however, individual variation makes it advisable to consider fertility preservation measures even when treatment may fall into the low to moderate risk category. (Reprinted, with permission, from Meirow et al., 2010). *Vertical arrows represent the level of risk, with the greater number of arrows indicating greater risk; the horizontal arrow indicating negligible or unknown risk. **Dashed arrows represent the reduction in ovarian reserve that occurs following chemothereapy
Alkylating agents have an extremely damaging effect and are responsible for the highest age-adjusted odds ratio of ovarian failure rates[13]
Platinum-based compounds such as cisplatin cause DNA damage. They carry a medium risk of amenorrhea[14]
Anthracycline antibiotics such as doxorubicin (DXR) induce oxidative stress. The amenorrhea and fertility risk is medium to low with this group of drugs. Mailhes in 1995[15] reported that DXR administration in female mice caused dominant lethal mutations and aneuploidy in maturing/preovulatory oocytes in female mice
Vinca alkaloids do not seem to increase the risk of ovarian failure[16] though animal experiments show a high rate of oocyte aneuploidy[15]
Anti-metabolites like methotrexate and 5-fluorouracil do not seem to affect the ovary based on the limited current data available. Methotrexate is commonly used to treat the ectopic pregnancy without any effect on subsequent fertility
Taxanes – the data available are controversial with some studies showing increased risk of ovarian failure[11,15] others suggest that there is no increased risk[17,18]
Biological targeted therapies (herceptin, tamoxifen, rituximab) are anti-cancer treatments that are derived from living organisms. These agents are designed to interfere with specific molecules expressed by tumors (herceptin or tamoxifen), or act via the immune system (rituximab). Fertility risk data for these drugs are limited, but since they target specific cells, it is believed that the risk should be low. They are generally given as adjuvant therapy for 5 or more years after cancer treatment, this delay however might by itself pose a fertility risk.
Effect on oocytes
In animals, most cytotoxic drugs have been found to be mutagenic and teratogenic when oocytes are exposed during maturation, an increase in congenital malformations is seen when conception occurs within 3 months of treatment. In humans, live birth rates from pregnancies in cancer survivors are similar to those of siblings. No significant increase in miscarriage, congenital malformations, genetic abnormalities, or malignant neoplasms have been found when conception has taken place long after completion of therapy.[19,20] Risk of mutagenesis is maximum during the maturation phase of the oocyte, which is approximately 6 months[21] and minimum during the dormant stage. It is, therefore, recommended that patients be asked to delay conception for 6 months after completing the treatment, and fertility preservation techniques such as oocyte and embryo cryopreservation are carried out 6 months after treatment and not between treatments.[22] The exact safe interval from completion of treatment to oocyte collection for preservation has not been established.
Effect of radiotherapy
Unlike chemotherapy, radiotherapy affects both the ovary and the uterus.
Ovarian effects
Human oocyte is sensitive to radiation, with an estimated median lethal dose (LD50) of <2 Gy. Damage to the ovary by radiotherapy is dependent on the age of the patient and dose of the ovarian exposure. The effective sterilizing dose (ESD) is the dose of fractionated radiotherapy (Gy) at which POF occurs immediately after treatment in 97.5% of patients. ESD decreases with increasing age, being 20.3 Gy at birth, 18.4 Gy at 10 years, 16.5 Gy at 20 years, and 14.3 Gy at 30 years, with only 6 Gy being required to cause permanent ovarian failure in women over 40. The number of primordial follicles present at the time of treatment and the dose of radiation received by the ovaries determines the fertility “window.” Ovarian failure has been reported in 90% of patients following total body irradiation (TBI) (10–15.75 Gy) and in 97% of females treated with total abdominal irradiation (20–30 Gy) during childhood.[23]
Uterine effect
Uterine growth starts at puberty and is completed almost 7 years after menarche that is, around the age of 20.[24] Uterine blood flow also increases during puberty.[25] Exposure to radiation leads to reduced vascularity, damage to myometrium leading to fibrosis and hormone dependent endometrial insufficiency, which results in adverse reproductive outcomes subsequently. The uterine volume is lower and endometrium atrophies completely if there is direct radiation. In adults, an exposure to TBI of 12 Gy is associated with significant uterine damage. Radiation doses of >25 Gy directly to the uterus in childhood appears to induce irreversible damage.[26]
Increased rates of infertility, miscarriage, preterm labor, intra-uterine growth retardation and low birth weight have been reported by Reulen et al. 2009[27] especially if conception occurs within a year of radiotherapy.[28] An increase in perinatal mortality has been reported by Chiarelli et al. 2000,[29] though this group did not find any increase in spontaneous abortions or birth defects.
Teh et al. 2014[30] have suggested that patients receiving >45 Gy during adulthood and >25 Gy in childhood should be counseled to avoid attempting pregnancy. There is no clarity on the dose of radiation to the uterus, above which a pregnancy would not be sustainable. The first successful delivery after transplantation of cryopreserved ovarian cortical tissue and subsequent in vitro fertilization (IVF) has been reported in a patient of Ewing's sarcoma who had received sterilizing pelvic radiotherapy (54 Gy) and 40 weeks intensive high-dose chemotherapy for the treatment of Ewing's sarcoma 14 years earlier. Repeated transplantation procedures were required to obtain fully functional follicular development. Enlargement of the transplants over time and increase of the size of the uterus were demonstrated on sequential ultrasonographic exams.[31]
In colorectal cancers, radiation damage to the gonads and uterus is inevitable hence fertility preservation techniques should be strongly advised. Ovarian transposition needs to be performed to get the ovary out of the radiation field.
FERTILITY PROTECTION – MEDICAL AND SURGICAL STRATEGIES
Fertility-sparing surgery
Ovarian transposition
Protects ovarian function by moving the ovaries out of the field of radiation.[32] In craniospinal irradiation, the ovaries are fixed as laterally as possible, away from the spine; for pelvic irradiation, they are moved outside the pelvis and anchored as high as possible above the pelvic brim either in the paracolic gutter or anterior abdominal wall. This requires mobilization of the ovary by cutting the utero-ovarian ligaments. Titanium clips are placed on the two opposite borders of the ovaries for radiological identification. Ovarian transposition does carry certain risks such as increased ovarian cyst formation, postoperative adhesions, chronic pelvic pain, migration of the ovaries back to their native position and POF, apart from the surgical risk. There is also the concern of metastatic disease in the ovaries though it exists in a minority of patients (1%).[33] Since this procedure does not prevent ovarian damage by cytotoxic drugs, it should be avoided if the patient has to undergo both chemo and radiotherapy. Transvaginal oocyte recovery becomes difficult because of ovarian transposition, and transabdominal oocyte retrieval (OR) may be required for IVF.
Fertility-sparing surgery for cervical cancer
Standard management for cervical cancer is radical hysterectomy with lymph node dissection for early disease and a combination of chemotherapy and radiotherapy when disease has progressed. Radical trachelectomy is performed for women with early-stage cervical cancer (<2 cm in size) who have not yet completed their childbearing. Conization and simple trachelectomy can also be offered in selected cases in very early stage cancer. Xu et al. 2011,[34] compared 587 patients with early cervical cancer who underwent either radical trachelectomy or radical hysterectomy in a systemic review. No significant differences were noted between the groups for rates of recurrence, mortality, 5 years recurrence-free survival, or 5 years survival. There is, however, an increased risk of cervical incompetence, preterm delivery, low birth weight and cesarean section associated with this procedure.[35]
Ovarian tumors
Approximately, 30% of borderline tumors of the ovary affect women under 40 years of age. Traditional management is total hysterectomy with bilateral salpingo-oophorectomy (BSO). Type of fertility-sparing surgery (FSS) depends upon the histology, stage of disease and preexisting ovarian reserve. Nonepithelial malignant ovarian tumors, particularly germ-cell tumors, do well with fertility-sparing surgery. Unilateral salpingo-oophorectomy or in some cases cystectomy is done with extensive staging and subsequent follow-up. Recurrence after cystectomy is high 25%. FSS has also been tried for early stage epithelial ovarian malignancy. Prerequisites for conservative surgery include well-differentiated unilateral disease, with no sign of extra-ovarian metastasis. Kajiyama et al. 2011[36] in a series of 572 women with stage (i) epithelial ovarian cancer showed no differences in 5 years overall survival (OS) or disease-free survival between women who had undergone radical hysterectomy and those who had undergone fertility-sparing surgery. Patients need to understand the risk of recurrence and give consent knowing this potential risk.
Endometrial carcinoma
Standard management is total hysterectomy with BSO. Progestational agents are offered in well-differentiated early disease to women desirous of conception. Majority (73–81%) patients respond well to treatment. Recurrence rates are 18–40%, concurrent ovarian malignancy is present in 11–29% patients.[37] Pregnancy rate (PR) of 40% and live birth rates up to 47% have been reported.[38] So far, no randomized controlled trials have compared this treatment with standard care. Strict follow-up should be carried out with endometrial biopsy and magnetic resonance imaging to look for recurrence. Definitive treatment is required after completing childbearing.
FERTO-PROTECTIVE ADJUVANT THERAPY
“Ferto-protective adjuvant therapy” is a term used for administration of adjuvant therapy during or prior to chemotherapy with an agent that can prevent loss of ovarian reserve.[39] Unfortunately, no drug fits the bill yet though research has identified a few promising drugs. The list of drugs under investigation are sphingosine-1-phosphate (S1P) and imatinib that inhibit apoptosis, thalidomide and granulocyte colony-stimulating factor mechanism of their action is unclear, tamoxifen which works as an antioxidant, and AS101 which causes modulation of follicle activation pathway.[12] So far, the only drug used in clinical practice is the gonadotropin-releasing hormone (GnRH) agonist.
Development of ovo-protective agents is an urgent need to improve the quality of life of cancer survivors. Such drugs would allow patients to retain their natural fertility, eliminating the need for invasive fertility preservation procedures. Additionally, other distressing side effects of POF could be avoided. Consideration has to be given to the fact that such agents should not interfere with the efficacy of cancer treatment.
Gonadotropin-releasing hormone agonist is frequently used in conjunction with chemotherapy; however, contradictory results have been reported regarding loss of the follicular pool. Though menstrual cyclicity and even ovulation may resume, benefits in terms of fertility outcome are controversial. GnRH agonist causes suppression of the gonadotropin levels to prepubertal levels and decreases utero-ovarian perfusion,[40] these actions are believed to protect the follicles from destruction. GnRH analogs may also up-regulate anti-apoptotic molecules such as S1P.[41] Cochrane database review of 2011[42] concludes that the “use of GnRH agonists should be considered in women of reproductive age receiving chemotherapy. Intramuscular or subcutaneous GnRH analogs seem to be effective in protecting ovaries during chemotherapy and should be given before or during treatment, although no significant difference in PRs was seen.” Del Mastro et al. 2013[43] did a systemic review and meta-analysis of 9 studies (765 patients) and reported a significant protective effect of GnRH analogs in young cancer patients. The pooled OR estimate indicated a highly significant reduction in the risk of POF (OR = 0.43; 95% confidence interval: 0.22–0.84; P = 0.013) in patients receiving GnRHa. There was statistically significant heterogeneity among studies, and further studies are required to reach a conclusion.
Gonadotropin-releasing hormone-a administration should begin at least 10 days before the beginning of chemotherapy because of the initial flare-up effect and should continue till 2 weeks after the end of chemotherapy. In the case of estrogen-sensitive tumors, a tamoxifen therapy can be initiated after the GnRH-a treatment. GnRH analogs are not currently Food and Drug Administration approved for fertility preservation, but may be used “off label.”
FERTILITY PRESERVATION TECHNIQUES IN FEMALES
Embryo cryopreservation
Oocyte cryopreservation
Ovarian tissue cryopreservation (OTC)
In vitro maturation (IVM).
Embryo cryopreservation
This requires the patient to go through IVF. Since a sperm sample is required for oocyte fertilization, the woman must be married or should have a partner. Embryo cryopreservation is an established technology that provides a good success rate depending on the number and quality of embryos stored. Data on pregnancy and live birth rates in cancer patients after frozen embryo transfer are limited. A live birth rate of 38.7% per embryo transfer is reported for frozen embryo transfer in nononcological patients <35 years of age and 34.8% for oocyte donor cycles.[44] Cardozo et al. 2015[45] in a retrospective analysis compared PR in cancer patients who had a frozen embryo transfer with patients of tubal factor infertility undergoing IVF. Cumulative PR per transfer for cancer patients compared to controls was similar, 37 versus 43% respectively (P = 0.49) and cumulative live birth rate per transfer too did not show a difference 30 versus 32% respectively (P = 0.85). Cancer patients had a higher likelihood of live birth resulting in twins (44 vs. 14%; P = 0.035) possibly because there was no underlying infertility factor in these patients.
Limitations of the procedure:
Controlled ovarian stimulation (COS) takes approximately 2 weeks from the 2nd day of the period, and this may delay cancer treatment
High estradiol levels during stimulation may have a negative effect on estrogen-sensitive tumors
Partner's or donor sperm required which limits reproductive autonomy in the future and increases stress levels
Ethical, legal and religious implications regarding disposal of embryos in case patient dies before she can use the embryos or there is a separation of the partners
Cannot be used in prepubertal patients.
Mature oocyte cryopreservation
When a woman is unmarried or does not have a partner mature oocyte cryopreservation is carried out. In fact, it has been suggested that oocyte preservation is a better option for all women to maintain reproductive autonomy. Oocyte cryopreservation also requires the patient to go through ovarian stimulation and OR. Data on pregnancy and live birth rates from oocyte cryopreservation in cancer patients are scarce, so success rates extrapolated from other populations, such as young oocyte donors, have to be used for patient counseling.[46]
Due to improved freezing and thawing techniques PRs with oocyte cryopreservation have improved considerably. Cobo et al. in 2008 and in 2010,[47,48] reported an implantation rate (IR) of 40% and clinical PR of 55% with vitrified oocytes which was similar to that with fresh oocytes. In the study by Rienzi 2010[49] on self oocytes, the IR was 20% versus 21% and PR was 38% versus 45% vitrified versus fresh oocytes.
Both embryo and oocyte cryopreservation cannot be performed on prepubertal girls. Another disadvantage is that only a limited number of oocytes/embryos can be collected/generated in one attempt, which in turn restricts the number of attempts for pregnancy.
Ovarian stimulation for embryo or mature oocyte cryopreservation
This procedure should be recommended only if the patient's medical condition allows the COS and OR to be carried out safely and if there is a fair chance of a good ovarian response. Time is also a constraint since ovarian stimulation has to be started from day 2 of the menstrual cycle. The implications of delaying cancer therapy to complete the IVF procedure have to be taken into account. To avoid delay in treatment, random start of COS has been suggested. Oocyte recovery rates are not compromised prior to cancer therapy, but ovarian reserve may be compromised in women who have undergone prior gonadotoxic therapy.
Ovarian stimulation regimes
Gonadotropin-releasing hormone antagonist protocols afford more flexibility and are favored because of lower estradiol rise and lower gonadotropin usage. Dose of gonadotropins can be decided based on ante-mullerian hormone (AMH) levels, antral follicle count (AFC), age and body mass index to get an appropriate response.
To overcome time constraints, luteal phase stimulation and random start protocols have been proposed. In the luteal phase protocol, GnRH antagonist is given for 3–4 days to achieve a quick down-regulation and COS is started subsequently with or without the onset of a menstrual bleed. Cakmak et al. 2013[50] proposed the random start protocol where COS is started irrespective of the phase of the cycle in which the patient presents. GnRH antagonist is started when the follicles secondary to the lead follicle reaches a size of 12 mm. Normal follicular growth and development is observed despite the increased progesterone levels seen in the luteal phase or a spontaneous luteinizing hormone surge, which may occur when the initial lead follicle reaches maturity. The authors concluded that the number of total OR, oocyte maturity rate, mature oocyte yield, and fertilization rates were similar in random-(n = 35) and conventional-start (n = 93) cycles. No superiority was noted when comparing COS started in late follicular (n = 13) or luteal phase (n = 22). Duration of stimulation was increased in both phases compared to conventional start of COS on day 2 of the cycle.
Anti-estrogens letrozole and tamoxifen have been added to OS regimes to lower the peak estradiol levels in patients with estrogen-sensitive tumors. Oktay et al.[51] showed substantially reduced peak estradiol levels after stimulation with tamoxifen, letrozole, or tamoxifen and low-dose gonadotropins (peak estradiol level: 419 pg/mL, 380 pg/mL, and 1182 pg/mL respectively) than with traditional COS with gonadotropins. In a follow-up of 5-48 months there was no increase in the rate of cancer recurrence for the treated women compared with untreated controls. Letrozole in a dose of 2.5-5 mg is started from cycle day 2 and given up to the day of human chorionic gonadotropin trigger. The addition of letrozole did not adversely affect oocyte maturity and competence in either random or conventional-start protocols however it has been reported to reduce total oocyte numbers available for cryopreservation.[52] Tamoxifen, a selective estrogen receptor (ER) modulator binds with ER in target tissue, e.g. breast tissue and prevents proper binding of estrogen and subsequent transcription of DNA to mRNA. It is used as adjuvant therapy in breast cancer patients, and its use in OS protocols in breast cancer patients is seen as protective.
Complications
Since there is generally only a single attempt possible for IVF there is a temptation to go for heavy stimulation to recover the maximum number of oocytes. Such decisions should be taken with extreme caution as ovarian hyperstimulation syndrome (OHSS) in these women can pose a real danger apart from delaying cancer treatment. Antagonist regimes with GnRH agonist trigger should be the method of choice in patients at risk of OHSS. Other risks include delay of cancer therapy, theoretic stimulation of estrogen-sensitive cancers, a risk of thromboembolic phenomena.
In vitro maturation
Involves aspiration of immature oocytes after minimal or no stimulation followed by IVM and cryopreservation of mature oocytes or embryos generated after fertilization. Immature oocytes can also be collected in the luteal phase and from antral follicles in the ovarian tissue removed for cryopreservation. This technique has been performed experimentally and with good success in girls as young as 5 years.[53] So far, this technique has mainly been used in polycystic ovary syndrome patients, and data on efficacy and safety of IVM in cancer patients are not available. IR's with IVM are low being between 10% and 15%.
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue that is rich in primordial follicles, prior to ovarian failure by laparoscopy or laparotomy. Ovarian tissue is dissected into small fragments, and cryopreserved by slow-cooling technique or vitrification. The tissue is transplanted after completion of cancer therapy[54] into the pelvis (orthoptic transplant) or outside the pelvis-abdominal wall, and fore-arm have been used (heterotopic transplant). Spontaneous pregnancies can occur after orthotopic pelvic transplant[55] but IVF is necessary when a heterotropic transplant is carried out. Orthotopic transplantation has been more successful in humans, and many successful pregnancies have been reported. The first ongoing pregnancy from a heterotopic implantation of ovarian tissue has been reported recently by Stern et al.[56] from Melbourne, in a patient who had both ovaries removed because of ovarian cancer. The tissue was transplanted into the abdominal wall, two oocytes were recovered after mild stimulation and embryos implanted into the uterus. No live births have been reported so far in females who cryopreserved tissue before puberty.
Low follicular survival rate after ovarian transplantation, precludes its use in women over 40 years. In younger patients, the amount of ovarian tissue cryopreserved theoretically should be proportional to the risk of age-related diminished follicular reserve. Based on the current evidence, removal of both ovaries for cryopreservation is not justified at this time unless the chemotherapy regimen has an extremely high likelihood of inducing complete ovarian failure.
This technique has many advantages over oocyte and embryo cryopreservation. It does not delay the start of cancer therapy and avoids the risk of ovarian stimulation. There is no need for partner or donor sperm. It preserves a larger pool of follicles and allows for the resumption of ovarian function. Ovarian function generally resumes between 60 and 240 days posttransplant and lasts for up to 7 years.[57] It is the only technique available for preserving fertility in prepubertal girls. Although no transplantations of tissue harvested from prepubertal girls have yet been reported in humans, the procedure is well-tolerated and holds great promise for the future.
Reseeding tumor cells following ovarian tissue transplantation is a major concern especially for malignancies like leukemias that are systemic in nature autologous transplantation is contraindicated in situations where cancer cells may be present in the cryopreserved ovarian tissue. It is unclear whether screening with histologic evaluation or with tumor markers is reliable and reduces the risk of reseeding tumor cells.[58] Patients harboring the BRCA1 or BRCA2 gene may also be at risk. A temporary heterotopic transplantation followed by removal of the tissue after childbearing can be an option for at-risk specimens.[59]
Currently, OTC is considered experimental though more than 30 live births have been reported so far. It can be recommended in carefully selected patients and should be offered only by centers with the necessary laboratory and surgical expertise.
SPECIAL CLINICAL CONDITIONS
Breast cancer
Patients with breast cancer can undergo IVF while waiting for chemotherapy after surgery. The challenge here is the hyperestrogenemia caused by COS. Aromataze inhibitors, mild stimulation protocols have been used to avoid high estradiol levels. IVM or ovarian tissue preservation can be offered to patients not willing for OS. Carriers of BRCA mutations may be offered BSO as a risk reduction strategy for ovarian cancer. They can have multiple OR's to preserve oocytes/embryos before BSO. Preimplantation genetic diagnosis can be carried out on the embryos for BRCA mutation before transfer. OTC for transplantation is not advisable in patients carrying a BRCA mutation given the increased risk of ovarian cancer in this population. Ovarian tissue harvesting for IVM of oocytes or follicles may be considered.
Concerns
Cryopreserving ovarian tissue may prevent thorough pathologic examination of the ovaries and, therefore, miss an occult epithelial malignancy.
Hematologic malignancies
Patients with hematologic malignancies (leukemias ad lymphomas) often present after having already been exposed to gonadotoxic therapy because of the immediate need for therapy. Due to abnormal hematological parameters risk of surgical complications increases, pregnancy outcomes using embryos created after recent exposure to chemotherapy are not known. Animal data suggest that there may be an increased risk of miscarriage and birth defects. Patients with leukemia may be good candidates for GnRH agonist co-administration in order to manage the ovulation and menstrual bleeding during chemotherapy.
Children and adolescents children and adolescents represent a special patient group there is a need for extreme sensitivity when broaching the topic of fertility preservation. Parents have to be given full information of the process, associated risks and success rates. Thorough psychological counseling is required, and ethical considerations have to be kept in mind.
Postpubertal girls under the age of 18 may be candidates for mature oocyte cryopreservation following OS. This also may be an option for adolescents who are peripubertal, but still premenarchal.[60] IVM and OTC can also be offered. In prepubertal girls, OTC is currently the only way to cryopreserve gametes. Careful counseling and informed consent is especially recommended.
THE INTERNATIONAL SOCIETY FOR FERTILITY PRESERVATION RECOMMENDATIONS FOR FERTILITY PRESERVATION
All patients who desire to preserve fertility should be counseled and informed about currently available fertility preservation options by fertility specialists. Recommendations should be individualized and should not violate the ethical principles. In general, fertility preservation before cancer treatment is strongly recommended if the chance of losing fertility is over 30% with cancer therapy. In pediatric patients, the risk of gonadal failure with chemotherapy is very low in the absence of hematopoietic stem cell transplantation (HSCT).[61]
Lymphoma
Postpubertal female
Cryopreservation of embryos or cryopreservation of oocytes is recommended if cancer treatment can be delayed. However, immediate treatment is required in most of the lymphoma patients, and thus cryopreservation of ovarian tissue should be considered as a fertility preservation option. Alternatively, immature OR followed by IVM and cryopreservation of oocytes or embryos can be considered. The protective effect of GnRHa is questionable and controversial. However, GnRHa co-treatment can be considered for female patients undergoing chemotherapy (not for HSCT) if there is no other option.
Prepubertal female
Ovarian tissue cryopreservation, if the risk of ovarian failure after cancer treatment is high enough to justify the procedure.
Leukemia
Postpubertal female
No ideal option to date. However, cryopreservation of ovarian tissue should be considered before HSCT.
Prepubertal female
Ovarian tissue cryopreservation before HSCT.
Any harvested tissue from leukemia patients should not be used for auto-transplantation because of high risk of cancer cell reintroduction. In the absence of HSCT, fertility preservation before chemotherapy is not necessary.
Breast cancer
It is recommended that fertility preservation consultation is arranged at the time of initial diagnosis. In many cases, young breast cancer patients require adjuvant chemotherapy after surgery (mastectomy or lumpectomy). The best time for fertility preservation is after surgery and before adjuvant therapy. Cryopreservation of embryos or cryopreservation of oocytes is recommended as a fertility preservation option before chemotherapy. As cryopreservation of embryos or oocytes requires COS, the risk of increased peak estradiol levels with COS in breast cancer patients (especially with ER + tumor) should be discussed before the procedure. The COS strategy using tamoxifen or letrozole in conjunction with gonadotropin may be safer for women with ER + tumor. For women who require urgent cancer treatment such as neo-adjuvant chemotherapy, cryopreservation of ovarian tissue should be considered. Alternatively, immature OR followed by IVM and cryopreservation of oocytes or embryos can be considered.
Criteria for ovarian tissue banking
Age: Under 37 years (may be individualized based on the status of ovarian reserve)
Ovarian function: Premenopausal by follicle-stimulating hormone, AFC or AMH
Communication with oncologists: Cancer treatment plan, prognosis
When embryo freezing or oocyte freezing is not indicated: Delaying cancer treatment is not acceptable, hormonal stimulation is not permitted, assisted reproductive technology (ART) is not allowed
Prepubertal girls who do not have any other options
High risk for POF (when significant loss of ovarian follicles is anticipated with cancer therapy)
Informed consent from adult patients
Informed consent from parents/guardians as well as informed assent from minors, if the patient is <18 years.
Physically and mentally healthy enough for surgery
Desires to have a child in the future (preferably before the age 50)
Thorough patient counseling: Currently available fertility preservation options including embryo and oocyte cryopreservation, how to use cryobanked ovarian tissue for fertility restoration
Should understand the experimental nature and potential risks of cancer cell transmission.
FERTILITY PRESERVATION SERVICES
The practice committee of ASRM recommendations[6] for fertility preservation services are summarized below.
Programmatic requirements for a fertility preservation program
Rapid access
It should be available as there is a shortage of time.
Interdisciplinary medical team
Interdisciplinary medical team is required which should include oncologists, reproductive endocrinologists and urologists, and reproductive surgeons trained in Fertility preservation techniques.
Laboratory requirements
Fertility preservation programs should be associated with an experienced ART program capable of providing a full complement of Fertility preservation techniques all the year round. Ideally, programs also should be able to counsel prepubertal patients and provide access to procedures (under Institutional Review Board-approved protocols) such as ovarian and testicular tissue cryopreservation, both of which are still considered experimental.
Counselors
Mental health professionals: To counsel patients and help them in the decision-making process
Genetic counselors: Some diseases are heritable so a genetic counselor should be available to discuss any potential risks of transmission of the disease to the resulting offspring and available genetic testing
Financial counselors.
Interdisciplinary collaboration
Collaboration between medical and surgical oncologists, reproductive endocrinologists, and urologists is important. Oncologists have the initial responsibility to discuss the reproductive risks of intended cancer therapies. An experienced reproductive endocrinologist or urologist should discuss in detail the appropriate Fertility preservation techniques. Ideally all adolescents and individuals of reproductive age should be referred.
Medical considerations
Patients in need of fertility preservation should be given all the options available for preservation of their gametes, as well as alternatives such as the use of donor gametes, donor embryos, surrogacy, and adoption. The potential safety of future pregnancy after cancer should be addressed, taking into account the type of cancer and proposed treatment. Consent forms should include options for gamete disposition in the event of demise of the patient.
CONCLUSION
In “oncomedicine” disease-free state is becoming a reality in a significant number of young men and women. These patients would look at the prospects of reproduction once they achieve disease free status. If appropriate action is not taken in time to preserve their fertility, the toxic effects of chemo and radiation therapy may render them sterile. A number of techniques are available for fertility preservation and they can be used individually or together in the same patient to maximize efficiency for e.g. IVM and OTC, ovarian transposition and OTC or OTC followed by ovarian stimulation and oocyte preservation.[62] Oocyte and embryo cryopreservation are now established techniques but have their limitations. OTC has a wider application and the advantage of keeping the fertility window open for a longer time. The need for fertility preservation has to be weighed against morbidity and mortality associated with cancer. There is thus a need for a multidisciplinary collaboration between oncologists and reproductive specialists to improve awareness and availability.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.National Cancer Registry of India. http://www.icmr.nic.in .
- 2.Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999;33:29–33. doi: 10.1002/(sici)1096-911x(199907)33:1<29::aid-mpo6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Noyes N, Melzer K, Druckenmiller S, Fino ME, Smith M, Knopman JM. Experiences in fertility preservation: Lessons learned to ensure that fertility and reproductive autonomy remain options for cancer survivors. J Assist Reprod Genet. 2013;30:1263–70. doi: 10.1007/s10815-013-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn GP, Vadaparampil ST, Bell-Ellison BA, Gwede CK, Albrecht TL. Patient-physician communication barriers regarding fertility preservation among newly diagnosed cancer patients. Soc Sci Med. 2008;66:784–9. doi: 10.1016/j.socscimed.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 6.Practice Committee of American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril. 2013;100:1214–23. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci U S A. 2005;102:737–42. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higdon RE, Marchetti F, Mailhes JB, Phillips GL. The effects of cisplatin on murine metaphase II oocytes. Gynecol Oncol. 1992;47:348–52. doi: 10.1016/0090-8258(92)90138-9. [DOI] [PubMed] [Google Scholar]
- 9.Meirow D, Wallace WH. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:2682. [PubMed] [Google Scholar]
- 10.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–43. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–92. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 12.Roness H, Kalich-Philosoph L, Meirow D. Prevention of chemotherapy-induced ovarian damage: Possible roles for hormonal and non-hormonal attenuating agents. Hum Reprod Update. 2014;20:759–74. doi: 10.1093/humupd/dmu019. [DOI] [PubMed] [Google Scholar]
- 13.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–39. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 14.Nozaki Y, Furubo E, Matsuno T, Fukui R, Kizawa K, Kozaki T, et al. Collaborative work on evaluation of ovarian toxicity 6) Two- or four-week repeated-dose studies and fertility study of cisplatin in female rats. J Toxicol Sci. 2009;34(Suppl 1):SP73–81. doi: 10.2131/jts.34.s73. [DOI] [PubMed] [Google Scholar]
- 15.Mailhes JB. Important biological variables that can influence the degree of chemical-induced aneuploidy in mammalian oocyte and zygotes. Mutat Res. 1995;339:155–76. doi: 10.1016/0165-1110(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 17.Davis AL, Klitus M, Mintzer DM. Chemotherapy-induced amenorrhea from adjuvant breast cancer treatment: The effect of the addition of taxanes. Clin Breast Cancer. 2005;6:421–4. doi: 10.3816/CBC.2005.n.046. [DOI] [PubMed] [Google Scholar]
- 18.Ganz PA, Land SR, Geyer CE, Jr, Cecchini RS, Costantino JP, Pajon ER, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29:1110–6. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, et al. Pregnancy outcome of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol. 2002;187:1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 20.Green DM, Sklar CA, Boice JD, Jr, Mulvihill JJ, Whitton JA, Stovall M, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–81. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meirow D, Schiff E. Appraisal of chemotherapy effects on reproductive outcome according to animal studies and clinical data. J Natl Cancer Inst Monogr. 2005;34:21–5. doi: 10.1093/jncimonographs/lgi025. [DOI] [PubMed] [Google Scholar]
- 22.Chung K, Donnez J, Ginsburg E, Meirow D. Emergency IVF versus ovarian tissue cryopreservation: Decision making in fertility preservation for female cancer patients. Fertil Steril. 2013;99:1534–42. doi: 10.1016/j.fertnstert.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–44. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Holm K, Laursen EM, Brocks V, Müller J. Pubertal maturation of the internal genitalia: An ultrasound evaluation of 166 healthy girls. Ultrasound Obstet Gynecol. 1995;6:175–81. doi: 10.1046/j.1469-0705.1995.06030175.x. [DOI] [PubMed] [Google Scholar]
- 25.Laursen EM, Holm K, Brocks V, Jarden M, Müller J. Doppler assessment of flow velocity in the uterine artery during pubertal maturation. Ultrasound Obstet Gynecol. 1996;8:341–5. doi: 10.1046/j.1469-0705.1996.08050341.x. [DOI] [PubMed] [Google Scholar]
- 26.Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Müller J, Andersen AN. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand. 2004;83:96–102. doi: 10.1111/j.1600-0412.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 27.Reulen RC, Zeegers MP, Wallace WH, Frobisher C, Taylor AJ, Lancashire ER, et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2239–47. doi: 10.1158/1055-9965.EPI-09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenig E, Mishaeli M, Kalish Y, Lishner M. Pregnancy and radiation. Cancer Treat Rev. 2001;27:1–7. doi: 10.1053/ctrv.2000.0193. [DOI] [PubMed] [Google Scholar]
- 29.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–54. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 30.Teh WT, Stern C, Chander S, Hickey M. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int. 2014;2014:482968. doi: 10.1155/2014/482968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Wallberg KA, Karlström PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2014 Dec 24; doi: 10.1111/aogs.12568. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisharah M, Tulandi T. Laparoscopic preservation of ovarian function: An underused procedure. Am J Obstet Gynecol. 2003;188:367–70. doi: 10.1067/mob.2003.38. [DOI] [PubMed] [Google Scholar]
- 33.Ronn R, Holzer HE. Oncofertility in Canada: Gonadal protection and fertility-sparing strategies. Curr Oncol. 2013;20:e602–7. doi: 10.3747/co.20.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L, Sun FQ, Wang ZH. Radical trachelectomy versus radical hysterectomy for the treatment of early cervical cancer: A systematic review. Acta Obstet Gynecol Scand. 2011;90:1200–9. doi: 10.1111/j.1600-0412.2011.01231.x. [DOI] [PubMed] [Google Scholar]
- 35.Rasool N, Rose PG. Fertility-preserving surgical procedures for patients with gynecologic malignancies. Clin Obstet Gynecol. 2010;53:804–14. doi: 10.1097/GRF.0b013e3181f97d02. [DOI] [PubMed] [Google Scholar]
- 36.Kajiyama H, Shibata K, Mizuno M, Umezu T, Suzuki S, Nawa A, et al. Long-term survival of young women receiving fertility-sparing surgery for ovarian cancer in comparison with those undergoing radical surgery. Br J Cancer. 2011;105:1288–94. doi: 10.1038/bjc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorais J, Dodson M, Calvert J, Mize B, Travarelli JM, Jasperson K, et al. Fertility-sparing management of endometrial adenocarcinoma. Obstet Gynecol Surv. 2011;66:443–51. doi: 10.1097/OGX.0b013e31822f8f66. [DOI] [PubMed] [Google Scholar]
- 38.Kalogiannidis I, Agorastos T. Conservative management of young patients with endometrial highly-differentiated adenocarcinoma. J Obstet Gynaecol. 2011;31:13–7. doi: 10.3109/01443615.2010.532249. [DOI] [PubMed] [Google Scholar]
- 39.Woodruff TK. Preserving fertility during cancer treatment. Nat Med. 2009;15:1124–5. doi: 10.1038/nm1009-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22:1626–33. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 41.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy. The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries? Oncologist. 2007;12:1044–54. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Li J, Cui T, Hu L. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2011;11:CD008018. doi: 10.1002/14651858.CD008018.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Del Mastro L, Giraudi S, Levaggi A, Pronzato P. Medical approaches to preservation of fertility in female cancer patients. Expert Opin Pharmacother. 2011;12:387–96. doi: 10.1517/14656566.2011.522568. [DOI] [PubMed] [Google Scholar]
- 44.Society for Assisted Reproductive Technology. Clinic Summary Report. 2010 [Google Scholar]
- 45.Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: A 17-year experience. J Assist Reprod Genet. 2015 doi: 10.1007/s10815-015-0428-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: A guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Cobo A, Domingo J, Pérez S, Crespo J, Remohí J, Pellicer A. Vitrification: An effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol. 2008;10:268–73. doi: 10.1007/s12094-008-0196-7. [DOI] [PubMed] [Google Scholar]
- 48.Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–64. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: A prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: Random-start controlled ovarian stimulation. Fertil Steril. 2013;100:1673–80. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 51.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: A prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–53. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 52.Revelli A, Porcu E, Levi Setti PE, Delle Piane L, Merlo DF, Anserini P. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol. 2013;29:993–6. doi: 10.3109/09513590.2013.819083. [DOI] [PubMed] [Google Scholar]
- 53.Revel A, Revel-Vilk S, Aizenman E, Porat-Katz A, Safran A, Ben-Meir A, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92:458–63. doi: 10.1016/j.fertnstert.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Morris SN, Ryley D. Fertility preservation: Nonsurgical and surgical options. Semin Reprod Med. 2011;29:147–54. doi: 10.1055/s-0031-1272477. [DOI] [PubMed] [Google Scholar]
- 56.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod. 2013;28:2996–9. doi: 10.1093/humrep/det360. [DOI] [PubMed] [Google Scholar]
- 57.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29:489–93. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosendahl M, Andersen MT, Ralfkiær E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94:2186–90. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 59.Practice Committee of the American Society for Reproductive Medicine. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2004;82:993–8. doi: 10.1016/j.fertnstert.2004.07.925. [DOI] [PubMed] [Google Scholar]
- 60.Reichman DE, Davis OK, Zaninovic N, Rosenwaks Z, Goldschlag DE. Fertility preservation using controlled ovarian hyperstimulation and oocyte cryopreservation in a premenarcheal female with myelodysplastic syndrome. Fertil Steril. 2012;98:1225–8. doi: 10.1016/j.fertnstert.2012.07.1056. [DOI] [PubMed] [Google Scholar]
- 61.ISFP Practice Committee. Kim SS, Donnez J, Barri P, Pellicer A, Patrizio P, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet. 2012;29:465–8. doi: 10.1007/s10815-012-9786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huober-Zeeb C, Lawrenz B, Popovici RM, Strowitzki T, Germeyer A, Stute P, et al. Improving fertility preservation in cancer: Ovarian tissue cryobanking followed by ovarian stimulation can be efficiently combined. Fertil Steril. 2011;95:342–4. doi: 10.1016/j.fertnstert.2010.07.1074. [DOI] [PubMed] [Google Scholar]


