Abstract
Aim:
The present study deals with the formulation of fast dissolving films of Rizatriptan benzoate that is used for the treatment of Migraine. The concept of fast-dissolving drug delivery emerged from the desire to provide patient with more conventional means of taking their medication. Materials and Methods: In the present research work, various trials were carried out using film forming agents such as maltodextrin, gum karaya and xanthan gum to prepare an ideal film. Emulsion evaporation method was used for the preparation of films. The prepared films were evaluated for weight uniformity, drug content, film thickness, folding endurance, dispersion test and curling. The in vitro dissolution studies were carried out using simulated salivary fluid (pH 6.8 phosphate buffer).
Results:
About 97% of the drug was found to be released from the film within 10 min that is a desirable character for fast absorption. The drug excipient interaction studies carried out by differential scanning calorimetry analysis and Fourier transform infrared studies revealed that there were no major interactions between the drugs and excipients used for the preparation of films.
Conclusion:
Fast dissolving films of Rizatriptan benzoate prepared by emulsion evaporation technique were found to be suitable for eliciting better therapeutic effect in the treatment of migraine.
Keywords: Emulsion evaporation method, fast dissolving films, gum karaya, maltodextrin, rizatriptan benzoate, xanthan gum
INTRODUCTION
The oral cavity has been investigated as a site for drug delivery for a long period. Drug delivery through the oral cavity offers many advantages. Among all the routes of administration, the oral route is most preferred route in the designing of dosage form than drug delivery design by other routes of administration.[1,2] The oral mucosa is conveniently and easily accessible and therefore allows uncomplicated application of various dosage forms. Furthermore, the oral mucosa is robust against local stress or damage and shows fast cellular recovery. Active Substances can be administered locally to treat oral diseases such as periodontal disease, bacterial and fungal infections. A systemic action can be achieved via drug permeation through the mucosal epithelium.[3,4] The concept of fast-dissolving drug delivery emerged from the desire to provide patient with more conventional means of taking their medication. Fast dissolving films recently have acquired great importance in the pharmaceutical industry due to their unique properties and specific advantages like no need of water for disintegration, accurate dosing, rapid onset of action, ease of transportability, ease of handling, pleasant taste and improved patient compliance.[5,6] Fast dissolving film is a type of drug delivery system, which when placed in the oral cavity it rapidly disintegrates and dissolves to release the medication for oromucosal and intragastric absorption, without chewing and intake of water.[7,8] This technology evolved over the past few years from the confection and oral care markets in the form of breath strips and became a novel and widely accepted form by consumers. These films have the potential to deliver the drug systemically through intragastric, sublingual or buccal route of administration and also has been used for local action.[9,10,11] Rizatriptan benzoate is a selective 5-hydroxy tryptamine receptor subtype agonist indicated for the acute treatment of migraine with or without aura in adults. The half-life of Zolmitriptan is 2-3 h and it undergoes hepatic metabolism, the absolute oral bioavailability is about 45%.[12] The present work was aimed to improve the bioavailability and efficacy of rizatritan benzoate by preparing rapidly dissolving buccal films.
MATERIALS AND METHODS
Rizatriptan benzoate was obtained as gift sample from Apotex labs, Bangalore. Maltodextrin was obtained from Mylan Hyderabad. Xanthum gum and Gum karaya were obtained from Yarrow chem. Ltd., Mumbai. Cinnamon oil, mannitol, saccharin, starch and citric acid were obtained from Qualigens Fine Chemicals., Mumbai.
Preparation of rizatriptan film
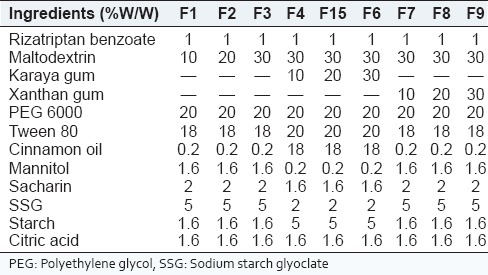
Rizatriptan benzoate buccal films were prepared by emulsion evaporation method. Cinnamonoil (oil phase) and Tween 80 were taken in a 50 ml beaker and mixed well by using magnetic stirrer to get a clear solution which is labeled as solution A. Solution B is prepared by taking sufficient quantities of polyethylene glycol (PEG) 6000 and starch in another beaker, sufficient amount of water was added and heated on heating mantle until a clear solution was obtained (aqueous phase). Film-forming polymers, sweetening agents, and citric acid was dissolved in distilled water to obtain solution C. Solution B were carefully added to the solution A with continuous stirring for 10 min to get a milky white emulsion. Then, the accurately weighed amount of drug was added to the emulsion and was stirred continuously for 10 min. Finally, the solution C was added to the above and was stirred continuously for 1 h. The emulsion obtained was casted on the nonadhesive base plate and dried under infrared lamp for 24 h. After complete drying the films, were cut into required sizes. Various trials were carried out to optimize the formula for the preparation of Rizatriptan benzoate films. The compositions of prepared films were given in Table 1.
Table 1.
Composition of rizatriptan benzoate buccal fast dissolving films along with maltodextrin
Evaluation of physical parameters for rizatriptan benzoate buccal fast dissolving films
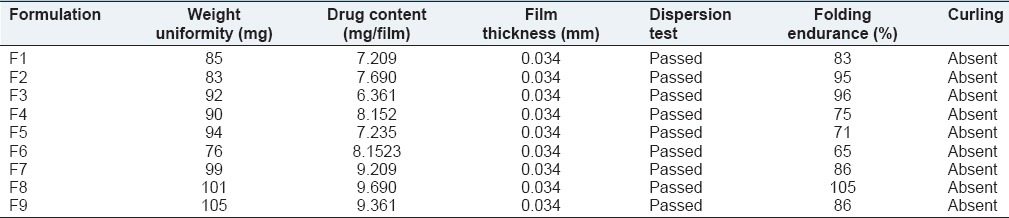
The film formulations were further evaluated for physical parameters. Thickness of the film was measured using screw gauge. Folding endurance of the film was determined by repeatedly folding a small strip of the film at one place. Air bubble entrapment is a common phenomenon that we see in all liquid formulations Presence of air bubbles leads to improper drug content and content uniformity. Entrapment of air bubbles is completely absent in all formulations before casting the film and after drying the film. Curling of the films is observed normally in film formulations prepared using solvent casting method in order to minimize curling of film starch (1.6%) is used as anticurling agent. The results of physical evaluation test were given in Table 2.
Table 2.
Evaluation of physical parameters for rizatriptan benzoate buccal fast dissolving films
In vitro dissolution studies
Dissolution studies were performed on all the film formulation by using Franz diffusion cell apparatus containing simulated salivary fluid (pH 6.8 phosphate buffer) as a medium. The dissolution studies were carried over a period of 15 min for all the formulations. Dissolution studies were carried out in triplicate, maintaining the sink conditions for all the formulations. A 5 ml aliquot of samples was withdrawn at regular time intervals, filtered and assayed spectrophotometrically at 280 nm. The drug release profiles for all the film formulations were shown in Figures 1–3.
Figure 1.
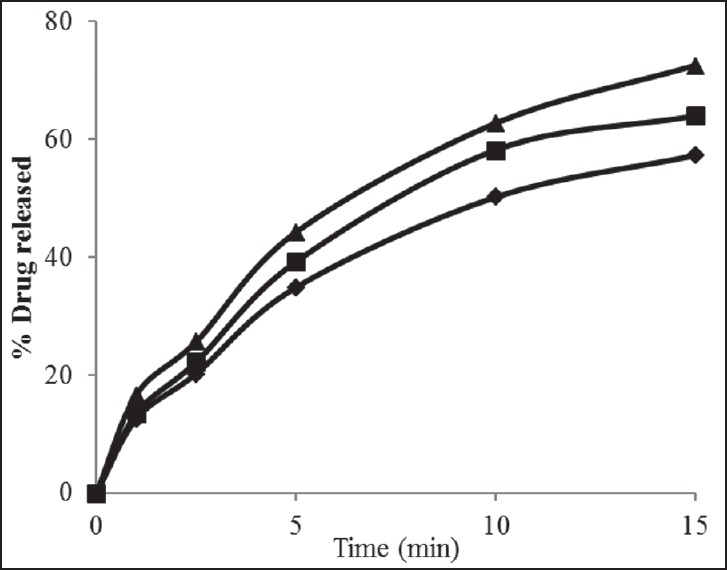
Drug release profiles for rizatriptan benzoate buccal fast dissolving films along with maltodextrin (F1-F3). Formulation F 1 (-♦-), F2 (-■-) F3 (-▲-)
Figure 3.
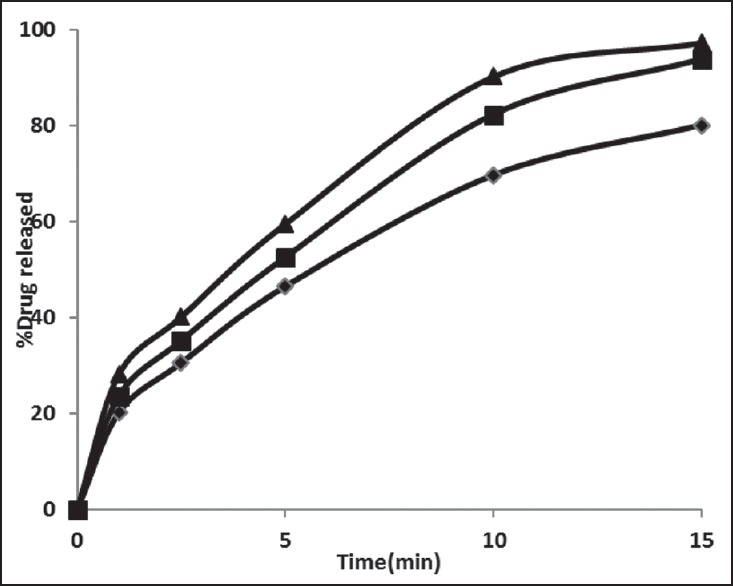
Drug release profiles for rizatriptan benzoate buccal fast dissolving films along with maltodextrin and xanthan gum (F7-F9). Formulation F7 (-♦-), F8 (-■-) F9 (-▲-)
Figure 2.
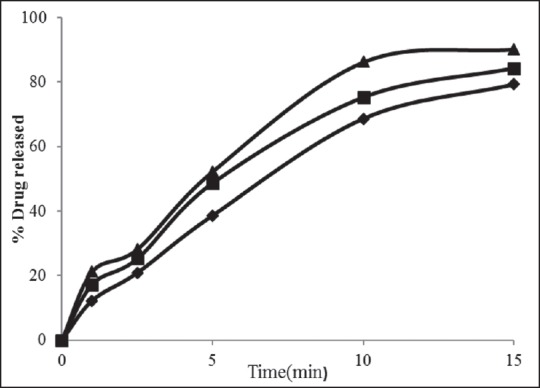
Drug release profiles for rizatriptan benzoate buccal fast dissolving films along with maltodextrin and karayan gum (F4-F6). Formulation F4 (-♦-), F5 (-■-) F6 (-▲-)
Evaluation of various dissolution parameters
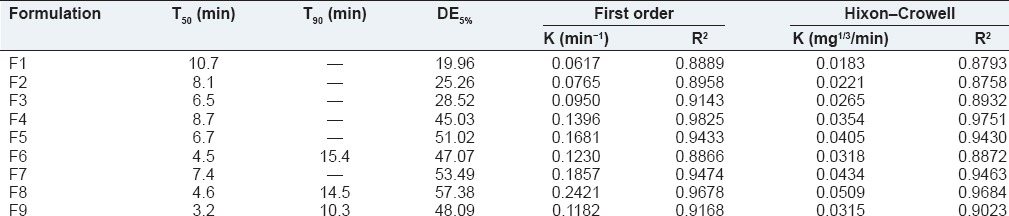
Dissolution parameters such as T50, T90, DE5% first order rate constant and Hixon-Crowell and were calculated from the dissolution data obtained, and the results were given in Table 3.
Table 3.
Evaluation of in vitro dissolution parameters for rizatriptan benzoate buccal fast dissolving films
Characterization
Based on the dissolution studies performed on all the formulations formulation F9 were further evaluated by Fourier transform infrared (FTIR), differential scanning calorimetry (DSC) and scanning electron microscopy (SEM) analysis.
Fourier transform infrared spectroscopy
The FTIR spectra of rizatriptan benzoate, maltodextrin, xanthan gum and on optimized formulation F9 were obtained using Brucker FTIR spectrophotometer to study the interaction between drug and carrier in films. The samples were prepared in KBr discs (2 mg sample in 200 mg KBr) and the sampling range was 400-4000 cm−1 and the resolution was 4 cm−1. The FTIR spectra were shown in Figures 4 and 5.
Figure 4.
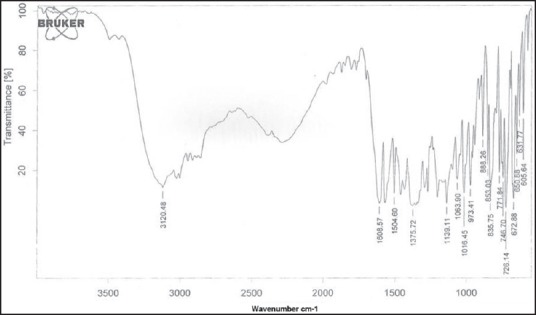
Fourier transform infrared spectrum of rizatriptan benzoate pure drug
Figure 5.
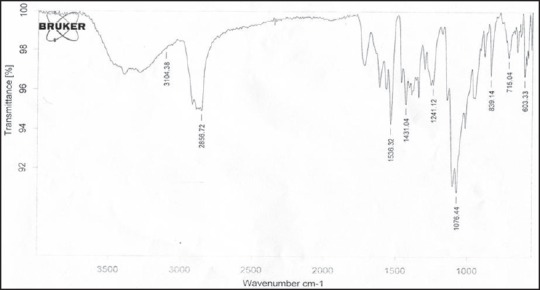
Fourier transform infrared spectrum of optimized film formulation (F9)
Differential scanning calorimetry
Differential scanning calorimetry measurements were performed on rizatriptan benzoate, maltodextrin, xanthan gum and on optimized formulation F9 using differential scanning calorimeter (METTLER TOLEDO INDIA Pvt Ltd Make DSC 1 with eSTAR software). The samples were placed in a sealed aluminum crucible and evaluated with a heating rate of 20°C/min at a temperature range of 25-250°C. The thermograms were recorded and were shown in Figures 6–9.
Figure 6.
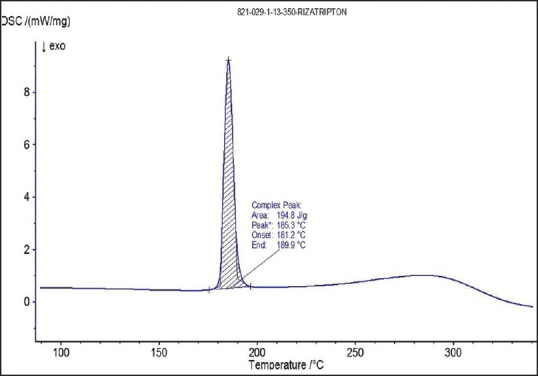
Differential scanning calorimetry thermogram of rizatriptan benzoate
Figure 9.
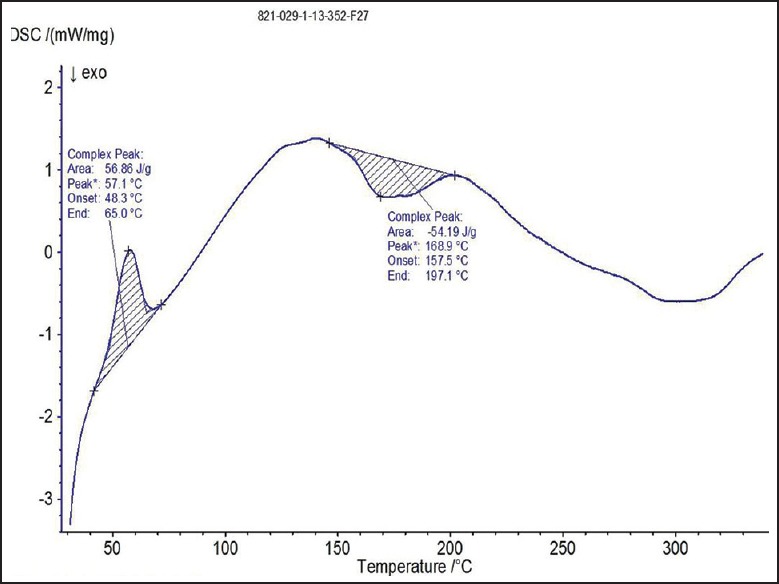
Differential scanning calorimetry thermogram of optimized film formulation (F9)
Figure 7.
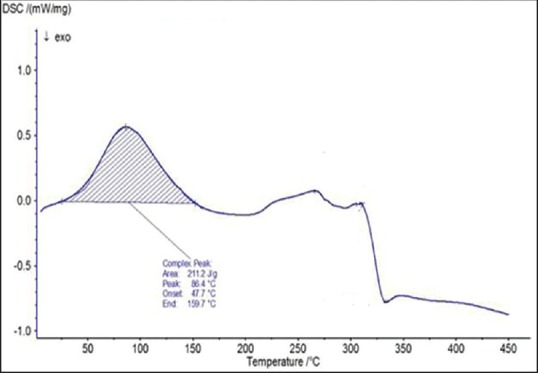
Differential scanning calorimetry thermogram of maltodextrin
Figure 8.
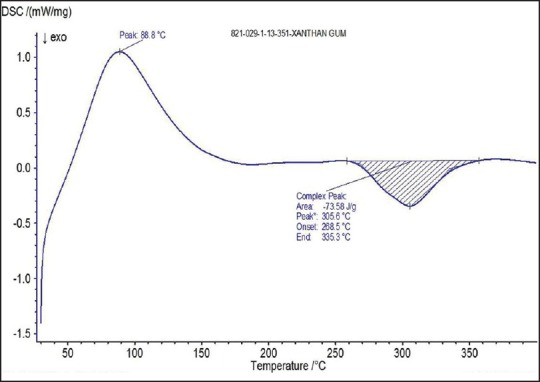
Differential scanning calorimetry thermogram of xanthan gum
Scanning electron microscopy analysis
The SEM photographs were taken for the optimized film formulation F9 and rizatriptan benzoate pure drug. The SEM photographs were shown in Figures 10 and 11.
Figure 10.

Scanning electron microscopy photograph of rizatriptan benzoate pure drug
Figure 11.
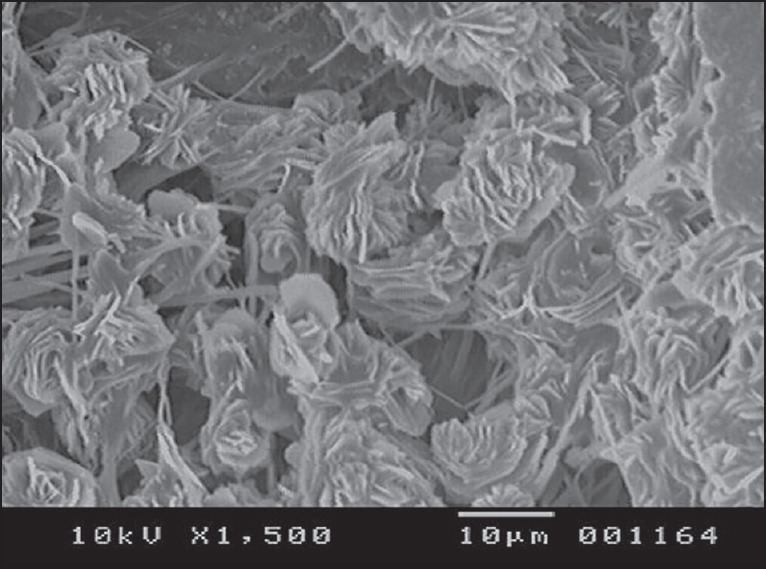
Scanning electron microscopy photograph of optimized film formulation (F9)
Stability studies
The optimized film formulation were further subjected to accelerated stability studies up to 6 months at 40°C with 75% RH and evaluated for any physical changes and for drug release patterns.
RESULTS AND DISCUSSION
In the present study, rizatriptan benzoate was selected as the drug candidate; rizatriptan benzoate is 5-HT receptor subtype agonist for the acute treatment of migraine. It is available as white color and is soluble in alcohol, water and pH 6.8 phosphate buffer. Mean absolute oral bioavailability is approximately 45%. Based on the physiochemical and biopharmaceutical properties this study was taken with an aim to prepare fast dissolving films, using emulsion evaporation method, which offered a suitable and practical approach in serving desired objective of faster disintegration and dissolution characteristics with increased bioavailability.
Maltodextrin, Xanthan Gum and Karaya gum were selected as the film forming agents, PEG 6000 as plasticizer, starch as anticurling agent, sodium starch glycolate as super disintegrant. Moreover, the investigation mainly concentrated on optimizing PEG 6000 concentration and optimizing the best film forming agents. Drug and excipients used in the formulations were found to be compatible. No drug-excipient reactions were observed. 20%w/w concentration of plasticizer for the film formulation was elegant and did not break by applying pressure. Hence, optimum strength was obtained. The thickness of all formulations was maintained at the range of 0.33 ± 0.03 mm. It is observed that formulation F9 showed good folding endurance compared with other films. Entrapment of Air Bubbles was completely absent in all formulations before casting the film and after drying the film. To minimize curling of film starch (1.6%) was used as an anticurling agent. All the film formulations were found to be stable, and they were further evaluated for physical parameters such as weight uniformity, drug content, film thickness, dispersion test, folding endurance and curling. The average drug content of all formulations was measured as 10.0 ± 0.5 mg. The compositions of rizatriptan benzoate buccal films were given in the Table 1.
Rizatriptan benzoate fast dissolving films were prepared by emulsion evaporation method. Emulsion evaporation method was suitable for drugs and polymers used. Dissolution studies were performed on all the film formulations using Franz diffusion cell apparatus containing simulated salivary fluid (pH 6.8 phosphate buffer) as a medium.
The film formulations F1-F3 which were formulated by using maltodextrin showed an average drug release of 57-72% within 15 min. The film formulations F4-F6, which were formulated by using maltodextrin and karaya gum showed an average drug release of 79-90% within 15 min. The film formulations F7-F9 which were formulated by using maltodextrin and xanthan gum showed an average drug release of 80-97% within 15 min. Among all the film formulations the films that were prepared by using xanthan gum, showed better drug release. The film formulation F9 prepared using xanthan gum showed 97.27% drug release in 15 min. The drug release profiles were showed in the Figures 1–3. All the film formulations were found to be linear with first order release rate with R2 values in the range of 0.97-0.98. Thus, the rates of drug release from all the film formulations were concentration dependent and was linear with first order release rate constant (K1). The Hixson Crowell constants for all the film formulations were in the range of 0.0163-0.0509. W01/3–Wt1/3 versus time plots were found to be linear with R2 values in the range of 0.96-0.97. Thus, the drug release from the film formulations was by diffusion of the drug from the polymeric matrix, followed by erosion of the polymer. The results of the evaluation of physical parameters for rizatriptan benzoate films were given in the Table 2.
The spectra of optimized film formulation F9 exhibited all the principle peaks present in the rizatriptan benzoate pure drug. Hence, there is no interaction between drug and polymers used in the formulation. The FTIR spectra were shown in the Figures 4 and 5. The endothermic peak for the pure drug was obtained at 183.5°C, whereas for pure polymer Maltodextrin at 86.4°C respectively. The broad endothermic peak for the F9 film formulation was observed at 168.9°C The broad endothermic peak of the maltodextrin and drug may overlap, hence interaction between drug and polymer was studied using IR spectra only. The DSC thermograms were shown in Figures 6–9. The F9 formulation with drug showed smooth, even surface in SEM analysis. The SEM images were shown in the Figures 10 and 11.
The accelerated stability studies indicated that there were no visible and physical changes observed in the films after storage. It was also observed that there was no significant change in drug release from the films. The drug release characteristics of the films remained unaltered. Thus, the drug release characteristics of fast dissolving films designed were found to be quite stable.
CONCLUSION
Fast dissolving films prepared in the study exhibited good film characteristic features as indicated by thickness measured, folding endurance, dispersion test, drug content, etc. The prepared films were found to be uniform, flexible, and 97% of drug was released from F9 film formulation within 10 min that was desirable for fast absorption. Hence, fast dissolving films of Rizatriptan benzoate were found to be suitable for eliciting better therapeutic effect in the treatment of migraine.
ACKNOWLEDGMENTS
The authors express their gratitude to Apotex labs Bangalore and Yarrow chem. Products Mumbai for providing the gift samples. The authors are thankful to the management of Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Guntur for providing the facilities to carry out the research work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Vyas SP, Khar RK. Controlled Drug Delivery Concepts and Advances. 1st ed. Delhi: Vallab Prakasham Publications; 2002. Controlled oral administration. [Google Scholar]
- 2.Borsadia S, O'Halloran D, Osborne JL. Quick dissolving films – A novel approach to drug delivery. Drug Deliv Technol. 2003;3:63–6. [Google Scholar]
- 3.Parakh SR, Gothoskar AV. Review of mouth dissolving tablet technologies. Pharm Technol. 2003;27:92–100. [Google Scholar]
- 4.Satishbabu BK, Shrinivasan BP. Preparation and evaluation of buccoadhesive film of atenolol. Indian J Pharm Sci. 2008;20:175–9. doi: 10.4103/0250-474X.41451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seager H. Drug-delivery products and the Zydis fast-dissolving dosage form. J Pharm Pharmacol. 1998;50:375–82. doi: 10.1111/j.2042-7158.1998.tb06876.x. [DOI] [PubMed] [Google Scholar]
- 6.Mishra R, Amin A. Formulation and characterization of rapidly dissolving films of cetirizine hydrochloride using pullulan as a film forming agent. India J Pharm Educ Res. 2011;45:75–6. [Google Scholar]
- 7.Prabhu P, Malli R, Koland M, Vijaynarayana K, D'Souza U, Harish N, et al. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int J Pharm Investig. 2011;1:99–104. doi: 10.4103/2230-973X.82417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tora GJ, Gorahowski SR. Principles of Anatomy and Physiology. Vol. 7. Wiley & Sons, Incorporated, John: Harpet Tora Tora Gorahowski; 1992. pp. 770–4. [Google Scholar]
- 9.Yoshifumi G, Ryosei K. Preparation of fast dissolving films for oral dosage from natural polysaccharides. Materials. 2010;3:4291–4296. doi: 10.3390/ma3084291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathi V, Senthil V, Kammili L, Hans R. A brief review on oral film technology. Int J Res Ayurvedic Pharm. 2011;2:1138–47. [Google Scholar]
- 11.Barnhart SD, Sloboda MS. The future of dissolvable films. Drug Deliv Technol. 2007;7:34–7. [Google Scholar]
- 12.Tripathi KD. Essentials of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brothers Medical Publishers; 2005. p. 531. [Google Scholar]


