Abstract
Introduction:
The aim of the present work was to develop controlled release, floating and mucoadhesive beads of glipizide by using the polyionic complexation technique. Plasma half-life of glipizide being 2–4 h was selected for development of controlled release dosage form.
Methods:
Formulation batches were designed by employing chitosan as cationic and xanthan gum as anionic polymers. In vitro drug release was evaluated for the period of 24 h in phosphate buffer pH 7.4.
Results:
Sustained release of drug was observed in all formulation batches with % drug release ranging from 87.50% to 100.67%, no significant effect on the drug release was observed after varying chitosan to xanthan gum ratio. Encapsulation efficiency was found to be in the range of 79.48 ± 1.10–94.48 ± 1.52. In vitro bioadhesion studies showed that beads had satisfactory bioadhesive strength ranging from 67.11% ± 1.73% to 93.12% ± 1.56%. Buoyancy studies revealed that beads possess comparable floating capacity in the gastric fluids. Swelling kinetics was carried in pH 1.2 and 7.4 buffers. Significant difference (P < 0.05) in swelling kinetics was observed. Drug to polymer interaction was analyzed by Fourier transform infrared spectroscopy and differential scanning calorimetry studies. Scanning electron microscopy studies revealed that formed beads were discrete with rough and wrinkled surfaces.
Conclusions:
In conclusion, beads were successfully formed by employing chitosan and xanthan gum and showed to possess sustained release effect. Beads also showed pH dependent swelling kinetics, this property can also be applied for the drugs which are susceptible to the acidic environment in the stomach, and comparable bioadhesive and floating properties were also observed.
Keywords: Beads, chitosan, polyelectrolyte complexation, sustained release, xanthan gum
INTRODUCTION
Hydrogels are three-dimensional, hydrophilic, polymeric networks capable of soaking large amounts of water or biological fluids. Homopolymers or copolymers are used in the preparation; these networks are insoluble due to the presence of chemical crosslinks. Thermodynamic compatibility of hydrogels with water allows them to swell in an aqueous media, further three-dimensional structure is an additional advantage for mechanical and chemical stability to the network due to gelation.[1,2]
Development of controlled or sustained release drug delivery systems with biopolymers has achieved importance and interest during the last decade.[3] Mixing of two oppositely charged polyelectrolytes in aqueous solutions reacts in polyelectrolyte complex formation. Polyelectrolyte complexes being highly hydrophilic materials form highly swollen systems in water. Xanthan gum and chitosan have preferentially ionizable groups and thus form polyelectrolyte complex when in solution. When dissolved in a solvent, ionizable groups dissociate into polyions and counterions and thus form complex with strong electric interactions, display unique physical and chemical properties through these interactions which are stronger than secondary binding interactions.[4,5] Formation of beads from biopolymers employs aqueous solvents which minimizes toxicity issues and environmental problems associated with organic solvents.[6] Several natural, biodegradable polysaccharides like chitosan, alginate, pectin, guar gum, and xanthan gum for controlled drug delivery were reported earlier.[7]
The major disadvantage of controlled drug delivery systems is its inability to remain localized within the region of drug absorption in the gastrointestinal tract and variability in gastric emptying process from patient to patient. These variations result in unpredictable bioavailability and unpredictability in time to achieve peak plasma levels. Several unique advantages are associated with site-specific controlled release systems, which includes localized delivery of drug to a particular part of the body, drug stability, maximum absorption of drugs, reduced need for follow-up care, assurance of treatment continuity in the nocturnal phase. Multiunit systems are not subjected to “all or nothing” gastric emptying nature of single unit systems. Gastric retentive drug delivery systems are highly useful for delivery of different types of drugs which include drugs that acts locally in the stomach, drugs that are absorbed from the proximal part of a small intestine, these drugs exhibit improved bioavailability with this type of drug delivery systems in patients.[8,9,10,11,12]
Xanthan gum is a high molecular weight extracellular heteropolysaccharaide, a cellulose derivative, produced by fermentation with the Gram-negative bacteria Xanthomonas campestris. The primary structure consists of the cellulosic backbone (β-D 1,4 glucose residue), which has a branching trisaccharide side chain of β-D (1,2)-mannose attached to β-D (1,4) glucuronic acid, which extinguish in β-D-mannose. The terminal residue may carry a pyruvate function, distribution of which is totally dependent on the bacterial strain and fermentation conditions. The nonterminal D-mannose unit in the side chain contains an acetyl function. The anionic nature of this polymer is due to the presence of both glucuronic and pyruvic acid groups in the side chain. The molecular weight of xanthan gum can reach up to 6 million Daltons; resulting in viscous solutions with lower concentrations. Apart from the enzymatic resistance, xanthan gum display stability over a wide range of temperatures and pH.[5,13,14]
Chitosan is a linear cationic polysaccharide consisting of units of glucosamine and N-acetyl-glucosamine which are linked by (1 → 4) β-glycosidic bonds. It is obtained by the alkaline deacetylation of chitin, which is the second abundant polysaccharide after cellulose. Chitosan is a weak base, and it is insoluble in water and organic solvents, but soluble in dilute aqueous acidic solutions (pH < 6.5) which can convert glucosamine units into a soluble form with protonated amine groups. Chitosan precipitates in alkaline solution or with polyanions and forms gel at lower pH. Commercially available chitosan has molecular weight ranging from 3800 to 2,000,000 Daltons and deacetylation degree between 66% and 95%. Chitosan is nontoxic, biodegradable and biocompatible in nature due to which it has several pharmaceutical applications. Particle size, density, viscosity, degree of deacetylation, and molecular weight are vital properties of chitosan for its effect on pharmaceutical formulations of importance.[15,16,17,18]
The hydrogel network formed by ionic interactions between the amino groups of chitosan and carboxyl groups of xanthan gum shows pH-sensitive swelling characteristics, which ensures the controlled release of entrapped materials.[5]
Glipizide is a weak acid (pKa = 5.9), practically insoluble in water, in an acidic environment, and highly permeable (BCS class II). Oral absorption is uniform, rapid and complete; its bioavailability is nearly 100%, and elimination half-life is 2-4 h.[19]
Mucoadhesive microcapsules, microspheres for glipizide are reported by several research groups earlier,[20,21,22] also floating microspheres[23] and sustained release microspheres of glipizide were reported.[24] Controlled release biodegradable nanoparticles of glipizide were also reported.[25] Drug delivery system based on pulsatile release of glipizide was formulated and evaluated earlier.[26] Osmotically controlled drug delivery system for sustained release of glipizide was also reported in the past.[27]
The aim of the present work was to develop sustained release mucoadhesive and floating beads of glipizide for prolonged gastrointestinal release at the site of its absorption by employing a mechanism of polyelectrolyte complexation between chitosan and xanthan gum. We here report floating beads prepared by combining these two oppositely charged polymers for the 1st time.
MATERIALS AND METHODS
Materials
Glipizide was obtained as gift sample from USV limited (Mumbai, India). Xanthan gum was purchased from Loba Chemie (Mumbai, India). Chitosan with deacetylation degree >90% was purchased from Marine chemicals (Kerala, India). All the other reagents used were of analytical reagent grade.
Preparation of beads
Initially, chitosan was dissolved in 75 ml of 0.1M glacial acetic acid, with stirring using three blade propeller at 200 rpm until complete solubilization of chitosan and the volume was made up to 100 ml by adding 25 ml of distilled water. Xanthan gum was dissolved in water (25 ml) with gentle heating by using magnetic stirrer, glipizide was dispersed in it with magnetic stirring. Chitosan solution was continuously stirred at 200 rpm, and the above mixture was added drop wise from the constant distance with syringe. Beads were formed due to the complex coacervation mechanism.[5] Further, beads were stirred for 2 h for complete crosslinking and were separated by filtration by using Whatmann's filter paper. The beads were hardened with 1% (25% aqueous dispersion) glutaraldehyde solution for 5 min, further dried at atmospheric conditions for 24 h and stored in a moisture free condition until further use.
Percent encapsulation efficiency
Beads were crushed using mortar and pestle, and 50 mg powder was accurately weighed and diluted to 25 ml with 0.05 N sodium hydroxide solution. This solution was ultrasonicated on bath ultrasonicator (UCB-40, Spectra Lab, India) for 30 min for complete extraction of the drug from the polymer matrix. Later, 1 ml sample was pipetted out, and this was again diluted to 50 ml in a volumetric flask with 0.05N sodium hydroxide solution. Solutions were then filtered through 0.45 μ syringe filter and absorbance was measured at 276 nm, which is the wavelength of maximum absorbance for glipizide.
Encapsulation efficiency (%) was calculated by following formula:
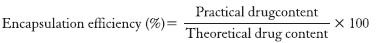
Fourier transform infrared spectroscopy
Samples were weighed (2 mg) and with 10 mg of potassium bromide. The samples were then compressed using a hydraulic press to obtain a translucent pellet. The pellet was placed in Fourier transform infrared spectrometer (Model-8400-S, Shimadzu, Japan), and the samples were scanned between 4000 and 450 cm1 .
Differential scanning calorimetry
Differential scanning calorimetric studies were carried out for pure polymers, drug and for formulations by using differential scanning calorimeter (Schimadzu, DCS-50 cell Japan). About 1 mg of powder was weighed and sealed into the aluminum pan with a lid. Samples were heated between 25°C and 400°C at the interval of 5°C/min.
Scanning electron microscopy
The surface morphology of formed beads was observed by scanning electron microscopy (Jeol JFC-1600, Japan). Beads were mounted on to metal stubs using a double side adhesive tape and made conductive by coating with platinum.
Swelling studies
The percent swelling (equilibrium water uptake) of the beads was determined by measuring the extent of swelling of the polymer matrix in the medium of pH 1.2, and 7.4 buffers. To achieve the equilibrium, the beads were allowed to swell for 8 h. The surface adhered liquid drops were removed by blotting with soft tissue papers, and the swollen beads were weighed using an electronic balance. The percent swelling was calculated as:

Buoyancy studies
Prepared beads were evaluated for buoyancy and floating time by USP type 2 dissolution apparatus. One hundred beads of each batch were placed in 900 ml of 0.1N hydrochloric acid containing 0.02% w/v tween 80 and agitated at 100 rpm, during study temperature was maintained at 37°C ± 2°C. Number of sinking beads were observed visually.[28]
In vitro mucoadhesive test
Beads (50 mg) were placed on albino rat stomach (4 cm) mounted on the glass slide and kept for 20 min in a humidity temperature control cabinet at 75% relative humidity and temperature of 25°C ± 2°C to allow hydration of beads. This was followed by thorough washing with pH 6.8 buffer. Finally, mucosa was washed off, and beads were then dried at 70°C in a hot air oven. Percent bioadhesion was determined by the following formula.[29]
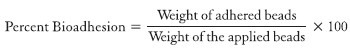
In vitro drug release
Drug release studies were carried out in phosphate buffer pH 7.4 (900 ml) using dissolution test apparatus basket method. Stirring speed was maintained at 50 rpm. Samples of dissolution fluid were withdrawn at different time intervals and filtered through 0.45 μm syringe filter and after suitable dilutions assayed at 276 nm for drug content using a double-beam spectrophotometer.[21]
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 5.03 (GraphPad®, CA, USA). Unpaired Student's t-test was performed to evaluate statistical significance among different formulations. Results were considered as statistically significant if P < 0.005.
RESULTS AND DISCUSSION
Preparation of beads
All the batches of beads were prepared by drop wise addition of xanthan gum solution to chitosan solution. A concentration range of two polymers was selected based on the formation of beads [Table 1]. When chitosan was used below 0.7% w/v, there was no formation of beads. Hence, amount of chitosan was fixed 0.7% w/v. Similarly, when chitosan concentration above 1.3% w/v and xanthan gum concentration below 1.8% w/v was used, did not form beads. Therefore, chitosan was used in the concentration range of 0.7-1.3% w/v and xanthan gum concentration range was fixed between 1.8% and 2.6% w/v.
Table 1.
Formulation composition, encapsulation efficiency profiles and % bioadhesion of beads
Acetic acid was selected as a solvent for chitosan, based on the observation that, when 0.1 M hydrochloric acid was used as a solvent for chitosan, formed beads were not hard and was easily breakable. The pH of chitosan solution was controlled, ranging from 4.5 to 6.2, where the ionization degree of chitosan is unity, because chitosan precipitates above its pKa = 6.3.
Percent encapsulation efficiency
As shown in Table 1, percent encapsulation efficiency was observed in the range of 79.48% ± 1.10% to 94.48% ± 1.52% for all the batches. Effect of change in concentration of either polymers on encapsulation efficiency was not observed. The reason for good encapsulation efficiency may be low solubility of glipizide in water and in acidic solutions. Hence, the maximum amount of drug dispersed into xanthan gum solution got entrapped within the beads.
Fourier transform infrared spectroscopy
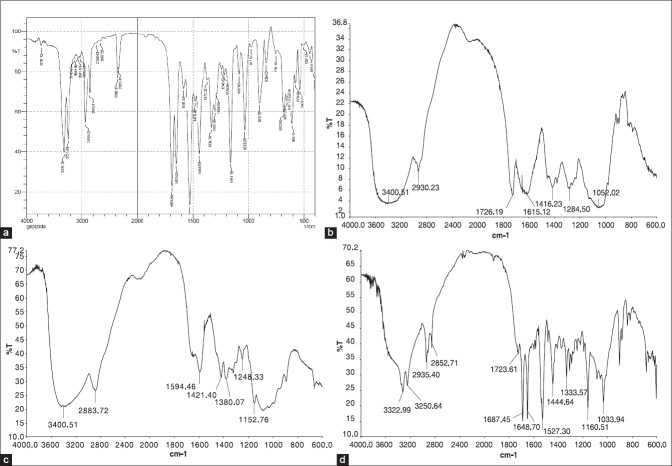
Fourier transform infrared spectroscopy (FTIR) spectrum of glipizide showed characteristic bands [Figure 1a] at 1689 cm1 which is due to C = O stretch of amides, a band at 1151 cm1 is present because of C-N stretching vibrations of amines, bands at 1653 cm1, and 1444 cm1 were assigned to C = C stretching of aromatic ring, a peak observed at 1663 cm1 attributed to C = N stretching, strong band at 1540 cm1 was assigned to N-H bending vibrations. A band at 3325 cm1 could probably be assigned to N-H stretching vibrations. A band present at 1033 cm1 is due to S = O.[30]
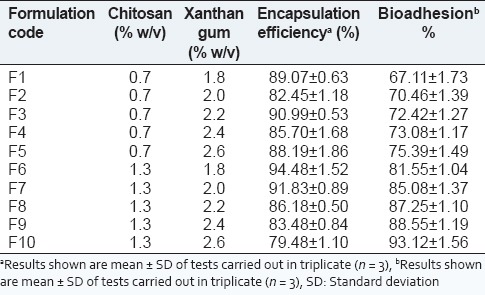
Figure 1.
Fourier transform infrared spectroscopy spectra glipizide (a), xanthan gum (b), chitosan (c), and formulation (d)
In the spectrum of xanthan gum [Figure 1b], peak observed at 1416 cm1 is due to O-H bending. The peak at 1615 cm1 is probably due to bound water present in xanthan gum. Band at 2930 cm1 is due to C-H stretching and a broad peak observed at 3400 cm1 is because of hydrogen bonded hydroxyl group (O-H) could be attributed to complex vibrational stretching, connected with free inter and intra molecular bound hydroxyl group.[31]
Similarly in the spectrum of chitosan [Figure 1c], a band at 1380 cm1 was observed which is due to–CH3 bend in the amide group. A band at 1594 cm1 can be assigned to NH2 in the amino group. Band at 2883 cm1 could be assigned to symmetric or asymmetric CH2 stretching vibrations attributed to pyranose ring. And the band at 3400 cm−1 is due to the hydroxyl group (O-H). Finally, a band at 1152 cm1 is probably due to -C-O-C- in glycosidic linkage.[32]
Fourier transform infrared spectroscopy spectrum of formulation batch [Figure 1d] showed prominent peak at 1033 cm1 which could be assigned to S = O group of glipizide, 1160 cm1 would probably be assigned to C-N stretching vibrations of amines, which are present in the structure of glipizide, similarly peaks at 1648 cm1 and 1444 cm1 may be due to C = C stretching of aromatic ring of glipizide, and bands at 1527 cm1, and 1687 cm1, may be present due to N-H bending vibrations, and C = O stretch of amide groups present in the structure of glipizide respectively. Peaks at 3250 cm1 and 3322 cm1 are observed in the FTIR spectrum of formulation batch are also evident in the structure of glipizide. Due to the hydrogel network formed through the ionic interactions between the amino groups of chitosan and carboxyl groups of xanthan gum,[5] two oppositely charged polymers, peaks corresponds to of the polymers are not evident in the formulation spectrum.
Differential scanning calorimetry
The differential scanning calorimetry (DSC) thermograms of glipizide, chitosan, xanthan gum and formulation are shown in Figure 2. Thermogram of glipizide showed a sharp endothermic peak at 215.20°C [Figure 2a]. No peak was observed for chitosan [Figure 2b]. And a broader endothermic peak was observed for xanthan gum at 262.08°C [Figure 2c]. Similarly, DSC thermogram of formulation showed endothermic peak of glipizide at 221.45°C [Figure 2d] and peak of xanthan gum was absent. From this data, a conclusion can be drawn about interaction between chitosan and xanthan gum and complete crosslinking of both the polymers.
Figure 2.
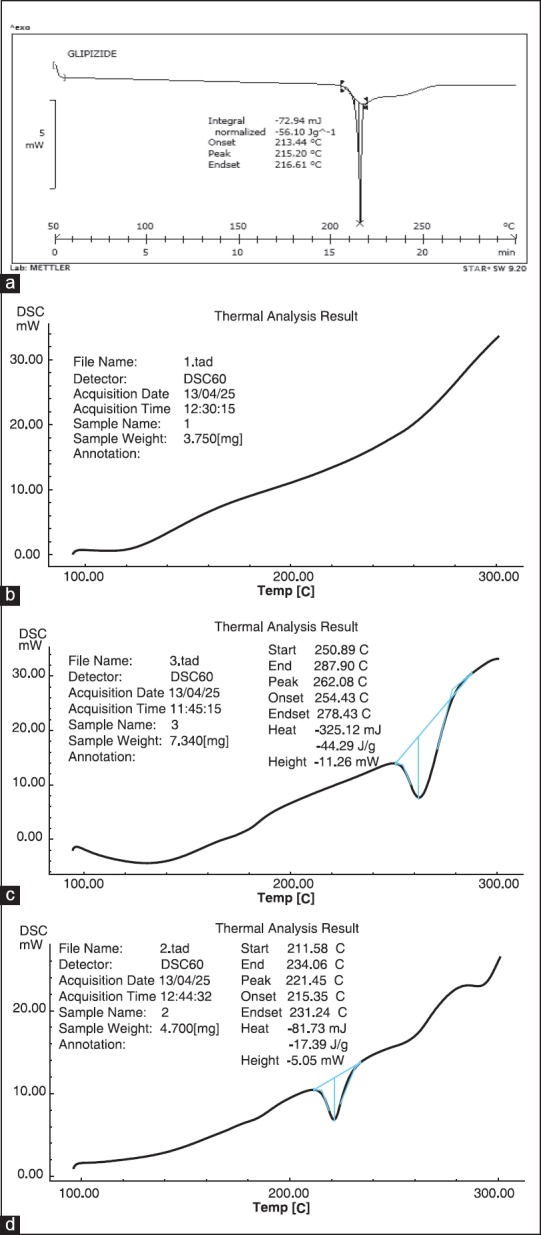
Differential scanning calorimetry thermogram of glipizide (a), chitosan (b), xanthan gum (c) and formulation (d)
Scanning electron microscopy
Scanning electron microscopy illustrations revealed that beads are having rough, wrinkled surfaces [Figure 3]. This may be due to loss of water from the surface. The particle size observed is above 500 μm, few smaller beads are also evident in the images.
Figure 3.
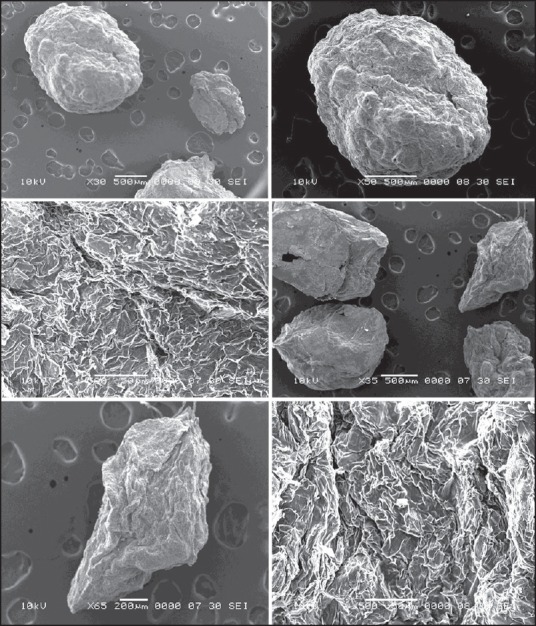
Scanning electron microscopy images of beads
Swelling studies
Swelling kinetic studies was carried out in solutions of pH 1.2 and 7.4. In pH 1.2 [Figure 4] buffer, equilibrium in water uptake was achieved in longer duration of time, and there was a gradual increment in water absorption with time. However, at pH 7.4 buffer the equilibrium was achieved quickly as evident from the graph shown in Figure 5. After the equilibrium, there was no pronounced rise in water uptake. With the water uptake breaking tendencies of beads was commonly observed in formulation batches F6 to F10 in pH 7.4, due to which it was difficult to weigh the broken parts of beads, hence for these formulations evaluation was carried out up to 2 h [Figure 5]. This may probably be due to higher proportions of chitosan as well as xanthan gum in these formulation batches. A significant (P < 0.05) increase in the percent swelling in formulation batches F1 to F5 at pH 7.4 was observed as shown in Figure 5 than at pH 1.2 as evident in Figure 4. Formulation F10 was observed to show maximum water uptake at pH 1.2 and formulation F9 at pH 7.4.
Figure 4.
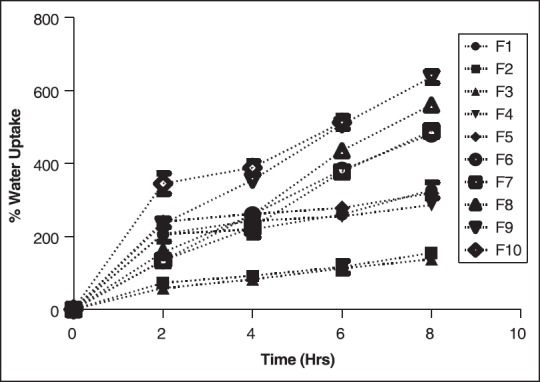
Swelling kinetics in pH 1.2
Figure 5.
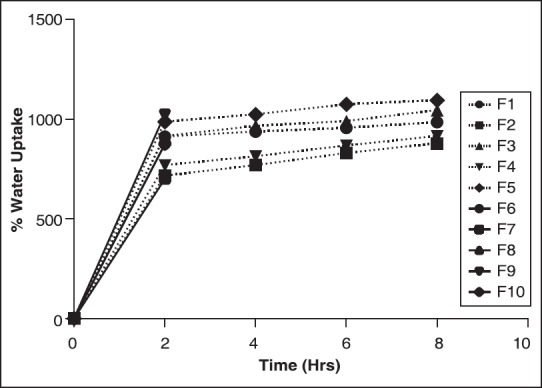
Swelling kinetics in pH 7.4
This may probably be due to the highest concentration of two hydrophilic polymers used in both the formulations.
Previously, it was reported that hydrogel network formed through the ionic interactions between the amino groups of chitosan and carboxyl groups of xanthan gum shows pH-sensitive swelling characteristics, which enable the controlled release of entrapped materials.[4,5] Increase in pH corresponds to a decrease in the concentration of H+, A in the solution, which is accompanied by decrease in–NH3+, A coupling in the number of connectivity links. In the case of basic media, when pH increases, the number of Me+ OH increases in the solution. Me+ OH− can interact with–NH3+, Cl− by forming NH2, H2 O and Me+, Cl−. Decrease in the connectivity results in an increase in swelling. It was also reported that at the same conditions of preparation, the spheres swell more than the bulk preparation. This phenomenon can be explained by rapid formation of the membrane. During the time of coacervation, the chitosan must diffuse through the bead membrane and interact with the xanthan gum. The membrane decreases the rate of diffusion of chitosan, and it is possible that some macromolecules of xanthan gum have not interacted with chitosan. Hence, beads of hydrogel swell more than the bulk hydrogel preparation.[2] Statistical comparison with other formulation batches could not be carried out, as stated above, because of breaking tendencies, and as equilibrium swelling studies were observed for 2 h. Earlier, it was reported that in solutions with higher pH values xanthan-chitosan hydrogel showed significant % water uptake.[4] Here, we report that percent water uptake was not significantly affected when there was an increase in concentration of xanthan gum. These results are not consistent with the finding reported earlier.[5]
Buoyancy studies
Floating studies were carried out to investigate the floating ability of the beads. The beads were spread over the surface of an acid medium and the number of beads settled down as a function of time was counted. No effect of encapsulation efficiency was observed on floating ability of beads. Lower percentage of floating beads was observed specifically for formulations F9 and F10 [Figure 6]. Above mentioned batches contains the highest proportion of chitosan and xanthan gum if compared with other preparations in the series of batches. This probably makes beads denser and reduces the floating time. Another reason may be a slight increase in water uptake as evident from the swelling studies shown in Figure 5. Formulation F1 showed best results in terms of the maximum number of beads observed to remain floating at the end of 8 h. As shown in Table 1, formulation F1 contain a minimum concentration of both the hydrophilic polymers and because of that there may be less amount of water uptake which resulted into maximum number of beads remain floating for longer duration of time. The percent floating was observed to be 84.66% ± 3.78%. Thus for the 1st time we report floating beads prepared from a combination of chitosan-xanthan polyelectrolyte complex.
Figure 6.
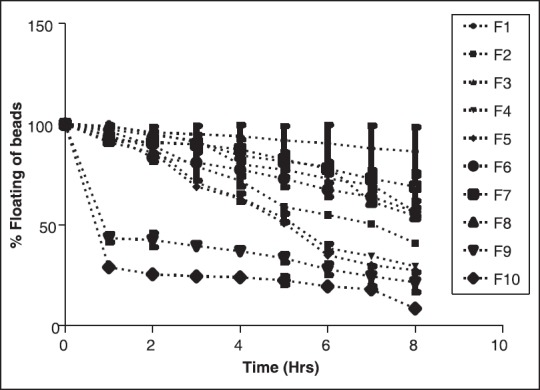
Floating behavior of beads
In vitro bioadhesion test
“Bioadhesion” in simple terms can be described as the attachment ofsynthetic or biological macromolecule to a biological tissue. An adhesive bond may form either with the epithelial cell layer, the continuous mucus layer or a combination of the two. Coupling of bioadhesive properties to microspheres has additional advantages, e.g., efficient absorption and enhanced bioavailability due to the high surface to volume ratio, much more intimate contact with the mucus layer. Specific targeting of the drug to the absorption site is achieved by anchoring plant lectins, bacterial adhesions and antibodies etc., on the surface of the microspheres.[33] The positive charge on chitosan polymer gives strong electrostatic interaction with the mucus.[17] The in vitro bioadhesion studies showed that all the batches of beads had satisfactory bioadhesive strength ranging from 67.11% ± 1.73% to 93.12% ± 1.56% [Table 1]. Increase in proportion of chitosan as well as xanthan gum (Formulations F6 to F10) showed enhanced percent bioadhesion in comparison with formulations F1 to F5. This may be due to the strong interaction between mucin and chitosan.[15] Chitosan also inhibits mucin turnover.[33] Earlier published reports revealed that xanthan gum also showed mucoadhesive properties[34] and increased charge density gives better adhesion. It was also reported that polyanion polymers are more effective as bioadhesive than polycation polymers or nonionic polymers.[35] Formulation F10 showed maximum bioadhesive strength that is 93.12% ± 1.56%, as shown in Table 1, again which could be assigned to the highest concentration of chitosan and xanthan gum. Many research groups reported that the good mucoadhesion is shown by polymers with highly flexible backbone structure and of its polar functional groups. Such flexibility of the polymer chains, however, is reduced if the polymer molecules are highly cross-linked.[36]
In vitro drug release
Hydrogels are soft tissue biocompatible polymers and able to disperse drugs within the network easily with a high degree of controlling on release, extensive efforts has been dedicated to use them in pharmaceutical applications.[37]
Sustained release of drug was achieved for 24 h for all the batches [Figure 7a and b]. From the evidence of swelling studies, beads showed maximum swelling in pH 7.4 [Figure 5] may be attributed to the formation of a barrier for diffusion of the drug from the beads. This matrix retards release of the drug for prolonged time period. There was no significant effect on sustained release of drug was observed upon increase in concentration of chitosan and xanthan gum. Chitosan-xanthan gum based hydrogels were reported earlier for controlled release of theophylline.[38]
Figure 7.
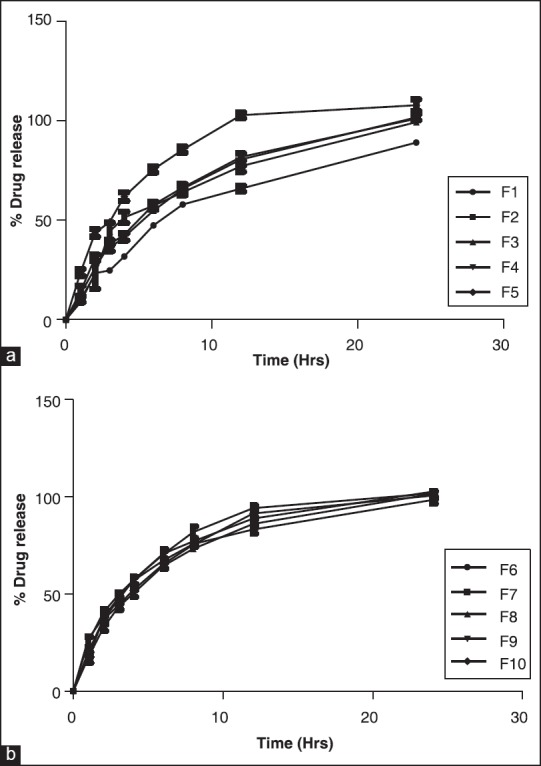
In vitro dissolution profiles, formulations F1–F5 (a), formulations F6–F10 (b)
CONCLUSION
It can be concluded that Chitosan-xanthan hydrogel beads could successfully be used as a means for controlled release of micromolecular drugs. Because of the floating and mucoadhesive properties, drug release can be targeted in the upper region of the small intestine to avoid erratic absorption of many micromolecules. It was also observed that extent of swelling of beads was less in the acidic conditions; hence drugs which might degrade in acidic conditions in the stomach could be protected from the harsh acidic environment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 2.Magnin D, Lefebvre J, Chornet E, Dumitriu S. Physicochemical and structural characterization of a polyionic matrix of interest in biotechnology, in the pharmaceutical and biomedical fields. Carbohydr Polym. 2004;55:437–53. [Google Scholar]
- 3.Desai KG, Park HJ. Preparation of cross-linked chitosan microspheres by spray drying : e0 ffect of cross-linking agent on the properties of spray dried microspheres. J Microencapsul. 2005;22:377–95. doi: 10.1080/02652040500100139. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Ruvalcaba A, Chornet E, Rodrigue D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr Polym. 2007;67:586–95. [Google Scholar]
- 5.Argin-Soysal S, Kofinas P, Lo YM. Effect of complexation conditions on xanthan-chitosan polyelectrolyte complex gels. Food Hydrocoll. 2009;23:202–9. [Google Scholar]
- 6.Wang K, He Z. Alginate-konjac glucomannan-chitosan beads as controlled release matrix. Int J Pharm. 2002;244:117–26. doi: 10.1016/s0378-5173(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 7.Ray S, Maiti S, Sa B. Preliminary investigation on the development of diltiazem resin complex loaded carboxymethyl xanthan beads. AAPS PharmSciTech. 2008;9:295–301. doi: 10.1208/s12249-007-9012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risbud MV, Hardikar AA, Bhat SV, Bhonde RR. pH-sensitive freeze-dried chitosan-polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Control Release. 2000;68:23–30. doi: 10.1016/s0168-3659(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 9.Jain SK, Awasthi AM, Jain NK, Agrawal GP. Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery : Preparation and in vitro characterization. J Control Release. 2005;107:300–9. doi: 10.1016/j.jconrel.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Joseph NJ, Lakshmi S, Jayakrishnan A. A floating-type oral dosage form for piroxicam based on hollow polycarbonate microspheres : In vitro and in vivo evaluation in rabbits. J Control Release. 2002;79:71–9. doi: 10.1016/s0168-3659(01)00507-7. [DOI] [PubMed] [Google Scholar]
- 11.Singh BN, Kim KH. Floating drug delivery systems : An approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235–59. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. In vitro and in vivo evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. Int J Pharm. 2004;275:97–107. doi: 10.1016/j.ijpharm.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Pongjanyakul T, Puttipipatkhachorn S. Xanthan-alginate composite gel beads: Molecular interaction and in vitro characterization. Int J Pharm. 2007;331:61–71. doi: 10.1016/j.ijpharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Talukdar MM, Kinget R. Swelling and drug release behaviour of xanthan gum matrix tablets. Int J Pharm. 1995;120:63–72. [Google Scholar]
- 15.He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88. [Google Scholar]
- 16.Carreno-Gomez B, Duncan R. Evaluation of the biological properties of soluble chitosan and chitosan microspheres. Int J Pharm. 1997;148:231–40. [Google Scholar]
- 17.Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274:1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero S, Teijon C, Muniz E, Teijon JM, Blanco MD. Characterization and in vivo evaluation of ketotifen-loaded chitosan microspheres. Carbohydr Polym. 2010;79:1006–13. [Google Scholar]
- 19.Jamzad S, Fassihi R. Development of a controlled release low dose class II drug-Glipizide. Int J Pharm. 2006;312:24–32. doi: 10.1016/j.ijpharm.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Chowdary KP, Rao YS. Design and in vitro and in vivo evaluation of mucoadhesive microcapsules of glipizide for oral controlled release: A technical note. AAPS PharmSciTech. 2003;4:E87–92. doi: 10.1208/pt040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel JK, Patel RP, Amin AF, Patel MM. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS PharmSciTech. 2005;6:E49–55. doi: 10.1208/pt060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaba P, Singh S, Gaba M, Gupta GD. Galactomannan gum coated mucoadhesive microspheres of glipizide for treatment of type 2 diabetes mellitus : I0 n vitro and in vivo evaluation. Saudi Pharm J. 2011;19:143–52. doi: 10.1016/j.jsps.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandya N, Pandya M, Bhaskar VH. Preparation and in vitro characterization of porous carrier-based glipizide floating microspheres for gastric delivery. J Young Pharm. 2011;3:90 7–104. doi: 10.4103/0975-1483.80292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phutane P, Shidhaye S, Lotlikar V, Ghule A, Sutar S, Kadam V. In vitro evaluation of novel sustained release microspheres of glipizide prepared by the emulsion solvent diffusion-evaporation method. J Young Pharm. 2010;2:35–41. doi: 10.4103/0975-1483.62210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S, Saraf S. Influence of processing variables and in vitro characterization of glipizide loaded biodegradable nanoparticles. Diabetes Metab Synd Clin Res Rev. 2009;3:113–7. [Google Scholar]
- 26.Yadav D, Survase S, Kumar N. Dual coating of swellable and rupturable polymers on glipizide loaded MCC pellets for pulsatile delivery : f0 ormulation design and in vitro evaluation. Int J Pharm. 2011;419:121–30. doi: 10.1016/j.ijpharm.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Verma RK, Garg S. Development and evaluation of osmotically controlled oral drug delivery system of glipizide. Eur J Pharm Biopharm. 2004;57:513–25. doi: 10.1016/j.ejpb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Badve SS, Sher P, Korde A, Pawar AP. Development of hollow/porous calcium pectinate beads for floating-pulsatile drug delivery. Eur J Pharm Biopharm. 2007;65:85–93. doi: 10.1016/j.ejpb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Rastogi R, Sultana Y, Aqil M, Ali A, Kumar S, Chuttani K, et al. Alginate microspheres of isoniazid for oral sustained drug delivery. Int J Pharm. 2007;334:71–7. doi: 10.1016/j.ijpharm.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Maiti S, Ranjit S, Mondol R, Ray S, Sa B. Al +3 ion cross-linked and acetalated gellan hydrogel network beads for prolonged release of glipizide. Carbohydr Polym. 2011;85:164–72. [Google Scholar]
- 31.Shalviri A, Liu Q, Abdekhodaie MJ, Wu XY. Novel modified starch-xanthan gum hydrogels for controlled drug delivery : S0 ynthesis and characterization. Carbohydr Polym. 2010;79:80 98–907. [Google Scholar]
- 32.Pawlak A, Mucha M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta. 2003;396:153–66. [Google Scholar]
- 33.Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255:10 3–32. doi: 10.1016/s0378-5173(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Huwaij R, Obaidat RM, Sweidan K, Al-Hiari Y. Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine. AAPS PharmSciTech. 2011;12:21–7. doi: 10.1208/s12249-010-9534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs : a0 lginate and chitosan - A review. J Control Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Patil SB, Sawant KK. Chitosan microspheres as a delivery system for nasal insufflation. Colloids Surf B Biointerfaces. 2011;84:384–9. doi: 10.1016/j.colsurfb.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Hezaveh H, Muhamad II. Controlled drug release via minimization of burst release in pH-response kappa-carrageenan/polyvinyl alcohol hydrogels. Chem Eng Res Des. 2013;91:508–19. [Google Scholar]
- 38.Popa N, Novac O, Profire L, Lupusoru CE, Popa MI. Hydrogels based on chitosan-xanthan for controlled release of theophylline. J Mater Sci Mater Med. 2010;21:1241–8. doi: 10.1007/s10856-009-3937-4. [DOI] [PubMed] [Google Scholar]