Abstract
Background:
Nanoscale solid lipid particles of rasagiline mesylate (RM) were fabricated by microemulsion technique. The nanoscale particle must be sterile for intravenous administration, and several approaches are available for sterilization. However, the selection of sterilization technique for the fabricated RM loaded nanoscale solid lipid particles mainly depends on the nature of the drug that needs to be encapsulated and release pattern of the polymer.
Materials and Methods:
We have preferred moist heat sterilization, as it is the most convenient and the composition of the carrier and incorporated drug should remain unchanged and the incorporated drug should not leak out of the drug carrier. The physical and chemical stability of RM loaded nanoscale solid lipid particles investigated during sterilization and to determine the average mean particle size, polydispersity index, zeta potential (ZP), transmission electron microscopy (TEM), entrapment efficiency (EE), and drug content after autoclaving.
Result:
There were no significant changes in the average mean particle size, polydispersity index, ZP, TEM, EE, and drug content of RM loaded nanoscale solid lipid particles after autoclaving (121°C for 20 min [15 lbs]).
Conclusion:
These observations suggest that the moist heat sterilization by autoclaving is the most suitable method for nanoscale solid lipid formulations.
Keywords: Entrapment efficiency, photon correlation spectroscopy, selected area electron diffraction, transmission electron microscopy, zeta potential
INTRODUCTION
In designing a delivery system, it is important to notice the route of administration and drug dose, as well as a mechanism and duration of the desired effect of the drug.[1] Nanoscale solid lipid particles as drug carriers can be utilized for the treatment of neurodegenerative disorders. The nanoscale solid lipid particles and their physiological ingredients are best suitable for systemic delivery, and they have respectable storage stabilities after lyophilization and/or sterilization.[2] Sterilization is an important stage in the fabrication of nanoscale solid lipid particles intended for parenteral administration, and the size of the particles should be controlled. Among modern drug delivery carriers, nanoscale solid lipid particles have been extensively investigated for targeted drug delivery system because their particle size (10-1000 nm) is agreeable for intravenous administration and this carrier size is also desirable for intramuscular and subcutaneous routes.[3,4,5] However, the bioacceptable and biodegradable nanoscale solid lipid particles enhance the therapeutic significance of diverse drugs and biological agents.[6] Many approaches have been implemented to fabricate the nanoscale solid lipid particles includes, high-pressure homogenization, solvent emulsification, microemulsion, double emulsion, and solvent injection method.[7] Among these approaches, the microemulsion technique was most suitable for the fabrication of nanoscale solid lipid particles, which is optically isotropic systems, thermodynamically stable, obtained spontaneously by mixing surfactant, co-surfactant, oil, and water.[8]
The intravenous administration of nanoscale solid lipid particles size should be below 1 μm and sterilization is mandatory for parenteral drug carriers, which can be detrimental to the stability of the nanoscale solid lipid preparations.[9] However, the selection of sterilization technique for nanoscale solid lipid particles mainly depends on the nature of drug and excipients. In general, sterilization can be reached using heat, filtration, and g-irradiation. The filtration and γ-radiation methods were limited mainly due to particle size and unacceptable chemical itemization of formulations. The filtration techniques are not applicable to particles size below 0.2 μm, and the gamma radiation causes the unacceptable chemical itemization.[10,11] Fabrication of nanoscale solid lipid particles under aseptic conditions is conceivable but very complex and expensive. To overcome these limitations, the moist heat sterilization seemed to be a convenient method for fabricating nanoscale solid lipid particles. Cavalli et al. and Heiati et al. was intensively studied the sterilization of fabricated nanoscale solid lipid particles and they reported the particle size, composition of the carrier and incorporated drug should remain unchanged and the incorporated drug should not leak out of the drug carrier.[12,13]
Rasagiline mesylate (RM) is a second-generation of propargylamine used for the treatment of Parkinson disease, which is the second most common neurodegenerative disorder in the world. RM is a secondary cyclic benzylamine and indane derivative, novel irreversible monoamine oxidase type B-inhibitor.[7]
The purpose of the present study was to attest the effect of RM-loaded nanoscale solid lipid particles sterilization by Moist heat sterilization. The physical and chemical stability of RM loaded nanoscale solid lipid particles investigated in term particle size, polydispersity index, zeta potential (ZP), transmission electron microscopy (TEM), entrapment efficiency (EE), and RM content after the moist heat sterilization using autoclave.
MATERIALS AND METHODS
Materials
Rasagiline mesylate was a kind gift from Orchid health care Pvt. Ltd (Chennai, India). Stearic acid, Poloxamer 407 and Tween 80 were purchased from Sigma Chemical Labs (Bangalore, India). The analytic grade chemicals and reagents were used for all the experiments. Double distilled water was used after filtration through a 0.45 μm membrane (cellulose acetate).
Methods
Fabrication of nanoscale solid lipid particles
Rasagiline mesylate loaded solid lipid nanoparticles (SLNs) were prepared according to the microemulsion technique.[7,14] Briefly, the lipid phase containing stearic acid was melted above its melting point. RM was added in the melted lipid. The surfactant (Poloxamer 407) and co-surfactant (Tween 80) were dissolved in 10 ml of the aqueous phase. The aqueous phase was mixed with the lipid phase and then subjected to magnetic stirrer for 15 min at 300 rpm. The obtained o/w microemulsion was dispersed in cold water under probe sonicator for 20 min to solidify the nanoparticles.
Sterilization of nanoscale solid lipid particles
Rasagiline mesylate loaded nanoscale solid lipid particles were sterilized by moist heat sterilization using autoclave (Technico Autoclave, India) at 121°C, 2 bar, (15 lbs) for 15 min following the Indian pharmacopoeia. The physical and chemical stability of formulations was investigated during autoclave and to determine the average mean particle size, polydispersity index, ZP, TEM, EE, and drug content after the moist heat sterilization.[12,15]
CHARACTERIZATION OF NANOSCALE SOLID LIPID PARTICLES
Photon correlation spectroscopy
The mean particle size and polydispersity index of fabricated RM SLNs was determined by dynamic light scattering, also known as photon correlation spectroscopy or quasi-elastic light scattering is used to records the variation in the intensity of scattered light on the microsecond timescale using Malvern Mastersizer 2000MS device (Malvern Instruments, Worcestershire, UK) and laser diffraction with a beam length of 2.40 mm, range lens of 300 RF mm, at 10.14% obscuration.
Polydispersity index, a parameter calculated from the width of the particle size of the distribution using the equation = D (0.9)-D (0.1)/D (0.5). Where, D (0.9), D (0.5), and D (0.1) are corresponding to particle size immediately above 90%, 50%, and 10% of the sample. The measuring range of the Malvern Mastersizer is from 0.02 μm to 2000 μm.[16]
Zeta potential measurement
The fabricated RM nanoscale solid lipid particle surface charge and electrostatic stabilization was measured by laser Doppler electrophoresis using Malvern Zetasizer Nano ZS (Malvern Instruments, UK). The instrument quantifies the electrophoretic mobility of the particles, which was converted into the ZP using the Helmholtz-Smoluchowski equation built into the Malvern Zetasizer software. The measured ZP of particles depends on the dispersion medium; therefore, the surface charge has been measured in deionized distilled water with a conductivity adjusted to 50 μS/cm with sodium chloride.[17]
Transmission electron microscopy
Morphological evaluation of nanoscale solid lipid particles was studied by TEM using JEOL JEM-2000 EXII TEM (Tokyo, Japan). The particulate carriers were negatively stained with 0.5% (w/v) phosphotungstic acid solution and fixing on coated copper grids with carbon film and dried under vacuum pressure. The samples were diluted to 5 mL in buffer to obtain a clear solution and scanned in all zones before the image is taken.
Determination of drug content
The RM loaded nanoscale solid lipid particle assay was determined using high-performance liquid chromatography (HPLC) system (Thermoscientific, spectra system P-4000, USA) with ultraviolet (UV) detector (Kromosil 100) and C18 column (particle size 5 μm, 250 mm × 4 mm). 2 ml of sample was diluted with 10 ml of mobile phase consisted of acetonitrile: Water (5:95, v/v) and filtered through 0.45 μm nylon millipore membranes (Millipore, USA). The absorbance was recorded at 265 nm. Under these conditions, the retention time of RM was at 4.672 min. RM concentrations were calculated relative to a calibration curve.[7,18]
Determination of entrapment efficiency
Entrapment efficiency was determined to access the extent of RM incorporation in the nanoscale solid lipid particles. The EE of the RM-SLNs was determined by measuring the concentration of free drug in the supernatant obtained after centrifuging at 16,000 rpm for 30 min at 0°C (Remi C 24, Mumbai, India). The amount of free drug was quantified by HPLC method (Thermoscientific, spectra system P-4000, USA) and the incorporated drug was calculated through following equation:
EE (%, W/W) = W1 − W2 /W1
Where, W1 - the total amount of drug in the formulation and W2 - amount of drug in the supernatant.[7]
In vitro drug release studies
In vitro drug release from RM loaded SLNs was investigated using phosphate buffer at pH 7.4 at 37°C ± 0.5°C. RM-SLNs equivalent to 1 mg was transmitted into a conical flask containing 50 ml of phosphate buffer (pH - 7.4) and the flask was kept in incubator shaker (Lark, India) at 50 rpm horizontal shakes per min at 37°C ± 0.5°C. The incubator shaker condition was maintained for the whole experiment. One milliliter of the release medium was withdrawn at regular intervals and the same volume of prewarmed (37°C ± 0.5°C) fresh release medium was replaced to maintain the volume constant. The withdrawn samples were filtered through a 0.45 μm membrane filter (Elix, Mill-Q) and the RM content in each sample was estimated after suitable dilution with a Thermoscientific HPLC (spectra system P-4000, USA) with UV detector (Kromosil 100) and C18 column (particle size 5 μm, 250 mm × 4 mm) at 265 nm. RM dissolved at specified time periods was plotted as cumulative percent release versus time (h) curve.[18]
RESULTS AND DISCUSSION
Rasagiline mesylate is an Anti-Parkinson drug used for the treatment of Parkinson disease. The RM loaded nanoscale solid lipid particles were fabricated by microemulsion technique. The lipid, surfactant, and co-surfactant were chosen based on a preliminary study. Co-surfactant plays an important role in the formation of microemulsion. An o/w microemulsion was obtained by adding an aqueous phase into the lipid phase at the same temperature. The resultant emulsion was dispersed into cold water, which assisted the rapid lipid crystallization and prevented lipid aggregation. Fabricated RM loaded nanoscale solid lipid particles must be sterile for intravenous administration, so the formulation was sterilized by moist heat sterilization using autoclaving, which is a convenient and cost effective technique for SLNs. Because, the sterilization by filtration requires a high pressure which is not applicable to SLNs. Sterilization by γ-radiation cause unacceptable chemical breakdown of bilayer components in phospholipid. During the process, the rasagiline loaded SLNs formulation reached high temperature probably causes a warm o/w microemulsion to form in the autoclave and presumably modifies the particle size of the formulation. On subsequent slow cooling, the RM loaded nanoscale solid lipid particles were reformed.
The physical and chemical stability of formulations was investigated during autoclave and to determine the average mean particle size, polydispersity index, ZP, TEM, EE drug content, and in vitro drug release after the moist heat sterilization by autoclave.
Before sterilization, the average mean particle size of RM loaded nanoscale solid lipid particles range from 186 nm to 212.04 nm and their curves were unimodal particle size distribution [Figure 1a]. The polydispersity index is a ratio, which determined as a measure of the homogeneity. Ideally, it should be <0.3.[19] RM loaded solid lipid particles had the polydispersity index in the range of 0.394-0.486, indicating narrow size distribution and suggesting particles are monodispersity. The value of ZP is a factor to evaluate the stability of RM loaded solid lipid particles. When the ZP is more positive than 30 mV or more negative than 30 mV are electrochemically stable.[20] The ZP of RM loaded solid lipid particles range from −32.7 mV to −38.5 mV, and it indicates the formulation was electrochemically stable [Figure 2b].
Figure 1.
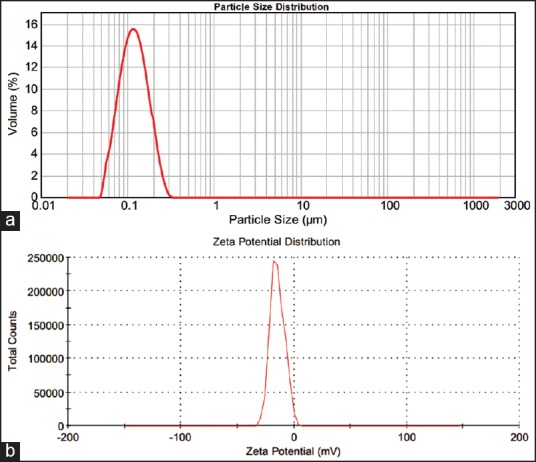
(a) Particle size distribution and (b) Zeta potential measurements before sterilization
Figure 2.
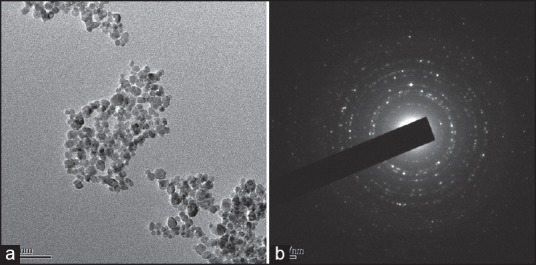
(a) Transmission electron microscopy micrograph and (b) The selected area electron diffraction pattern of rasagiline mesylate loaded solid lipid particles before sterilization
Figure 2a shows the representative TEM photomicrograph, which get more insight about the morphology of the RM loaded nanoscale solid lipid particles before sterilization. The observation shows that the formulation was monodisperse, uniform size, and quasispherical shape with a smooth surface. Figure 2b indicates the corresponding selected area electron diffraction (SAED) pattern. It obtained using the smallest selected-area aperture. The results show that the RM loaded nanoscale solid lipid particles are polycrystalline structure. The RM loaded solid lipid particles EE and drug content were 87.32% and 98.57%, respectively
After sterilization, the RM loaded solid lipid of particle mean particle size was increased, but to a negligible extent [Figure 3a]. The particle size range from 198.45 nm to 232.17 nm and a polydispersity index calculated through volumetric distribution of particles and the results of polydispersity index (Pdi) from 0.317 to 0.426. The polydispersity index measurements diverse faintly after sterilization, showing that the variation in the population distribution was only small. The zeta potential value and their effects were shown in Table 1. The ZP value range from −35.6 mV to −38.1 mV, which shows the electrochemical stability of the formulations [Figure 3b] and the measurements of ZP before and after sterilization was greater, as shown in Table 2. The TEM studies were carried out to get more insight about the morphology of the RM loaded nanoscale solid lipid particles.
Figure 3.
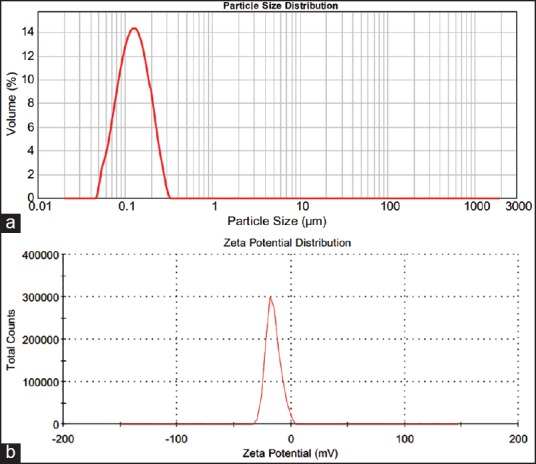
(a) Particle size distribution and (b) Zeta potential measurements after sterilization
Table 1.
Measurements of zeta potential
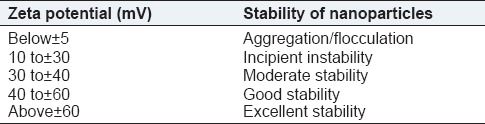
Table 2.
The influence of a sterilization process on rasagiline mesylate loaded nanoscale solid lipid particles

After, sterilized RM loaded SLNs Transmission electron microscope [Figure 4a] results show the particles are almost uniform size and spherical shape and few small particles aggregate into secondary particles because of their very small dimensions and high-surface energy. Figure 4b shows the SAED pattern, which was obtained using the smallest selected-area aperture. The results of SAED indicate the RM loaded nanoscale solid lipid particles are polycrystalline structure.
Figure 4.
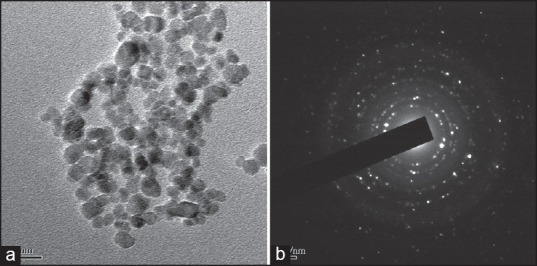
(a) Transmission electron microscopy micrograph and (b) The selected area electron diffraction pattern of rasagiline mesylate loaded solid lipid particles after sterilization
After sterilized RM-SLNs show a slight in the average mean particle size as compared to before sterilized RM-SLNs. But there are no morphological changes between before sterilized and after sterilized of RM loaded SLN. The surfactant seems to protect the RM loaded SLNs by acting as a steric stabilizer and avoiding the coalescence of SLNs during autoclaving processes.
In vitro release of RM from SLNs before and after sterilization results is shown in Figure 5. Before and after sterilization of the formulation released 91.06 ± 1.03% and 90.13 ± 2.26%, respectively, of their RM content at the end of 24 h. Each data point in the drug release profile represents the mean of three determinations.
Figure 5.
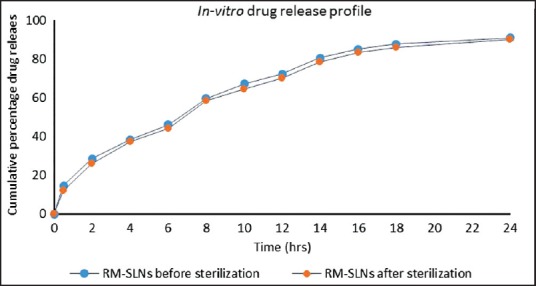
In vitro release profile of rasagiline mesylate (RM) loaded solid lipid nanoparticle before and after sterilization. In vitro cumulative release of RM-solid lipid nanoparticles from before sterilization (—·—) and after sterilization (—·—). Each point represents mean ± standard deviation, n = 3
Rasagiline mesylate loaded SLN before sterilization and after sterilization released 72.45 ± 1.65% and 70.32 ± 0.45% of RM at the end of 12 h and 91.06 ± 1.03% and 90.13 ± 2.26% at the end of 24 h, respectively. The results of in vitro drug release profile after sterilization indicate the effect of autoclave did not influence the release from RM loaded SLNs
CONCLUSION
In this study, nanoscale solid lipid particles using RM as model drug were successfully fabricated by microemulsion method. The fabricated RM loaded nanoscale solid lipid particles were sterilized by moist heat sterilization using autoclaving, maintaining an almost quasispherical shape with a smooth surface as confirmed by TEM analysis. The physical-chemical stability of RM loaded nanoscale solid lipid particles was investigated during autoclave and the average mean particle size, polydispersity index, ZP, EE, and drug content was determined after sterilization by autoclaving. The obtained in vitro drug release profile after sterilization indicates the effect of autoclave did not influence the release from RM loaded SLNs. There were no significant changes in the parameters and the observations suggest that the moist heat sterilization by autoclaving is the most suitable method for nanoscale solid lipid formulations.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Thassu D, Dellers M, Pathak Y. Drugs and Pharmaceu tical Science; Nanoparticles Drug Delivery System. Vol. 166. New York, London: Informa Health Care; 2007. p. 237. [Google Scholar]
- 2.Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Allémann E, Leroux J, Gurny R. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Deliv Rev. 1998;34:171–189. doi: 10.1016/s0169-409x(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 4.Jeon HJ, Jeong YI, Jang MK, Park YH, Nah JW. Effect of solvent on the preparation of surfactant-free poly(DL-lactide-co-glycolide) nanoparticles and norfloxacin release characteristics. Int J Pharm. 2000;207:99–108. doi: 10.1016/s0378-5173(00)00537-8. [DOI] [PubMed] [Google Scholar]
- 5.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 6.Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int J Pharm. 2005;293:261–70. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Viveksarathi K, Kannan K. Multi criteria decision making to select the best method for the preparation of solid lipid nanoparticles of rasagiline mesylate using analytic hierarchy process. J Adv Pharm Tech Res. 2014;5:1–7. doi: 10.4103/2231-4040.137410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourrel M, Schecter RS. Microemulsion and Related Systems. Vol. 30. New York: Marcel Dekker; 1988. p. 26. [Google Scholar]
- 9.Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: Effect of formulation variables on size distribution. Int J Pharm. 2005;290:137–44. doi: 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein JN, Leserman LD. Liposomes as drug carriers in cancer chemotherapy. Pharmacol Ther. 1984;24:207–33. doi: 10.1016/0163-7258(84)90035-4. [DOI] [PubMed] [Google Scholar]
- 11.Sculier JP, Coune A, Brassinne C, Laduron C, Atassi G, Ruysschaert JM, et al. Intravenous infusion of high doses of liposomes containing NSC 251635, a water-insoluble cytostatic agent. A pilot study with pharmacokinetic data. J Clin Oncol. 1986;4:789–97. doi: 10.1200/JCO.1986.4.5.789. [DOI] [PubMed] [Google Scholar]
- 12.Heiati H, Tawashi R, Phillips NC. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J Microencapsul. 1998;15:173–84. doi: 10.3109/02652049809006847. [DOI] [PubMed] [Google Scholar]
- 13.Cavalli R, Caputo O, Carlotti ME, Trotta M, Scarnecchia MC, Gasco R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int J Pharm. 1997;148:47–54. [Google Scholar]
- 14.Gasco MR. Method for producing solid lipid microspheres having a narrow size distribution. US Patent No. 5250236. 1993 [Google Scholar]
- 15.Indian Pharmacopoeia. Vol. 1. Delhi: Published by the Controller of Publication; 1996. pp. 256–7. [Google Scholar]
- 16.Kashanian S, Azandaryani AH, Derakhshandeh K. New surface-modified solid lipid nanoparticles using N-glutaryl phosphatidylethanolamine as the outer shell. Int J Nanomedicine. 2011;6:2393–401. doi: 10.2147/IJN.S20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorthi C, Krishnan K, Manavalan R, Kathiresan K. Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles. Asian Pac J Trop Biomed. 2012;2:841–8. doi: 10.1016/S2221-1691(12)60241-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández M, Negro S, Slowing K, Fernández-Carballido A, Barcia E. An effective novel delivery strategy of rasagiline for Parkinson's disease. Int J Pharm. 2011;419:271–80. doi: 10.1016/j.ijpharm.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Pathak P, Nagarsenker M. Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech. 2009;10:985–92. doi: 10.1208/s12249-009-9287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashanian S, Azandaryani AH, Derakhshandeh K. New surface-modified solid lipid nanoparticles using N-glutaryl phosphatidylethanolamine as the outer shell. Int J Nanomedicine. 2011;6:2393–401. doi: 10.2147/IJN.S20849. [DOI] [PMC free article] [PubMed] [Google Scholar]


