Abstract
The ranges of mechanisms by which exercise affects energy balance remain unclear. One potential mechanism may be that exercise reduces intake and preference for highly palatable, energy dense fatty foods. The current study used a rodent wheel running model to determine whether and how physical activity affects HF diet intake/preference and reward signaling. Experiment 1 examined whether wheel running affected the ability of intracerebroventricular (ICV) µ opioid receptor agonist D-Ala2, NMe-Phe4, Glyol5-enkephalin (DAMGO) to increase HF diet intake. Experiment 2 examined the effects of wheel running on the intake of and preference for a previously preferred HF diet. We also assessed the effects of wheel running and diet choice on mesolimbic dopaminergic and opioidergic gene expression. Experiment 1 revealed that wheel running decreased the ability of ICV DAMGO administration to stimulate HF diet intake. Experiment 2 showed that wheel running suppressed weight gain and reduced intake and preference for a previously preferred HF diet. Furthermore, the mesolimbic gene expression profile of wheel running rats was different from that of their sedentary paired-fed controls but similar to that of sedentary rats with large HF diet consumption. These data suggest that alterations in preference for palatable, energy dense foods play a role in the effects of exercise on energy homeostasis. The gene expression results also suggest that the hedonic effects of exercise may substitute for food reward to limit food intake and suppress weight gain.
Keywords: physical activity, wheel running, high fat diet, mesolimbic system, opioid, dopamine
1. Introduction
Reduced physical activity and unhealthy diet choices are predominant factors that contribute to the global obesity crisis (Lenard and Berthoud, 2008, Berthoud, 2012). Physical activity has multiple beneficial effects on general health. Data from animal models and clinical studies have suggested that sufficient physical activity can improve cardiovascular function (Park et al., 2012), glucose metabolism (Boersma et al., 2012, Cadeddu et al., 2014), mood disorders (Cunha et al., 2013, Wegner et al., 2014), and lead to healthy weight maintenance and weight loss (Anderson et al., 2001, Swift et al., 2014). While regular physical exercise can sustain a healthy weight or a weight loss regimen, the range of mechanisms by which exercise contributes to the control of body weight remains unclear.
One potential mechanism is through reducing intake of and preference for highly palatable, energy dense foods. According to National Weight Control Registry of the USA, individuals who successfully maintain ≥ 30 lbs (13.6 kg) weight loss for over one year exercise persistently e.g., an average weekly energy expenditure of 2853.6 kcal from physical activity. The data also indicate a negative relationship between physical activity and fat intake i.e. the more exercise the less fat intake (Ogden et al., 2012). Nonetheless, the few studies on exercise and macronutrient intake in human subjects have not provided consistent results (Elder and Roberts, 2007, Donnelly et al., 2014). The primary limitations of studies involving human subjects include the control of the amount and intensity of exercise between and within subjects and food intake assessment. Furthermore, considerable variation in daily food intake and energy expenditure in humans has been reported (Edholm et al., 1955, Bray et al., 2008). The studies that have investigated the effects of exercise on food intake/selection, however, normally measured food intake of only a single meal each prior to and after exercise regimen. Taken together, the fact that no consistent conclusions can be drawn from those studies may reflect the difficulty of using human subjects to elucidate physiological interactions among exercise, appetite, and the multiple regulatory mechanisms controlling food intake. Thus, the current study applied a diet choice (high carbohydrate chow vs. high fat diet) protocol in a well-developed rat wheel running model to examine the effects of voluntary physical activity on high fat (HF) diet intake and preference.
Previous studies have demonstrated that infusion of a µ opioid receptor agonist D-Ala2, NMe-Phe4, Glyol5-enkephalin (DAMGO) into the brain increases HF diet intake and preference (Bakshi and Kelley, 1993, Zhang and Kelley, 2000). Furthermore, opioid agonists and antagonists have been reported to enhance or attenuate wheel running activity and the effects produced by it (Boer et al., 1990, Lett et al., 2001, Sisti and Lewis, 2001). Despite these effects, no study has examined how highly palatable food and physical activity interact to alter µ opioid receptor stimulated behaviors and related gene expression. This study examined whether and how exercise affects HF diet preference from two perspectives. Experiment 1 tested whether wheel running alters DAMGO induced increases in HF diet intake/preference. Because µ opioid receptors undergo several post-translational modifications (Zheng et al., 2010), using a selective µ opioid receptor agonist, such as DAMGO, will allow us to accurately assess the interaction between wheel running and highly palatable food intake on µ opioid receptor stimulated behaviors. Multiple studies have reported that wheel running is rewarding (Belke, 1997, Greenwood et al., 2011, Meijer and Robbers, 2014) and may alter the drive for other rewards e.g. drugs of abuse (Smith et al., 2008, Smith and Pitts, 2012) and palatable food (Satvat and Eikelboom, 2006, Scarpace et al., 2010). Thus, Experiment 2 examined whether wheel running reduces preference for a previously preferred HF diet. Both the dopaminergic and opioid systems mediate the intensity/amount (Boer et al., 1990, Sisti and Lewis, 2001, Knab et al., 2012, Roberts et al., 2012, Klinker et al., 2013) as well as the rewarding effects of wheel running in rodents (Lett et al., 2001, Vargas-Perez et al., 2008, Greenwood et al., 2011, Rasmussen and Hillman, 2011). Thus, Experiment 2 also determined whether wheel running alters the mRNA expression of dopaminergic and opioidergic genes in reward pathways.
2. Experiment 1: methods
2.1 Subjects
Sixteen male Sprague-Dawley (Harlan, Frederick, MD) rats weighing 250-275 g upon arrival were the subjects of this experiment. The rats were housed in a climate-controlled vivarium with a 12 h on//off light (0100)/dark (1300) cycle. Rats were individually housed either in metal wire-meshed hanging cages (the sedentary, Sed group) or conventional tubs equipped with a locked wheel (Mini Mitter, Philips Respironics, OR, USA; the wheel running, WR group). Due to limited number of running wheel cages, the Sed controls were housed in metal wire-meshed hanging cages. A standard chow diet (Harlan 2018, 3.1 kcal/g, 58% carbohydrate, 24% protein and 18% fat; Harlan Laboratories, USA) and tap water were available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University and conformed to the guidelines of the National Institutes of Health.
2.2 Lateral intracerebroventricular (ICV) cannulation and verification
After two days of habituation, rats were anesthetized with a mixture of ketamine (100 mg/kg, ip) and xylazine (20 mg/kg, ip) and mounted in a stereotaxic instrument. A 23-gauge stainless steel guide cannula (Plastics One, VA, USA) aimed at the lateral ventricle was implanted 4.5 mm below the skull, 0.8 mm caudal to bregma, and 1.6 mm lateral to the midline. A stainless steel dummy (Plastics One, VA, USA) was inserted into the cannula to maintain patency. After 8 days of postoperative recovery, body weight was more than preoperative level, and cannula placements were assessed by examining water intake without food or water deprivation in response to ICV angiotensin II (Sigma, MO, USA) administration. Rats were injected with 5 nmol angiotensin II in 5 μl of saline or saline alone and allowed 30-min access to bottles of water with drinking tubes. The criterion for correct placement and cannula patency was defined as 5 ml more water intake after angiotensin II than after saline vehicle administration. The same angiotensin II test was done again at the end of the experiment. Only data from the rats that met the criterion during both of the angiotensin II tests were included in the statistical analyses.
2.3. Procedures
Two-diet choice test. Three days after the cannulation surgery, rats had daily exposure to two diets simultaneously at the first 3 hrs of the dark cycle for 5 consecutive days. During this period, Harlan 2018 chow was replaced by a Prolab chow (RMH 1000, 3.37 kcal/g, 65.6% carbohydrate, 18.6% protein and 15.8% fat, LabDiet, MO, USA) and a HF diet (D12492, 5.24 kcal/g, 20% carbohydrate, 20% protein and 60% fat; Research Diets, NJ, USA). Intakes of these two diets were measured at 1 and 3 hr time point. The positions of the diets were alternated daily to prevent bias in the results if the rats develop a side preference. After 5 training days, the two-diet choice test occurred once when the WR rats had run for 2 wks and on the days the rats received an ICV injection.
Wheel running schedule
Two to three days after the initial angiotensin II placement verification, the wheels for the WR rats were unlocked and their voluntary running was recorded and analyzed through a computer and the Vital View Software System (Philips Respironics, OR, USA). All rats were provided with water ad libitum. Except for during the two-diet choice test, the Harlan chow diet was available at all times. The WR rats had free access to the running wheels until the end of the experiment.
ICV injection
After one week of wheel running access, ICV injections of DAMGO (Sigma, MO, USA) or saline vehicle (bacteriostatic 0.9% saline, HOSPIRA, IL, USA) began. The doses of DAMGO injected were administered in a randomized order of 3.2, 10, and 1 nmol (in 3 μl/injection). Saline injections occurred twice, once before and once between the DAMGO injections. To ensure the washout of DAMGO, there were at least 72 hrs between each injection. The injections occurred between 30 min before and 30 min after the dark onset. Before the injections, Harlan chow diet was removed and immediately after the injection, the Prolab chow and HF diets were placed into the cage. One and 3 hr intakes of the two diets were measured. A summary of the experimental time line is listed in Table 1.
Table 1.
Timeline of Experiment 1.
| Time | Day -16 and -15 | Day -11 to -7 | Day -4 and -3 | Day 1-14 | Day 15-38 | Day 43 |
|---|---|---|---|---|---|---|
| Procedures | cannula implantation |
Diet Choice Training |
ICV verification 1 |
Sedentary vs. wheel running |
Sedentary vs. wheel running |
ICV verification 2 |
| Diet test | ad lib Harlan chow |
daily 3 hrs Prolab and HF diet choice training |
ad lib Harlan chow |
saline injection with diet choice on day 7 |
injections with diet choice on days 18, 25, 29, 34 |
ad lib Harlan chow |
2.4 Statistical analysis
Data were analyzed by one-way ANOVA, repeated-measures ANOVA, and post hoc Fisher LSD tests as appropriate using Statistica 7.1 (Tulsa, OK). Average intakes from the two saline ICV injections were used to compare with DAMGO effects on intakes. High fat diet preference was calculated as HF intake (g) divided by HF + Prolab chow intake (g). Data are presented as mean ± standard error of the mean (SEM).
3. Experiment 1: Results
Diet choice tests
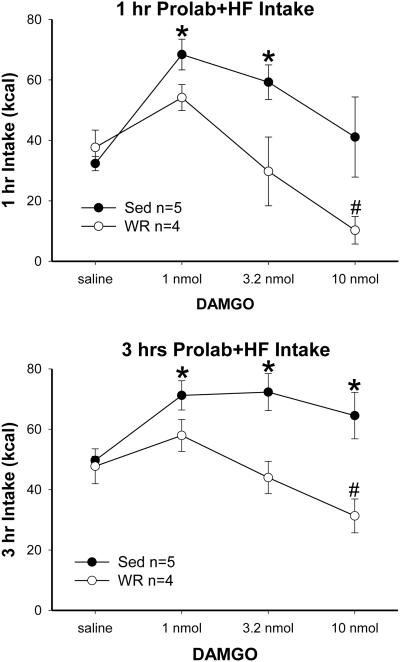
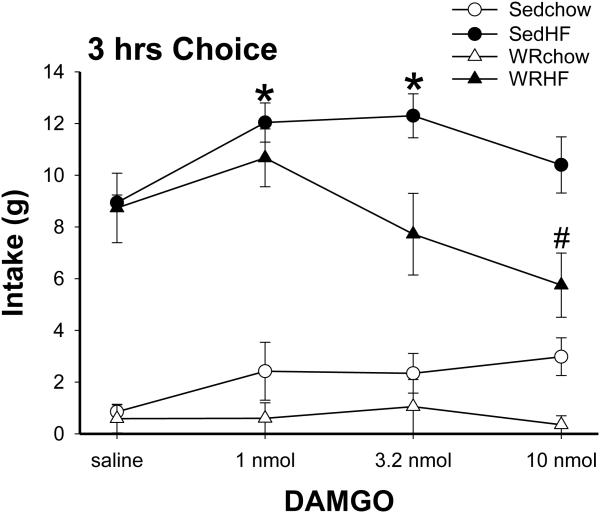
At baseline training, both Sed (n=5) and WR (n=4) rats preferred the HF diet (average preference ratio > 0.98). There were no baseline differences in Prolab, HF or total energy intake (data not shown). Lateral DAMGO ICV injections differently affected total energy intake during the 3 hrs diet choice tests in the Sed and WR groups (Fig. 1). Total energy intake significantly increased in the Sed rats but remained unchanged or decreased in the WR rats after lateral DAMGO ICV injections [1 hr vs. 3 hr for group effect, F(1,7)=7.43 vs. 11.94, both P<0.03 ; for DAMGO effect, F(3,21)=9.18 vs. 6.03, both P<0.004; for group χ DAMGO, F(3, 21)= 2.84 vs. 4.82, P=0.06 vs. <0.02]. The differences in total energy intake were due to differences in HF diet intakes because DAMGO administration did not significantly affect chow intakes in either the Sed and WR rats. DAMGO significantly increased the HF diet intake in Sed but not in WR rats at both 1 hr and 3 hr [1 hr vs. 3 hr, for group, F(1, 7)=6.29 vs. 6.56, both P<0.05; for DAMGO, F(3, 21)=9.64 vs. 5.69, both P<0.006; for group χ DAMGO, F(3, 21)=2.80 vs. 3.54, P=0.07 vs. <0.04]. In Sed rats, DAMGO administration significantly increased the HF diet intake at 1 nmol (post hoc 1 hr and 3 hr, both P<0.02) and 3.2 nmol (post hoc 1 hr and 3 hr, both P<0.009). In contrast, DAMGO administration in WR rats did not change HF diet intake at the low and median doses but decreased intake significantly at 10 nmol (post hoc 1 hr and 3 hr, both P<0.03). As a result, HF diet intakes differ significantly between the Sed and WR groups at the dose of 3.2 and 10 nmol (post hoc 1 hr and 3 hr, both P<0.02). Fig 2 shows the effects of DAMGO on 3 hrs intakes of chow and HF diet. Finally, the WR rats weighed less and so the intake data were also analyzed by normalizing to body weight. Results of repeated measure ANOVA with normalized intakes during the 3 hr intake test [group effect, F(1,7)=9.19, P<0.02; DAMGO effect, F(3,21)=4.2, P<0.02; group χ DAMGO, F(3, 21)=4.9, P<0.01] were consistent with the effects mentioned above. That is, high dose DAMGO reduced intakes during the 3 hrs test duration in WR rats.
Fig. 1.
First (top) and third (bottom) hour total energy intake of the chow and high fat (HF) diet. Rats were either sedentary (Sed) or with wheel running (WR) access. They received access to both a high carbohydrate chow and a HF diet for 3 hrs immediately after lateral ICV DAMGO ((µ-opioid receptor agonist) injection. Intakes (mean ± SEM) after 10 nmol DAMGO injection during 1 and 3 hrs were reduced in WR rats. *: saline vs. DAMGO in Sed rats; #: saline vs. DAMGO in WR rats, P< 0.05.
Fig. 2.
Three hours intakes of the chow and HF diet. Lateral ICV 1 nmol and 3.2 nmol DAMGO administration significantly increased HF diet intake in Sed rats. In contrast, 10 nmol DAMGO administration significantly decreased HF diet intake in WR rats. *: saline vs. DAMGO in SedHF, P<0.05; #: saline vs. DAMGO in WRHF, P< 0.05.
Although HF diet intakes differed between groups after DAMGO treatment, it did not alter HF diet preference at 1 hr or 3 hr. Repeated-measures ANOVA revealed no effect of group [1 hr vs. 3 hr: F(1, 7)= 0.38, P=0.56 vs. F(1, 7)= 0.84, P=0.39], DAMGO dose [1 hr vs. 3 hr: F(3, 21)=0.78, P=0.52 vs. F(3, 21)=0.61, P=0.61] or the group and DAMGO interaction [1 hr vs. 3 hr: F(3, 21)=0.21, P=0.89 vs. F(3, 21)=0.79, P=0.51].
Daily total intakes, wheel running activity and body weight
At baseline, the Sed and WR groups had similar total energy intake (average ranging 70 – 83 kcal/day). Energy intake was reduced to 47.59 ± 2.19 kcal after the first day of wheel running. Daily energy intakes during the first 11 days of running were significantly less than not only the baseline intake of WR rats but were also significantly below the intakes of the Sed rats during the same period [Repeated measure ANOVA: group, F(1, 7)=136.78, P<0.0001; intake, F(14, 98)=4.07, P<0.0001; group × intake, F(14, 98)=2.50, P<0.005 ]. After 2 weeks of running, daily energy intake of the WR rats were comparable to that of the Sed rats and group differences only reappeared during DAMGO injection. That is, DAMGO injection increased total daily intake (sum of Harlan, Prolab and HF diets) in the Sed rats (average ranging 84.8 – 94.0 kcal), but the increases were not statistically significant. As mentioned above, DAMGO injection in the WR rats significantly decreased HF intake during the 3 hrs of the diet choice test at the doses of 3.2 and 10 nmol. Such decreases in HF intake were not compensated for since the total energy intakes during these two days were significantly less than baseline (3.2 vs. 10 nmol = 49.13 ± 5.26 vs. 49.29 ± 5.94 kcal). Repeated measure ANOVA comparing daily intakes at baseline and during DAMGO injection revealed significant effects of group [F(1, 7)=39.98, P<0.0004], intake [F(3, 21)=8.61, P<0.0007] and group × intake [F(3, 21)=6.99, P<0.002].
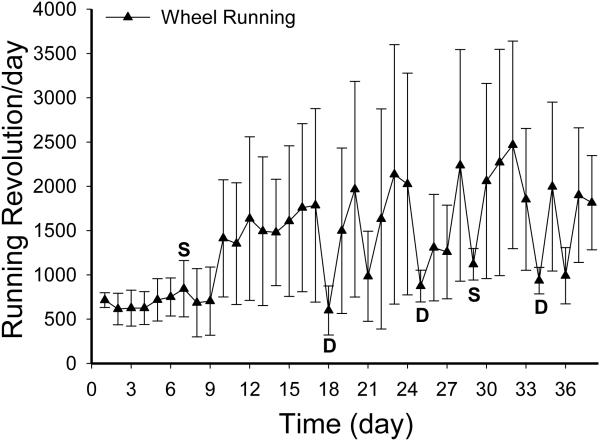
Wheel running activity varied greatly among the WR rats (lowest vs. highest runner: 645.7 ± 53 vs. 3283.8 ± 289.6 revolution/day; Fig. 3.). One way repeated measure ANOVA of the degree of wheel running suppression after ICV DAMGO administration revealed a significant dose effect [F(3, 9)=8.38, P<0.006]. That is, DAMGO at 1 (pre vs. post ICV: 1852.8 ±801 vs. 935.5 ± 149.9 revolution/day) and 3.2 nmol (pre vs. post ICV: 1785.3 ± 1092.8 vs. 597.8 ± 277.4 revolution/day) significantly decreased wheel running (both post hoc P<0.02). Although the 24 hr running revolutions were reduced after DAMGO administration, the reduction in running appeared to occur outside the 3-hr chow and HF diet intake test. That is, repeated measure ANOVA comparing the 3-hr running activity on the day prior to and on the day of DAMGO administration revealed no significant effects of DAMGO injection [F(1, 6)=0.24, P=0.64]. During this 3-hr period, running activity also varied greatly among WR rats (lowest vs. highest runner: 92.6 ± 30.7 vs. 1566 ± 284.9 revolution/3-hr). Among the WR rats, the three low runners increased (average prior day vs. the day of DAMGO injections: 111.2 ± 24.2 vs. 215.9 ± 75.2 revolution/3-hr) whereas the one high runner decreased (average prior day vs. the day of DAMGO injections: 1825.3 ± 166.7 vs. 636.3 ± 134.6 revolution/3-hr) the 3-hr running activity in response to DAMGO injections.
Fig. 3.
Average daily wheel running activity from 4 WR rats with verified functioning ICV cannulas in Experiment 1. The letter S and D indicates saline and DAMGO injection, respectively. Rats received ad lib access to standard chow diet during non-injection days. On the injection days, they received simultaneous access to a Prolab and a HF diet for 3 hours after the injection (see the description in Section 2).
Wheel running suppressed weight gain. Repeated-measures ANOVA comparing weekly body weight indicates significant effects of group [F(1,7)=7.48, P<0.03] and time [F(4, 28)=16.04, P<0.0001] but not group × time interaction [F(4, 28)=1.51, P=0.23]. At pre-running baseline, the two groups of rats had similar weight (average of 343 – 344 g). At the end of DAMGO experiment, WR rats weighed significantly less than the Sed ones (401.88 ± 7.13 vs. 375.1± 6.66 g, P<0.02).
4. Experiment 2: methods
4.1 Subjects
A total of 44 male Sprague-Dawley (Harlan, Frederick, MD) rats weighing 250-275 g upon arrival were used in this experiment. Rats were housed in a climate-controlled vivarium with a 12 h on/off light (0400)/dark (1600) cycle. Due to the limited numbers of running wheels, the experiment was conducted in two cohorts. The first cohort included 3 groups: Naïve, sedentary (Sed) and wheel running (WR). The group assignment of the second cohort was similar to the first cohort except that a Paired-Fed group was included. Together, the subject number for each group was 13 for Naïve, 12 for Sed, 12 for WR, and 7 for Paired-Fed groups.
4.2 Procedures
Upon arrival, rats in the Naïve group were individually housed in conventional tubs with water and the standard Harlan chow (3.1 kcal/g, the same as in Experiment 1) available ad libitum. Naïve rats remained in the tubs throughout the experiment. Sed and WR rats were individually housed in tubs equipped with a running wheel (Mini Mitter, Philips Respironics, OR, USA) and, during habituation the wheels were locked. These rats had free access to water, the standard chow and a 60% HF diet (5.24 kcal/g, D12492, Research Diets, NJ, USA; the same as in Experiment 1). The locations of the chow and HF diet were alternated daily to prevent side preference. Initial side preferences were observed in approximately 90% of the rats. This behavior did not differ between groups. Once intakes of chow and the HF diet stabilized (2 weeks), the Sed rats remained sedentary and the wheels were unlocked for the WR group. Voluntary running was recorded and analyzed through a computer and the Vital View Software System (Philips Respironics, OR, USA). The two-diet choice and free running regimen continued for 16 days. Daily body weight and food intakes were measured during the light cycle between 0700 and 0900. The Paired-Fed rats were individually housed in conventional tubs. They were acclimated with free access to water and chow during the first week. From the 2nd week, their intakes were paired to those of the WR group. That is they received the amount of daily average intakes of chow and HF diet consumed by the WR group. The allotted foods for the paired-fed rats were provided at the time when body weight and intakes were measured.
At the end of 16 days running, foods were removed at 0700 and wheels for the WR rats were locked. After 3 hrs, rats were sacrificed with rapid decapitation. Sacrificing order was organized so that the timing of sacrifice for each group was distributed evenly. Brains were collected and instantly frozen on powdered dry ice and then stored at −80°C until further processing for RNA extraction.
4.3 Brain punch and real-time QPCR
The prefrontal cortex (PFC), nucleus accumbens (NAc) and ventral tegmental area (VTA) were punched from frozen coronal sections (300 – 600 µm) using a blunted 16G stainless steel needle (inner diameter = 1.65 mm) based on the coordinates described in Paxinos and Watson (Paxinos and Watson, 2007). Taking Bregma as reference, PFC and NAc were punched between +2.28 and +0.88 mm. The PFC punches were dorsal to the midline of the corpus callosum and the NAc punches were from the shell of the NAc located medial to the anterior commissure. The VTA was punched between −4.80 and −6.00 mm. The punches were dorsal to mammillary nucleus and medial to substantia nigra. Punches of PFC, NAc and VTA were put into QIAzol Lysis Reagent (QIAGEN, Valencia, CA) and homogenized immediately with a sterile pipet tip. RNA was extracted with the RNeasy Mini Kit (QIAGEN). For each individual sample, 500ng total RNA was used in reverse transcription using the QuantiTect Rev. Transcription Kit (QIAGEN). mRNA expression levels of target genes was determined by real-time QPCR using gene-specific TaqMan probes (Applied Biosystems, Foster City, CA) with TaqMan Gene Expression Master Mix (Applied Biosystems) on the ABI 7900HT Fast Real Time PCR system set for 40 PCR cycles. Probes used for RT-PCR are listed in Table 2. We measured mRNA for Actb (beta actin), DAT (dopamine transporter), D1R (dopamine receptor 1), D2R (dopamine receptor 2), OPRM1 (opioid receptor µ1), and PENK (proenkephalin). To determine relative expression values, the 2−ΔΔCt method (Applied Biosystems) was used, where triplicate Ct values for each sample were averaged and subtracted from those derived from the housekeeping gene Actb. Data are expressed as relative fold change to levels of the Naïve controls.
Table 2.
List of TaqMan probes used for gene expression assays.
| Gene | Gene | Refseq | TaqMan |
|---|---|---|---|
| Symbol | Name | mRNA | Assay ID |
| Actb | actin, beta | NM_031144.2 | Rn00667869_m1 |
| DAT | dopamine transporter | NM_012694.2 | Rn00562224_m1 |
| D1R | dopamine receptor 1 | NM_012546.2 | Rn03062203_s1 |
| D2R | dopamine receptor 2 | NM_012547.1 | Rn00561126_m1 |
| OPRM1 | opioid receptor μ1 | NM_013071.2 | Rn01430371_m1 |
| PENK | proenkephalin | NM_017139.1 | Rn00567566_m1 |
4.4 Data analysis
Data included in the analysis were from 13 Naïve, 11 Sed, 10 WR and 7 paired-fed rats. One rat in the Sed group was water deprived by mistake at the beginning of the experiment and two in the WR group did not run once the wheels were unlocked. Thus, data from these rats were excluded. Although the locations of chow and HF diet were alternated daily to prevent side preference, signs of side preference i.e. significant higher HF intake on alternative days were observed in some rats. To minimize such effect in statistical analysis, intake data are presented as 2 days blocks. The HF diet preference ratio was calculated as the kcal intake of the HF diet divided by total kcal intake within that block. Data were analyzed by ANOVA, repeated-measures ANOVA, or Student’s t tests for independent samples as appropriate using Statistica 7.1 (Tulsa, OK). Subsequent comparisons between groups were performed using Fisher LSD tests. Data are presented as the mean ± standard error of the mean (SEM).
5. Experiment 2: results
HF and chow diet intakes
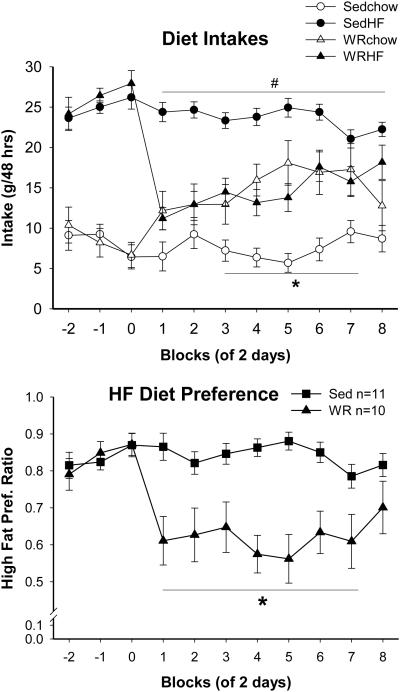
After two weeks of exposure, all rats consumed significantly more of the HF than the chow diet (Fig. 4, top) and the HF diet preference ratio were >0.85 (Fig. 4, bottom). Wheel running significantly increased chow intake and decreased HF diet intake and preference. Two-way (group, block) repeated measures ANOVA revealed significant effects of group [chow vs. HF: F(1,19)=5.5 vs. 19.0, P<0.03 vs. 0.001], block [chow vs. HF: F(10,190)=4.5 vs. 19.1, both P<0.001], and group × block interaction [chow vs. HF: F(10,190)=5.8 vs. 14.0, both P<0.001]. As soon as the wheels were unlocked for the WR group, the HF intake reduced significantly. The reduced HF intake continued throughout the period of running (post hoc P<0.05 for blocks 1-8). The HF intakes were significantly less in the WR than in the Sed group for as long as the WR rats were running (post hoc P<0.05 for blocks 1-8). The chow intake in the WR group increased gradually and became significantly more than baseline level by block 4 (post hoc P<0.003 for blocks 4-7) and more than that of the Sed group by block 3 (post hoc P<0.05 for blocks 3-7). In contrast, the chow and HF intakes in the Sed rats remained constant during the same period. As a result, the HF preference ratios were significantly reduced in the WR group but remain unchanged in the Sed controls. These results are supported by two-way (group, block) repeated measures ANOVA with significant effects of group [F(1,19)=9.8, P<0.006], block [F(10,190)=6.9, P<0.001] and group × block interaction [F(10,190)=8.1, P<0.001]. The HF preference ratios of the WR group were significantly lower than at baseline (post hoc P<0.04, blocks 1-8) and those of the Sed controls in blocks 1-7 (post hoc P<0.009).
Fig. 4.
Intakes of chow and HF diet (top) and HF diet preference (bottom) in Sed and WR rats. All rats were initially sedentary and wheel running access was provided to the WR rats after block 0. Wheel running significantly reduced HF diet and increased chow intakes. *, Sed vs. WR in chow intake (top) or in HF diet preference, P<0.05. #, Sed vs. WR in HF diet intake, P<0.05.
Energy intake, wheel running and body weight
Rats with access to both chow and HF diets (Sed and WR) had significantly higher energy intake per 2 day block than did the Naïve group at baseline (mean=124.9 – 133.8 kcal/block). During this baseline period, total energy intakes did not differ between the Sed (mean=145.7 – 159.7 kcal/block) and WR (mean=152.3 – 167.1 kcal/block) groups. Two-way repeated measures ANOVA comparing energy intakes throughout the experiment indicates significant effects of group [F(2, 31)=17.7, P<0.001], block [F(13, 403)=15.7, P<0.001] and group × block interaction [F(26,403)=11.6, P<0.001]. Immediately after wheels were unlocked, energy intake of WR rats significantly reduced to less than their baseline consumption and was also less than the intake levels in both the Sed and Naïve groups. The energy intakes for the WR group were significantly less than those of the Naïve group for 4 blocks (mean=96.4 – 118.4 kcal/block, post hoc P<0.03). At the end of the experiment, energy intake of the WR (last block=134.8 ± 4.8 kcal) group did not differ from either the Naïve (last block= 124.9 ± 2.7 kcal) or Sed, (last block=143.5 ±4.3 kcal) but the intakes of Sed were significantly more than those of the Naïve ones (post hoc, P<0.001). Finally, because body weigh differed between groups (see below), energy intakes were normalized to body weight and the main effects remain when the normalized intakes were analyzed. Two-way repeated measures ANOVA comparing body weight normalized energy intakes throughout the experiment indicates significant effects of group [F(2, 31)=25.7, P<0.001], block [F(13, 403)=64.7, P<0.001] and group × block interaction [F(26,403)=9.9, P<0.001].
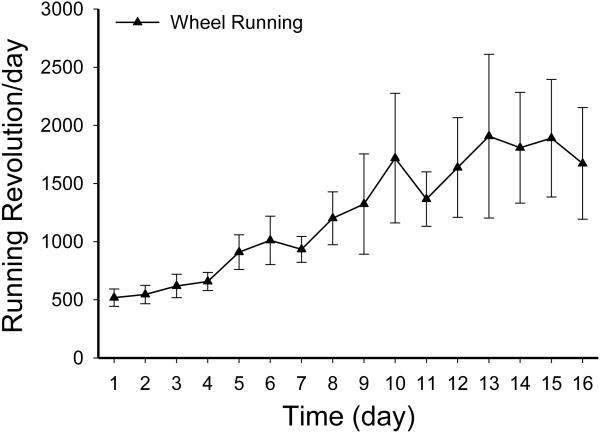
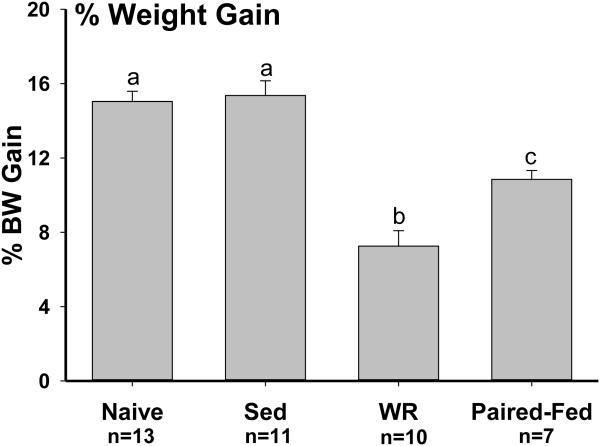
Running activity in the WR rats initially increased over days and then stabilized (Fig. 5). One-way repeated measure ANOVA revealed a significant time effect [F(15, 135)=4.9, P<0.001]. During the 16-day running period, the mean running activity rose from 518.4 ± 74.5 revolutions on day 1 to a peak of 1907.8 ± 704.4 revolutions on day 13. Body weights on the day before wheels were unlocked were significantly different among groups [one-way ANOVA, F(2, 31)=6.1, P<0.006]. At this point, Naïve rats (320.1 ± 2.7 g) with chow access weighted significantly less than the sedentary Sed (330.9 ± 4.1 g) and WR (336.5 ± 3.6 g) rats with access to both the chow and HF diets. Running not only decreased energy intake but also suppressed weight gain. That is, after 16 days running, body weights of the WR rats (361.0 ± 4.7 g) became significantly less than that of the Sed rats (381.9 ± 6.6 g) but were not different from the body weight of Naïve controls [368.3 ± 4.2 g; one-way ANOVA, F(2, 31)=3.9, P<0.04]. The Paired-Fed rats arrived in the laboratory on the same days as the rats in the other groups. However, their experimental procedure began 1 week later, a time at which they weighed more than the rats in the other groups. During the entire experimental period, their intakes were first matched with average intakes of chow and HF diet in the sedentary Sed and WR rats. The last 16 days were matched with the average intakes of the running WR rats. Comparison of the percent weight gain during this 16-day period with one-way ANOVA revealed a significant group effect [F(3,37)=30.8, P<0.001]. That is, during this period percent weight gain from highest to lowest was: Naïve = Sed > Paired-Fed > WR (Fig. 6).
Fig. 5.
Average daily wheel running activity from 10 WR rats in Experiment 2. Rats received simultaneous ad lib access to a standard chow and a HF during this running period (see the description in Section 4).
Fig. 6.
Percent weight gain during the 16 days period when the WR rats run voluntarily. Rats with wheel running access (WR) showed significantly less percent weight gain compared to their sedentary Paired-Fed controls, Naïve with only chow diet and Sed with both chow and HF diet. Different letters indicate significant difference between the two groups (P<0.05).
Gene expression
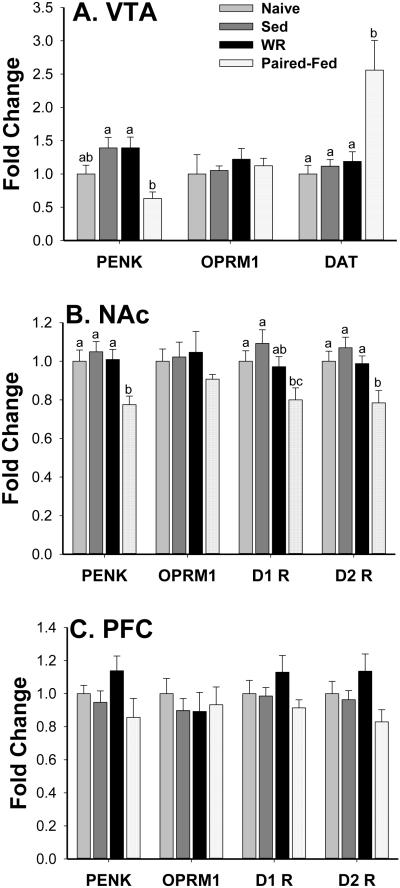
Data on gene expression of PENK, OPRM1 and DAT in the VTA are presented in Fig. 7A. One way ANOVA indicated a significant group effect on PENK expression [F(3,33)=4.9, P<0.007]. There was a trend for mRNA expression in the Sed and WR groups to be similarly elevated relative to that of the Naïve group (for both groups vs. Naïve, post hoc, P=0.06). While PENK gene expression in the Paired-Fed rats did not differ significantly from the Naïve levels, the level was significantly less than those in both the Sed and WR. There were also group differences in the mRNA expression of DAT in the VTA [F(3,30)=11.8, P<0.001]. Levels were similar in Naïve, Sed and WR groups and these levels were significantly lower than those in the Paired-Fed group. The mRNA expression of OPRM1 in the VTA was not affected by the various treatments [F(3, 33)=0.96, P=0.4].
Fig. 7.
Reward relative gene expression profile in the ventral tegmental area (VTA, A), nucleus accumbens (NAc, B) and prefrontal cortex (PFC, C). Data is expressed as relative fold change to the Naïve, chow only controls. The Sed and WR groups had simultaneous ad lib access to chow and HF diet. The Paired-Fed group was sedentary and their chow and HF diet intakes were matched with the WR group. Sed and WR brains had a trend of increased proenkephalin (PENK) gene expression in the VTA (for both groups vs. Naïve, post hoc, P=0.06). The Paired-Fed group showed decreased expression of PENK in the VTA and NAc, decreased dopamine receptor 1 (D1R) and dopamine receptor 2 (D2R) expression in the NAc and increased dopamine transporter (DAT) expression in the VTA. Overall no significant opioid receptor µ1 (OPRM1) expression changes were found. The Different letters indicate significant difference between the two groups (P<0.05).
Data on mRNA expression of PENK, OPRM1, D1R and D2R in the NAc and PFC are presented in Fig. 7B and Fig. 7C, respectively. In the NAc, the mRNA levels of PENK and D2R were significantly lower in the Paired-Fed group than those in the other 3 groups [F(3,36)=3.9 and 4.2, both P<0.02]. Furthermore, the one way ANOVA also revealed a significant group effect for the gene expression of D1R [F(3,36)=3.2, P<0.04]. The mRNA expression of D1R in the Paired-Fed group was similar to that of the WR group but was significantly lower than those of the Naïve and Sed controls. Expression of OPRM1 mRNA did not differ among all four groups [F(3, 36)=0.5, P=0.7]. Finally, in the PFC, one-way ANOVA revealed no significant effect in any of the genes [PENK, OPRM1, D1R and D2R: F(3,36)=2.1, 0.3, 1.1 and 2.1; P=0.12, 0.82, 0.35 and 0.12] measured.
6. Discussion
Wheel running alters HF diet intake and/or preference under different feeding schedules. In Experiment 1, all rats preferred the HF diet to the high carbohydrate diet during intermittent 3-hr diet choice schedule. Lateral ICV infusion of DAMGO significantly increased HF diet intake at the doses of 1 and 3.2 nmol in the sedentary rats. In contrast, the same DAMGO infusions not only failed to increase but actually significantly decreased HF diet intake at the dose of 10 nmol in rats with running wheel access. It is possible, however, that the reduction in HF diet intake in the WR group was caused by an aversive effect of DAMGO at the 10 nmol dose. Nonetheless, these data indicate that running wheel activity can prevent hyperphagia induced by µ-opioid receptor stimulation. The use of a selective µ opioid agonist, such as DAMGO, to assess the intake of a more preferred diet compared with a less preferred diet allowed to determine the role of central µ opioid stimulation on diet intake and preference.
Experiment 2 examined whether wheel running access and the resulting activity alter intake of a preferred HF diet in a continuous ad lib access feeding schedule. Prior to wheel running access, rats developed persistent preferences to the HF diet and consumed significantly more HF than the high carbohydrate chow diet. As soon as wheel running access was allowed, the rats ran and reduced their HF diet intake significantly. Over the 16 days running period, the chow diet intake gradually increased. Consequently, the HF diet was less preferred by the WR rats that resulted in a HF diet preference ratio less than that of the Sed controls. Although Paired-Fed rats consumed the same amount of HF and chow diet as the WR rats, they had reduced expression of PENK in the VTA and NAc and D2R in the NAc but showed increased expression of DAT in the VTA. Intriguingly, the gene expression profiles of the WR and Sed rats were similar; both had elevated PENK expression in the VTA, despite differences in HF and chow diet consumption between the two groups. Overall, the results indicate that running exercise can reduce exogenous opioid agonist stimulated or daily non-stimulated HF diet intakes. Furthermore, running may maintain the gene expression profile of PENK and dopamine (DA) receptors at levels similar to those of rats maintained in sedentary condition without consuming comparably large amount of HF diet – the reward from wheel running activity appears to substitute for the reward derived from consuming a HF diet.
The findings that wheel running activity reduces the consumption of highly palatable foods are consistent with results from previous studies. The reduced intake of palatable foods as a result of wheel running has been demonstrated in different exercise and feeding paradigms as well as in different strains of rats. Daily or alternative-day access to running suppresses intake of a 24% sucrose solution in Sprague-Dawley rats (Satvat and Eikelboom, 2006). In Fisher 344 and Brown Norway hybrid rats, wheel running not only reduces intake/preference to a previously preferred HF diet (Scarpace et al., 2010) but also results in relative anorexia to both a familiar chow and a novel HF diet leading to severe weight loss (Scarpace et al., 2012). Furthermore, treadmill running reduces fat intakes of weight cycling female rats (Gerardo-Gettens et al., 1991). Taken together, previous findings and this current data indicate that, unlike the inconclusive results in human studies (Elder and Roberts, 2007), exercise reduces intake and preference for highly palatable, energy dense food in rats. Data from this study further demonstrate that running suppresses weight gain even with HF diet available. The suppression of weight gain is not simply due to reduced intakes of chow and HF diet in WR rats compared to Sed rats with similar diet availability because sedentary Paired-Fed rats that consumed equal amount of chow and HF diets also gained significantly more weight than rats with wheel running access (Fig. 4). Accordingly, the results of percent weight gain and energy intake among Sed, WR and Paired-Fed groups indicate that running sustains a relative negative energy balance even with ad lib access to chow and HF diets.
The reduced responses to DAMGO stimulated HF diet intake in WR rats do not appear to be a result of general behavioral inhibition produced by DAMGO in wheel running condition. As mentioned above, the opioid system has been shown to modulate the intensity/amount of wheel running (Boer et al., 1990, Sisti and Lewis, 2001) in rodents. Depending on the dosage, the opioid receptor agonist morphine has been shown to have biphasic effects on wheel running activity (Schnur et al., 1983, Schnur et al., 1983). Low dose morphine enhances and high dose decreases wheel running activity (Schnur et al., 1983, Schnur et al., 1983, Sisti and Lewis, 2001). The current result that ICV DAMGO administration reduced daily wheel running activity is consistent with these data and suggests that the alteration of wheel running activity by peripheral opioid agonist is a central effect. Furthermore, in combination with the result that WR rats also had reduced responses to DAMGO stimulated hyperphagia/HF intake, one would suspect that DAMGO administration in the wheel running condition may result in general behavioral inhibition. The answer to this question is complicated by the fact that the ICV injections themselves may affect wheel running activity and the effects of ICV injections on running may vary dependent on the activity level. At low running activity (<1000 revolution/day), saline injection did not alter running activity. As the running activity increased to above 1500 revolution/day, both saline and DAMGO injections suppressed running (Fig. 3). While a general behavioral inhibition may be excluded by the fact that running activity during the 3-hr intake test was not changed by DAMGO treatment, a conclusion cannot be made due to the low numbers of WR animals (n=4) with verified ICV DAMGO injection. The low subject numbers and lack of a running group without HF diet competition are limitations of Experiment 1.
This is one of the very few studies (Scarpace et al., 2010, Scarpace et al., 2012) that monitored daily, ad libitum intakes and choices between a standard high carbohydrate and a 60% HF diet throughout a period of running wheel access. Sedentary rats exposed to simultaneous access to the high carbohydrate and HF diets highly preferred the HF diet during the first 2 wks of exposure i.e. HF diet preference ratio > 0.8. After two weeks of exposure, HF diet preference ratio may fluctuate but in general the ratio remained between 0.7-0.8 (Fig. 3). Experiment 2 demonstrated that wheel running decreased preference to a previously preferred HF diet in male rats. Overall, we demonstrated that chronic wheel running in rats produces a decreased HF diet preference and the running rats maintain significantly lower body weight as compared to sedentary rats with the same simultaneous access to the high carbohydrate and HF diets. The effects of exercise in human subjects are variable with some subjects responding to exercise and others not. Responders show significant reductions in energy intake and body weight whereas the non-responders increase energy intake with little weight loss (Hopkins et al., 2011). Furthermore, our results support the concept that wheel running is rewarding in rodents (Greenwood et al., 2011, Meijer and Robbers, 2014) and suggest that the rewarding property of wheel running may contribute to the decreased HF diet preference. In humans, however, only about half of the studies recording fat intake reported decreased fat intake in subjects with higher levels of physical activity and the rest studies failed to show any relationship (Donnelly et al., 2014). Given that the rewarding effects of exercise may be an important factor, one future approach for human studies may be to determine whether the responders and non-responders have differences in diet choice and in perceived reward during exercise.
To our knowledge, this is the first study to examine how wheel running affects opioid-stimulated HF diet intake (Experiment 1). This is also the first study to assess changes in dopaminergic and opioidergic gene expression after simultaneous free access to wheel running and HF diet (Experiment 2). Both the dopaminergic and opioidergic systems underlie highly palatable food intake. As mentioned above, administration of opioid analogs in the brain increases intakes of foods and particularly those high in fat content (Zhang and Kelley, 2000, Gosnell and Levine, 2009). Compared to controls, rats that are prone to overconsume HF diet and diet-induced-obesity (DIO) have higher expression of PENK mRNA in multiple forebrain regions including the NAc (Chang et al., 2010). DAT function, D1R and D2R expression decrease after prolonged HF diet consumption and DIO in rats (Narayanaswami et al., 2013). In this study, although a significant change was not found in the NAc, both the Sed and WR rats with a history of HF diet consumption showed a trend toward increased PENK mRNA expression in the VTA. Dopaminergic mRNA expression in Sed and WR rats with 4 wks ad lib access to HF diet did not differ from that of chow Naïve controls. While not consistent with previous reports mentioned above, these results are consistent with the unchanged dopaminergic gene expression after short term (days – 2 wks) and prolonged (> 6 wks) HF diet exposure reported in other studies (South et al., 2012, Cone et al., 2013, Martire et al., 2014). Differences in types of HF diets, feeding schedule and brain collection protocol may contribute to differences in gene expression changes among the current and previous studies.
The results of this study also suggest that altered behavioral responses to exogenous opioid stimulation may not be accompanied by changes in mRNA expression in the mesolimbic opioidergic system. Although the running and feeding schedules between Experiment 1 and 2 were not identical, the timing of DAMGO test and gene expression measurement both occurred after 2 wks of running. While Experiment 2 showed that PENK and OPRM1 mRNA expression in WR brains did not differ from Naïve or Sed brains, Experiment 1 revealed that the ability for DAMGO to increase HF diet intake were compromised in WR rats. Similar phenomenon of altered behavioral responses to exogenous agents without changes in endogenous mRNA expression has also been reported in the dopaminergic system (Cone et al., 2013). Furthermore, previous studies have demonstrated that wheel running can reduce the rewarding and analgesic effects of morphine (Kanarek et al., 1998, Mathes and Kanarek, 2001, Lett et al., 2002). In combination with the current result of decreased responses to DAMGO-stimulated HF intake after wheel running, these data support the notion that cross-tolerance develops between exogenous opioid drugs and the endogenous opioid released during wheel running (Mathes and Kanarek, 2001).
Perhaps the potential mechanisms by which wheel running reduces HF diet intake/preference are better understood by examining the gene expression results of the brain from Paired-Fed rats in Experiment 2. The data of Paired-Fed rats would indicate the effects of restricting intake of a previous abundant HF diet because the HF diet intake was matched with the WR rats and was significantly reduced for the two weeks period before the brains were collected. This two-week restriction resulted in increased gene expression of DAT in the VTA and decreased expression of PENK and D2R in the NAc. Such a gene expression profile is consistent with previous reports of the same genes in respective brain regions after restricted consumption of palatable HF, high sugar foods (Bello et al., 2003, Kelley et al., 2003, Alsio et al., 2010). These changes in gene expression appear to be neural adaptive responses to limited availability of food rewards. The differences in mesolimbic dopaminergic and opioidergic gene expression profiles between WR and Paired-Fed rats that had the same chow and HF intake indicate the neural effects of wheel running. The expression profile of WR brains was actually similar to that of Sed brains that experienced more HF diet. Together with prior reports (Greenwood et al., 2011, Meijer and Robbers, 2014), these data suggest that wheel running produces rewarding effects and furthermore, such effects may substitute for food reward.
The suggestion that wheel running may substitute for food reward does not necessarily imply a correlation between the levels of running activity and the degree of HF diet intake suppression i.e. the more the running activity the less the HF diet intake. Under ad libitum feeding and free running access conditions, physiological mechanisms to maintain balance between energy intake and expenditure play important roles in food intake and weight maintenance. Rats reduce food intake when initially provided with wheel running access. As the running access continues, running activity levels increase and food intakes also increase to maintain body weight i.e. no further weight loss. These phenomena are shown in the current study as well as previous studies (Tokuyama et al., 1982, Bi et al., 2005, Chao et al., 2011). The results in Experiment 2 that HF diet intake increased toward the end of the experiment were not unpredicted because intakes of both the high carbohydrate and HF diets increased (Fig. 4) as energy expenditure increased with increased running (Fig. 5) and the HF diet preference ratio in the WR rats was maintained lower than that of the Sed rats. In Experiment 2, the Sed rats with access to both the high carbohydrate and HF diets were housed in the same wheel running cages as were the WR rats. The difference was that the wheels were locked for the Sed rats. Compared to the Naïve rats housed in cages without wheels, the Sed rats were housed in an enriched environment and they were often seen climbing the wheels. These Sed rats, however, did not show significant reduction in HF diet preference. Taken together, it does not appear that the changes in HF diet preference in the WR rats were due to the existence of the running wheel that may provide the opportunity for a competing behavior e.g. eating or interacting with the wheel. Nonetheless, the interpretation of the results in Experiment 2 is limited by not including Sed rats with two-diet choice and without locked wheels and WR rats without access to the HF diet. Because our goal was to assess the impact of physical activity on diet preference, we did not investigate the independent (i.e., under non-competing conditions) effects of DAMGO on wheel running or diet preference. Thus, the suppressive effects of DAMGO could have been influences by the conditions of our paradigm. In a similar fashion, the gene expression profile could represent a response to other aspects of our paradigm, such as response to diet novelty, and scheduling and entrainment consequences. Future experiments will be designed to account for these possible influences.
Highlights.
Wheel running decreases high-fat diet intake after DAMGO injection.
Wheel running decreases preference for previously preferred high-fat diet.
Wheel running suppresses weight gain during high-fat diet exposure.
Exercised and sedentary rats had similar reward gene expression profile.
Acknowledgment
The authors thank Mr. Kevin Gormley at the NIDA Drug Supply Program for providing DAMGO and Dr. Bo Sun and Miss Jenny Albertz and Mr. Ryan Purcell for assisting with animal care and tissue collection. This research was supported by the Klarman Family Foundation and NIH DK-19302.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsio J, Olszewski PK, Norback AH, Gunnarsson ZE, Levine AS, Pickering C, Schioth HB. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171(3):779–787. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265(3):1253–1260. [PubMed] [Google Scholar]
- Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 1997;67(3):337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1260–1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012;71(4):478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146(4):1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- Boer DP, Epling WF, Pierce WD, Russell JC. Suppression of food deprivation-induced high-rate wheel running in rats. Physiol Behav. 1990;48(2):339–342. doi: 10.1016/0031-9384(90)90324-w. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Barf RP, Benthem L, van Dijk G, Scheurink AJ. Forced and voluntary exercise counteract insulin resistance in rats: the role of coping style. Horm Behav. 2012;62(1):93–98. doi: 10.1016/j.yhbeh.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Bray GA, Flatt JP, Volaufova J, Delany JP, Champagne CM. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr. 2008;88(6):1504–1510. doi: 10.3945/ajcn.2008.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadeddu C, Nocco S, Lucia C, Deidda M, Bina A, Fabio O, Bandinu S, Cossu E, Baroni MG, Mercuro G. Effects of metformin and exercise training, alone or in association, on cardiopulmonary performance and quality of life in insulin resistance patients. Cardiovasc Diabetol. 2014;13:93. doi: 10.1186/1475-2840-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Barson JR, Chang SY, Leibowitz SF. Increased enkephalin in brain of rats prone to overconsuming a fat-rich diet. Physiol Behav. 2010;101(3):360–369. doi: 10.1016/j.physbeh.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PT, Terrillion CE, Moran TH, Bi S. High-fat diet offsets the long-lasting effects of running-wheel access on food intake and body weight in OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1459–1467. doi: 10.1152/ajpregu.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One. 2013;8(3):e58251. doi: 10.1371/journal.pone.0058251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MP, Oliveira A, Pazini FL, Machado DG, Bettio LE, Budni J, Aguiar AS, Jr., Martins DF, Santos AR, Rodrigues AL. The antidepressant-like effect of physical activity on a voluntary running wheel. Med Sci Sports Exerc. 2013;45(5):851–859. doi: 10.1249/MSS.0b013e31827b23e6. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS One. 2014;9(1):e83498. doi: 10.1371/journal.pone.0083498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edholm OG, Fletcher JG, Widdowson EM, McCance RA. The energy expenditure and food intake of individual men. Br J Nutr. 1955;9(3):286–300. doi: 10.1079/bjn19550040. [DOI] [PubMed] [Google Scholar]
- Elder SJ, Roberts SB. The effects of exercise on food intake and body fatness: a summary of published studies. Nutr Rev. 2007;65(1):1–19. doi: 10.1111/j.1753-4887.2007.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Gerardo-Gettens T, Miller GD, Horwitz BA, McDonald RB, Brownell KD, Greenwood MR, Rodin J, Stern JS. Exercise decreases fat selection in female rats during weight cycling. Am J Physiol. 1991;260(3):R518–524. doi: 10.1152/ajpregu.1991.260.3.R518. Pt 2. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes (Lond) 2009;33(Suppl 2):S54–58. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins M, Jeukendrup A, King NA, Blundell JE. The relationship between substrate metabolism, exercise and appetite control: does glycogen availability influence the motivation to eat, energy intake or food choice? Sports Med. 2011;41(6):507–521. doi: 10.2165/11588780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D'Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1998;61(1):19–27. doi: 10.1016/s0091-3057(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18(9):2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Klinker F, Hasan K, Paulus W, Nitsche MA, Liebetanz D. Pharmacological blockade and genetic absence of the dopamine D2 receptor specifically modulate voluntary locomotor activity in mice. Behav Brain Res. 2013;242:117–124. doi: 10.1016/j.bbr.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Hamilton AT, Lightfoot JT. Pharmacological manipulation of the dopaminergic system affects wheel-running activity in differentially active mice. J Biol Regul Homeost Agents. 2012;26(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72(3):355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72(1-2):101–105. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- Martire SI, Maniam J, South T, Holmes N, Westbrook RF, Morris MJ. Extended exposure to a palatable cafeteria diet alters gene expression in brain regions implicated in reward, and withdrawal from this diet alters gene expression in brain regions associated with stress. Behav Brain Res. 2014;265:132–141. doi: 10.1016/j.bbr.2014.02.027. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol Behav. 2001;74(1-2):245–251. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci. 2014;281(1786) doi: 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond) 2013;37(8):1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden LG, Stroebele N, Wyatt HR, Catenacci VA, Peters JC, Stuht J, Wing RR, Hill JO. Cluster analysis of the national weight control registry to identify distinct subgroups maintaining successful weight loss. Obesity (Silver Spring) 2012;20(10):2039–2047. doi: 10.1038/oby.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Booth FW, Lee S, Laye MJ, Zhang C. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol. 2012;590:4255–4268. doi: 10.1113/jphysiol.2012.234856. Pt 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. Academic Press; 2007. [Google Scholar]
- Rasmussen EB, Hillman C. Naloxone and rimonabant reduce the reinforcing properties of exercise in rats. Exp Clin Psychopharmacol. 2011;19(6):389–400. doi: 10.1037/a0024142. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol Behav. 2012;105(3):661–668. doi: 10.1016/j.physbeh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Satvat E, Eikelboom R. Dissociation of conditioned and unconditioned factors in the running-induced feeding suppression. Physiol Behav. 2006;89(3):428–437. doi: 10.1016/j.physbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Scarpace ET, Matheny M, Strehler KY, Shapiro A, Cheng KY, Tumer N, Scarpace PJ. Simultaneous introduction of a novel high fat diet and wheel running induces anorexia. Physiol Behav. 2012;105(4):909–914. doi: 10.1016/j.physbeh.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Zhang Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiol Behav. 2010;100(2):173–179. doi: 10.1016/j.physbeh.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur P, Bravo F, Trujillo M. Tolerance and sensitization to the biphasic effects of low doses of morphine in the hamster. Pharmacol Biochem Behav. 1983;19(3):435–439. doi: 10.1016/0091-3057(83)90116-8. [DOI] [PubMed] [Google Scholar]
- Schnur P, Bravo F, Trujillo M, Rocha S. Biphasic effects of morphine on locomotor activity in hamsters. Pharmacol Biochem Behav. 1983;18(3):357–361. doi: 10.1016/0091-3057(83)90454-9. [DOI] [PubMed] [Google Scholar]
- Sisti HM, Lewis MJ. Naloxone suppression and morphine enhancement of voluntary wheel-running activity in rats. Pharmacol Biochem Behav. 2001;70(2-3):359–365. doi: 10.1016/s0091-3057(01)00624-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Wheel running decreases the positive reinforcing effects of heroin. Pharmacol Rep. 2012;64(4):960–964. doi: 10.1016/s1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98(1-2):129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T, Westbrook F, Morris MJ. Neurological and stress related effects of shifting obese rats from a palatable diet to chow and lean rats from chow to a palatable diet. Physiol Behav. 2012;105(4):1052–1057. doi: 10.1016/j.physbeh.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–447. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama K, Saito M, Okuda H. Effects of wheel running on food intake and weight gain of male and female rats. Physiol Behav. 1982;28(5):899–903. doi: 10.1016/0031-9384(82)90211-6. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Sellings LH, Paredes RG, Prado-Alcala RA, Diaz JL. Reinforcement of wheel running in BALB/c mice: role of motor activity and endogenous opioids. J Mot Behav. 2008;40(6):587–593. doi: 10.3200/JMBR.40.6.587-593. [DOI] [PubMed] [Google Scholar]
- Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of Exercise on Anxiety and Depression Disorders: Review of Meta-Analyses and Neurobiological Mechanisms. CNS Neurol Disord Drug Targets. 2014 doi: 10.2174/1871527313666140612102841. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. Agonist-selective signaling of G protein-coupled receptor: mechanisms and implications. IUBMB Life. 2010;62(2):112–119. doi: 10.1002/iub.293. [DOI] [PMC free article] [PubMed] [Google Scholar]