Abstract
Background
Several studies suggested that vaccines could have non-specific effects on mortality depending on the type of vaccine. Non-specific effects seem to be different in boys and girls. In this study we want to investigate whether there are differences in gender-specific mortality among Dutch children according to the last vaccination received. We tested the hypothesis that the mortality rate ratio for girls versus boys is more favourable for girls following MMR ± MenC vaccination (from 14 months of age) compared with the ratio following DTP-IPV vaccination (2–13 months of age). Secondarily, we investigated whether there were gender-specific changes in mortality following booster vaccination at 4 years of age.
Methods
This observational study included all Dutch children aged 0–11 years from 2000 until 2011. Age groups were classified according to the last vaccination offered. The mortality rates for all natural causes of death were calculated by gender and age group. Incidence rate ratios (IRRs) were computed using a multivariable Poisson analysis to compare mortality in boys and girls across different age groups.
Results
The study population consisted of 6,261,472 children. During the study period, 14,038 children (0.22%) died, 91% of which were attributed to a known natural cause of death. The mortality rate for natural causes was higher among boys than girls in all age groups. Adjusted IRRs for girls compared with boys ranged between 0.81 (95% CI 0.74-0.89) and 0.91 (95% CI 0.77-1.07) over the age groups. The IRR did not significantly differ between all vaccine-related age groups (p = 0.723), between children 2–13 months (following DTP-IPV vaccination) and 14 months - 3 years (following MMR ± MenC vaccination) (p = 0.493) and between children 14 months - 3 years and 4–8 years old (following DTP-IPV vaccination) (p = 0.868).
Conclusions
In the Netherlands, a high income country, no differences in gender-specific mortality related to the type of last vaccination received were observed in DTP-IPV- and MMR ± MenC eligible age groups. The inability to detect this effect indicates that when non-specific effects were present the effects were not reflected in changes in the differences in mortality between boys and girls. The findings in this large population-based study are reassuring for the continued trust in the safety of the national vaccination programme.
Keywords: Vaccination, DTP-IPV, MMR, MenC, Mortality, Sex-differential
Background
The Dutch National Immunisation Programme (NIP) has contributed greatly to reduced mortality among infants and (young) children. Since its introduction in 1957, the number of deaths due to diseases targeted by the NIP has drastically declined [1]. Notwithstanding this important contribution of vaccinations, several studies in recent decades have suggested that vaccinations could also give non-specific effects, i.e. effects on non-targeted diseases, on mortality, with differential effects in boys and girls [2-17]. It is therefore of great public health relevance to explore the overall impact of vaccination on mortality.
Studies in countries with high childhood mortality observed that live attenuated vaccines, such as the measles vaccine and BCG-vaccine, might reduce mortality far more than can be attributed to the target disease [2,3,7,8,10,12-16]. In contrast, it has been suggested that inactivated vaccines, such as DTP, can increase the mortality from infections other than the target disease [2-8,10,11,13-17]. Such non-specific effects can be significant, with changes in all-cause mortality of more than fifty percent. Both positive and negative effects were stronger in girls than in boys [2,3,5,6,9,10,13-17]. Several mechanisms driving potential sex-differential effects of vaccines have been suggested, including differences in adaptive and innate immunity [2,18].
In the Netherlands, no research has been performed to date on possible non-specific effects of vaccination on mortality. However, such potential effects of vaccinations need to be explored proactively for public health purposes to guide vaccination policy, including ensuring accurate information to the public and maintaining public trust in public health interventions such as vaccinations. To assess whether there are such non-specific effects of vaccines, mortality in vaccinated and unvaccinated children should be compared, ideally in a randomised controlled trial. However, the use of randomised controlled trials is not feasible or ethical in this case, as the direct benefits of vaccination have been demonstrated very convincingly. In the Netherlands, vaccination coverage is high, and childhood mortality is low. Therefore, in this study, we retrospectively investigated whether there were indications for differences in gender-specific mortality among children with respect to the ages of last vaccination offered. We hypothesized that the mortality rate ratio for girls versus boys is more favourable for girls following MMR ± MenC vaccination (after 14 months of age) compared with the ratio after DTP-IPV vaccination (2–14 months of age). Secondary we investigated whether there are gender-specific changes in mortality following booster vaccination at 4 years of age.
Methods
Study design and population
For this observational study, a dynamic cohort was used that included all Dutch children who were 0-11-years-old in the years 2000 to 2011. The date of study entry was 01/01/2000 or the date of birth, whatever was last. The end date of follow up was 31/12/2011, the date of 12th birthday or the date of death, whatever came first. The data were available from Statistics Netherlands, who linked demographic data of persons included in the Municipal Personal Records Database (GBA) with causes of death derived from Statistics Netherlands.
Dutch national immunisation programme
The Dutch NIP was started in 1957 with childhood vaccinations against diphtheria, tetanus, pertussis and poliomyelitis. The programme has been regularly updated and modified ever since its inception. The current programme contains childhood vaccinations against diphtheria, tetanus, pertussis, polio, Haemophilus influenzae serotype b, mumps, measles, rubella, meningococcal serogroup C (MenC), hepatitis B, pneumococcal disease and human papillomavirus (Table 1). Table 2 presents the main changes in the childhood NIP programme in the period 1988 to 2011. Some of the important changes are the introduction of meningococcal C vaccination in 2002 at 14 months of age (i.e., administered together with the MMR vaccine) with in addition a catch-up campaign up to 19 years of age, the expansion of the DTP-IPV vaccine with Haemophilus infuenzae type b and hepatitis b components in 2003 and 2011, respectively, and the introduction of pneumococcal vaccination in 2006 at 2, 3, 4 and 11 months of age (simultaneous with DTP-IPV/Hib).
Table 1.
Vaccination scheme and coverage of the Dutch NIP in 2011
| Age | Vaccination 1 | Coverage vaccine 1 (%) | Vaccination 2 | Coverage vaccine 2 (%) |
|---|---|---|---|---|
| 2 months | DTaP-HBV-IPV/Hib | Pneumo | ||
| 3 months | DTaP-HBV-IPV/Hib | Pneumo | ||
| 4 months | DTaP-HBV-IPV/Hib | Pneumo | ||
| 11 months | DTaP-HBV-IPV/Hib | 95.4/96.0 | Pneumo | 94.8 |
| 14 months | MMR | 95.9 | MenC | 95.9 |
| 4 years | DTaP-IPV | 92.0 | ||
| 9 years | DT-IPV | 92.2 | MMR | 92.1 |
| 12 years | HPV (3 doses)* | 52.5 |
*Only for girls; three doses on 0 days, 1 month and 6 months.
Table 2.
Major changes in the Dutch NIP from 1988 to 2011
| Month/Year | Change |
|---|---|
| July 1993 | Introduction of vaccination against Haemophilus influenzae type b (Hib) disease for all children born on or after 1st April 1993 |
| January 1999 | Advanced age for start of primary vaccinations from 3 to 2 months |
| July 2001 | Acellular pertussis vaccine added at 4 years of age for all children born on or after 1st January 1998 |
| September 2002 | Meningococcal serogroup C vaccine added at 14 months of age for all children born on or after 1st June 2001 and a catch-up campaign for everyone up to the age of 19 years |
| March 2003 | Hepatitis B vaccination added for infants in specified risk groups at 2, 4, and 11 months of age for all children born on or after 1st January 2003 |
| Haemophilus influenza serotype b vaccine given with the DTwP-IPV vaccine at 2, 3, 4, and 11 months of age for all children born on or after 1st April 2002 | |
| January 2005 | Whole cell pertussis component of the DTwP-IPV vaccine was replaced by the acellular pertussis component at 2, 3, 4, and 11 months of age for all children born after the 1st February 2004 |
| January 2006 | Hepatitis B vaccination added at birth for children of mothers who tested positive for HBsAg |
| June 2006 | Pneumococcal vaccination added at 2, 3, 4, and 11 months of age for children born on or after 1st April 2006 |
| Introduction of combined vaccine DTaP-HBV-IPV/Hib at 2, 3, 4, and 11 months of age for children in specified risk groups born on or after 1st April 2006 | |
| July/August 2006 | Transition to combined DTaP-IPV vaccine for children at 4 years of age born from July/August 2002 onwards |
| January 2008 | Hepatitis B vaccination added for children with Down syndrome born on or after 1st January 2008 |
| March 2009 | Human papillomavirus (HPV) catch-up campaign for girls born 1993-1996 |
| January 2010 | HPV vaccination for 12-year-old girls born on or after 1st January 1997 |
| March 2011 | The 7-valent pneumococcal vaccine was replaced by the 10-valent pneumococcal vaccine for children born on or after 1st March 2011 |
| August 2011 | Hepatitis B vaccination introduced for all children born on or after 1st August 2011 |
For many years, the childhood vaccination coverage has been high (92-96%; measured at the age of 2 years for newborns, at the age of 5 years for toddlers and at the age of 10 years for schoolchildren [19]) for all target diseases (Table 1), except for HPV, resulting in an highly effective NIP [1]. Therefore, in this study, we assumed all children to be vaccinated according to the Dutch schedule. In addition to the NIP, children with parents originating from tuberculosis endemic countries are invited for vaccination with Bacillus Calmette-Guérin (BCG) vaccine.
Absolute contraindications for vaccination are a proven serious allergy for one of the components of the vaccine or a proven serious allergic reaction following the administration of the same vaccine. Relative contraindications, in which the administration of the vaccine should be considered, include cases of fever (≥38.5 C) and serious immune disorders (disease, treatment with corticosteroids or cytostatics, radiation), following the administration of blood products or immunoglobulins, and patients with an increased tendency toward clotting or bleeding, pregnancy, or planned anaesthesia. However, these cases are quite rare, i.e., 5–1000 cases per year.
Analysis
The following age groups were created: 0–1 months (i.e., unvaccinated), 2–13 months (i.e., following DT(a)P(−HBV)-IPV(/Hib) (and pneumococcal) vaccination), 14 months to 3 years (i.e., following MMR (and meningococcal C) vaccination (Figure 1)), 4–8 years (i.e., following DT(aP)-IPV booster vaccination) and 9–11 years (i.e., following DT-IPV booster and MMR booster vaccination).
Figure 1.
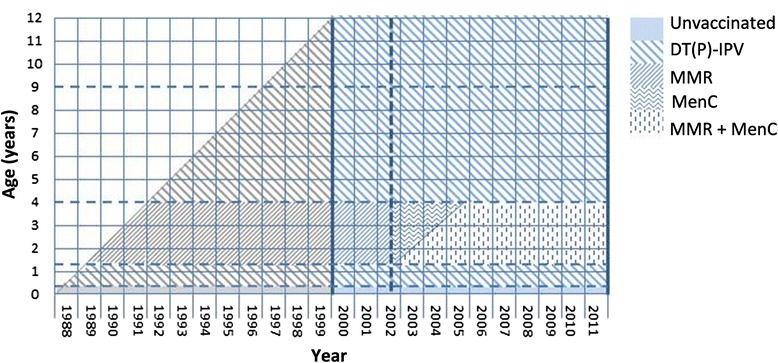
Lexis diagram of the analysed cohort. Diagram that represents the type of last vaccination received for the analysed cohort by age and year. The bold vertical lines represent the study period and the dashed vertical line represents the introduction of Meningococcal C vaccination. All changes in the vaccination schedule are presented in Table 2.
Mortality rates and 95% confidence intervals for all natural causes of death, which was defined as all causes of death other than unknown and external causes of injury and poisoning, were calculated by gender and by age group. Children who died from unknown causes or external causes of injury and poisoning were censored in the analyses. Crude monthly incidence rate ratios (IRR) for girls compared to boys were visualised. IRR, with accompanying 95% confidence intervals, by age group for girls compared with boys were computed using multivariable Poisson regression adjusted for calendar year. To assess whether the IRR differed between age groups, the interaction between age group and gender was tested (degrees of freedom (df) = 4). Furthermore, we tested this interaction for both hypotheses specifically (df = 1), i.e. for 14 months – 3 years of age versus 2–13 months of age and for 4–8 years of age versus 14 months – 3 years of age. In addition, we conducted several sensitivity analyses: 1) including only the indigenous Dutch population, as defined by Statistics Netherlands as children of whom both parents were born in the Netherlands; 2) comparing the periods before and from September 2002 onwards, i.e. before and after the introduction of vaccination against meningococcal C at 14 months of age; 3) including only natural causes of death other than complications of pregnancy, childbirth and the puerperium, conditions originating in the perinatal period and congenital abnormalities; 4) including only infectious and parasitic diseases.
Ethical statement
The data were made available by Statistics Netherlands. The provided data was fully anonymised. Due to this, approval of an ethics committee was not required and therefore not obtained.
Results
Descriptive statistics
The total study population consisted of 6,261,472 individual children, accounting for 30,023,460 person-years. During the study period, 14,038 children (0.22%; mortality rate 4.7/10,000 person years) died. For approximately 9% of the deceased children, an unknown or non-natural cause of death was recorded (Table 3), and these children were censored in the analysis.
Table 3.
Characteristics of the study population
| Characteristic | Boys | Girls | Total |
|---|---|---|---|
| n (%) | n (%) | N (%) | |
| Population 0–11 yr | 3,188,004 | 3,073,468 | 6,261,472 |
| Indigenous Dutch | 2,292,021 (71.9) | 2,189,282 (71.2) | 4,481,303 (71.6) |
| 1st generation immigrant | 306,934 (9.6) | 322,167 (10.5) | 629,101 (10.0) |
| 2nd generation immigrant | 589,049 (18.5) | 562,019 (18.3) | 1,151,068 (18.4) |
| Deaths | 7,924 | 6,114 | 14,038 |
| 0-1 monthsa | 4,713 (59.9) | 3,688 (60.3) | 8,401 (59.8) |
| 2-13 monthsb | 1,143 (14.4) | 870 (14.2) | 2,013 (14.3) |
| 14 months – 3 yearsc | 902 (11.4) | 671 (11.0) | 1,573 (11.2) |
| 4-8 yearsd | 766 (9.7) | 557 (9.1) | 1,323 (9.4) |
| 9-11 yearse | 400 (5.0) | 328 (5.4) | 728 (5.2) |
| Primary cause of death | |||
| Unknown | 128 (1.6) | 122 (2.0) | 250 (1.8) |
| Infectious and parasitic diseases | 203 (2.6) | 171 (2.8) | 374 (2.7) |
| Neoplasms | 512 (6.5) | 393 (6.4) | 905 (6.4) |
| Diseases of the blood and blood forming organs and certain disorders involving the immune mechanism | 53 (0.7) | 46 (0.8) | 99 (0.7) |
| Endocrine, nutritional and metabolic diseases | 196 (2.5) | 148 (2.4) | 344 (2.5) |
| Mental and behavioural disorders | 23 (0.3) | 19 (0.3) | 42 (0.3) |
| Diseases of the nervous system | 383 (4.8) | 337 (5.5) | 720 (5.1) |
| Diseases of the circulatory system | 176 (2.2) | 134 (2.2) | 310 (2.2) |
| Diseases of the respiratory system | 162 (2.0) | 125 (2.0) | 287 (2.0) |
| Diseases of the digestive system and the genitourinary system | 99 (1.2) | 61 (1.0) | 160 (1.1) |
| Diseases of the skin, subcutaneous tissue, musculoskeletal system and connective tissue | 13 (0.2) | 17 (0.3) | 30 (0.2) |
| Complications of the pregnancy, childbirth and the puerperium and conditions originating in the perinatal period | 2,973 (37.5) | 2,192 (35.9) | 5,165 (36.8) |
| Congenital anomalies | 1,926 (24.3) | 1,630 (26.7) | 3,556 (25.3) |
| Symptoms, signs and ill-defined conditions | 426 (5.4) | 332 (5.4) | 758 (5.4) |
| External causes of injury and poisoning | 651 (8.2) | 387 (6.3) | 1,038 (7.4) |
aMost recent vaccination: none.
bMost recent vaccinations: DTaP(−HBV)-IPV/Hib and pneumococcal vaccination.
cMost recent vaccinations: MMR and meningococcal C vaccination (only after September 2002).
dMost recent vaccination: DTaP-IPV booster vaccination.
eMost recent vaccinations: DT-IPV booster and MMR booster vaccination.
Mortality rates
The mortality rate due to natural causes was highest for children 0 to 1 months old. This high mortality rate was mainly caused by the relatively high mortality during the first day after birth, which accounted for 25% of all childhood mortality occurring in the first month of life. In all age groups, the mortality rate was higher among boys than girls (Table 4). Mortality rates declined over the years from 2000 to 2011 (for boys 4.0 in 2000 to 2.8 in 2011; for girls 3.4 in 2000 to 2.1 in 2011).
Table 4.
Crude mortality rates due to natural causes in children with respect to gender and age group, from 2000–2011, and adjusted incidence rate ratios (IRR) for girls compared with boys
| Age group | Boys | Boys | Girls | Girls | IRR a | p-value |
|---|---|---|---|---|---|---|
| Number of deaths | Mortality rate/10,000 person | Number of deaths | Mortality rate/10,000 person | (95% CI) | ||
| (N = 7145) | Years (95% CI) | (N = 5605) | Years (95% CI) | |||
| 0-1 months | 4578 | 32.1 (31.2-33.1) | 3555 | 26.2 (25.3-27.0) | 0.81 (0.78-0.85) | |
| 2-13 months | 1035 | 4.6 (4.3-4.8) | 797 | 3.7 (3.4-3.9) | 0.81 (0.74-0.89) | |
| 14 months – 3 years | 680 | 1.4 (1.3-1.5) | 551 | 1.2 (1.1-1.3) | 0.85 (0.76-0.95) | 0.493b |
| 4-8 years | 552 | 0.7 (0.7-0.8) | 441 | 0.6 (0.5-0.7) | 0.84 (0.74-0.95) | 0.868c |
| 9-11 years | 300 | 0.6 (0.5-0.6) | 261 | 0.5 (0.5-0.6) | 0.91 (0.77-1.07) | |
| 0.723d |
aAdjusted for calendar year.
bp-value for interaction (df = 1) between age group and gender for 14 months – 3 years of age versus 2–14 months of age.
cp-value for interaction (df = 1) between age group and gender for 4–8 years of age versus 14 months – 3 years of age.
dp-value for interaction (df = 4) between age group and gender for all age groups.
The plot of female-to-male IRR shows that IRR fluctuated over age in months, however, intervals were overlapping (Figure 2). IRRs fluctuated over the years, especially for children 9–11 years of age (data not shown). The adjusted IRRs for girls compared with boys ranged between 0.81 (95% CI 0.74-0.89) and 0.91 (95% CI 0.77-1.07) in the age groups (Table 4). In particular, the IRR for children 2 to 13 months-old was 0.81 (95% CI 0.74-0.89) and for children 14 months-old to 3 years-old the IRR was 0.85 (95% CI 0.76-0.95). Importantly, no statistically significant differences in IRRs were observed between all age groups (p = 0.723), between children 2–13 months and 14 months - 3 years (p = 0.493) and between children 14 months - 3 years and 4–8 years old (p = 0.868). In sensitivity analyses for indigenous Dutch children only, for periods before and from September 2002 onwards, for natural causes of death other than complications of pregnancy, childbirth, perinatal period and congenital abnormalities, and for infectious and parasitic diseases only, no statistically significant differences in IRR between vaccine-related age groups were found (Table 5).
Figure 2.
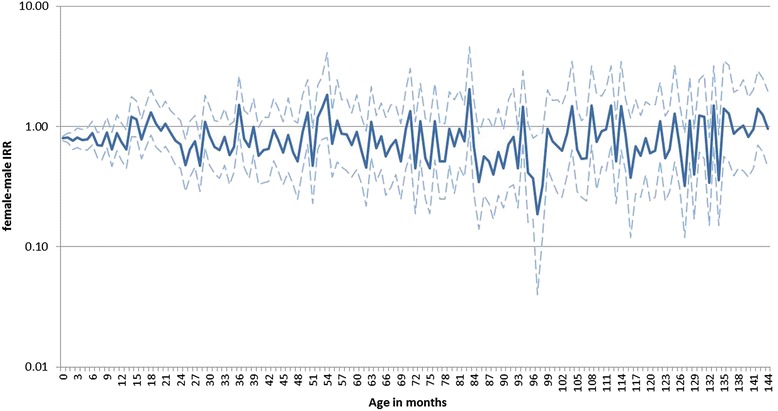
Female-to-male incidence rate ratios up to 12 years of age. Plot of crude incidence rate ratios (IRR), including 95% confidence intervals, for girls compared to boys for mortality due to natural causes, from 2000–2011, by month of age up to 12 years.
Table 5.
Incidence rate ratios (IRR) for girls compared with boys for indigenous Dutch children only, for the periods before and from September 2002 onwards, and for natural causes of death other than complications of pregnancy, childbirth, perinatal period and congenital abnormalities
| Age group | IRR a | p-value | IRR a | p-value | IRR a | p-value | IRR a | p-value | IRR a | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) for natural causes for indigenous Dutch children only (N deaths = 9,328) | (95% CI) for natural causes before September 2002 (N deaths = 3,529) | (95% CI) for natural causes from September 2002 onwards (N deaths = 9,221) | (95% CI) for natural causes other than complications of pregnancy, childbirth, perinatal period and congenital abnormalities (N deaths = 4,029) | (95% CI) for infectious and parasitic diseases (N deaths = 374) | ||||||
| 0-1 months | 0.80 (0.76-0.84) | 0.81 (0.74-0.88) | 0.82 (0.78-0.86) | 0.97 (0.82-1.15) | 1.13 (0.67-1.90) | |||||
| 2-13 months | 0.78 (0.70-0.87) | 0.86 (0.73-1.02) | 0.79 (0.71-0.88) | 0.74 (0.65-0.84) | 0.79 (0.55-1.13) | |||||
| 14 months – 3 years | 0.79 (0.69-0.90) | 0.882b | 0.81 (0.66-0.99) | 0.637b | 0.87 (0.76-0.99) | 0.266b | 0.82 (0.73-0.93) | 0.789b | 0.90 (0.63-1.27) | 0.621b |
| 4-8 years | 0.83 (0.72-0.96) | 0.637c | 0.75 (0.59-0.96) | 0.652c | 0.87 (0.75-1.01) | 0.973c | 0.85 (0.74-0.97) | 0.217c | 0.95 (0.52-1.75) | 0.863c |
| 9-11 years | 0.92 (0.75-1.11) | 0.72 (0.52-0.98) | 1.00 (0.82-1.21) | 0.89 (0.75-1.06) | 0.71 (0.33-1.54) | |||||
| 0.673d | 0.835d | 0.248d | 0.112d | 0.817d |
aAdjusted for calendar year.
bp-value for interaction (df = 1) between age group and gender for 14 months – 3 years of age versus 2–14 months of age.
cp-value for interaction (df = 1) between age group and gender for 4–8 years of age versus 14 months – 3 years of age.
dp-value for interaction (df = 4) between age group and gender for all age groups.
Discussion
Childhood mortality in the Netherlands was very low and declined further over the period from 2000–2011. Mortality from natural causes between birth and 12 years of age was consistently lower among girls than among boys. We observed no significant difference in the female-to-male IRR in DTP-IPV- and MMR ± MenC-eligible children with respect to the last scheduled vaccination.
A major strength of this study is that near complete mortality data were obtained for a long time period from the national registry for causes of death and included the entire Dutch population. However, this study also has some limitations. We could not verify the individual vaccination status of the children and assumed that vaccinations were given as scheduled. A recent study observed that 82% of the vaccinated population received the first DTaP-IPV within 9 weeks of age (<70 days) [20]. The median age at vaccination was 62 days (5th to 95th percentile 51–81 days). Vaccination coverage (personal communication A. van Lier) and delay in vaccination [20] in the Netherlands does not differ by sex. However, delay in vaccination may have resulted in an underestimation of the effect of the last received vaccination on mortality.
Furthermore, changes in the vaccination schedule were made during the study period. Haemophilus influenzae type b and hepatitis b components were added to the DTP-IPV vaccine in 2003 and 2011, respectively and in 2006 pneumococcal vaccination was introduced simultaneously. In September 2002 vaccination against meningococcal C was introduced for children 14 months of age together with a catch-up campaign for children up to 19 years of age. This could possibly have interfered with the potential effect of MMR vaccination and therefore sensitivity analysis was done to compare the periods before and from September 2002 onwards. This analysis did not change our conclusions. Additionally, children with parents originating from tuberculosis endemic countries are offered vaccination with the BCG vaccine in addition to vaccinations through the NIP. BCG vaccination could potentially interfere with the results of the study. To exclude this, we conducted a sensitivity analysis including indigenous Dutch children only, which resulted in similar findings. Also, we conducted sensitivity analysis for natural causes of death except for pregnancy, childbirth and perinatal complications and congenital abnormalities to exclude causes of death which were not related to immune system complications. This analysis did also not change our conclusion. Finally, analysis for children who died due to infectious or parasitic diseases only also has not led to other results, although numbers were low.
We did not observe higher mortality among girls than boys following DTP-IPV vaccination in conjunction with a significant declining female-to-male IRR after measles vaccination as reported previously in countries with high childhood mortality [6,10,13-15,17]. In our study, female-to-male IRRs were less than 1 for all age groups and did not differ between age groups. A possible explanation for the different findings could be the major difference in populations and in setting between our study and previous studies. Our study was conducted in a high-income country with low mortality among children, whereas the other studies were conducted in low-income settings with high childhood mortality. In the latter setting, healthcare is less accessible, infectious disease burden from non-vaccine preventable diseases can be significantly higher, and childhood nutrition might be less balanced. These conditions may exhibit a differential impact on girls versus boys or on vaccinated versus unvaccinated children, as reasons for non-vaccination are unlikely to be random, leading to a potential selection bias [21]. Furthermore, it might be more difficult to collect complete validated data on childhood mortality as well as detailed and reliable information on vaccination status, leading to survival bias [8,21,22]. In addition, the non-specific effects of vaccination are less likely to influence overall mortality in high-income settings because of the low prevalence of infectious diseases and the small number of children who die due to infectious diseases. It would be more sensitive to assess these non-specific effects on morbidity. In Denmark, a high-income county, receipt of the MMR vaccine versus the DTaP-IPV-Hib vaccine as the most recent vaccine was found to be associated with a lower rate of hospital admissions due to infections [23]. However, no difference was observed between boys and girls, which indicate that non-specific effects are not necessarily sex-differential.
Conclusions
In conclusion, we did not find indications for sex-differential non-specific effects on mortality related to the last scheduled vaccination in DTP-IPV- and MMR ± MenC-eligible children in the Netherlands. These study results are important for public health policies. Ongoing evaluation of specific and potential non-specific effects of vaccinations on mortality and morbidity in high-income countries is needed to guide policy, to provide accurate information of the benefits and risks of vaccination and to maintain public trust in public health interventions, such as vaccination.
Acknowledgements
We thank Statistics Netherlands, who have made the data available and made it possible to link demographic data of persons included in the Municipal Personal Records Database (GBA) with causes of death.
Abbreviations
- BCG
Bacillus Calmette-Guérin
- CI
Confidence interval
- Df
Degrees of freedom
- DTP
Diphtheria, tetanus, pertussis
- DTaP
Diphtheria, tetanus, acellular pertussis
- GBA
Municipal Personal Records Database
- HBV
Hepatitis B virus
- Hib
Haemophilus influenza type b
- HPV
Human papillomavirus
- IPV
Inactivated polio vaccine
- IRRs
Incidence rate ratios
- MMR
Mumps, measles, rubella
- MenC
Meningococcal serotype C
- NIP
National Immunisation Programme
- Pneumo
Pneumococcal
Footnotes
Mirjam J Knol, Hester E de Melker and Marianne AB van der Sande contributed equally to this work.
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
TMS contributed to conception and design of the study, carried out acquisition, statistical analysis and interpretation of the data and drafted the manuscript. MJK contributed to the analysis and interpretation of the data and have been involved in drafting the manuscript. HEM participated in the design and coordination of the study and helped to draft the manuscript. MABS conceived the study, and participated in its design and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Tessa M Schurink-van’t Klooster, Email: tessa.schurink@rivm.nl.
Mirjam J Knol, Email: mirjam.knol@rivm.nl.
Hester E de Melker, Email: hester.de.melker@rivm.nl.
Marianne AB van der Sande, Email: marianne.van.der.sande@rivm.nl.
References
- 1.Schurink VT, Klooster T, De Melker H. The National Immunisation Programme in the Netherlands: Developments in 2013. Bilthoven: RIVM; 2013. [Google Scholar]
- 2.Flanagan KL, van Crevel R, Curtis N, Shann F, Levy O. Heterologous (“nonspecific”) and Sex-differential effects of vaccines: epidemiology, clinical trials, and emerging immunologic mechanisms. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57:283–289. doi: 10.1093/cid/cit209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirve S, Bavdekar A, Juvekar S, Benn CS, Nielsen J, Aaby P. Non-specific and sex-differential effects of vaccinations on child survival in rural western India. Vaccine. 2012;30:7300–7308. doi: 10.1016/j.vaccine.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Welaga P, Nielsen J, Adjuik M, Debpuur C, Ross DA, Ravn H, et al. Non-specific effects of diphtheria-tetanus-pertussis and measles vaccinations? An analysis of surveillance data from Navrongo, Ghana. Tropical Med Int Health TM IH. 2012;17(12):1492–505. doi: 10.1111/j.1365-3156.2012.03093.x. [DOI] [PubMed] [Google Scholar]
- 5.Benn CS, Aaby P. Diphtheria-tetanus-pertussis vaccination administered after measles vaccine: increased female mortality? Pediatr Infect Dis J. 2012;31:1095–1097. doi: 10.1097/INF.0b013e318263135e. [DOI] [PubMed] [Google Scholar]
- 6.Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ open. 2012;2:3. doi: 10.1136/bmjopen-2011-000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaby P, Benn CS. Non-specific and sex-differential effects of routine vaccines: what evidence is needed to take these effects into consideration in low-income countries? Hum Vaccin. 2011;7:120–124. doi: 10.4161/hv.7.1.13848. [DOI] [PubMed] [Google Scholar]
- 8.Shann F. The non-specific effects of vaccines. Arch Dis Child. 2010;95:662–667. doi: 10.1136/adc.2009.157537. [DOI] [PubMed] [Google Scholar]
- 9.Benn CS, Fisker AB, Rodrigues A, Ravn H, Sartono E, Whittle H, et al. Sex-differential effect on infant mortality of oral polio vaccine administered with BCG at birth in Guinea-Bissau. A natural experiment. PLoS One. 2008;3:e4056. doi: 10.1371/journal.pone.0004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aaby P, Vessari H, Nielsen J, Maleta K, Benn CS, Jensen H, et al. Sex differential effects of routine immunizations and childhood survival in rural Malawi. Pediatr Infect Dis J. 2006;25:721–727. doi: 10.1097/01.inf.0000227829.64686.ae. [DOI] [PubMed] [Google Scholar]
- 11.Moulton LH, Rahmathullah L, Halsey NA, Thulasiraj RD, Katz J, Tielsch JM. Evaluation of non-specific effects of infant immunizations on early infant mortality in a southern Indian population. Tropical Med Int Health TM IH. 2005;10:947–955. doi: 10.1111/j.1365-3156.2005.01434.x. [DOI] [PubMed] [Google Scholar]
- 12.Aaby P, Jensen H. Do measles vaccines have non-specific effects on mortality? Bull World Health Organ. 2005;83:238. [PMC free article] [PubMed] [Google Scholar]
- 13.Veirum JE, Sodemann M, Biai S, Jakobsen M, Garly ML, Hedegaard K, et al. Routine vaccinations associated with divergent effects on female and male mortality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine. 2005;23:1197–1204. doi: 10.1016/j.vaccine.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 14.Aaby P, Jensen H, Rodrigues A, Garly ML, Benn CS, Lisse IM, et al. Divergent female–male mortality ratios associated with different routine vaccinations among female–male twin pairs. Int J Epidemiol. 2004;33:367–373. doi: 10.1093/ije/dyh004. [DOI] [PubMed] [Google Scholar]
- 15.Aaby P, Jensen H, Garly ML, Bale C, Martins C, Lisse I. Routine vaccinations and child survival in a war situation with high mortality: effect of gender. Vaccine. 2002;21:15–20. doi: 10.1016/S0264-410X(02)00441-3. [DOI] [PubMed] [Google Scholar]
- 16.Shann F. Non-specific effects of vaccines in developing countries. We need evidence about the effect of vaccines on mortality from all causes. BMJ (Clin Res Ed) 2000;321:1423–1424. doi: 10.1136/bmj.321.7274.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaby P, Ravn H, Roth A, Rodrigues A, Lisse IM, Diness BR, et al. Early diphtheria-tetanus-pertussis vaccination associated with higher female mortality and no difference in male mortality in a cohort of low birthweight children: an observational study within a randomised trial. Arch Dis Child. 2012;97:685–691. doi: 10.1136/archdischild-2011-300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan KL, Klein SL, Skakkebaek NE, Marriott I, Marchant A, Selin L, et al. Sex differences in the vaccine-specific and non-targeted effects of vaccines. Vaccine. 2011;29:2349–2354. doi: 10.1016/j.vaccine.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 19.Van Lier EA, Oomen PJ, Giesbers H, Drijfhout IH, De Hoogh PAAM, De Melker HE. Immunization Coverage National Immunization Programme in the Netherlands Year of Report 2011. Bilthoven: RIVM; 2011. [Google Scholar]
- 20.Woestenberg PJ, van Lier A, van der Maas NA, Drijfhout IH, Oomen PJ, de Melker HE. Delayed start of diphtheria, tetanus, acellular pertussis and inactivated polio vaccination in preterm and low birth weight infants in the Netherlands. Pediatr Infect Dis J. 2014;33:190–198. doi: 10.1097/INF.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 21.Farrington CP, Firth MJ, Moulton LH, Ravn H, Andersen PK, Evans S. Epidemiological studies of the non-specific effects of vaccines: II–methodological issues in the design and analysis of cohort studies. Tropical Med Int Health TM IH. 2009;14:977–985. doi: 10.1111/j.1365-3156.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 22.Fine PE, Smith PG. ‘Non-specific effects of vaccines’–an important analytical insight, and call for a workshop. Tropical Med Int Health TM IH. 2007;12:1–4. doi: 10.1111/j.1365-3156.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA. 2014;311:826–835. doi: 10.1001/jama.2014.470. [DOI] [PubMed] [Google Scholar]


