Abstract
Purpose
This study examined the reliability and validity of the Test of Infant Motor Performance Screening Items (TIMPSI) in infants with type I spinal muscular atrophy (SMA).
Methods
After training, 12 evaluators scored 4 videos of infants with type I SMA to assess interrater reliability. Intrarater and test-retest reliability was further assessed for 9 evaluators during a SMA type I clinical trial, with 9 evaluators testing a total of 38 infants twice. Relatedness of the TIMPSI score to ability to reach and ventilatory support was also examined.
Results
Excellent interrater video score reliability was noted (intraclass correlation coefficient, 0.97–0.98). Intrarater reliability was excellent (intraclass correlation coefficient, 0.91–0.98) and test-retest reliability ranged from r = 0.82 to r = 0.95. The TIMPSI score was related to the ability to reach (P ≤ .05).
Conclusion
The TIMPSI can reliably be used to assess motor function in infants with type I SMA. In addition, the TIMPSI scores are related to the ability to reach, an important functional skill in children with type I SMA.
Keywords: child development, female, humans, infant, male, motor skills/physiology, observer variation, physical therapy specialty/instrumentation, physical therapy specialty/standards, reproducibility of results, spinal muscular atrophies of childhood/diagnosis, spinal muscular atrophies of childhood/physiopathology, video recording
INTRODUCTION AND PURPOSE
Proximal spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disorder caused by loss or mutation of the survival motor neuron 1 (SMN1) gene, with retention of its paralog, SMN2. Spinal muscular atrophy is the most common genetic cause of mortality for infants younger than 2 years, affecting 1 in 10000 live births.1 All patients with SMA have at least 1 copy of SMN2 that produces a small amount of the identical full-length SMN protein produced by SMN1. In addition, studies in both mouse models and humans have clearly demonstrated that SMN dosage has a modifying effect on disease severity. Therefore, strategies that either increase or stabilize SMN are very promising.2 New therapies specifically designed to target SMN have now entered phase 1 and 2 clinical trials.3,4 Thus, the need to validate tools capable of measuring meaningful changes in motor function, especially in young infants with type I SMA as soon as possible after its diagnosis, is now critical.
Diagnosis of SMA is based on a classification scheme that includes age of onset and maximal level of motor function achieved. Infants with type I SMA are typically diagnosed by 6 months of age and never achieve the ability to sit independently. Infants with type II SMA present between 6 and 18 months of age, and although they achieve the ability to sit unsupported, they are unable to ambulate independently. Children with type III SMA typically present after 24 months of age and may stand and take steps.5 The variability of motor presentation across ages and across the disease spectrum is wide and uniquely challenging when developing motor scales and outcome measures to assess function in infants and young children, especially early in the disease process. This issue is most challenging in those with type I SMA, who often present with profound generalized hypotonia, neuromuscular weakness, and respiratory compromise. Within the first few months of life, these infants manifest diminished head control and poor antigravity limb control, severely limiting functional abilities such as reaching, playing, and interacting within their environment.5
The importance of well-defined, reliable, and validated outcomes for any clinical trial cannot be overemphasized. While current studies have led to the development and validation of multiple strength and functional outcome measures for those with the less-severe forms of SMA (SMA types II and III),6–13 there are as yet few validated, reliable functional outcome tests for those with the more severe form of SMA (SMA type I).3,14,15 Currently, for infants with type I SMA, an appropriate primary endpoint for success or failure within a clinical trial is longevity (time to death), which may also be measured using a respiratory surrogate for survival such as tracheostomy or greater than 1 month of noninvasive ventilation for 16 hours or more per day.3 However, secondary endpoints that are reliable and valid are needed to assess motor function in this population of infants and children to allow for meaningful assessment of change over time.3
The Test of Infant Motor Performance (TIMP) is a psychometrically valid, well-constructed scale that is useful as an evaluative and predictive tool to assess motor performance in infants born preterm through 4 months of age. It is used to assess the postural and selective control of movement typically used by infants younger than 5 months.16 It has demonstrated excellent reliability and validity when used in infants born prematurely and at high risk for poor motor performance.16–21 It has also been previously demonstrated that the TIMP can be administered reliably in a small number of infants with type I SMA.14 However, the test proved stressful because of the length of the test and positions the infant was required to tolerate.14 On the contrary, tests of motor function that are developed on the basis of normal motor development such as the Alberta Infant Motor Scale and Bayley Scales of Infant Development III are self-limiting for those with type I SMA because the test items are beyond the infant’s motor capabilities. Such tests will have notable floor effects and will be not be able to discriminate among infants within the spectrum of SMA type I.
Therefore, in the SMA Carni-Val type I study, we explored the use of the shorter, screening version of the TIMP, the Test of Infant Motor Performance Screening Items (TIMPSI). Because of severe weakness from motor unit loss, infants with type I SMA have difficulty with both postural control and volitional extremity movement required for functional motor performance. Measurement of these components of motor performance is embedded in the construct of the TIMPSI. The test items and item scoring seemed well suited to assess strength, endurance, and antigravity movement in all body segments in various planes and directions, which are significant impairments for these infants. The TIMPSI is also based on extensive psychometrics, including Rasch analysis. It has demonstrated strong predictability for scores on the TIMP when used with infants at high risk for poor motor performance, and has demonstrated reliability, validity, and sensitivity when used in that population.22 The TIMPSI is shorter and thus can be administered quickly with less stress to the infant at high risk for poor motor performance.
Reliability and use of the TIMPSI in infants with SMA type I have not previously been explored. Items on the test cover a span of motor function that is appreciably affected by weakness in these infants, while covering a range of functions deemed clinically relevant. In addition, the items capture the range of skills that infants with type I SMA typically achieve and allow for change in either direction. Because of the shorter nature of the test, we predicted it would be well tolerated and feasible to administer to infants with SMA type I and hypothesized that the test could be reliably administered across sites and would demonstrate test-retest stability as well as intrarater reliability over a short nonintervention period. In addition, as the ability to reach appears to vary across the spectrum of SMA type I and seems indicative of underlying strength, we predicted that higher total TIMPSI scores would be related to improved ability to lift the arms, bend the elbows, and reach from the supine and supported-sitting positions, as reported on a type I caregiver questionnaire, the Project Cure Functional Rating Scale for SMA Type I: A Primary Caregiver Questionnaire (PCFRS-I). We also hypothesized that the items from the PCFRS-I assessing need for ventilatory support (bilevel positive airway pressure [BiPAP], continuous positive airway pressure, mechanical ventilation) and/or frequency of urgent respiratory support (repositioning to improve breathing, cough assist, secretion clearance) might discriminate infants who are weaker from those who are stronger. (See Tables 1 and 2 for PCFRS-I reaching and respiratory function items and scoring.)
TABLE 1.
Reaching Ability From Supine and Supported-Sitting Positionsa
| Score | Which of the Following Motor Abilities Has Your Child Been Able to Perform Most Days Over the Past Month?
|
|
|---|---|---|
| When My Child Is Lying on His or Her Back | When My Child Is Placed in a Supported Upright Sitting Position in a Chair or Stroller, With Arms Resting at His or Her Sides, Elbows Straight, Hands Not on Lapb | |
| 3 | My child lifts either arm (ie, one or the other) entirely off the bed to reach for a toyc | My child can lift one or both arm(s) from the shoulder to reach for an object placed in front of his or her at shoulder height (arm outstretched, elbow unsupported) |
| 2 | My child lifts either hand off the bed but with elbow still on the bed | My child can lift arm or forearm/hand, by bending at the elbow, to reach lap |
| 1 | My child is unable to reach for a toy but can grasp a toy when it is placed in his or her hand | My child can lift arm or forearm/hand but unable to reach lap |
| 0 | My child is unable to grasp a toy placed in his or her hand | My child is unable to elevate either the arm or the forearm/hand in this position |
| NS | My child is unable to tolerate inclined sitting position | |
Abbreviation: NS, not scored.
Items from the Project Cure Functional Rating Scale for SMA Type I: A Primary Caregiver Questionnaire.
Upright sitting position is defined as at least 75° inclined from horizontal; 90° is considered fully upright sitting position; and 0° is flat position while asleep or awake.
Lifting arm means elbow elevates off the surface while hand is unsupported.
TABLE 2.
Respiratory Function and Need for Urgent Ventilation Scoring Criteriaa
| Score | Respiratory Function: Ventilator Support (BiPAP, CPAP or Mechanical Ventilation via Tracheostomy Tube) | Need for Urgent Ventilation: My Child Has Respiratory Needs Requiring Urgent Intervention/Attentionb |
|---|---|---|
| 3 | My child requires/receives <12 h per day of ventilator support (this choice includes no ventilator support) | Never or rarely |
| 2 | My child requires/receives 12–16 h per day of ventilator support | Only during an upper respiratory illness |
| 1 | My child requires/receives >16 but ≤20 h per day of ventilator support | Occasionally |
| 0 | My child requires/receives >20 h per day of ventilator support | Daily |
Abbreviations: BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure.
Items from the Project Cure Functional Rating Scale for SMA Type I: A Primary Caregiver Questionnaire.
For example, need to reposition to improve breathing, need for clearing of secretions, need for urgent intervention with cough assist machine.
METHODS
The Institutional Review Board (all sites in the United States) or Institutional Ethics Board (Germany and Canada) at each participating institution approved this study. The parent and/or legal guardian of each infant who participated provided informed consent.
Test Instruments
The TIMPSI is a 29-item evaluation that contains 3 item sets: a Screening set, an Easy set, and a Hard set. The Screening set consists of 11 items from the TIMP, each with a 5- to 7-point rating scale; the Easy set has 6 items with 5-or 6-point rating scales and 4 dichotomously scored items; the Hard set has 8 items, 3 with 5-point rating scales and 5 items that are scored dichotomously. The Total score is derived from all subset scores and is the sum of those subset scores.
The PCFRS-I was developed as a disease-specific scale to assess oral or bulbar function, respiratory function, motor function, and quality of life for those with SMA type I (K. J. Swoboda, K. J. Krosschell, T. O. Crawford, unpublished observations, 2008). The questionnaire demonstrated good reliability and validity to assess change in those with SMA type I (K. J. Swoboda et al, manuscript in preparation). Items in each subset are scored with a 4-point rating scale. For this study, we specifically used the respiratory function (Table 1) and reach items (Table 2) from the PCFRS-I to assess convergent and discriminant validity of the TIMPSI.
Feasibility
All infants’ behaviors were closely monitored throughout testing, using Brazelton’s behavioral states, and testing was carried out only during states 4 to 5 to capture the infants’ best and most consistent performances.23 If an infant was noted to be in states 1, 2, 3, or 6, testing was attempted again when the infant was in a more optimal behavioral state. In addition, the infants were monitored for signs of stress and irritability, such as increased respiratory rate, changes in color or tone or inability to be consoled following testing of a specific item. Time to complete the TIMPSI during the baseline visits of each infant was recorded in minutes. Time to complete included any necessary rest periods between items and time needed for suctioning, reassurance, or other medical interventions if required. Need for BiPAP or suctioning during the TIMPSI assessment was also recorded. In addition, in a previous study using the full version of the test (TIMP) with infants with type I, Finkel et al14 noted that infants demonstrated limited tolerance for several test items, particularly those items that were tested in the prone position (items 36–39). They also noted that head control (items 15–17) and defensive reaction items (items 25–26) on the TIMP were likely to provoke irritability in weaker infants. Of these 9 items, only 4 (number 15, head control in supported-sitting position; number 36, head lift in the prone position; number 37, crawling; and number 38, head turn in the prone position to the right side) remain a part of the TIMPSI. Qualitative assessment of these 4 items was undertaken by viewing test video recordings to assess whether the item provoked irritability or stress for the infant. An independent rater, blinded to the TIMPSI scores, rated these items for invoked stress or discomfort, using a dichotomous rating scale (0, no stress or discomfort and 1, any stress or discomfort).
Evaluator Training
Before the initiation of the Carni-Val type I clinical trial (an open-label, phase 1/2 trial designed to obtain additional safety parameters of valproic acid and carnitine in infants with type I SMA), the study evaluator training was conducted to establish standardized assessment and reliability of the TIMPSI in infants with type I SMA. A total of 16 physical and occupational therapists were trained. Training materials included the standard TIMP training CD (version 4.0; IMPS, LLC, Chicago, Illinois) and manual, as well as a TIMPSI-specific manual of procedures. The TIMPSI training CDs that were specific to infants with SMA were used at an in-person training session. Videos of infants with type I SMA exhibiting a range of functional levels were used in discussion to clarify technique, standardize administration of items, and standardize scoring criteria. The manual of procedures and scoring forms were reviewed item by item. Infants with SMA were available at the training session to allow examiners to practice assessment skills, review item criteria, and clarify scoring. Of the total 29 items, 2 items (lateral straightening of the head and body with arm support and lateral hip abduction reaction) were deemed poorly tolerated by infants with type I SMA, during test administration. Raters were advised that these items should not be administered and should be scored as a zero, as per instructions for scoring the test.19
Interrater Reliability of the TIMPSI Evaluators Using Video
Posttraining interrater reliability for the group was established using video assessment. Twelve of the 16 evaluators who had attended the in-person training session scored 4 videos of infants being assessed with the TIMPSI, using standardized techniques posttraining to allow for assessment of interrater scoring reliability. The intraclass correlation coefficient (ICC = 3, 1) was used to assess interrater reliability.
Intrarater Reliability of the TIMPSI Evaluators
Clinical evaluators at each of the 8 Project Cure clinical sites who had completed evaluator training and participated in the Carni-Val type I clinical trial (n = 9) took part in this study. Each evaluator tested 2 to 7 infants with type I SMA at their clinical site, during that clinical trial. Using the Carni-Val trial’s baseline visits (before any intervention), intrarater reliability for the TIMPSI Total, Screening, and Easy scores was assessed using the ICC (2, 1).
Test-Retest Reliability of the TIMPSI
A multicenter cohort of 38 infants with type I SMA had the TIMPSI assessments at baseline while participating in the Carni-Val type I trial, intended to assess safety, tolerability, and potential efficacy of a combined regimen of oral valproic acid and L-carnitine. All testing took place at 8 Project Cure clinical research sites within the United States, Canada, and Germany, with 9 total participating raters. The TIMPSI assessments were performed twice (screening visits 1 and 2) for each infant during the baseline period, before pharmacological intervention. Each assessment was scheduled at the same time of day, 1 to 28 days apart (mean, 4.3 days or 0.14 months [SD = 0.18 months]; median, 1 day). Test-retest reliability for the complete TIMPSI as well as for the Screening and Easy subsets were assessed using the Pearson product-moment correlation if the data were normally distributed or Spearman rank correlation for data that were not normally distributed (both noted as r).
Convergent Validity
Correlation of the Total and Screening TIMPSI scores to several other items deemed important to outcome and well-being and clinically relevant to function was explored. Across the spectrum of type I SMA, we hypothesized that those with better functional reaching abilities would be stronger overall. Therefore, we compared the total TIMPSI score to reaching items from the PCFRS-I (Table 1).
Discriminant Validity
As infants with type I SMA typically manifest respiratory compromise and insufficiency within the first year of life, one could theorize that those requiring more ventilatory support (measured by the time the child required ventilatory support such as BiPAP, continuous positive airway pressure, or mechanical ventilation via tracheostomy tube) and those with greater frequency of urgent respiratory needs (eg, need for repositioning to improve breathing, need for clearing of secretions, or need for urgent intervention with cough assist machine) may be weaker. Therefore, we also explored the relationship between the TIMPSI scores and the frequency of ventilatory support and urgent respiratory needs. We examined such using the ventilatory support questions from the PCFRS-I (Table 2).
Correlation between the TIMPSI scores and age were also explored. Whereas developmental maturity may play a role in score improvement, we suspected that the TIMPSI scores would likely decrease with increasing age in the absence of a disease-modifying treatment, reflective of increased weakness at older ages and decreased ability to move against gravity as body segments and full body movement, and antigravity control, in particular, more difficult.
Data Analysis
Interrater reliability analysis for the 12 raters scoring the 4 videos of infants with type I SMA was performed for Total, Screening, Easy, and Hard subsets of the TIMPSI, using ICC (3, 1). Intrarater reliability of the TIMPSI (Total, Screening, and Easy scores) administered to 38 infants during a clinical trial baseline period was analyzed using ICC (2, 1). Normality of the data was examined using the Shapiro-Wilk test. To examine test-retest stability of the TIMPSI scores administered to 38 infants during the trial’s baseline period, Pearson product-moment correlation coefficients (for normally distributed data) were used to examine test-retest stability of the TIMPSI (Total, Screening, and Easy scores). Spearman ρ was used to examine test-retest stability of Hard test scores because of nonnormality of the data. Analysis of variance was used to examine correlation of the TIMPSI scores with the reach ability scores and age. Ratings of stress or discomfort were calculated for each of the 4 items of concern by totaling the ratings for each item and dividing by the number of assessments (n) of each item to get a percentage of discomfort score. A score closer to 0 indicates no concern, while a score closer to 1 indicates significant concern.
RESULTS
Feasibility
No adverse events occurred during testing, and only 1 infant required use of BiPAP during testing. This infant used BiPAP on a regular basis as per report. Five infants required suctioning 1 to 4 times each, during testing. Test time was recorded for 65 of 78 test sessions. Average test time was 44.01 minutes (range, 5–125 min; median, 38 min; SD, 21 min). Time to test varied, but adequate rest periods were allotted (eg, after suctioning or when the infant appeared fatigued) and calculated into actual test times. Items suggested to invoke stress or discomfort (item numbers 15, 36, 37, and 38) were rated for all 38 infants during both assessments. Ratings for each item were as follows: item 15 = 0.013; item 36 = 0.118; item 37 = 0.134; item 38 = 0.026.
Interrater Reliability of the TIMPSI Evaluators Using Video
Posttraining interrater reliability using video assessment ranged from 0.97 to 0.98 (ICC = 3, 1) for 12 evaluators. The TIMPSI Total score ICC was 0.097; Screening, Easy, and Hard score ICCs were 0.98.
Intrarater Reliability of the TIMPSI Evaluators
Thirty-eight infants were tested twice during baseline visits before study intervention. Infants’ ages during baseline visits ranged from 0.50 to 10.60 months (mean, 5.52 ± 2.66 months; median, 5.65 ± 2.66 months). Intrarater reliability for the Total TIMPSI score was excellent (ICC = 0.85–0.97) when infants with type 1 SMA were examined at the evaluator’s clinical site. Intrarater reliability for the TIMPSI Screening score was also excellent (ICC = 0.94–0.97) for all but 1 rater, who had poor reliability (0.25). The TIMPSI Easy score reliability ranged from good to excellent (ICC = 0.76–0.98) (Table 3). Although 9 raters were trained and carried out the TIMPSI assessments at their sites, 3 raters completed too few assessments (n = 2) to calculate their intrarater reliability.
TABLE 3.
Intrarater Reliability of Evaluators of Infants With Type I SMA
| Rater | n | Range of TIMPSI Scores | Mean | Median | SD | ICC (2,1) |
|---|---|---|---|---|---|---|
| TIMPSI Total score intrarater reliability | ||||||
| A | 6 | 15–49 | 30.2 | 28.5 | 11.7 | 0.94 |
| B | 3 | 10–45 | 27 | 26 | 17.5 | 0.97 |
| C | 6 | 7–52 | 33.3 | 32 | 13.3 | 0.96 |
| D | 6 | 12–45 | 30.8 | 30.5 | 12.4 | 0.97 |
| E | 7 | 10–41 | 28.9 | 32 | 12.5 | 0.94 |
| F | 4 | 21–41 | 29.9 | 28.7 | 8.3 | 0.85 |
| TIMPSI Screening score intrarater reliability | ||||||
| A | 6 | 9–26 | 16.2 | 14.5 | 5.8 | 0.97 |
| B | 3 | 2–17 | 9 | 8 | 7.5 | 0.95 |
| C | 6 | 5–26 | 15.4 | 13 | 8 | 0.95 |
| D | 6 | 1–20 | 13.5 | 15 | 6.9 | 0.99 |
| E | 7 | 5–20 | 12 | 12 | 5.6 | 0.94 |
| F | 4 | 12–17 | 13.7 | 14.1 | 2.3 | 0.25 |
| TIMPSI Easy score intrarater reliability | ||||||
| A | 6 | 4–20 | 12.5 | 12.3 | 5.5 | 0.77 |
| B | 3 | 7–25 | 16 | 16 | 9 | 0.95 |
| C | 6 | 2–26 | 15.5 | 16 | 8.6 | 0.97 |
| D | 6 | 7–22 | 15 | 14.8 | 6.1 | 0.98 |
| E | 7 | 5–21 | 18 | 18.4 | 6.4 | 0.76 |
| F | 4 | 7–19 | 14 | 13.5 | 4.9 | 0.98 |
Abbreviation: TIMPSI, Test of Infant Motor Performance Screening Items.
Test-Retest Reliability of the TIMPSI
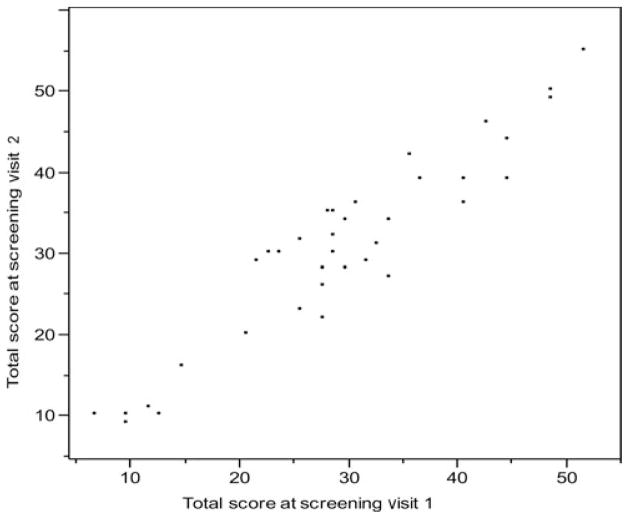
Thirty-eight subjects had 2 screening visits. The average time between visits was 4.3 days or 0.14 months (SD = 0.18 months). Infants’ ages during baseline visits ranged from 0.50 to 10.60 months (mean, 5.52 ± 2.66 months; median, 5.65 ± 2.66 months). The TIMPSI total score (P = .35), Screening score (P = .45), and Easy scores (P = .29) were all normally distributed for visits 1 and 2. The test-retest reliability of TIMPSI Total, Screening, and Easy scores, using Pearson product-moment correlation for normality of data, was excellent (r = 0.946, r = 0.909, r = 0.815, respectively; all P ≤ .001). The Hard score had a smaller range of 0 to 5 and only whole number scores made it behave like a categorical rather than continuous scale, with a P = .005 normality test for both visits. The means were equal from screening visit 1 to 2, so the t test was 0 and the P value was 1.000. The data were categorical, and the Spearman rank correlation was low at 0.65 (Table 4; Figure 1).
TABLE 4.
Test-Retest Reliability of the TIMPSI
| TIMPSI Score | Range of Scores S1; Range of Scores S2 (Total Possible Score) | Mean S1, Mean S2 (SD) | Reliability, r (P ≤ .001) |
|---|---|---|---|
| Total | 7–52; 9–55 (96) | 30.4, 31.0 (11.6,11.7) | 0.95 |
| Screening | 1–26; 0–29 (51) | 13.7, 14.2 (5.7, 6.2) | 0.91 |
| Easy | 2–26; 4–26 (31) | 14.7, 14.9 (6.3,6.0) | 0.82 |
| Hard | 0–5; 0–4 (19) | 1.9, 1.9 (1.2, 1.0) | 0.65 |
Abbreviations: S1, screening visit 1; S2, screening visit 2; TIMPSI, Test of Infant Motor Performance Screening Items.
Fig. 1.
Test-retest of the TIMPSI Total scores at screening visits 1 and 2.
Discriminant and Convergent Validity
The TIMPSI score was not related to the need for ventilation (P ≤ .001) (discriminant validity), but was related to the ability to lift the arms and bend the elbows to reach (convergent validity). The TIMPSI Total and Screening scores were related to the ability to reach when assessed by using reaching items from the PCFRS-I. Infants with higher TIMPSI scores demonstrated improved ability to reach from both the supine and supported-sitting positions (P ≤ .05) (Tables 5 and 6). The TIMPSI Total and Screening scores were not related to age (r = 0.123, P = .282) (Figure 2).
TABLE 5.
TIMPSI Scores Relatedness to PCFRS-I Ability to Reach From Supine Position
| Level | Screening Score
|
Total Score
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Lift either arm | 16.5 | 4.2 | 36.1 | 8.8 |
| Lift either hand | 11.7 | 4.4 | 24.5 | 10.2 |
| Unable to reach toy | 6.7 | 5.1 | 22 | 8.7 |
| P | .0016 | .0045 | ||
Abbreviations: PCFRS-I, Project Cure Functional Rating Scale for SMA Type I: A Primary Caregiver Questionnaire; TIMPSI, Test of Infant Motor Performance Screening Items.
TABLE 6.
TIMPSI Scores Relatedness to PCFRS-I Ability to Reach From Supported-Sitting Position
| Level | Screening Score
|
Total Score
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Lift arm(s) | 17.7 | 4.4 | 39.7 | 5.5 |
| Bend elbow | 13.6 | 3.9 | 29.9 | 8.7 |
| Unable to reach lap | 8 | 6.6 | 18.3 | 8.5 |
| Unable to elevate arm | 12.5 | 6.4 | 24 | 2.8 |
| Unable to sit supported | 10.2 | 7.1 | 22.5 | 17.6 |
| P | .0438 | .0134 | ||
Abbreviations: PCFRS-I, Project Cure Functional Rating Scale for SMA Type I: A Primary Caregiver Questionnaire; TIMPSI, Test of Infant Motor Performance Screening Items.
Fig. 2.
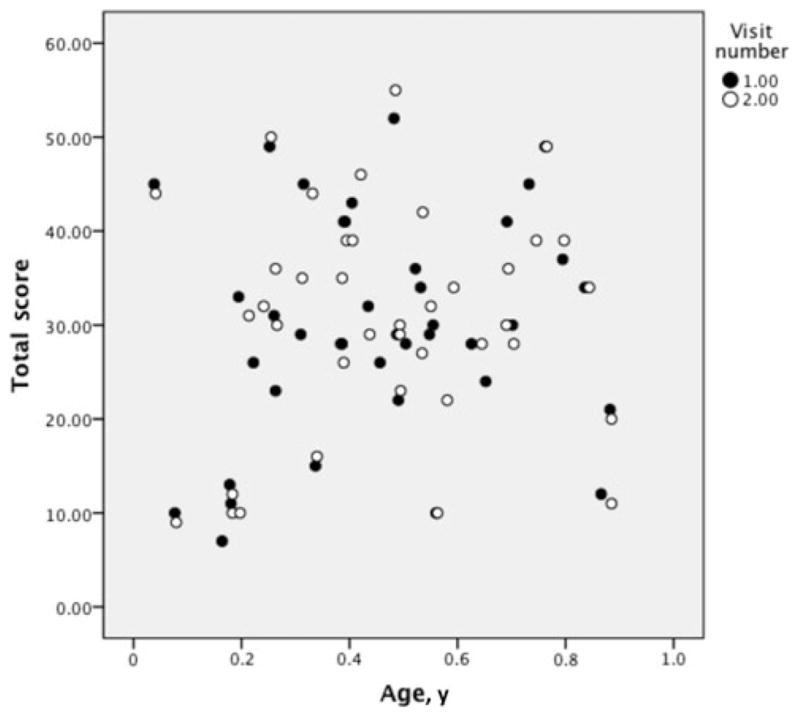
Age versus TIMPSI Total scores.
DISCUSSION
Infants with SMA type I are living longer, secondary to proactive respiratory support and management.24,25 However, since significant denervation is already present at the time of diagnosis, improved survival has not translated to significantly improved motor function.24 If opportunities for clinical trial enrollment could be facilitated efficiently, improvement in motor function in response to more potent therapies might be feasible, although the best outcomes will undoubtedly require presymptomatic diagnosis. Assessment of motor function is necessary clinically to help guide choices for appropriate equipment to facilitate mobility and communication to achieve the best long-term outcomes. In addition, assessment of motor function over time is critical for use in multicenter clinical trials to explore the potential benefit of interventions that may modify but not fully correct the phenotype. The construct of the TIMPSI and its reliability in infants with SMA type I facilitate its use as a secondary outcome measure for use in assessing potential change in a clinical trial or study to assess an intervention specifically intended to improve motor function.
In this study, we demonstrate that the TIMPSI can be safely and feasibly administered to infants with SMA type I, with a wide range of ages and overall severity of weakness. However, sufficient time is necessary to ensure that breaks are provided for rest, reassurance, or suctioning if needed. Overall test time varied among infants, as did time for breaks. Whereas this might suggest that the time to test more fragile infants could be longer, that hypothesis would require further study. Despite medical fragility and profoundly appreciable weakness, all enrolled infants were able to tolerate testing, without undue stress or irritability. In a previous study using the TIMP in infants with SMA type I, the authors reported limited tolerance for several test items, particularly those items that were tested in the prone position (items 36–39). It was also reported that head control items (15–17) and defensive reaction items (25–26) on the TIMP were likely to provoke irritability in weaker infants.14 Of these 9 items, only 4 (15, 36, 37, 38) remain a part of the TIMPSI. These items are easily administered and do not appear to provoke undue irritability or stress as compared with other TIMPSI test items. In addition, the wide range of scores across ages and degree of weakness suggests that the test can capture functional variability along with increases and decreases in motor function in the patients who are youngest and weakest, as well as in older children with type I SMA who are limited to an infant’s motor repertoire because of their weakness and medical fragility.
Training materials and sessions incorporating the use of already available materials as well as newly developed materials demonstrating testing in infants with type I SMA allowed evaluators to achieve multisite agreement or reliability as well as intrarater reliability. In addition, the TIMPSI demonstrated good to excellent test-retest reliability over a brief nonintervention interval in this multicenter clinical trial and captured a range of motor functions across the spectrum of motor ability for infants with type 1 SMA. One evaluator was noted to be an outlier, as demonstrated by lower overall Total and Screening TIMPSI intrarater reliability (Table 2, evaluator F). This observation emphasizes the importance of careful site monitoring and training over the length of the trial to minimize such differences and optimize reliable performance.
The TIMPSI appears to capture motor function across the spectrum of motor ability without floor or ceiling effects. In addition, the TIMPSI scores correlate with upper extremity function in reaching, as independently assessed with the PCFRS-I, administered in parallel with each visit. The TIMPSI scores were related to an infant’s ability to reach from both the supine and supported-sitting positions. The reaching tasks were indicative of motor strength, as higher item scores indicated increased ability for selective antigravity movement and movement of more proximal limb segments in both reaching tasks. Thus, initial validation of the TIMPSI in infants with SMA type I is supported by the independent correlation of motor function via the PCFRS-I. The correlation between the reach scores and the TIMPSI scores supports the convergent validation of the TIMPSI for those with SMA. This finding also supports preliminary observations from the recently completed STOP SMA study that demonstrates a correlation between the TIMPSI scores and other measures of disease progression, including ulnar compound muscle action potential amplitudes and PCFRS-I scores in infants with SMA followed longitudinally (NCT00528268, K.J.S).
Discriminant validity was supported by the use of ventilatory support questions from the PCFRS-I. Need for urgent ventilation and daily respiratory support did not correlate with the TIMPSI scores, which theoretically makes sense, as the TIMPSI test construct does not measure respiratory need or compromise. We further explored the possibility that age and the need for and urgency of respiratory support might correlate to the TIMPSI score, as a measure of disease severity; however, no relationship was noted. This may be because of the small cross-sectional range of ages represented in our sample and the fact that most infants were recently diagnosed and younger than 11 months at the time of testing. Families may not have yet been introduced to or made supportive or proactive pulmonary care decisions. However, the lack of a correlation between the TIMPSI scores and the reported need for respiratory support on a regular basis, along with the finding that few infants required respiratory support (suction, BiPAP) during baseline testing could also suggest that the test was well tolerated and did not induce respiratory compromise. Assessment by using O2 saturation as an additional measure of respiratory sufficiency could be of value in future studies to determine whether various positions or tasks provoke stress.
The clinical phenotype of SMA type I is notable for discrete differences in age of onset, severity of weakness, and natural history, particularly with regard to respiration-related morbidity and mortality. Those with SMA type Ia have the earliest generalized hypotonia and weakness and a poor prognosis, while those with type Ic achieve some degree of head control and have the best prognosis.3 Our study results suggest that the TIMPSI captures those gross motor skills that are present in a broad spectrum of infants with SMA type I, and that the TIMPSI scores may help distinguish between these 3 subgroups, providing valuable information about prognosis. In addition, the relatedness of the ability to reach and head control items should be further explored to examine whether these items can predict the overall TIMPSI scores. If so, they may be better suited to assess the infant’s overall motor function, without causing the infant to become uncomfortable because of fatigue or respiratory compromise as may occur with longer testing in the most fragile infants.
Continued assessment of validity as well as sensitivity and responsiveness of the measure to change over time in this patient population is ongoing and is necessary to fully assess its potential usefulness in future clinical trials. A longer study looking at change over time in infants with SMA type I will be important to determine the ability of the TIMPSI to responsively capture small degrees of change. Known sensitivity to change could serve as a useful predictor of decline tracked longitudinally, offering additional information on natural history. Currently, the dichotomous measure of time to more than 16 hours per day of ventilatory support and/or tracheostomy or death has been suggested as an endpoint for clinical trials. However, this can only be assessed when an endpoint is reached. Following further validation in other settings in this population, the TIMPSI may serve as a feasible and useful outcome measure of change over time for infants with SMA type I.
CONCLUSIONS
The TIMPSI can reliably assess motor function in infants with type I SMA. A range of scores across patients and ages was noted suggesting that the TIMPSI may capture the functional variability demonstrated by these infants without floor or ceiling effects and may distinguish between SMA types 1a through 1c. The test was well tolerated and can be safely administered in infants with SMA type 1. Scores are not related to the need for ventilation, but are related to the ability to reach. Validity and sensitivity to change require further assessment.
Acknowledgments
Grant Support: This project was fully funded by Families of Spinal Muscular Atrophy in the United States and its affiliate in Canada. The German site was supported by a grant from Initiative und Forschung fuer SMA.
We thank all Project Cure Team members who made this study possible. We especially thank the families and infants with SMA who participated and contributed to this study and Dr Suzanne Campbell for her support early on in this project.
Project Cure SMA Investigators Network members
Kathryn J. Swoboda, MD, Departments of Neurology and Pediatrics, Primary Children’s Medical Center, and University of Utah School of Medicine, Salt Lake City; Gyula Acsadi, MD, PhD, Division of Neurology, Connecticut Children’s Medical Center, Hartford, Connecticut; Thomas Crawford, MD, Departments of Neurology and Pediatrics, Johns Hopkins Hospital, Baltimore, Maryland; Guy D’Anjou, MD, Department of Pediatrics, Hôpital Sainte-Justine, Montreal, Quebec, Canada; Bakri Elsheik, MD, Department of Neurology, Ohio State University, Columbus; John T. Kissel, MD, Department of Neurology, Ohio State University, Columbus; Priya Kishnani, MD, Division of Medical Genetics, Department of Pediatrics, Duke University Medical Center, Durham, North Carolina; Kristin J. Krosschell, PT, DPT, MA, PCS, Department of Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, Illinois; Bernard LaSalle, PhD, Biomedical Informatics, University of Utah, Salt Lake City; Sandra P. Reyna, MD, Department of Neurology, University of Utah School of Medicine, Salt Lake City; Mary K. Schroth, MD, Department of Pediatrics, University of Wisconsin Children’s Hospital, Madison; Charles Scott, PhD, CBS Squared, Fort Washington, Pennsylvania; Louise R. Simard, PhD, Departments of Biochemistry and Medical Genetics, University of Manitoba, Winnepeg, Mannitoba, Canada; Edward C. Smith, MD, Division of Pediatric Neurology, Department of Pediatrics, Duke University Medical Center, Durham, North Carolina; Brunhilde Wirth, Institute of Human Genetics, University Hospital of Cologne, Cologne, Germany, Jürgen-Christoph von Kleist-Retzow, MD, Department of Pediatrics, Hospital of the University of Cologne, Cologne, Germany.
Footnotes
The authors declare no conflict of interest.
References
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Sumner CJ. Therapeutics development for spinal muscular atrophy. Neuro Rx. 2006;3(2):235–245. doi: 10.1016/j.nurx.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertini E, Burghes A, Bushby K, et al. Neuromuscul Disord; 134th ENMC International Workshop: outcome measures and treatment of spinal muscular atrophy; 11–13 February 2005; Naarden, the Netherlands. 2005. pp. 802–816. [DOI] [PubMed] [Google Scholar]
- 4.Kolb SJ, Kissel JT. Spinal muscular atrophy: a timely review. Arch Neurol. 2011;68(8):979–984. doi: 10.1001/archneurol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CH, Bonnemann CG, Rutkowski A, et al. Consensus statement on standard of care for congenital muscular dystrophies. J Child Neurol. 2010;25(12):1559–1581. doi: 10.1177/0883073810381924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krosschell KJ, Maczulski JA, Crawford TO, Scott C, Swoboda KJ. A modified Hammersmith Functional Motor Scale for use in multi-center research on spinal muscular atrophy. Neuromuscul Disord. 2006;16(7):417–426. doi: 10.1016/j.nmd.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krosschell K, Scott C, Maczulski J, et al. Reliability of the Modified Hammersmith Functional Motor Scale in young children with spinal muscular atrophy. Muscle Nerve. 2011;44(2):246–251. doi: 10.1002/mus.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Main M, Kairon H, Mercuri E, Muntoni F. The Hammersmith Functional Motor Scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol. 2003;7(4):155–159. doi: 10.1016/s1090-3798(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 9.O’Hagen JM, Glanzman AM, McDermott MP, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. 2007;17(9–10):693–697. doi: 10.1016/j.nmd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Montes J, McDermott MP, Martens WB, et al. Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010;74(10):833–838. doi: 10.1212/WNL.0b013e3181d3e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson L, Owens H, Hynan LS, Iannaccone ST. The gross motor function measure is a valid and sensitive outcome measure for spinal muscular atrophy. Neuromuscul Disord. 2006;16(6):374–380. doi: 10.1016/j.nmd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Merlini L, Mazzone ES, Solari A, Morandi L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve. 2002;26(1):64–70. doi: 10.1002/mus.10166. [DOI] [PubMed] [Google Scholar]
- 13.Iannaccone ST, Hynan LS. Reliability of 4 outcome measures in pediatric spinal muscular atrophy. Arch Neurol. 2003;60(8):1130–1136. doi: 10.1001/archneur.60.8.1130. [DOI] [PubMed] [Google Scholar]
- 14.Finkel RS, Hynan LS, Glanzman AM, et al. The test of infant motor performance: reliability in spinal muscular atrophy type I. Pediatr Phys Ther. 2008;20(3):242–246. doi: 10.1097/PEP.0b013e318181ae96. [DOI] [PubMed] [Google Scholar]
- 15.Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOPINTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155–161. doi: 10.1016/j.nmd.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell SK, Kolobe TH, Osten ET, Lenke M, Girolami GL. Construct validity of the test of infant motor performance. Phys Ther. 1995;75(7):585–596. doi: 10.1093/ptj/75.7.585. [DOI] [PubMed] [Google Scholar]
- 17.Noble Y, Boyd R. Neonatal assessments for the preterm infant up to 4 months corrected age: a systematic review. Dev Med Child Neurol. 2012;54(2):129–139. doi: 10.1111/j.1469-8749.2010.03903.x. [DOI] [PubMed] [Google Scholar]
- 18.Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol. 2008;50(4):254–266. doi: 10.1111/j.1469-8749.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 19.Campbell SK. The Test of Infant Motor Performance: Test Users Manual Version 2.0. Chicago, IL: IMPS LLC; 2005. [Google Scholar]
- 20.Campbell SK, Hedeker D. Validity of the Test of Infant Motor Performance for discriminating among infants with varying risk for poor motor outcome. J Pediatr. 2001;139(4):546–551. doi: 10.1067/mpd.2001.117581. [DOI] [PubMed] [Google Scholar]
- 21.Campbell SK, Kolobe TH, Wright BD, Linacre JM. Validity of the Test of Infant Motor Performance for prediction of 6-, 9- and 12-month scores on the Alberta Infant Motor Scale. Dev Med Child Neurol. 2002;44(4):263–272. doi: 10.1017/s0012162201002043. [DOI] [PubMed] [Google Scholar]
- 22.Campbell SK, Swanlund A, Smith E, Liao PJ, Zawacki L. Validity of the TIMPSI for estimating concurrent performance on the test of infant motor performance. Pediatr Phys Ther. 2008;20(1):3–10. doi: 10.1097/PEP.0b013e31815f66a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brazelton T, Nugent J. Neonatal Behavioral Assessment Scale. 3. London, England: Mac Keith Press; 1995. [Google Scholar]
- 24.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69(20):1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 25.Lemoine TJ, Swoboda KJ, Bratton SL, Holubkov R, Mundorff M, Srivastava R. Spinal muscular atrophy type 1: are proactive respiratory interventions associated with longer survival? Pediatr Crit Care Med. 2012;13(3):e161–e165. doi: 10.1097/PCC.0b013e3182388ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]