Abstract
Developing effective techniques for the cryopreservation of human adipose-derived adult stem cells could increase the usefulness of these cells in tissue engineering and regenerative medicine. Unfortunately, the use of serum and a commonly used cryoprotectant chemical, dimethyl sulphoxide (DMSO), during cryopreservation storage restricts the direct translation of adult stem cells to in vivo applications. The objective of this study was to test the hypothesis that the stromal vascular fraction (SVF) of adipose tissue can be effectively cryopreserved and stored in liquid nitrogen, using a freezing medium containing high molecular weight polymers, such as methylcellulose (MC) and/or polyvinylpyrollidone (PVP), as the cryoprotective agent (CPA) instead of DMSO. To this end, we investigated the post-freeze/thaw viability and apoptotic behaviour of SVF of adipose tissue frozen in 16 different media: (a) the traditional medium containing Dulbecco’s modified Eagle’s medium (DMEM) with 80% fetal calf serum (FCS) and 10% DMSO; (b) DMEM with 80% human serum (HS) and 10% DMSO; (c) DMEM with 0%, 2%, 4%, 6%, 8% or 10% DMSO; (d) DMEM with 1% MC and 10% of either HS or FCS or DMSO; (e) DMEM with 10% PVP and varying concentrations of FCS (0%, 10%, 40% or 80%); (f) DMEM with 10% PVP and 10% HS. Approximately 1 ml (106 cells/ml) of SVF cells were frozen overnight in a −80 °C freezer and stored in liquid nitrogen for 2 weeks before being rapidly thawed in a 37 °C water bath (1–2 min agitation), resuspended in culture medium and seeded in separate wells of a six-well plate for a 24 h incubation period at 37 °C. After 24 h, the thawed samples were analysed by brightfield microscopy and flow cytometry. The results suggest that the absence of DMSO (and the presence of MC) significantly increases the fraction of apoptotic and/or necrotic SVF cells. However, the percentage of viable cells obtained with 10% PVP and DMEM was comparable with that obtained in freezing medium with DMSO and serum (HS or FCS), i.e. ~54 ± 14% and ~63 ± 10%, respectively. Adipogenic and osteogenic differentiation behaviour of the frozen thawed cells was also assessed, using histochemical staining. Our results suggest that post-thaw SVF cell viability and adipogenic and osteogenic differentiability can be maintained even when they are frozen in the absence of serum and DMSO but with 10% PVP in DMEM.
Keywords: cryopreservation, cryoprotective agents, human subcutaneous adipose tissue, stromal vascular fraction cells, adipogenesis, ostoegenesis, necrosis, apoptosis
1. Introduction
Recent reports indicate that adipose tissue is a novel source of multipotent stem cells. As adipose tissue allows extraction of a large volume of tissue with limited morbidity, this tissue could be an exciting alternative stem cell source. Intriguingly, it has also been reported that human adipose tissue contains a population of cells, harvested by liposuction, that are able in vitro to undergo adipogenic, osteogenic, chondrogenic and myogenic differentiation (Vogel, 2000; Zuk et al., 2001; Halvorsen et al., 2001; Zuk et al., 2002; Gimble and Guilak, 2003; Rodriguez et al., 2004; Awad et al., 2004; Hicok et al., 2004). The nature of the adipose-derived cells changes as a function of isolation and passage. For example, Mitchell et al. (2006) showed that the immunophenotype of the adipose-derived cells based on flow cytometry changed progressively with adherence and passage. Multiple studies report that primary pre-adipocytes derived from the stromal-vascular fraction (SVF) of human adipose tissue, although being able to differentiate in adipocytes under appropriate conditions, decrease their ability to differentiate after multiple passages; this coincides with a prolonged doubling time and replicative senescence (Rubio et al., 2005). Furthermore, human adipose-derived cells maintained for >3 months in culture transformed spontaneously, giving rise to sarcomas when transplanted as xenografts into immunodeficient murine recipients (Rubio et al., 2005). Kang et al. (2004) found that non-human primate adipose-derived stem cells have markedly reduced multilineage and self-renewal potentials after 12 passages, reaching senescence by passage 20. Studies by De Ugarte et al. (2003) suggested that the adipogenic potential of adipose-derived adult stem cells decreased with passaging. In a more recent study Wall et al. (2007) found that adipose-derived stem cells are capable of both adipogenic and osteogenic differentiation through 10 passages, but that osteogenic differentiation may start to dominate at later passages. These variable results indicating the genotype and phenotype may not remain stable during growth and passage of adult stem cells suggest that passaged cells for higher generations may not be suitable for regenerative medical therapies. Moreover, serial passaging exposes cell cultures to the repeated risk of contamination by environmental microorganisms. These shortcomings, associated with in vitro stem cell expansion, can be partly alleviated by cryopreservation (freezing storage) of SVF of human adipose tissue (Thirumala et al., 2005a, 2005b; Devireddy et al., 2005; Goh et al., 2007; Fuller and Devireddy, 2008).
In general, freezing storage requires the addition of a class of chemicals known as cryoprotective agents (CPAs), such as dimethyl sulphoxide (DMSO) or glycerol (Lovelock, 1954; Benson and Bremner, 2004; Fuller, 2004). Although widely used, these chemicals need to be removed prior to transplantation and are not safe for in vivo use (Beaujean et al., 1991; Rubinstein et al., 1995; Rodriguez et al., 2005). Alternative cryoprotective agents need to be identified and developed for use with SVF cells to further their use in in vivo clinical settings (Farrant, 1969; Franks et al., 1977; Merten et al., 1995; Hubálek 2003). The objective of this study was to test the hypothesis that the SVF of adipose tissue containing adult stem cells can be effectively cryopreserved and stored in liquid nitrogen using a freezing medium containing either methylcellulose (MC) or polyvinylpyrollidone (PVP) as the CPA instead of DMSO. The choice of MC or PVP is cell-specific and their effectiveness to act as a CPA cannot be adjudicated a priori. Thus to assess the relative merits and de-merits associated with the use of MC and PVP as a CPA, we investigated the post-freeze/thaw viability and apoptotic behaviour of SVF cells cryopreserved in 16 different media preparations: (a) the traditional medium containing Dulbecco’s modified Eagle’s medium (DMEM) with 80% fetal calf serum (FCS) and 10% DMSO; (b) DMEM with 80% human serum (HS) and 10% DMSO; (c) DMEM with 0%, 2%, 4%, 6%, 8% or 10% DMSO; (d) DMEM with 1% MC and 10% of either HS or FCS or DMSO; (e) DMEM with 10% PVP and varying concentrations of FCS (0%, 10%, 40% or 80%); (f) DMEM with 10% PVP and 10% HS. The thawed samples were analysed by brightfield microscopy and flow cytometry to determine the percentage of viable, necrotic and apoptotic cells. Adipogenic and osteogenic differentiation behaviour of the frozen cells post-thawing was also assessed, using histochemical staining. Our results suggest that post-thaw SVF cell viability and adipogenic and osteogenic differentiability can be maintained when they are frozen in DMEM in the absence of serum and DMSO but with 10% PVP substituted as an alternative CPA.
2. Materials and methods
2.1. Materials
All materials were obtained from Sigma-Aldrich (St. Louis, MO, USA: www.sigmaaldrich.com) or Fisher Scientific (Pittsburgh, PA, USA; www.fisherscientific.com), unless otherwise stated.
2.2. Cell isolation
Protocols were reviewed and approved by the Pennington Biomedical Research Center Institutional Research Board. Lipoaspirate samples were obtained from local surgeons performing elective liposuction procedures. The lipoaspirate samples were localized to subcutaneous adipose tissue sites and were processed within 24 h of sample collection. The tissue was washed three or four times with pre-warmed phosphate-buffered saline (PBS) to remove erythrocytes and white blood cells. Floating adipose tissue fragments were separated from unwanted erythrocytes by removal of the infranatant solution. The tissue was then suspended in an equivalent volume of PBS containing 1% bovine serum albumin and 0.1% collagenase type I (Worthington Biochemical Corporation, Lakewood, NJ, USA; www.worthington-biochem.com) that was pre-warmed to 37 °C. The solution was then placed in a 37 °C incubator with continuous agitation for 1 h to enhance the digestion of the adipose tissue fragments. After digestion, the solution was centrifuged at 300 × g for 5 min at room temperature to separate mature adipocytes from the stromal-vascular fraction (SVF). The solution was then homogenized by shaking and centrifuged again under the same conditions to enhance separation. The supernatant containing lipids and primary mature adipocytes was then aspirated, while the pellet was identified as the stromal-vascular fraction containing adipose-derived adult stem cells. The SVF was suspended in stromal medium [Dulbecco’s modified Eagle’s medium (DMEM)/F-12 Ham’s, 10% FBS, 100 U penicillin/100 µg streptomycin/0.25 µg fungizone] and centrifuged at 300 × g for 5 min at room temperature to remove the remaining collagenase solution. A portion of the cells was resuspended in the various cryopreservation media at a concentration of 1.0 × 106 cells/ml cryopreservation medium. The SVF cells were then frozen at −80 °C in an ethanol-jacketed closed container overnight.
2.3. Preparation of freezing solutions
The CPAs used were: MC (methocel), PVP (average molecular weight 40 000) and DMSO (average molecular weight 78.14). PVP and MC were individually autoclaved at 121 °C for 30 min before being added to DMEM. The DMEM–PVP solutions (PVP concentration of 10%) and DMEM–MC solutions (MC concentration of 1%) were prepared by dissolving weighed PVP and MC in DMEM at room temperature and the solutions were then stored overnight at 4°C to obtain a homogeneous preparation. Concentrations above 1% MC and 10% PVP were found to be highly viscous and hard to handle, and hence were not used in the present study.
2.4. Freezing (and thawing) experiments
As described earlier, the SVF cells in the various media were frozen to −80 °C in an ethanol-jacketed closed container overnight. The temperature/time history experienced by the cells in the ethanaol-jacketed container were measured by using a type-T hypodermic needle thermocouple (Omega Technologies, Stamford, CT, USA). Thermocouple voltages were read by a precision temperature data logger (Veriteq Instruments Inc, Richmond, BC, Canada) and transferred to a personal computer for further reduction and data analysis. The cells were then subsequently stored in liquid nitrogen for at least 2 weeks until use. Prior to the brightfield microscopy and the flow cytometric analysis, individual cryovials of cells were rapidly thawed in a 37 °C water bath (1–2 min agitation), resuspended in the stromal culture medium and seeded into the separate wells of a six-well plate for 24 h of incubation at 37 °C.
2.5. Cell viability and apoptosis/necrosis assessment
A well-established annexin V apoptosis assay was analysed by quantitative flow cytometry. As a chemically-induced apoptotic control, SVF cells were incubated in fresh medium enriched with 40 mm etoposide (24 h). For a necrotic control, SVF cells were incubated for 24 h in fresh medium with 5 mm hydrogen peroxide (H2O2). The no-treatment control consisted of SVF cells treated in fresh medium, free from inducing agents. For each treatment, detached and attached cells were pooled, harvested by trypsinization (0.25% trypsin), washed with 10 ml culture medium and resuspended in 100 µl 1 × annexin-binding buffer (included in annexin V-FITC/PI kit). Approximately 100 µl cell suspension was mixed with 8 µl annexin–V-FITC and 8 µl 100 mg/ml propidium iodide (PI) and incubated in the dark at room temperature for 15 min. Liquid volume was removed by centrifugation and aspiration, and the cells were resuspended by gentle vortexing in 300–500 µl 1× annexin-binding buffer for analysis on the flow cytometer. Apoptotic analyses for SVF cells were performed on a FACS Caliber flow cytometer (BD Biosciences, San Jose, CA, USA), utilizing 488 nm laser excitation and fluorescence emission at 530 nm (FL1) and >575 nm (FL3). Forward and side scatter measurements were made using linear amplification, and all fluorescence measurements were made with logarithmic amplification. A total of 20 000 cells/sample were acquired using Cell Quest software (BD Biosciences).
Apoptosis is characterized by phosphatidylserine (PS) translocation from the inner leaflet to the outer leaflet of the lipid bilayer, while the cell membrane remains intact. Annexin V-positive cells correspond to cells that have experienced PS translocation. PI staining of the cells indicates that the integrity of the cell membrane has been compromised and is used to distinguish living and early apoptotic cells from necrotic cells. The fluorescent dot-plots show three cell populations: live [annexin V-FITC-negative and PI-negative (annexin V− and PI−)], necrotic [(annexin V-FITC-positive and PI-positive (annexin V+ and PI+)] and apoptotic [annexin V-FITC-positive and PI-negative (annexin V+ and PI−)]. Quadrant analysis was performed on the gated fluorescence dot-plot to quantify the percentage of live, necrotic and apoptotic cell populations. The quadrant positions were placed according to the no-treatment control and 5 mm H2O2 necrotic control.
2.6. Adipogenesis
Confluent cultures of SVF cells were induced to adipogenesis by replacing the medium with an adipocyte induction cocktail containing DMEM/F-12 Ham’s with 3% FBS, 33 µm biotin, 17 µm pantothenate, 1 µm bovine insulin, 1 µm dexamethasone, 0.5 mm isobutylmethylxanthine (IBMX), 5 µm rosiglitazone and 100 U penicillin/100 µg streptomycin/0.25 µg fungizone. After 72 h the adipocyte induction medium was replaced with adipocyte maintenance medium, which contains the same components as the induction medium except for IBMX and rosiglitazone. Induced cells were maintained in culture for 9 days, with adipocyte maintenance medium replacement every 3 days. Upon the ninth day, the cultures were washed twice with pre-warmed PBS and fixed in formalin at 4 °C. Adipocyte quantification was determined by staining neutral lipids with oil red O.
2.7. Osteogenesis
Confluent cultures of SVF cells were induced to osteogenesis by replacing the medium with an osteogenic induction cocktail containing DMEM/F-12 Ham’s, 10% FBS, 10 mm β-glycerophosphate, 50 µg/ml sodium ascorbate 2-phosphate and 100 U penicillin/100 µg streptomycin/0.25 µg fungizone. The induced cells were fed fresh osteogenic induction medium every 3 days for 3 weeks. The cultures were then washed with 0.9% sodium chloride solution and fixed in 70% ethanol. Osteoblast quantification was determined by alizarin red staining for calcium phosphate.
3. Results and discussion
The various cooling rates experienced by the cells in the ethanol-jacketed container placed in a −80 °C freezer are shown in Figure 1. The plot suggests that the cells were subjected to different cooling rates at different time points within the ethanol-jacketed container. Ice nucleation was observed around −8 °C and subsequently a cooling rate of ~0.4 °C/min was imposed to a temperature of −15 °C. Further temperature drop occurred at a rate of ~1.2 °C/min to a temperature of ~−50 °C. The cooling rates experienced by the cells then further dropped to 0.4 °C/min before reaching −80 °C.
Figure 1.
Representative cooling rates experienced by the cells in the ethanol-jacketed closed container placed in the −80 °C freezer in DMEM with 10% DMSO. The temperature (°C) experienced by the cells is shown on the y axis while the time (min) is shown on the x axis
As described earlier, the use of DMSO with concentrations in the range 0–10% as the sole CPA in DMEM on the post-freeze/thaw viability, apoptotic and necrotic response of SVF cells was assessed using flow cytometry. Characteristic flow cytometer fluorescence dot-plots for SVF cells frozen/thawed with different concentrations of DMSO, as determined by annexin V staining and PI uptake, are shown in Figure 2. The fluorescence dot-plots for cells frozen in the presence of 2% and 10% DMSO (Figure 2B, F) showed that a majority of the cells were in the lower left quadrant (annexin V− and PI−), corresponding to live cell population. However, at 0% DMSO, the number of cells present in the upper right quadrant (annexin V+ and PI+), which corresponded to a necrotic cell population, increased. It is also important to note that the number of discrete events collected was approximately 20 000 events (or cells)/run, but with 0% DMSO this value was reduced to 8000–9000 events, presumably because of the significantly larger necrotic cells within the population that were collected as debris during cell processing for flow cytometry. Similar population quadrant analysis was performed on all other experimental treatments to obtain quantitative information on the condition of the cells following the freeze/thaw process for the different media combinations investigated as part of this study.
Figure 2.
Characteristic flow cytometer fluorescence dot-plots showing fluorescence-activated cell sorting (FACS) analysis of SVF cells frozen/thawed in the presence of various concentrations (0%, 2%, 4%, 6%, 8% and 10%) of DMSO in DMEM (n = 3 individual donors). (A–F) 0%, 2%, 4%, 6%, 8% and 10% DMSO in DMEM, respectively. The fluorescent dot-plots show three cell populations: live (annexin V-FITC-negative and PI-negative; annexin V− and PI−) necrotic (annexin V-FITC-positive and PI-positive; annexin V+ and PI+) and apoptotic (annexin V-FITC-positive and PI-negative; annexin V+ and PI−). For easier comprehension, the quadrants are labelled within (2A). The quadrant positions were placed according to the no treatment control, 40 µm etoposide apoptotic control and 5 mm H2O2 necrotic control (see text for further details)
The post-thaw results obtained from the most routinely used cryopreservation media, either 80% FCS with 10% DMSO in DMEM or 80% HS with 10% DMSO in DMEM, were used as controls. The post-thaw behaviour obtained for cells frozen using these traditional cryopreservation media is shown in Table 1. The viability of SVF cells cryopreserved in the control medium containing 80% FCS with 10% DMSO in DMEM was ~65 ± 3% (Table 1). Presumably, this routinely used cryopreservation medium produces maximum viability; therefore, these data was used as a control to compare with the data obtained from the freezing media supplemented with either MC or PVP. This initial ~35% reduction in the viability of SVF is attributed to cell damage occurring due to the lipoaspiration and/or the collagenase digestion procedures. The control data also suggest that the choice of the serum (HS or FCS) does not significantly alter the SVF cell survival when frozen/thawed in 10% DMSO and DMEM (Table 1). Additional data from assay experiments (unfrozen cells, apoptotic control treated with epotoside, necrotic cells treated with H2O2) are also shown in Table 1.
Table 1.
The percentage of viable, apoptotic and necrotic stromal vascular fraction (SVF) cells obtained using fluorescence-activated cell sorting (FACS) analysis for cells frozen in 10% DMSO in DMEM media with either 80% fetal calf serum (FCS) or 80% human serum (HS)
| Viable (%) (± SD) |
Apoptotic (%) (± SD) |
Necrotic (%) (± SD) |
|
|---|---|---|---|
| Media with serum | |||
| 80% HS with 10% DMSO in DMEM | 61.9 (± 2.4) | 11.2 (± 3.5) | 21.2 (± 5.0) |
| 80% FCS with 10% DMSO in DMEM | 65.3 (± 3.3) | 10.8 (± 6.9) | 18.6 (± 3.8) |
| Assay controls | |||
| Live (untreated, unfrozen control) | 67.3 (± 12.9) | 12.9 (± 2.4) | 11.5 (± 1.9) |
| Apoptotic (etoposide-treated) | 38.3 (± 9.3) | 32.8 (± 13.4) | 21.5 (± 10.6) |
| Necrotic (H2O2-treated) | 6.3 (± 2.2) | 9.7 (± 2.2) | 79.6 (± 6.3) |
The percentage of viable, apoptotic and necrotic SVF cells obtained using FACS analysis are also shown for untreated (unfrozen, controls), apoptotic control (treated with 40 µm etoposide/ml cell culture medium) and necrotic control (5 mm hydrogen pereoxide, H2O2). The data are from six individual donors.
Figure 3 shows the post-thaw results obtained for SVF cells frozen in DMEM with varying proportions of DMSO (0%, 2%, 4%, 6%, 8% and 10%). Although, the highest percentage of post-thaw survival was achieved with a concentration of 8% DMSO (72.9 ± 3.3%), the post-thaw survival values obtained with DMSO concentrations of 2%, 4%, 6% and 10% were similar (60.1 ± 4.4%, 62.8 ± 4.5%, 65.8 ± 2.4% and 69.8 ± 2.5%, respectively). However, freezing SVF in the absence of DMSO was detrimental to cell survival (the post-thaw cell survival dropped significantly to 11.4 ± 7.4%). Correspondingly, the percentages of apoptotic and necrotic cells were also comparable in DMEM containing 2–10% DMSO. Specifically, the apoptotic cells ranged from 14.1 ± 3.3% (with 8% DMSO) to 20.5 ± 4.1% (with 2% DMSO), while the necrotic cells ranged from 10.4 ± 5.5% (with 8% DMSO) to 16 ± 3.9% (with 4% DMSO). Statistically, the only significant differences in the data were found when the cells were frozen with and without DMSO, i.e. even the presence of 2% DMSO was significant, and this extremely low concentration was able to cryoprotect SVF cells. One possible explanation for this extraordinary cryoprotective ability of DMSO is its ability to stabilize the cell membrane bilayer gel phase rather than the interdigitated gel phase, even at low concentrations (Yamashita et al., 2000; Kennedy et al., 2003; Moldovan et al., 2007; Devireddy 2009).
Figure 3.
The effect of varying the dimethyl sulphoxide (DMSO) concentration (0%, 2%, 4%, 6%, 8% and 10%) on the post-thaw viability (open columns), apoptotic (partially shaded columns) and necrotic (completely shaded columns) response of SVF cells cryopreserved in DMEM. The error bars represent the standard deviation (SD) in the data (n = 3 individual donors). The percentage of cells (viable, apoptotic or necrotic) is shown on the y axis, while the percentage of DMSO in DMEM is shown on the x axis
Figure 4 shows the post-thaw results obtained for SVF cells frozen in DMEM with 1% MC supplemented with either no CPA or 10% HS or 10% FCS or 10% DMSO. As expected, the highest percentage of post-thaw cell survival was found for cells frozen with 10% DMSO (66.0 ± 2.1%) and the lowest values were found for cells frozen with 10% HS or 10% FCS (~21.0 ± 7%). The percentage of cell survival with 1% MC in DMEM was 37.3 ± 4.1% and was approximately half the value obtained earlier for cells frozen with 10% DMSO in DMEM. Thus, replacing DMSO with MC as the CPA leads to a significant reduction (~50%) in the post-thaw cell viability of SVF cells. It is also interesting to see the significant reduction (~50%) in cell viability when 10% HS or 10% FCS is added to the freezing medium when compared to the values obtained in their absence, i.e. the cell survival is further reduced from ~37% with 1% MC in DMEM to ~21% with either 10% HS or 10% FCS in DMEM with 1% MC. Conversely, the percentage of apoptotic (14.3 ± 1.5%) and necrotic (15.6 ± 2.3%) cells was the lowest for the cells frozen in 10% DMSO and 1% MC in DMEM. These values are comparable to the values obtained earlier (see Figure 3) for varying percentages of DMSO in solution (2–10%). The highest percentage of necrotic cells (~50 ± 4%) was found when they were frozen with 1% MC and either 10% HS or 10% FCS in DMEM. Clearly, the data suggest the following: (a) the addition of 1% MC increases the cell survival significantly from ~11% to ~37%, i.e. comparing the data with 100% DMEM (or 0% DMSO from Figure 3) and 1% MC with DMEM; (b) DMSO is more effective in preserving cell viability than MC, i.e. the maximum percantage of cell survival obtained with MC (~37%) is still only half of that obtained with media containing various concentrations of DMSO (~60–70%); (c) the addition of either HS or FCS with MC is extremely deleterious to post-thaw cell viability; (d) MC is significantly more viscous than DMSO and hence is harder to handle and mix with the cells. Thus, these results suggest that MC is not an optimal replacement CPA for DMSO for cryopreserving SVF cells.
Figure 4.
The post-thaw viability (open columns), apoptotic (partially shaded columns) and necrotic (completely shaded columns) response of SVF cells cryopreserved in the presence of 1% methyl cellulose (MC) in DMEM with either (a) no serum or dimethyl sulphoxide (DMSO), or (b) 10% human serum (HS), or (c) 10% fetal calf serum (FCS), or (d) 10% dimethyl sulphoxide (DMSO). The error bars represent SD in the data (n = 3 individual donors). The percentage of cells (viable or apoptotic or necrotic) is shown on the y axis, while the choice of chemical additive (none, 10% HS, 10% FCS or 10% DMSO) in the medium (1% MC in DMEM) is shown on the x axis
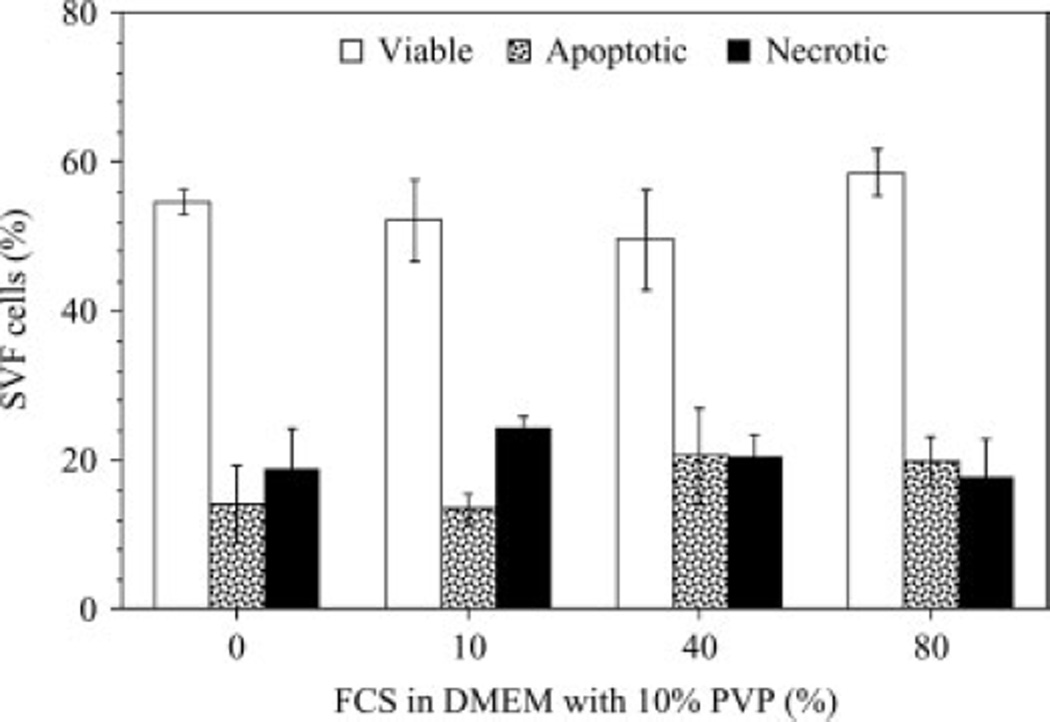
Figure 5 shows the post-thaw results obtained for SVF cells frozen in 10% PVP in DMEM with varying percentages of FCS (0%, 10%, 40% and 80%). Although, the highest percentage of post-thaw cell survival (58.6 ± 3.2%) was achieved with 10% PVP and 80% FCS in DMEM, the corresponding data for 10% PVP with either 40% FCS or 10% FCS or 0% FCS were comparable (49.7 ± 6.7%, 52.3 ± 5.7% and 54.6 ± 1.7%, respectively). Thus, the presence or absence of FCS did not significantly alter the post-thaw viability results for SVF cells frozen in the presence of 10% PVP. This observation is a direct contrast to the results presented earlier with MC (Figure 4). Additional experiments with SVF cells frozen in 10% PVP with DMEM in 10% HS showed the lack of sensitivity of the post-thaw results to the choice of serum (HS or FCS). Specifically, the percentages of viable SVF cells obtained with 10% PVP in DMEM with either 10% HS or 10% FCS in the freezing media were: 53.9 ± 5.5% and 52.3 ± 5.7%, respectively. Similarly, the percentages of apoptotic SVF cells obtained with 10% PVP in DMEM with either 10% HS or 10% FCS in the freezing media were 12.2 ± 2.1% and 13.5 ± 2.5%, respectively. Finally, the percentages of necrotic SVF cells obtained with 10% PVP in DMEM with either 10% HS or 10% FCS in the freezing media were 25.6 ± 4.3% and 24.2 ± 1.8%, respectively. Most significantly, the percentage of post-thaw cell viability (54.6 ± 1.7%) obtained with 10% PVP and DMEM is comparable (although statistically different at the 95% confidence level, using Student’s t-test) to that obtained (~64 ± 3%) with control medium (10% DMEM with 10% DMSO and either 80% FCS or 80% HS). Thus, these results suggest that PVP is a better cryoprotectant than MC for SVF cells and that it is possible to utilize PVP as the CPA instead of DMSO.
Figure 5.
The effect of varying the fetal calf serum (FCS) concentration (0%, 10%, 40%, 80%) on the post-thaw viability (open columns), apoptotic (partially shaded columns) and necrotic (completely shaded columns) response of SVF cells cryopreserved in the presence of 10% PVP in DMEM. The error bars represent SD in the data (n = 3 individual donors). The percentage of cells (viable, apoptotic or necrotic) is shown on the y axis, while the percentage of FCS in the medium (10% PVP in DMEM) is shown on the x axis
To address whether the presence of PVP and the absence of serum and DMSO during cryopreservation affects post-thaw adipogenic and osteogenic differentiation in SVF cells, we utilized toluidine blue, oil red O and alizarin red to stain the undifferentiated SVF cells, adipocytes and osteoblasts, respectively (Figure 6). The SVF cells after 14 days of adipogenic induction and 21 days of osteogenic induction displayed morphological features consistent with adipogenesis or osteogenesis from control (unfrozen) cells (data not shown). Representative photomicrographs in Figure 6 show undifferentiated SVF cells stained positive with toluidine blue (row 1), adipocytes stained positive with oil red O (row 2) and mineralized (osteoblast) cultures stained positive with alizarin red (row 3). Our experiments showed no qualitative morphological differences between the cells cry-opreserved in the presence of either 80% FCS with 10% DMSO in DMEM (column 1) or 0% FCS with 10% DMSO in DMEM (column 2), or 0% FCS with 2% DMSO in DMEM (column 3) or 0% FCS with 10% PVP in DMEM (column 4). More importantly, the results indicated that the adipogenic or osteogenic morphology of the cells cryopreserved in the presence of 10% PVP in DMEM and without any serum (column 4) is consistent with, and no different from, the control experiments with FCS (column 1) and with HS (data not shown).
Figure 6.
Representative phase contrast photomicrographs of SVF cells cultured under untreated (first row, toluidine blue staining), adipogenic (second row, oil red O staining) or osteogenic (third row, alizarin red staining) conditions (n = 3 individual donors). Adipogenic cultures were stained with oil red O 14 days after induction, while osteogenic cultures were stained with alizarin red after 21 days of culture. Images in column 1 represent cells that were cryopreserved in medium containing 80% FCS with 10% DMSO in DMEM. Images in column 2 represent cells that were cryopreserved in medium containing 0% FCS with 10% DMSO in DMEM. Images in column 3 represent cells that were cryopreserved in medium containing 0% FCS with 2% DMSO in DMEM. Images in column 4 represent cells that were cryopreserved in medium containing 0% FCS with 10% PVP in DMEM
The exact mechanism(s) for the cryoprotective action of PVP and MC (high molecular weight polymers) is still not clearly understood. Farrant (1969) advocated that high molecular weight polymers exhibit enhanced colligative properties at higher concentrations, and hence protect cells by lowering the external salt concentrations in a manner similar to low molecular cryoprotectants. Mazur (1970) proposed that polymeric CPAs prevent denaturing of the membranes due to high solute (salt) solutions at low temperatures, either by preventing seeding (nucleation) of the super-cooled water inside the cells or by coating the temperature- or salt concentration-sensitive plasma membranes. In yet another study, Williams (1983) suggested that the cryoprotective efficiency of polymers resides in their ability to alter the physical properties of solutions during freezing, rather than directly protecting and altering the cell membrane characteristics. To further investigate these claims, we have measured the depression in the freezing point of solutions containing PVP, using a vapour pressure osmometer (Thirumala et al., 2008). Typically, the freezing point depression can be correlated by a simple linear relationship with the concentration of solute present within the solution. However, as shown earlier (Lovelock, 1954), the presence of PVP significantly alters the linear relationship that one would expect between the freezing point depression and the concentration of additive. Additionally, cells frozen with PVP experienced significant super-cooling of the extracellular medium. Briefly, based on the measured osmolality, the phase change temperature of DMEM medium containing 10% PVP is −0.6 °C. However, in our freezing experiments (unpublished data) the phase change of the extracellular medium in the presence of PVP occurred at −15 °C, i.e. with a significant super-cooling. This super-cooling might have resulted in the formation of damaging ice crystals and hence the observed lower post-thaw viability of SVF cells frozen with PVP and DMEM when compared with cells frozen with DMSO in DMEM. Further studies are clearly needed to elucidate the exact mechanism by which PVP (and DMSO) is able to protect cells at low concentrations.
4. Conclusion
The results of this study demonstrate: (a) the remarkable cryoprotective ability of DMSO, even at the extremely low concentration of 2% in DMEM; (b) the extreme toxicity of MC to SVF cells; (c) the lack of sensitivity in the post-thaw data to the choice of serum, i.e. either FCS or HS, in the freezing medium; (d) the ability to successfully cryopreserve SVF cells in a freezing medium containing 10% PVP and DMEM (i.e. a freezing medium without either serum or DMSO). Most importantly, the SVF cells frozen/thawed solely in the presence of PVP (i.e. in the absence of both DMSO and serum) displayed similar phenotype and expression patterns of biomarkers (morphology and growth/differentiation characteristics) when compared to the cells cryopreserved in 80% serum with 10% DMSO in DMEM. We could thus hope that serum- (and DMSO)-free cryopreservation is a distinct possibility for adipose stem cell lines in the near future, and believe that using PVP as the sole cryoprotectant for freezing storage of SVF cells (and consequently, adult stem cells) could enhance and encourage their use in a wide range of tissue-engineering and regenerative medicine problems.
Acknowledgements
S.T. is supported by an EDA fellowship from LSU. J.M.G. was partially supported by a CNRU Center Grant (No. 1P30 DK072476) entitled ‘Nutritional programming: environmental and molecular interactions’, sponsored by NIDDK. The authors thank Dr Elizabeth Clubb and Dr James Wade at the Pennington Biomedical Research Center (PBRC) for supplying the liposuction aspirates, and their many patients for consenting to participate in this protocol; and Marilyn Dietrick, of the LSU School of Veterinary Medicine Flow Cytometry Core Facility, and Gang Yu MS, of the Stem Cell Biology Laboratory and CNRU Molecular Mechanism Core, for their technical assistance.
Footnotes
Conflict of interest statements
Dr Gimble has consulted for Toucan Capital and its adult stem cell companies.
References
- Awad HA, Wickham MQ, Leddy HA, et al. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Beaujean F, Hartmann O, Kuentz M, et al. A simple, efficient washing procedure for cryopreserved human hematopoietic stem cells prior to reinfusion. Bone Marrow Transpl. 1991;8:291–294. [PubMed] [Google Scholar]
- Benson EE, Bremner D. Oxidative stress in the frozen plant: a free radical point of view. In: Fuller BJ, Lane NJ, Benson EE, editors. Life in the Frozen State. Boca Raton, FL: CRC Press; 2004. pp. 205–242. [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Devireddy RV, Thirumala S, Gimble JM. Cellular response of adipose-derived passage-4 adult stem cells to freezing stress. J Biomech Eng. 2005;127:1081–1086. doi: 10.1115/1.2073673. [DOI] [PubMed] [Google Scholar]
- Devireddy RV. Statistical thermodynamics of biomembranes. Cryobiology. 2009 doi: 10.1016/j.cryobiol.2009.05.001. (in press;). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J. Is there a common mechanism of protection of living cells by polyvinylpyrrolidone and glycerol during freezing? Nature. 1969;222:1175–1176. doi: 10.1038/2221175a0. [DOI] [PubMed] [Google Scholar]
- Franks F, Asquith MH, Hammond CC, et al. Polymeric cryoprotectants in the preservation of biological ultrastructure. J Microsc. 1977;110:223–238. doi: 10.1111/j.1365-2818.1977.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett. 2004;25(6):375–388. [PubMed] [Google Scholar]
- Fuller R, Devireddy RV. The effect of two different freezing methods on the immediate post-thaw membrane integrity of adipose tissue-derived stem cells. Int J Heat Mass Transf. 2008;51:5650–5654. [Google Scholar]
- Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- Goh BC, Thirumala S, Kilroy G, et al. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322–324. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- Halvorsen YD, Franklin D, Bond AL, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- Hicok KC, Du Laney TV, Zhou YS, et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- Hubálek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46:205–229. doi: 10.1016/s0011-2240(03)00046-4. [DOI] [PubMed] [Google Scholar]
- Kang SK, Putnam L, Dufour J, et al. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells. Stem Cells. 2004;22(7):1356–1372. doi: 10.1634/stemcells.2004-0023. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Long CJ, Hmel PJ, et al. The interaction of DMSO with model membranes. II. Direct evidence of DMSO binding to membranes: an NMR study. J Liposome Res. 2003;13:259–267. doi: 10.1081/lpr-120026391. [DOI] [PubMed] [Google Scholar]
- Lovelock JE. The protective action of neutral solutes against haemolysis by freezing and thawing. Biochem J. 1954;56:265–270. doi: 10.1042/bj0560265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Physical factors implicated in the death of microorganisms at subzero temperatures. Ann NY Acad Sci. 1970;85:610–629. doi: 10.1111/j.1749-6632.1960.tb49986.x. [DOI] [PubMed] [Google Scholar]
- Merten OW, Petres S, Couve E. A simple serum-free freezing medium for serum-free cultured cells. Biologicals. 1995;23:185–189. doi: 10.1006/biol.1995.0030. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Moldovan D, Pinisetty D, Devireddy RV. Molecular dynamics simulation of pore growth in lipid bilayer membranes in the presence of edge-active agents. Appl Phys Lett. 2007;91:204104. [Google Scholar]
- Rodriguez AM, Elabd C, Delteil F, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315:255–263. doi: 10.1016/j.bbrc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Rodriguez L, Velasco B, Garcia J, et al. Evaluation of an automated cell processing device to reduce the dimethylsulfoxide from hematopoietic grafts after thawing. Transfusion. 2005;45:1391–1397. doi: 10.1111/j.1537-2995.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumala S, Zvonic S, Floyd E, et al. The effect of various freezing parameters on the immediate post-thaw membrane integrity of adipose tissue-derived adult stem cells. Biotech Prog. 2005a;21:1511–1524. doi: 10.1021/bp050007q. [DOI] [PubMed] [Google Scholar]
- Thirumala S, Gimble JM, Devireddy RV. Transport phenomena during freezing of adipose tissue-derived adult stem cells. Biotechnol Bioeng. 2005b;92:372–383. doi: 10.1002/bit.20615. [DOI] [PubMed] [Google Scholar]
- Thirumala S, Gimble JM, Devireddy RV. 19th National and 8th International ISHMT–ASME Heat and Mass Transfer Conference. Hyderabad, India: CD-ROM Publication; 2008. Post-freeze response of human adipose tissue-derived adult stem cells (huASCs) in the presence of polyvinylpyrrolidone (PVP) [Google Scholar]
- Vogel G. Can old cells learn new tricks? Science. 2000;287:1418. doi: 10.1126/science.287.5457.1418. [DOI] [PubMed] [Google Scholar]
- Wall ME, Bernacki SH, Loboa EG. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007;13(6):1291–1298. doi: 10.1089/ten.2006.0275. [DOI] [PubMed] [Google Scholar]
- Williams RJ. The surface activity of PVP and other polymers and their antihemolytic capacity. Cryobiology. 1983;20:521–526. doi: 10.1016/0011-2240(83)90040-8. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kinoshita K, Yamazaki M. Low concentration of DMSO stabilizes the bilayer gel phase rather than the interdigitated gel phase in dihexadecylphosphatidylcholine membrane. Biochim Biophys Acta. 2000;1467:395–405. doi: 10.1016/s0005-2736(00)00237-6. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]