Abstract
Diagnosis of pulmonary dysfunction is currently almost entirely based on a vast series of physiological changes, but comprehensive research is focused on determining biomarkers for early diagnosis of pulmonary dysfunction. Here we discuss the use of biomarkers of lung injury in cardiothoracic surgery and their ability to detect subtle pulmonary dysfunction in the perioperative period. Degranulation products of neutrophils are often used as biomarker since they have detrimental effects on the pulmonary tissue by themselves. However, these substances are not lung specific. Lung epithelium specific proteins offer more specificity and slowly find their way into clinical studies.
1. Introduction
Cardiothoracic surgery, defined as surgery on the heart and/or the lungs, has been performed since the 1950s and coronary artery bypass graft surgery, for instance, has increased to 415.000 procedures a year in the US (US Hospital Discharge Survey, 2009). From the beginning these procedures were associated with postoperative pulmonary complications [1]. These complications can partly be attributed to the unique aspects of cardiothoracic surgery, such as the sternotomy, cardioplegia, and use of cardiopulmonary bypass (CPB) with the exclusion of lung circulation. Despite continuous improvements in materials and surgical techniques, cardiothoracic surgery still causes lung injury, dysfunction, and delay of pulmonary recovery [2].
Lung dysfunction is common after cardiothoracic surgery [2] and varies between hypoxemia in all patients [3] and acute respiratory distress syndrome (ARDS) in 2% of the patients [4]. Lung dysfunction by itself may not influence the postoperative course of a patient; only when lung dysfunction evolves into a lung complication it becomes clinically relevant. Common pulmonary complications following cardiothoracic surgery are pleural effusion (38%) [5], atelectasis (20%) [6], phrenic nerve paralysis (32%) [7], and pneumonia (5%) [8]. Given the high incidence of pulmonary complications, it is important to monitor the onset and course of postoperative lung dysfunction.
Currently there is no gold standard for quantifying postoperative lung injury and dysfunction. Reported are a vast series of physiological changes (alveolar-arterial oxygen pressure difference, intrapulmonary shunt, degree of pulmonary edema, pulmonary compliance, and pulmonary vascular resistance) and measurement of inflammatory markers such as neutrophil elastase, myeloperoxidase, and interleukins [2]. Lung dysfunction, in the form of ARDS, is determined by specific diagnostic criteria recently revised according to the “Berlin criteria” [9]. Acute respiratory distress syndrome is characterized by the acute onset of lung injury within one week of a known clinical insult, bilateral opacities on chest imaging, respiratory failure not fully explained by cardiac failure or fluid overload, and decreased arterial PaO2/FiO2 ratio. Furthermore, ARDS can be divided into “mild” (PaO2/FiO2 ratio: 201–300 mmHg), “moderate” (PaO2/FiO2 ratio: 101–200 mmHg), and “severe” (PaO2/FiO2 ratio: ≤100 mmHg). Mild ARDS is comparable to the previous acute lung injury (ALI) definition of the American-European Consensus Conference [10].
Biomarkers, whether in serum, urine, or exhaled breath, have found limited use for identifying and quantifying postoperative lung injury. In this review, we focus on the field of cardiothoracic surgery, where serum biomarkers of lung injury are being used as a surrogate marker for (sub)clinical lung injury.
2. Postoperative Pulmonary Dysfunction
A distinction between pulmonary dysfunction and pulmonary complications following cardiothoracic surgery should be made [11], where pulmonary dysfunction refers to alterations in pulmonary function, such as shallow respiration and hypoxemia, and where pulmonary complications also require associated clinical findings such as atelectasis and chest radiographic infiltrates.
3. Biomarkers
Biomarkers are measurable parameters that reflect the state of a biological process. An often-cited definition of a biomarker is as follows: “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention [12].” Biomarkers are used to screen for, diagnose, or monitor disease and are also used to assess a therapeutic response [13]. The term biomarker is typically used for molecular biomarkers measured in blood, which is also the application we are focusing on in this review.
In cardiothoracic surgery research, biomarkers can serve as a surrogate endpoint for evaluating new procedures and/or medical equipment, giving more insight into a cellular level. Ideally biomarkers have features such as high sensitivity, high specificity, known reference values, and good predictive values. In case of a surrogate endpoint, biomarker values should have a good correlation with a clinical endpoint. However, when taking serial measurements during cardiothoracic surgery each patient serves as his or her own control making features such as high sensitivity and specificity less important.
Since sample sizes are usually limited in cardiothoracic surgery research, a biomarker could reveal a difference between study groups, whereas differences would not appear when clinical endpoints are considered. Another benefit of biomarkers is that they could give insight into the mechanism of disease, since the measurement is closer to the exposure/intervention of interest and it may be easier to relate causally than more distant clinical events. Being more sensitive, biomarkers could indicate subclinical benefits in a pilot study, supporting larger clinical studies afterwards.
4. Classification
The pathophysiology of postoperative lung injury is characterized by injury to the alveolar-capillary membrane, inflammation, increased permeability, and pulmonary edema [14]. Accordingly, biomarkers of lung injury can be classified as such. However, in this review we have focused on biomarkers originating from the alveolar compartment which can be measured in the circulation. We have organized the biomarkers using their cell of origin.
5. Polymorphonuclear Neutrophils
Polymorphonuclear neutrophils (PMNs) play an important role in lung injury following cardiothoracic surgery, as their release products can be detrimental to lung tissue. Moreover, the lungs harbour a number of leukocytes equal to or even more than the number of leukocytes present in the systemic circulation [15]. This is usually referred to as the marginated pool, which acts as a natural reservoir of leukocytes and is in dynamic equilibrium with the systemic circulation.
5.1. Neutrophil Elastase
Upon an inflammatory response PMNs degranulate and release an abundance of cytotoxic substances, such as serine proteases, metalloproteases, peroxidases, and reactive oxygen species (Figure 1) [16]. One of the serine proteases is neutrophil elastase (NE). Besides being a biomarker, NE is an enzyme that has an active role in the development of lung injury. It can degrade components of the endothelial basement membrane, such as elastin and collagen [17]. This has been shown by a loss in integrity of the endothelial vascular barrier, resulting in increased permeability of the alveolar-capillary membrane [18]. Neutrophil elastase is thought to hydrolyse junction proteins such as cadherins, which maintain cell-cell adhesion and diminish barrier function. Similarly, it has been shown that NE can disrupt the epithelial barrier [19]. Taken together, NE can be responsible for protein leakage from the blood stream to the alveolus and vice versa.
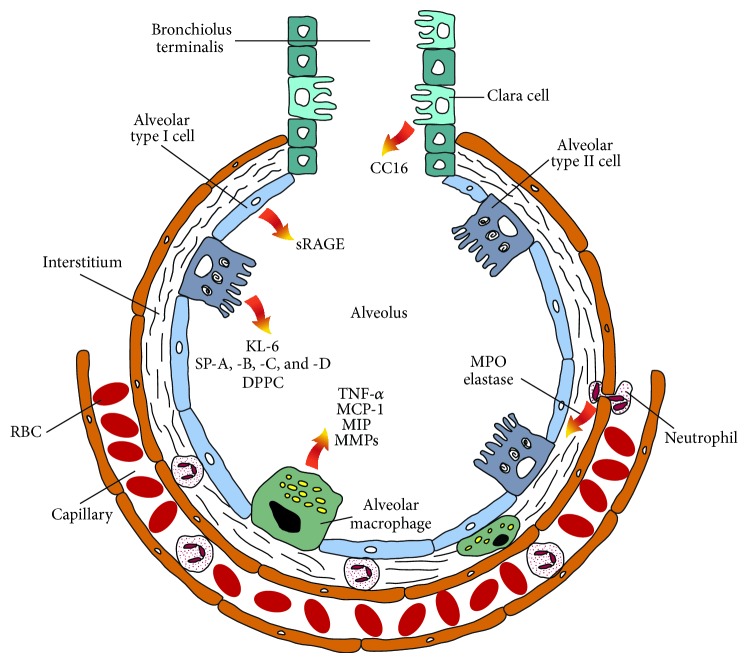
Figure 1.
Schematic representation of an alveolus with its various cell types and their secretion products which may serve as lung injury biomarkers.
Initially, NE was mostly used as a biomarker for the activation of PMNs in vivo after cardiopulmonary bypass [20], since extracorporeal circulation activates the complement system which in turn activates the PMNs [21].
A positive correlation has been observed between NE plasma concentrations after CPB and postoperative respiratory function, by changes in the respiratory index and increases in the intrapulmonary shunt [22]. In another study a positive correlation was found between the NE plasma concentration and the alveolar-arterial oxygen gradient and pulmonary vascular resistance [23].
In addition NE has been used to study the effects of leukocyte depletion during cardiopulmonary bypass [24, 25], NE inhibitors [26], pump types [27], and biocompatibility of leukocyte and fat removal filters [28].
Although NE can be a valuable biomarker in assessing PMNs induced lung injury, it is still only a measure of PMNs activation and not a specific lung biomarker.
5.2. Myeloperoxidase
Myeloperoxidase (MPO) is a peroxidase enzyme stored in the azurophilic granules of PMNs. Its primary function is to kill microorganisms in PMNs by forming halide derived oxidants in the phagosome [29]. Ischemia during cardiopulmonary bypass results in endothelial activation upon reperfusion [30]. The activated endothelium and the expression of specific surface adhesion molecules promote adherence of phagocytes [31], upon which MPO will be released. MPO measured in blood is a marker for degranulation of PMNs in plasma and for the infiltration of PMNs in tissue [32]. Besides being a marker for PMN degranulation, MPO is often implicated in lung injury. Pulmonary tissue is often the target of activated PMNs when it is being reperfused; therefore MPO concentrations are thought to be a marker of pulmonary injury.
During CPB, pulmonary endothelial permeability correlated with postoperative serum concentrations of MPO, implicating neutrophils having a central role in the development of lung injury [33]. However, we and others have shown that MPO shows a steep increase right after the administration of heparin [34, 35]. This increase of MPO after heparin administration is explained by liberation of MPO bound to the vessel wall [36], which suggests that an increase in plasma MPO does not necessarily represent activation/degranulation of leukocytes. This would also implicate that MPO is of limited use as a biomarker for assessing lung injury after CPB, which requires high dose heparin anticoagulation.
6. Lung Epithelium Specific Proteins
6.1. Soluble Receptor for Advanced Glycation End Products (sRAGE)
The receptor for advanced glycation end products (RAGE) was originally characterized for its ability to bind glycation end products of a carbohydrate to a protein. Besides advanced glycation end products, RAGE has the ability to bind several other ligands, for example, amphoterins, S100 proteins, Mac-1, phosphatidylserine, and complement C3a [37]. Expression of RAGE is encountered on multiple cell types such as smooth muscle cells, macrophages, and endothelial cells, but it is also highly expressed in the alveolar type I cells (Figure 1) of the lungs [38].
Soluble forms of RAGE can be formed by proteolytic cleavage of full length RAGE by metalloproteinases or by formation of a splice variant and can be measured in the blood stream [39]. Accordingly, this led to the potential application of (s)RAGE as a lung injury marker of alveolar type I cells. The function of circulating sRAGE is being investigated in various (clinical) studies and is not yet completely elucidated. However, sRAGE is thought to contribute to the removal and/or detoxification of proinflammatory products [39].
Indeed it has been shown that sRAGE is an injury marker of alveolar type I cells [40]. Uchida et al. found in patients with ALI that plasma concentrations were significantly higher than in patients with hydrostatic pulmonary edema or in healthy controls. Increased sRAGE plasma concentrations have also been associated with the use of CPB and mechanical ventilation in patients undergoing elective coronary artery bypass grafting [41]. More recently, Tuinman et al. showed that sRAGE plasma concentrations increased following valvular and/or coronary artery surgery and that they depicted an association with pulmonary leak index, indicating increased permeability of the alveolar-capillary membrane [42]. In young children, plasma concentrations of sRAGE were found to be an independent predictor of ALI after cardiac surgery with CPB [43]. Furthermore, in children as well as in adults increased plasma concentrations of sRAGE were associated with lower PaO2/FiO2 ratio, a higher radiographic lung injury score, longer mechanical ventilation time, and longer intensive care unit length of stay [43, 44].
Preoperative measurements of sRAGE are also of value. In a study where patients underwent elective cardiac surgery, preoperative sRAGE plasma concentrations were associated with duration of critical illness and length of hospital stay [45]. Furthermore, sRAGE was found to be an independent predictor of length of hospital stay.
Calfee et al. showed that sRAGE plasma concentrations measured four hours after allograft reperfusion were associated with poor short term outcome of lung transplantation, as indicated by longer duration of mechanical ventilation and longer intensive care unit length of stay [46]. This finding was supported by another study where an association was found between plasma concentrations of sRAGE and primary graft dysfunction at 6 and 24 hours following lung transplantation [47]. Furthermore an association between sRAGE plasma concentrations measured at 6 and 24 hours following transplantation and mechanical ventilation time was found. The same authors also established an association between sRAGE plasma levels measured at both 6 and 24 h postoperatively with long-term risk for bronchiolitis obliterans syndrome [48].
6.2. Clara Cell Secretory Protein
Clara cells are secretory epithelial cells lining the pulmonary airways (Figure 1). The exact role of these cells still remains unclear, although they are implicated in having a role in protecting and repairing the bronchial epithelium [49]. Clara cells are mainly located in the respiratory bronchioles and they have granules containing various proteins. One of these secretory proteins is Clara cell 16 kD secretory protein (CC16), which is referred to in literature by various names such as uteroglobin (UG), blastokinin, Clara cell secretory protein (CCSP), Clara cell-specific 10 kD protein (CC10), and secretoglobin 1A member 1 (SCGB1A1).
Clara cell secretory protein is believed to play a role in reducing inflammation of the airways [50] and protecting the respiratory tract against oxidative stress [51]. It is present in increasing density from the trachea to terminal bronchioles. Although there is evidence of extrapulmonary synthesis of the CC16 in the prostate, endometrium, and the kidney, these concentrations are on average twenty times lower than in the lungs [52]. This is the reason why CC16 is primarily ascribed to the respiratory tract and why it is considered to be lung specific.
With stable baseline serum concentrations of 10–20 ng/mL, an increase in serum is ascribed to injury to the alveolar-capillary membrane. When the membrane is known to be intact it could be related to the integrity of the Clara cell or the production and clearance of CC16.
Serum concentrations of CC16 have been associated with injury of the alveolar-capillary membrane and are nowadays often used as a biomarker of injury to the alveolar-capillary membrane in different models, such as ALI/ARDS [53, 54], cardiogenic pulmonary edema [54], chest trauma [55], chronic obstructive pulmonary disease [56, 57], primary graft dysfunction (PGD) [58], and injury due to fire exposure [59].
However, in the setting of cardiothoracic surgery CC16 has not often been used as a lung injury marker. Serum CC16 concentrations have been associated with bronchiolitis obliterans syndrome after lung transplantation [60] and primary graft dysfunction after lung transplantation [58]. In a more recent study, the same authors showed that even higher preoperative serum CC16 concentrations, measured in the recipient, were associated with primary graft dysfunction after lung transplantation [61]. Additionally, CC16 has been utilized as a lung injury marker for comparison of mechanical ventilation strategies during various surgical procedures [53], for comparison of a mini-extracorporeal circuit versus a conventional cardiopulmonary bypass [62–64], and for evaluation of pulsatile flow during CPB on lung function [65]. More recently, we have shown that CC16 concentrations correlate with pulmonary dysfunction (as indicated by the alveolar-arterial oxygen gradient) during cardiothoracic surgery and that it was possible to differentiate between off-pump and on-pump coronary artery bypass grafting [34]. In our opinion, given its small size which facilitates diffusion into the blood, this is a sensitive and very useful marker for detecting subclinical injury to the alveolar-capillary membrane.
6.3. Surfactant Proteins
Pulmonary surfactant is the main fraction of the epithelial lining fluid in the lungs. Its main function is to lower surface tension between air and the alveoli and thereby to prevent alveolar atelectasis at the end of expiration [66]. Pulmonary surfactant consists of lipids (90%) and proteins (5–10%). Type II alveolar epithelial cells are mainly responsible for synthesis and secretion of pulmonary surfactant (Figure 1), and before surfactant is secreted it is stored in organelles called “lamellar bodies.” The lipids of surfactant are mainly phospholipids, with phosphatidylcholine being the most abundant. Saturated phosphatidylcholine largely consists of dipalmitoylphosphatidylcholine (DPPC), which accounts for approximately 40% of total lipids and is the major surface-active component.
The protein fraction of surfactant is of more interest for this review. This fraction consists of four different surfactant proteins, SP-A, SP-B, SP-C, and SP-D. Surfactant proteins B (14 kDa) and C (6 kDa) are hydrophobic and are involved in phospholipid packaging, organization of surfactant, and lowering the surface tension at the air-liquid interface [67, 68]. Via its interaction with DPPC, SP-B has been considered to stabilize the phospholipid monolayer. Similarly, SP-C is also thought to be involved in stabilizing the phospholipid layers that form during film compression at low lung volumes [69].
The hydrophilic surfactant proteins, SP-A and SP-D, are predominantly involved in the innate host-defence system of the lung [70] and belong to the collectin family (along with mannose-binding lectin). They are assembled as a trimeric structure with the carbohydrate recognition domain connected to a collagenous domain [71]. The carbohydrate recognition domain has a high affinity for clustered oligosaccharides commonly found on the surface of viruses, bacteria, yeast, and fungi, which can lead to agglutination, phagocytosis, and removal by macrophages and neutrophils or by direct bacteriostatic and fungistatic effects [72].
So far, the use of surfactant protein leakage in blood during cardiothoracic surgery is limited. Agostoni et al. evaluated SP-B as a lung injury marker after elective coronary artery bypass grafting with the use of cardiopulmonary bypass [41]. Immediately after surgery, they found a fourfold increase of plasma SP-B, which returned to baseline within 48 hours. The authors concluded that SP-B could be a sensitive and rapid biomarker of lung distress. Unfortunately, due to small sample size and relatively healthy patients, they could not relate the change in SP-B to severity of lung injury. The same group has, however, shown that plasma SP-B levels are related to alveolar gas diffusion showing a link between SP-B plasma levels and injury to the alveolar-capillary membrane [73].
Sims et al. evaluated the use of SP-D as a lung injury marker in patients undergoing lung transplantation [74]. They found that SP-D serum concentrations were higher in idiopathic pulmonary fibrosis than in cystic fibrosis, chronic obstructive pulmonary disease, or pulmonary hypertension. During transplantation they found that SP-D concentrations decreased. However, postoperative values were higher in single lung transplantation as opposed to the bilateral lung transplantation. The authors suggested that postoperative SP-D concentrations were more likely to be determined by the inflamed native lung as opposed to the allograft, leaving the native lung as the source for SP-D translocation.
Determann et al. have used circulating plasma concentrations of SP-A and SP-D to evaluate mechanical ventilation strategies where a lower tidal volume was used [75]. They did not find differences in plasma concentrations of these surfactant proteins, which was consistent with clinical data as none of the patients showed signs of advanced lung injury.
Shah et al. used plasma SP-D, among other biomarkers, to better discriminate clinically graded primary graft dysfunction and to predict 90-day mortality after lung transplantation [76]. They found that SP-D together with plasminogen activator inhibitor-1 plasma concentrations, measured 24 h after transplantation, had an area under the curve of 0.76 for predicting grade 3 PGD in the first 72 h after transplantation. Furthermore, SP-D significantly increased prediction over PGD grading alone in 90-day mortality, although the other evaluated biomarkers performed even better.
In our experience SP-D can be a valuable marker during cardiothoracic surgery: we found that SP-D concentrations correlated with pulmonary (dys) function and that it was possible to differentiate surgical procedures (off-pump versus on-pump) [34].
6.4. Krebs von den Lungen 6
Krebs von den Lungen 6 (KL-6) is a mucinous sialylated sugar chain on human Mucin 1 [77]. Mucin 1 is a transmembrane protein with an extracellular domain, containing tandem repeat units that are heavily glycosylated. Mucins line the apical surface of epithelial cells in the bronchi, bronchioles, and alveoli where KL-6 is mainly expressed on alveolar type II cells [78] and expression is upregulated on regenerating alveolar type II cells [79]. KL-6 can be found in bronchoalveolar lavage fluid or in serum, and concentrations are elevated in patients with interstitial lung diseases, such as pulmonary sarcoidosis [80, 81]. Additionally, KL-6 seems to be a valuable biomarker in diagnosing bronchiolitis obliterans syndrome after lung transplantation [82, 83].
Although KL-6 plasma concentrations have been used as a marker of disease activity in a variety of respiratory illnesses, the use of this marker in cardiothoracic surgery remains limited. There is one report where it has been used for comparing between a mini cardiopulmonary bypass system and a conventional bypass system, but it failed to detect a difference between the two systems [63].
7. Inflammatory Secretion Products
The inflammatory secretion products discussed here are produced by a broad range of cell types; however in the alveoli the macrophage is one of the major sources. Alveolar macrophages are located at the luminal interface of the alveoli or in the interstitium and remove (dust) particles and/or microorganisms. Since the lungs are in contact with the outer world, they are exposed to a vast array of pathogens, chemicals, gasses, and particles. Besides a mucociliary layer for removal of these substances, alveolar macrophages are important for “cleaning” and defending the alveolar-capillary membrane. Upon activation, macrophages remove pathogens and foreign substances by phagocytosis and simultaneously secrete mediators of inflammation and complement proteins. Activated macrophages can be divided by activation state; these are known as M1 (or classically activated macrophages) and M2 (or alternatively activated macrophages) [84, 85]. While the M1 macrophages promote inflammation, extracellular matrix destruction, and apoptosis, the M2 macrophages promote extracellular matrix construction, cell proliferation, and angiogenesis. These two types of activation come with their own characteristic secretory profile of (anti)inflammatory cytokines, chemokines, and proteolytic enzymes. On the one hand, M1 macrophages will release proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α [86, 87]. Additionally, the chemokines IL-8, IL-10, MIP-1α, and MIP-1β and the matrix metalloproteases 1, 2, 7, 8, and 12 are released [88], which can degrade collagen, elastin, fibronectin, and other extracellular matrix components. On the other hand, M2 macrophages will release chemokines CCL17, CCL18, and CCL22 along with the anti-inflammatory cytokines Il-10 and TGF-β [89, 90].
In the setting of cardiothoracic surgery, with its more acute characteristics, the secretory products of the M1 macrophages are most interesting for assessing lung injury. During cardiothoracic surgery the lungs experience ischemia/reperfusion when cardiopulmonary bypass is used. Ischemia/reperfusion is known to be a strong stimulus to M1 macrophages [91, 92], upon which the aforementioned proinflammatory substances are released. These proinflammatory substances could be valuable predicting biomarkers for lung injury and/or lung dysfunction. And indeed, proteomic analysis showed that isolated alveolar macrophages, harvested during the course of ALI/ARDS, had an upregulated inflammatory profile [93]. Amongst these upregulated proteins was cathepsin B, a lysosomal cysteine proteinase, which the authors suggested to be a biomarker for early diagnoses of ALI/ARDS. During cardiothoracic surgery, however, this protein has not yet been used as a biomarker for lung injury.
Monocyte chemotactic protein 1 (CCL2), primarily secreted by monocytes, macrophages, and dendritic cells, was associated with complicated inflammatory lung or renal injury in patients undergoing primary elective coronary artery bypass grafting [94]. During lung transplantation, MCP-1 and interferon gamma-induced protein 10 (IP-10) were associated with the development of primary graft dysfunction, and from 6 to 72 hours following transplantation MCP-1 and IP-10 concentrations were significantly higher in patients with primary graft dysfunction [95]. In another lung transplantation study it was shown that interleukins 6, 8, and 10 were also associated with primary graft dysfunction [96], where IL-10 peaked at the start of reperfusion and IL-6 and IL-8 peaked 4 hours after transplantation.
In patients with moderate chronic obstructive pulmonary disease undergoing aortic valve surgery, leukocyte filtration during CPB resulted in lower plasma concentrations of IL-6, IL-8, and TNF-α from CPB discontinuation till 72 h postoperatively [97]. Furthermore, the authors found a linear correlation between IL-6 and TNF-α with the alveolar-arterial oxygen gradient (Aa-O2 gradient) and an inverse linear correlation between IL-6 and IL-8 with the arterial blood oxygenation (PaO2/FiO2).
8. Limitations
Besides the advantages described earlier, there are some limitations to the use of biomarkers. One limitation is the high variability in measuring biomarkers, which makes comparing studies more difficult. Most of the variability can be attributed to preanalytical and/or analytical variability [98], where preanalytical variability refers to stability over time and biological variability (e.g., age, sex, and ethnicity), and analytical variability refers to the performance of the test in the laboratory (validity, sensitivity, specificity, and reproducibility, amongst others). The high analytical variability is illustrated by a study where factors influencing the measurement of plasma SP-D by ELISA were examined [99]. It was found that the ELISA configuration (different manufactures) and the anticoagulant used could have serious effects on the measured SP-D concentration. For instance, the use of EDTA instead of heparin reduced the measured SP-D concentration by 50%.
Timing is critical when measuring biomarkers. Most biomarkers reviewed here show a postoperative increase. However, this increase can be of short duration, preventing possible detection when the time points for sampling are not optimally chosen. For instance, we studied SP-D and CC16 in patients undergoing either on- or off-pump coronary artery bypass grafting [34]. The largest difference between these two groups was at the end of CPB, while one hour after arrival on the intensive care unit the difference between these biomarkers was no longer significant. Having to sample many time points limits the cost effectiveness of biomarkers.
Failure to identify factors that can influence the measurement of a biomarker can lead to confounding effects. These effects can be patient characteristics, such as age, sex, weight, and use of medication, although groups are usually balanced for these potential confounding effects. An effect which, for instance, is often overlooked is the stability of a biomarker when it is stored for a prolonged period of time. When the inclusion of a study takes months or even years and the biomarker degrades when it is stored, large differences in measured biomarker concentrations between the first included patient and the last included patient can occur.
9. Concluding Remarks
In this review, we discussed different biomarkers to identify lung injury after cardiothoracic surgery, most often with the use of cardiopulmonary bypass. Though many biomarkers for lung injury are known, they are not often incorporated in clinical studies. For the several good biomarkers available for quantifying lung injury after cardiothoracic surgery, the clinical applications are significant. They enable early detection of patients with subtle injury when they are adequately sensitive and specific. In addition, it would assist in the development of improved surgical techniques to prevent injury after cardiothoracic surgery. For this purpose a panel of biomarkers is most informative, especially when biomarkers for alveolar types I and II cell injury are incorporated.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kolff W. J., Effler D. B., Groves L. K., Hughes C. R., McCormack L. J. Pulmonary complications of open-heart operations: their pathogenesis and avoidance. Cleveland Clinic Journal of Medicine. 1958;25(2):65–83. doi: 10.3949/ccjm.25.2.65. [DOI] [PubMed] [Google Scholar]
- 2.Ng C. S. H., Wan S., Yim A. P. C., Arifi A. A. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121(4):1269–1277. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 3.Taggart D. P., El-Fiky M., Carter R., Bowman A., Wheatley D. J. Respiratory dysfunction after uncomplicated cardiopulmonary bypass. The Annals of Thoracic Surgery. 1993;56(5):1123–1128. doi: 10.1016/0003-4975(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 4.Christenson J. T., Aeberhard J.-M., Badel P., et al. Adult respiratory distress syndrome after cardiac surgery. Cardiovascular Surgery. 1996;4(1):15–21. doi: 10.1016/0967-2109(96)83778-1. [DOI] [PubMed] [Google Scholar]
- 5.Vargas F. S., Cukier A., Terra-Filho M., Hueb W., Teixeira L. R., Light R. W. Relationship between pleural changes after myocardial revascularization and pulmonary mechanics. Chest. 1992;102(5):1333–1336. doi: 10.1378/chest.102.5.1333. [DOI] [PubMed] [Google Scholar]
- 6.Bonacchi M., Prifti E., Giunti G., Salica A., Frati G., Sani G. Respiratory dysfunction after coronary artery bypass grafting employing bilateral internal mammary arteries: the influence of intact pleura. European Journal of Cardio-Thoracic Surgery. 2001;19(6):827–833. doi: 10.1016/s1010-7940(01)00695-9. [DOI] [PubMed] [Google Scholar]
- 7.Efthimiou J., Butler J., Woodham C., Benson M. K., Westaby S. Diaphragm paralysis following cardiac surgery: role of phrenic nerve cold injury. Annals of Thoracic Surgery. 1991;52(4):1005–1008. doi: 10.1016/0003-4975(91)91268-z. [DOI] [PubMed] [Google Scholar]
- 8.Coleman C. I., Lucek D. M., Hammond J., White C. M. Preoperative statins and infectious complications following cardiac surgery. Current Medical Research and Opinion. 2007;23(8):1783–1790. doi: 10.1185/030079907X210570. [DOI] [PubMed] [Google Scholar]
- 9.Ranieri V. M., Rubenfeld G. D., Thompson B. T., et al. Acute respiratory distress syndrome: the Berlin definition. Journal of the American Medical Association. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Bernard G. R., Artigas A., Brigham K. L., et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Medicine. 1994;20(3):225–232. doi: 10.1007/bf01704707. [DOI] [PubMed] [Google Scholar]
- 11.Wynne R., Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. The American Journal of Critical Care. 2004;13(5):384–393. [PubMed] [Google Scholar]
- 12.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 13.Rifai N., Gillette M. A., Carr S. A. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nature Biotechnology. 2006;24(8):971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 14.Matthay M. A., Zimmerman G. A. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. American Journal of Respiratory Cell and Molecular Biology. 2005;33(4):319–327. doi: 10.1165/rcmb.f305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuebler W. M., Goetz A. E. The marginated pool. European Surgical Research. 2002;34(1-2):92–100. doi: 10.1159/000048894. [DOI] [PubMed] [Google Scholar]
- 16.Weiss S. J. Tissue destruction by neutrophils. The New England Journal of Medicine. 1989;320(6):365–376. doi: 10.1056/nejm198902093200606. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata K., Hagio T., Matsuoka S. The role of neutrophil elastase in acute lung injury. European Journal of Pharmacology. 2002;451(1):1–10. doi: 10.1016/S0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 18.Garden D., Xiao F., Moak C., Willis B. H., Robinson-Jackson S., Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. The American Journal of Physiology—Heart and Circulatory Physiology. 1998;275(2, part 2):H385–H392. doi: 10.1152/ajpheart.1998.275.2.H385. [DOI] [PubMed] [Google Scholar]
- 19.Ginzberg H. H., Cherapanov V., Dong Q., et al. Neutrophil-mediated epithelial injury during transmigration: role of elastase. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2001;281(3):G705–G717. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- 20.Faymonville M. E., Pincemail J., Duchateau J., et al. Myeloperoxidase and elastase as markers of leukocyte activation during cardiopulmonary bypass in humans. The Journal of Thoracic and Cardiovascular Surgery. 1991;102(2):309–317. [PubMed] [Google Scholar]
- 21.Edmunds L. H., Jr. Inflammatory response to cardiopulmonary bypass. The Annals of Thoracic Surgery. 1998;66(5, supplement 1):S12–S16. doi: 10.1016/s0003-4975(98)00967-9. [DOI] [PubMed] [Google Scholar]
- 22.Tonz M., Mihaljevic T., Von Segesser L. K., Fehr J., Schmid E. R., Turina M. I. Acute lung injury during cardiopulmonary bypass are the neutrophils responsible? Chest. 1995;108(6):1551–1556. doi: 10.1378/chest.108.6.1551. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto K., Miyamoto H., Suzuki K., et al. Evidence of organ damage after cardiopulmonary bypass: the role of elastase and vasoactive mediators. The Journal of Thoracic and Cardiovascular Surgery. 1992;104(3):666–673. [PubMed] [Google Scholar]
- 24.Mihaljevic T., Tönz M., Von Segesser L. K., et al. The influence of leukocyte filtration during cardiopulmonary bypass on postoperative lung function: a clinical study. The Journal of Thoracic and Cardiovascular Surgery. 1995;109(6):1138–1145. doi: 10.1016/s0022-5223(95)70197-4. [DOI] [PubMed] [Google Scholar]
- 25.Gu Y. J., de Vries A. J., Vos P., Boonstra P. W., van Oeveren W. Leukocyte depletion during cardiac operation: a new approach through the venous bypass circuit. The Annals of Thoracic Surgery. 1999;67(3):604–609. doi: 10.1016/s0003-4975(98)01262-4. [DOI] [PubMed] [Google Scholar]
- 26.Kadoi Y., Hinohara H., Kunimoto F., et al. Pilot study of the effects of ONO-5046 in patients with acute respiratory distress syndrome. Anesthesia & Analgesia. 2004;99(3):872–877. doi: 10.1213/01.ane.0000129996.22368.85. [DOI] [PubMed] [Google Scholar]
- 27.Driessen J. J., Dhaese H., Fransen G., et al. Pulsatile compared with nonpulsatile perfusion using a centrifugal pump for cardiopulmonary bypass during coronary artery bypass grafting: effects on systemic haemodynamics, oxygenation, and inflammatory response parameters. Perfusion. 1995;10(1):3–12. doi: 10.1177/026765919501000102. [DOI] [PubMed] [Google Scholar]
- 28.de Vries A. J., Vermeijden W. J., Gu Y. J., Hagenaars J. A. M., Van Oeveren W. Clinical efficacy and biocompatibility of three different leukocyte and fat removal filters during cardiac surgery. Artificial Organs. 2006;30(6):452–457. doi: 10.1111/j.1525-1594.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 29.Klebanoff S. J. Myeloperoxidase: friend and foe. Journal of Leukocyte Biology. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 30.Verrier E. D., Boyle E. M., Jr. Endothelial cell injury in cardiovascular surgery. The Annals of Thoracic Surgery. 1996;62(3):915–922. doi: 10.1016/s0003-4975(96)00528-0. [DOI] [PubMed] [Google Scholar]
- 31.Miller B. E., Levy J. H. The inflammatory response to cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia. 1997;11(3):355–366. doi: 10.1016/s1053-0770(97)90106-3. [DOI] [PubMed] [Google Scholar]
- 32.Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair D. G., Haslam P. L., Quinlan G. J., Pepper J. R., Evans T. W. The effect of cardiopulmonary bypass on intestinal and pulmonary endothelial permeability. Chest. 1995;108(3):718–724. doi: 10.1378/chest.108.3.718. [DOI] [PubMed] [Google Scholar]
- 34.Engels G. E., Gu Y. J., van Oeveren W., Rakhorst G., Mariani M. A., Erasmus M. E. The utility of lung epithelium specific biomarkers in cardiac surgery: a comparison of biomarker profiles in on- and off-pump coronary bypass surgery. Journal of Cardiothoracic Surgery. 2013;8(1, article 4) doi: 10.1186/1749-8090-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quaniers J. M., Leruth J., Albert A., Limet R. R., Defraigne J.-O. Comparison of inflammatory responses after off-pump and on-pump coronary surgery using surface modifying additives circuit. The Annals of Thoracic Surgery. 2006;81(5):1683–1690. doi: 10.1016/j.athoracsur.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 36.Baldus S., Rudolph V., Roiss M., et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113(15):1871–1878. doi: 10.1161/circulationaha.105.590083. [DOI] [PubMed] [Google Scholar]
- 37.Buckley S. T., Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. Journal of Biomedicine and Biotechnology. 2010;2010:11. doi: 10.1155/2010/917108.917108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirasawa M., Fujiwara N., Hirabayashi S., et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes to Cells. 2004;9(2):165–174. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 39.Mahajan N., Dhawan V. Receptor for advanced glycation end products (RAGE) in vascular and inflammatory diseases. International Journal of Cardiology. 2013;168(3):1788–1794. doi: 10.1016/j.ijcard.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Uchida T., Shirasawa M., Ware L. B., et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. The American Journal of Respiratory and Critical Care Medicine. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agostoni P., Banfi C., Brioschi M., et al. Surfactant protein B and RAGE increases in the plasma during cardiopulmonary bypass: a pilot study. The European Respiratory Journal. 2011;37(4):841–847. doi: 10.1183/09031936.00045910. [DOI] [PubMed] [Google Scholar]
- 42.Tuinman P. R., Cornet A. D., Kuipers M. T., et al. Soluble receptor for advanced glycation end products as an indicator of pulmonary vascular injury after cardiac surgery. BMC Pulmonary Medicine. 2013;13, article 76 doi: 10.1186/1471-2466-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X., Chen Q., Shi S., et al. Plasma sRAGE enables prediction of acute lung injury after cardiac surgery in children. Critical Care. 2012;16(3, article R91) doi: 10.1186/cc11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida T., Ohno N., Asahara M., et al. Soluble isoform of the receptor for advanced glycation end products as a biomarker for postoperative respiratory failure after cardiac surgery. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0070200.e70200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creagh-Brown B. C., Quinlan G. J., Hector L. R., Evans T. W., Burke-Gaffney A. Association between preoperative plasma sRAGE levels and recovery from cardiac surgery. Mediators of Inflammation. 2013;2013:7. doi: 10.1155/2013/496031.496031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calfee C. S., Budev M. M., Matthay M. A., et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. Journal of Heart and Lung Transplantation. 2007;26(7):675–680. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christie J. D., Shah C. V., Kawut S. M., et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. The American Journal of Respiratory and Critical Care Medicine. 2009;180(10):1010–1015. doi: 10.1164/rccm.200901-0118oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah R. J., Bellamy S. L., Lee J. C., et al. Early plasma soluble receptor for advanced glycation end-product levels are associated with bronchiolitis obliterans syndrome. The American Journal of Transplantation. 2013;13(3):754–759. doi: 10.1111/ajt.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh G., Katyal S. L. Clara Cells and Clara Cell 10 kD Protein (CC10) The American Journal of Respiratory Cell and Molecular Biology. 1997;17(2):141–143. doi: 10.1165/ajrcmb.17.2.f138. [DOI] [PubMed] [Google Scholar]
- 50.Lakind J. S., Holgate S. T., Ownby D. R., et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12(5):445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 51.Broeckaert F., Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clinical and Experimental Allergy. 2000;30(4):469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 52.Hermans C., Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. American Journal of Respiratory and Critical Care Medicine. 1999;159(2):646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 53.Determann R. M., Millo J. L., Waddy S., Lutter R., Garrard C. S., Schultz M. J. Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study. BMC Pulmonary Medicine. 2009;9, article 49 doi: 10.1186/1471-2466-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kropski J. A., Fremont R. D., Calfee C. S., Ware L. B. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–1447. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wutzler S., Lehnert T., Laurer H., et al. Circulating levels of clara cell protein 16 but not surfactant protein D identify and quantify lung damage in patients with multiple injuries. The Journal of Trauma—Injury, Infection and Critical Care. 2011;71(2):E31–E36. doi: 10.1097/ta.0b013e3181f6f0b4. [DOI] [PubMed] [Google Scholar]
- 56.Lomas D. A., Silverman E. K., Edwards L. D., Miller B. E., Coxson H. O., Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63(12):1058–1063. doi: 10.1136/thx.2008.102574. [DOI] [PubMed] [Google Scholar]
- 57.Pilette C., Godding V., Kiss R., et al. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. The American Journal of Respiratory and Critical Care Medicine. 2001;163(1):185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 58.Diamond J. M., Kawut S. M., Lederer D. J., et al. Elevated plasma Clara cell secretory protein concentration is associated with high-grade primary graft dysfunction. The American Journal of Transplantation. 2011;11(3):561–567. doi: 10.1111/j.1600-6143.2010.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greven F., Krop E., Burger N., Kerstjens H., Heederik D. Serum pneumoproteins in firefighters. Biomarkers. 2011;16(4):364–371. doi: 10.3109/1354750x.2011.578218. [DOI] [PubMed] [Google Scholar]
- 60.Nord M., Schubert K., Cassel T. N., Andersson O., Riise G. C. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation. 2002;73(8):1264–1269. doi: 10.1097/00007890-200204270-00013. [DOI] [PubMed] [Google Scholar]
- 61.Shah R. J., Wickersham N., Lederer D. J., et al. Preoperative plasma club (Clara) cell secretory protein levels are associated with primary graft dysfunction after lung transplantation. American Journal of Transplantation. 2014;14(2):446–452. doi: 10.1111/ajt.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Boven W.-J., Gerritsen W. B., Waanders F. G., Haas F. J., Aarts L. P. Mini extracorporeal circuit for coronary artery bypass grafting: initial clinical and biochemical results: a comparison with conventional and off-pump coronary artery bypass grafts concerning global oxidative stress and alveolar function. Perfusion. 2004;19(4):239–246. doi: 10.1191/0267659104pf746oa. [DOI] [PubMed] [Google Scholar]
- 63.van Boven W. J. P., Gerritsen W. B. M., Zanen P., et al. Pneumoproteins as a lung-specific biomarker of alveolar permeability in conventional on-pump coronary artery bypass graft surgery vs mini-extracorporeal circuit: a pilot Study. Chest. 2005;127(4):1190–1195. doi: 10.1378/chest.127.4.1190. [DOI] [PubMed] [Google Scholar]
- 64.van Boven W.-J. P., Gerritsen W. B., Driessen A. H., van Dongen E. P., Klautz R. J., Aarts L. P. Minimised closed circuit coronary artery bypass grafting in the elderly is associated with lower levels of rgan-specific biomarkers: a prospective randomised study. European Journal of Anaesthesiology. 2013;30(11):685–694. doi: 10.1097/eja.0b013e328364febf. [DOI] [PubMed] [Google Scholar]
- 65.Engels G. E., Dodonov M., Rakhorst G., et al. The effect of pulsatile cardiopulmonary bypass on lung function in elderly patients. The International Journal of Artificial Organs. 2014;37(9):679–687. doi: 10.5301/ijao.5000352. [DOI] [PubMed] [Google Scholar]
- 66.King R. J. Pulmonary surfactant. Journal of Applied Physiology: Respiratory Environmental & Exercise Physiology. 1982;53(1):1–8. doi: 10.1152/jappl.1982.53.1.1. [DOI] [PubMed] [Google Scholar]
- 67.Whitsett J. A., Weaver T. E. Hydrophobic surfactant proteins in lung function and disease. The New England Journal of Medicine. 2002;347(26):2141–2148. doi: 10.1056/nejmra022387. [DOI] [PubMed] [Google Scholar]
- 68.Kishore U., Greenhough T. J., Waters P., et al. Surfactant proteins SP-A and SP-D: structure, function and receptors. Molecular Immunology. 2006;43(9):1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Weaver T. E., Whitsett J. A. Function and regulation of expression of pulmonary surfactant-associated proteins. The Biochemical Journal. 1991;273(2):249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haagsman H. P., Diemel R. V. Surfactant-associated proteins: Functions and structural variation. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2001;129(1):91–108. doi: 10.1016/s1095-6433(01)00308-7. [DOI] [PubMed] [Google Scholar]
- 71.Sano H., Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Molecular Immunology. 2005;42(3):279–287. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Wright J. R. Immunoregulatory functions of surfactant proteins. Nature Reviews Immunology. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 73.Magrì D., Brioschi M., Banfi C., et al. Circulating plasma surfactant protein type B as biological marker of alveolar-capillary barrier damage in chronic heart failure. Circulation Heart Failure. 2009;2(3):175–180. doi: 10.1161/circheartfailure.108.819607. [DOI] [PubMed] [Google Scholar]
- 74.Sims M. W., Beers M. F., Ahya V. N., et al. Effect of single vs bilateral lung transplantation on plasma surfactant protein D levels in idiopathic pulmonary fibrosis. Chest. 2011;140(2):489–496. doi: 10.1378/chest.10-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Determann R. M., Wolthuis E. K., Choi G., et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2008;294(2):L344–L350. doi: 10.1152/ajplung.00268.2007. [DOI] [PubMed] [Google Scholar]
- 76.Shah R. J., Bellamy S. L., Localio A. R., et al. A panel of lung injury biomarkers enhances the definition of primary graft dysfunction (PGD) after lung transplantation. The Journal of Heart and Lung Transplantation. 2012;31(9):942–949. doi: 10.1016/j.healun.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shigemura M., Nasuhara Y., Konno S., et al. Effects of molecular structural variants on serum Krebs von den Lungen-6 levels in sarcoidosis. Journal of Translational Medicine. 2012;10(1, article 111) doi: 10.1186/1479-5876-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kohno N., Akiyama M., Kyoizumi S., Hakoda M., Kobuke K., Yamakido M. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Japanese Journal of Clinical Oncology. 1988;18(3):203–216. [PubMed] [Google Scholar]
- 79.Hirasawa Y., Kohno N., Yokoyama A., Inoue Y., Abe M., Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. The American Journal of Respiratory Cell and Molecular Biology. 1997;17(4):501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- 80.Kunitake R., Kuwano K., Yoshida K., et al. KL-6, surfactant protein A and D in bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Respiration. 2001;68(5):488–495. doi: 10.1159/000050556. [DOI] [PubMed] [Google Scholar]
- 81.Janssen R., Sato H., Grutters J. C., et al. Study of Clara cell 16, KL-6, and surfactant protein-D in serum as disease markers in pulmonary sarcoidosis. Chest. 2003;124(6):2119–2125. doi: 10.1378/chest.124.6.2119. [DOI] [PubMed] [Google Scholar]
- 82.Walter J. N., Fan L. L., Bag R., et al. Serum KL-6 as a marker for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2006;82(5):709–711. doi: 10.1097/01.tp.0000234952.46013.df. [DOI] [PubMed] [Google Scholar]
- 83.Ohshimo S., Bonella F., Sommerwerck U., et al. Comparison of serum KL-6 versus bronchoalveolar lavage neutrophilia for the diagnosis of bronchiolitis obliterans in lung transplantation. The Journal of Heart and Lung Transplantation. 2011;30(12):1374–1380. doi: 10.1016/j.healun.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 84.Hussell T., Bell T. J. Alveolar macrophages: plasticity in a tissue-specific context. Nature Reviews Immunology. 2014;14(2):81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 85.Mills C. D. M1 and M2 macrophages: oracles of health and disease. Critical Reviews in Immunology. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 86.Duffield J. S. The inflammatory macrophage: a story of Jekyll and Hyde. Clinical Science. 2003;104(1):27–38. doi: 10.1042/cs20020240. [DOI] [PubMed] [Google Scholar]
- 87.Gordon S. Alternative activation of macrophages. Nature Reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 88.Gibbs D. F., Shanley T. P., Warner R. L., Murphy H. S., Varani J., Johnson K. J. Role of matrix metalloproteinases in models of macrophage-dependent acute lung injury: evidence for alveolar macrophage as source of proteinases. The American Journal of Respiratory Cell and Molecular Biology. 1999;20(6):1145–1154. doi: 10.1165/ajrcmb.20.6.3482. [DOI] [PubMed] [Google Scholar]
- 89.Alber A., Howie S. E. M., Wallace W. A. H., Hirani N. The role of macrophages in healing the wounded lung. International Journal of Experimental Pathology. 2012;93(4):243–251. doi: 10.1111/j.1365-2613.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon S., Martinez F. O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 91.Den Hengst W. A., Gielis J. F., Lin J. Y., Van Schil P. E., De Windt L. J., Moens A. L. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. The American Journal of Physiology—Heart and Circulatory Physiology. 2010;299(5):H1283–H1299. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 92.Zhao M., Fernandez L. G., Doctor A., et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2006;291(5):L1018–L1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 93.Dong H., Li J., Lv Y., et al. Comparative analysis of the alveolar macrophage proteome in ALI/ARDS patients between the exudative phase and recovery phase. BMC Immunology. 2013;14(1, article 25) doi: 10.1186/1471-2172-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Onorati F., Rubino A. S., Cuda A., et al. Impact of endothelial activation on infective and inflammatory complications after cardiac surgery in type II diabetes mellitus. The International Journal of Artificial Organs. 2011;34(6):469–480. doi: 10.5301/ijao.2011.8329. [DOI] [PubMed] [Google Scholar]
- 95.Hoffman S. A., Wang L., Shah C. V., et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. American Journal of Transplantation. 2009;9(2):389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathur A., Baz M., Staples E. D., et al. Cytokine profile after lung transplantation: correlation with allograft injury. Annals of Thoracic Surgery. 2006;81(5):1844–1849. doi: 10.1016/j.athoracsur.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 97.Onorati F., Santini F., Mariscalco G., et al. Leukocyte filtration ameliorates the inflammatory response in patients with mild to moderate lung dysfunction. The Annals of Thoracic Surgery. 2011;92(1):111–121. doi: 10.1016/j.athoracsur.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 98.Vasan R. S. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/circulationaha.104.482570. [DOI] [PubMed] [Google Scholar]
- 99.Bratcher P. E., Gaggar A. Factors influencing the measurement of plasma/serum surfactant protein D levels by ELISA. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111466.e111466 [DOI] [PMC free article] [PubMed] [Google Scholar]