Abstract
Objective
Examine the association between perceived stress and hunger continuously over a week in free-living individuals.
Methods
Forty five young adults (70% women, 30% overweight/obese) ages 18 to 24 years (Mean = 20.7, SD = 1.5), with BMI between 17.4 and 36.3 kg/m2 (Mean = 23.6, SD = 4.0) provided between 513 and 577 concurrent ratings of perceived stress and hunger for 7 days via hourly, text messaging assessments and real-time eating records. Time-varying effect modeling was used to explore whether the within-day fluctuations in stress are related to perceived hunger assessed on a momentary basis.
Results
A generally positive stress–hunger relationship was confirmed, but we found that the strength of the relationship was not linear. Rather, the magnitude of the association between perceived stress and hunger changed throughout the day such that only during specific time intervals were stress and hunger significantly related. Specifically, the strength of the positive association peaked during late afternoon hours on weekdays (β = 0.31, p < .05) and it peaked during evening hours on weekend days (β = 0.56, p < .05).
Conclusion
This is the first empirical study to demonstrate potentially maladaptive, nonlinear stress–hunger associations that peak in the afternoon or evening hours. While we are unable to infer causality from these analyses, our findings provide empirical evidence for a potentially high-risk time of day for stress-induced eating. Replication of these findings in larger, more diverse samples will aid with the design and implementation of real-time intervention studies aimed at reducing stress-eating.
Keywords: Stress eating, Hunger, Ecological momentary assessments, Text-messaging, Time-varying effect modeling
Introduction
Nearly 70% of adults in the United States are overweight or obese (Ogden, Carroll, Kit, & Flegal, 2014). Although the causes of obesity are complex (McAllister et al., 2009), one potential contributor is psychosocial stress. A recent report by the American Psychological Association indicated that nearly all Americans have felt “moderately stressed” in the past month and 22% felt extremely stressed, with nearly 70% reporting altering their eating behavior in response to stress (American Psychological Association, 2012). The report revealed that 39% have a tendency to overeat when under high stress, and another 29% skip meals. These findings were consistent with other research showing changes in eating behavior and food choice in response to stress (Greeno & Wing, 1994). Furthermore, when examining the impact of psychosocial stress on weight change, Block, He, Zaslavsky, Ding, and Ayanian (2009) demonstrated that perceived stress was positively related to weight gain among U.S. adults, and among those classified as obese, in particular. While the biological mechanisms of this relationship are not completely understood, evidence suggests that psychosocial stress can trigger an overactive stress response leading to the increased production of cortisol and insulin, and to subsequent food intake, particularly among high cortisol reactors (Adam & Epel, 2007; Epel, Lapidus, McEwen, & Brownell, 2001). Thus, there is initial evidence that supports the adverse effects of stress on food intake and the growing rates of obesity in the U.S.
The stress-induced eating hypothesis has been supported by various laboratory studies in humans that demonstrate subjective feelings of hunger increase in intensity as perceived stress increases (Raspopow, Abizaid, Matheson, & Anisman, 2014; Sarker, Franks, & Caffrey, 2013). While of great value, it is unclear how generalizable these studies are to real-life conditions in which both perceived stress and hunger change in response to daily events. Preliminary studies support the notion of changing levels of stress throughout a day (Gellman & Turner, 2013). Research using momentary assessment techniques has shown that minor stressful daily events are associated with within-day changes in mood and perceived stress (Gellman & Turner, 2013). Similarly, levels of perceived hunger fluctuate considerably throughout a day as a function of a variety of homeostatic, hedonic, and conditioned or psychological factors that underlie a natural circadian rhythm. Peaks in hunger tend to occur around “mealtimes”; however, they may vary between individuals or days (weekdays vs. weekend days) and even within individuals (e.g., across time-sensitive contexts such as high- and low-stress valence). Collectively, these findings suggest that the relationship between perceived stress and hunger is likely better described as a non-constant, nonlinear association, and that it may vary in its magnitude and strength throughout the day in real life; however, this has yet to be tested empirically.
Research on within-person variations in the effects of psychosocial stress on perceived hunger in natural settings is generally limited (Newman, O’Connor, & Conner, 2007; O’Connor, Jones, Conner, McMillan, & Ferguson, 2008; Stone & Brownell, 1994). Using traditional assessment (daily diaries) and (linear) statistical methods, two studies conducted by O’Connor and colleagues (Newman et al., 2007; O’Connor et al., 2008) showed that daily stressors were significantly associated with high fat/high sugar snacking during the same day with the strongest effects among women with high cortisol reactivity (Newman et al., 2007). Though these studies have significantly contributed to our understanding of the association between stress and daily food intake, the use of modern assessment and non-linear statistical methods could enhance our understanding of the stress–hunger relationship. Using momentary assessment methods and an advanced statistical approach for intensive longitudinal data, one can determine whether the magnitude and direction of stress–hunger relationship is constant, independent of the contextual factors such as circadian rhythms (e.g., time of the day) or days of the week (e.g., week days, weekends). Contextual dynamics combined with biological fluctuations in hormonal cycles and circadian rhythms may manifest in novel, time-varying relationship patterns that can contribute to a deeper understanding of the effects stress has on eating behaviors.
Therefore, the overarching goal of the current study was to examine the association between perceived stress and hunger based on measures taken in situ several times a day over the course of a week (Shiffman, 2009). We compared this hunger–stress association between weekdays and weekends and tested the association using more traditional (i.e., constantly linear) and novel, nonlinear statistical approaches. We hypothesized (1) that a generally positive association between perceived stress and hunger would be detected via the traditional, general linear mixed and, via nonlinear time-varying effect modeling, (2) that the strength of the association would vary within a typical day, and (3) that the dynamics would manifest differently between weekdays vs. weekends.
Methods
Project TwEATs
Project TwEATs (Text with Ease Appetite Tracking System) was launched with an objective to test the use of Ecological Momentary Assessment (EMA) methodologies to collect hourly records of appetitive states and eating events. Results from Project TwEATs I demonstrated that automated text-messaging is an acceptable method to monitor perceived hunger ratings in a sample of adults over a consecutive week (Schembre & Yuen, 2011) representing an improvement over previously validated methodologies (Almiron-Roig et al., 2009; Mattes, Hollis, Hayes, & Stunkard, 2005; Stratton et al., 1998; Stubbs et al., 2000; Yeomans, Gray, Mitchell, & True, 1997). For Project TwEATs II we recruited a new sample of participants to explore the relationships between perceived psychological and physiological states, and eating behavior.
Participants
A convenience sample of 51 college students 18 to 24 years of age was recruited during the two weeks prior to the start of a spring semester. Eligibility requirements included being free from chronic diseases that could affect eating patterns (e.g. diabetes, Celiac disease), no history of a diagnosed eating disorder, no current pregnancy or lactation, and access to an unlimited text messaging plan with a personal mobile telephone service carrier. As part of Project TwEATs II, pre-prandial blood glucose data were collected via commercially available glucometers. Students who reported being unwilling to monitor their blood glucose levels prior to meals were additionally excluded.1 Two students, who initially met the screening requirements, were later deemed ineligible to participate in the study due to the impaired fasting blood glucose concentrations (>99 mg/dl). Of the 49 enrolled participants, four (8%) failed to provide momentary eating records. The final analytical sample included 45 participants. All participants provided informed consent, and study protocols were approved by the Institutional Review Board at the University of Hawaii Cancer Center, Honolulu, Hawaii.
Design
This was a seven-day observational study. Upon study enrollment, participants completed a number of online questionnaires and provided demographic data. Additionally, measures of weight, height, waist circumference, and fasting blood glucose concentrations were obtained in person. All participants were enrolled within 3 weeks of the start of the semester. To control for possible variations in mean stress levels from the beginning to the end of the college semester, participants were randomized to one of two cohorts beginning on the seven-day monitoring period either 1 week or 8 weeks after study enrollment. Notably, neither perceived stress (p = 0.37) nor perceived hunger (p = 0.99) varied significantly between the two cohorts. Randomization was stratified by sex and weight status.
EMA data sampling scheme
Interval- and event-contingent sampling methods were used to collect self-reported perceived stress and hunger data during the monitoring period. Interval-contingent data were collected using automated, reminder text messages delivered to the participant’s personal mobile phone at equally-spaced, 1-hour time intervals over the entire week to maximize the response rate. A total of 168 text message prompts were sent to each participant over the seven day period (24 hours by 7 days). Participants were requested to only respond to texts during their waking hours to maximize data collection. At each reminder prompt, participants were asked to “Reply with your hunger and stress rating with ‘D projecttweats’ (Rate 1–10)” (Shiyko, Lanza, Tan, Li, & Shiffman, 2012). Prior to initiating the study, research staff delivered practice prompts to train participants on how to respond correctly. Participants were asked to report their perceived hunger (PH) and stress (PS) rating at the time of their response with numerical ratings ranging from 1 = not at all to 10 = extremely.
In addition to hourly prompted responses, participants were instructed to report their stress and hunger levels within five-minutes prior to an eating event via paper-and-pen (event-contingent data). Standardized forms were provided to record eating events. An eating event was defined as any occasion a calorie-containing food or beverage, including meal-replacement beverages (e.g., shakes or smoothies), was consumed (Yannakoulia, Melistas, Solomou, & Yiannakouris, 2007). Consistent with previously published literature, eating episodes occurring >15 minutes apart were described as distinct, reportable events (Berteus Forslund, Torgerson, Sjostrom, & Lindroos, 2005; Yannakoulia et al., 2007). Participants recorded the date and time of each eating event and its general size (i.e., meal, snack, or smaller). During the monitoring period, participants were discouraged from any moderate-to-vigorous physical activity to minimize external influences on mood and hunger. Compliance with request was self-reported.
Interval-contingent text responses were collected on the Project TwEATs Twitter account, and event-contingent paper-and-pen eating records were returned by study participants at the conclusion of the monitoring period. All collected data were manually entered by research staff into a working database. Accuracy of the data entries was verified by an alternate staff member. For this study, all data, both interval- and event-contingent, collected between 10:00 and 23:59 were used for each day in order to maximize the number of available data points from hour to hour given the sparseness of the hour-to-hour data prior to 10:00 (2.8% from 7:00 to 7:59; 3.4% from 8:00 to 8:59; 4.0% from 9:00 to 9:59).
Statistical analysis
Mixed effects modeling of the average association between PH and PS
Initially, the overall association between PH and PS was examined with general linear mixed modeling. PS was included as a person-level predictor (an average of PS for a given individual across all time points) and prompt-level predictor (a deviation score from a person’s mean at a given moment calculated and standardized) (Tan, Shiyko, Li, Li, & Dierker, 2012) while controlling for BMI and gender, which were significantly associated with the main predictor, PS (p = .013 and p < .0001, respectively), and the outcome, PH (ps < .001). The dependent variable for our models, PH, neared normal distribution with slight positive skewness (skewness = 0.55 and kurtosis-3 = −.63). Variable transformation normalized the PH data distribution, but did not influence any of our findings. As such, the untransformed PH data were included in all of our analyses to aid with the interpretation of our findings.
Time-varying effect modeling of PS on PH within and across days
The time-varying association between PS and PH was examined with time-varying effect modeling (TVEM). TVEM is uniquely suitable for analysis of intensive longitudinal data (ILD) (Tan et al., 2012) collected with EMA methods that assess two, time-varying variables of interest repeatedly over time. The model is semi-parametric and assumes no traditionally used shapes (such as linear or quadratic). Rather, the pattern of the association is estimated from data and can follow a complex smooth non-linear curve. To accomplish this, the collected data are time-stamped. This temporal information is a key element to the TVEM approach. Unequal spacing between observations and unequal number of assessments across participants is accommodated.
The following TVEM was fit to the data:
The outcome, PHij, represents momentary assessments of perceived hunger for each person i assessed at unique time point j. Model parameters β0 through β3 represent parameter functions that change values depending on time (tij) Specifically, β0 (tij) is an intercept function that summarizes a level of hunger over the course of a weekday (e.g., continuously from 10:00 to 23:59) for individuals with an average level of stress (Wkend = 0 and PS = 0). Thus, instead of a single summary, the intercept is a function with gradually changing values over the course of a day, from morning to night. β2(tij) is a time-varying function of perceived stress (PS), portraying the impact of PS on PH during a weekday. The PS variable was centered at person-mean first, and then standardized with a 1 unit shift representing a 1 SD shift relative to one’s own mean. Further, β2(tij) represents the time-varying effect of weekends (Wkend, coded as a dummy variable) on the estimated level of PH, controlling for PS. β3(tij) represents differences of the effects of PS on weekends relative to weekdays. Gender and BMI were also included as relevant covariates in the model, and β4 and β5 represent traditional regression weights. The random errors, εij, are assumed to be normally and independently distributed.
The model was fitted in SAS 9.3 using the recommended P-spline estimation method with 10 knots (Yang, Tan, Li, & Wagner, 2012). The P-spline approach yields smoothed parameter functions that capture nuances of momentary fluctuations without over fitting the model to data. Technical details of model fitting can be found in Tan et al. (2012) and Shiyko and colleagues (Shiyko, Naab, Shiffman, & Li, 2013; Shiyko et al., 2012). To align the data points based on circadian rhythm, we used clock time (from 10:00 to 23:59) to model within-day changes in the associations between PS and PH rather than estimated wake time (from the first text response).
Results
Participant characteristics and compliance for EMA data
Participant’s characteristics are summarized in Table 1. A total of 3815 EMAs were collected, ranging from 20 to 127 observations per person (Mean = 84.8, Median = 87.0, SD = 22.8). A total of 2726 (71.5%) assessments were collected during weekdays, and 1089 (28.5%) during weekends. From all assessments, 3019 (79.1%) were interval-contingent. Over the course of 7 days, we expected a total of 98 assessments per person (14 responses by 7 days). On average, participants provided 67 assessments (68.8% compliance rate) (Median = 72.4, SD = 19.4, range = 9 to 95).
Table 1.
Sample characteristics (N = 45).
| Variable | M (SD) or n (%) |
|---|---|
| Age (years) | 20.7 (1.5) |
| BMI (kg/m2) | 23.6 (4.0) |
| Sex | |
| Women | 29 (64.4%) |
| Men | 16 (35.6%) |
| BMI category | |
| Overweight/obese | 14 (31.1%) |
| Non-overweight/obese | 31 (68.9%) |
| Race/Ethnicitya | |
| White | 18 (40.0%) |
| Asian | 16 (35.6%) |
| Hawaiian/Pacific Islander | 2 (4.4%) |
| Biracial/Mixed | 9 (20.0%) |
| Other | 5.1 (4.6%) |
| Year in college | |
| Freshman | 2 (4.4%) |
| Sophomore | 12 (26.7%) |
| Junior | 17 (37.8%) |
| Senior | 11 (24.4%) |
| Other (graduate students) | 3 (6.7%) |
| Living arrangement | |
| On-campus | 30 (66.7%) |
| Off-campus | 15 (33.3%) |
| Stress averaged over the week | 2.8 (1.1) |
| Hunger averaged over the week | 3.0 (1.0) |
The average association between perceived stress and hunger
Using both the event-contingent data (paper-and-pen eating event data) and interval-contingent data (hourly text responses), we initially used traditional general linear mixed models to account for the nested data structure (repeated measures within individual) and included gender and BMI as covariates (Table 2). The results confirmed that both the average and momentary PS are positively associated with PH. Specifically, those who reported higher average PS scores tended to report higher PH scores (β01 = .41, p < .001). In comparison, when the participant reported being more stressed than his/her usual level, increased PH was reported (β10 = .26, p < .001). Additional analyses showed that effects of momentary PS did not differ between weekdays and weekend days (p = .78). These findings were consistent when only the interval-contingent prompts were used in the mixed models.
Table 2.
General linear mixed model predicting hunger levels.
| Estimate | SE | P | |
|---|---|---|---|
| Intercept | 3.32 | 0.40 | <.001 |
| Time | −0.08 | 0.01 | <.001 |
| Between-subject stress | 0.41 | 0.11 | <.001 |
| Within-subject stress | 0.26 | 0.04 | <.001 |
| Weekend | 0.09 | 0.07 | 0.22 |
| Within-subject stress × Weekend | −0.02 | 0.08 | 0.78 |
| BMI | 0.35 | 0.26 | 0.20 |
| Gender | −0.79 | 0.27 | 0.01 |
General linear mixed model was used to estimate coefficients and standard errors for the model variables. In this model the intercept represents the level of hunger at 10:00 AM for a participant whose stress level is average when he/she feels stressed at her/his usual level on a weekday; Time represents time passage since 10:00 until 23:59; the between-subject stress represents grand mean-centered stress levels, the within subject stress variable represents individual mean-centered stress levels; the weekend variable represents weekend (=1) vs weekdays (=0) the and the Within-subject stress × Weekend variable represents the interaction between within-subject and weekend binary variable. BMI and gender were also included as control variables.
Time-varying levels of perceived hunger: weekdays vs. weekends
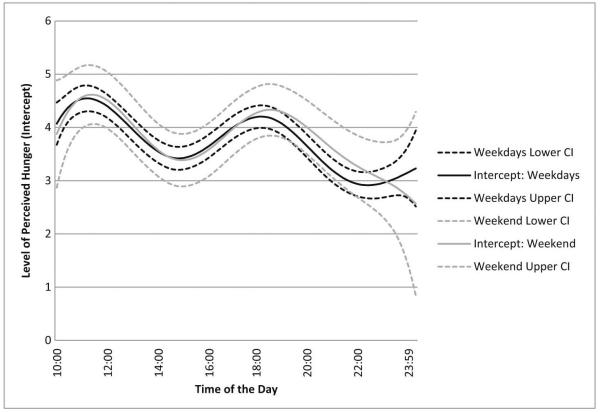
Graphical summaries of TVEM intercept parameter functions are summarized in Fig. 1 for weekdays (black curve) and weekend days (gray curve). The depicted intercept functions represent the mean PH within a day when a person’s PS is at his/her average level (as the stress variable was person-centered, then standardized), controlling for BMI and gender. Coefficient values ranged from approximately 2.9 to 4.6 on a 1 to 10 scale. Generally, for both week-days and weekend days, PH demonstrated strong circadian rhythms with peaks prior to noon and again in the evening around 17:00 to 19:00, then tapered off toward the end of the day with lowest levels of hunger after 22:00. Higher BMI was positively associated with PH (β = .51, p < .001) and men reported significantly lower levels of PH than did women (β = −0.86, p < .001). It should be noted that the within-day variation of PH was consistent between weekdays and weekend, as shown by the overlapped confidence intervals (Fig. 1). Again these findings were consistent when using only the interval-contingent prompts.
Fig. 1.
Graphical summary of intercept functions within a day; the average level of perceived hunger at a given moment at standardized person-mean stress level in a typical day. The Y-axis represents the intercept of perceived hunger when stress = 0 over time and the X-axis represents time of day.
Time-varying effects of stress on hunger: weekdays vs. weekends
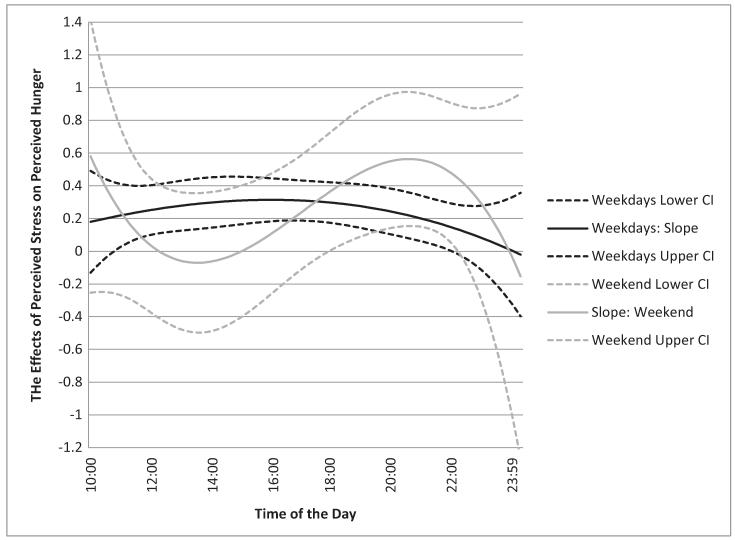
Figure 2 shows the momentary association between PS and PH for weekdays (black curve) and weekend days (gray curve). The curve for weekdays shows that PS and PH was associated in the afternoon and evening hours until about 22:00, represented by the functions being above the zero line at magnitudes ranging from β = 0.21 to 0.31. The PS and PH were most strongly linked during late afternoon (around 16:00) on weekdays. In contrast, the association between PS and PH on weekend days showed a different pattern (gray curve in Fig. 2). Overall, the relationship became positive later in the evening from 18:00 to 22:30 with the strongest association of .56 around 21:00. However, the shapes of the curves on weekdays and weekend days were not statically different evidenced by the overlapping confidence intervals of depicted slope curves.
Fig. 2.
Graphical summary of slope function within a day. The Y-axis represents the magnitude of momentary association between perceived hunger and stress and the X-axis represents time of day.
Discussion
The described findings empirically confirm our hypothesis that the nature of the association between PS and PH is better described as non-constant than as linear, which is most typically reported. To our knowledge, this is the first study to use modern EMA and statistical approaches to demonstrate the dynamic association between PS and PH during waking hours over a day. This study compliments past research in the area of stress-induced eating, which has demonstrated variations in food intake related to the perceived intensity of psychosocial stress. Consistent with previously published literature, our findings indicated a generally positive association between PS and PH. Yet, over a typical day, the stress–hunger association was especially predominant during late afternoon and evening hours. While we are unable to infer causality from these analyses, our findings provide empirical evidence for a potentially high-risk time of day for stress-induced eating.
As expected based on previous research (Newman et al., 2007; O’Connor et al., 2008; Stone & Brownell, 1994), we observed a generally positive perceived stress–hunger association. However, the finding that the strength of this association changes throughout a typical day with peaks in evening hours is relatively novel. In support of our observation, a recent study by Leblanc et al. (2012) demonstrated that emotional eating, which is closely related to stress eating, was positively associated with a higher proportion of energy intake from snacks after 5:00 pm. It was suggested that “this loss of control (overeating) later in the day” (p. 164) was likely related to declines in satiety over the course of the day (de Castro, 2001, 2004, 2009; de Castro, Bellisle, Feunekes, Dalix, & DeGraaf, 1997), but, from the current study, it can be suggested that stress-induced hunger could additionally contribute to excessive energy intake in the evening. It should be noted that similar stress–hunger association patterns were observed using only signal-contingent (i.e., non-eating) prompts. Future EMA research exploring the relationship between stress and eating will need to include a measure of dietary intake to confirm this hypothesis.
With regard to the contextual differences in the dynamic nature of the association, we observed a relatively constant, positive stress–hunger association during the weekdays versus a more dynamic association during the weekend days. One reason for this may be the result of PS being more labile on weekend days versus weekdays. This, in part, is evidenced by greater daily variation in mean PS levels during weekend days (SD = 1.4) versus weekdays (SD = 1.1). The “weekend effect” on decreasing negative moods and increasing positive moods is well documented and particularly pronounced in non-stressed samples such as this current sample of college students (Mean PS = 2.8 of 10) (Cranford et al., 2006). Interestingly, this effect tended to attenuate the strength of the stress–hunger association in the morning and enhance the effect in the evening. Unfortunately, we lacked statistical power to detect differences in the observed patterns of the stress–hunger association between weekdays and weekend days, likely due to the wide confidence intervals resulting from unavailable data, particularly on weekend days. To our knowledge, no research has been done to examine the specific influence of day (week versus weekend) on stress-eating. Future research will be needed to further explore the underlying reasons for this observation.
Despite the novelty of these findings, this study is limited by important factors. Young women were overrepresented in this sample (70% women vs. 30% men). Based on the gender differences in reported stress eating (Greeno & Wing, 1994) and emotional eating (Schembre & Geller, 2011), the strength and pattern of association may not be generalizable to populations of men, older adults, non-college or clinical populations. Requests to refrain from moderate-to-vigorous intensity activity may have disrupted the use of habitual stress-reduction strategies or natural circadian rhythms in a manner that could have affected our findings; however, we were unable to account for this in our models. Due to sample size restrictions, we were unable to explore differences in the stress–hunger association by sex or weight status. However, these covariates were controlled for in our final models. Similarly, statistically significant differences in the pattern of the observed stress–hunger association between weekdays and weekend days may have been observed with an increased number of participants and/or prompt-level observations (e.g., 2-week monitoring period), which would have yielded narrower confidence intervals around the function curves. Also, sparseness of the observations prior to 10:00 and after midnight prevented us from obtaining meaningful estimates of the PS–PH association for those hours, as observed in the wide confidence intervals at the both tails of the function curves. We are also unable to infer causality between perceived stress and subsequent hunger or eating behavior due to the use of concurrently measured perceived stress and hunger and the limited scope of the study as we were unable to collect data on dietary intake. Self-report data, as collected in this study, is subject to social desirability, reporting biases and measurement errors. However, due to the fact that we still found significant associations despite potential measurement error only strengthens our conclusion. Unanswered prompts were not recorded, therefore, compliance rates could not be directly modeled in terms of relevant predictors and covariates (ps > .46). Descriptive statistics showed that the compliance rates did not differ with respect to sex, age, race or BMI categories, although those who reported high levels of hunger, on average, tended to show lower compliance rates (r = −.49, p = < .001). In future studies, we plan to increase the sample to stabilize the estimates (i.e., decreased CI) and to examine between-subject differences with sufficient statistical power (Shiyko et al., 2012; Tan et al., 2012) as well as collect more specific data on dietary intake. Additionally, low reported levels of stress that help to assure reactivity bias, which could have falsely inflated an individual’s perceived stress ratings, were limited; however, we are unable to generalize our findings to a population who may be more stressed. Lastly, we chose to assess perceived hunger as a measure of appetite. We purposefully provided little training on the meaning of hunger, as we did not want to bias the participants’ personal perception of hunger. Therefore, we are unable to distinguish between homeostatic hunger and non-homeostatic hunger (e.g., desire to eat). However, a reliable and valid measure of homeostatic vs. non-homeostatic hunger is currently not available. Furthermore, the within-person standardization helped to account for between person differences in the perception of hunger and stress.
Despite these limitations, one strength of this research was the use of EMA to collect ILD. Repeated measurement of perceived stress and hunger at the micro-level allowed us to conduct TVEM analyses. Using this new statistical methodology, we have demonstrated the temporal variation of the association between perceived stress and hunger levels that would otherwise be overlooked with traditional linear methodologies. We are confident in the quality of our data particularly due to the relatively low burden, low recall bias, and frequency of prompts. Another strength of this study is the automated text-messaging system designed specifically for Project TwEATs (Schembre & Yuen, 2011). The system, which was supported by social media websites, utilized the participants’ own mobile phones and service plans, which drastically reduced the cost burden that can be associated with EMA data collection methods that require application programming on specified platforms that quickly become obsolete due to the high-speed pace of technological advancements.
Using ILD collected by a mobile phone-aided EMA methodology and TVEM, a modern and innovative statistical technique, we confirmed that the relationship between perceived stress and hunger levels is generally positive but dynamic among free-living individuals in a natural setting. We believe this is the first study to empirically demonstrate late afternoon to evening peaks in the stress–hunger association supporting this time of day as a high-risk window of time for stress-induced eating. Future research using these techniques, in addition to real-time assessment of dietary intake, will greatly inform our knowledge about stress eating and open doors to the exploration of other related research questions and intervention strategies to reduce excessive weight gain and obesity.
Acknowledgments
Data collection and the senior author SMS were supported by postdoctoral training grants at the University of Hawaii Cancer Center (NCI R25CA90956) and the Institute for Prevention Research at the University of Southern California (NCI T32CA009492). Work of author JH was supported by ACS MRSG-13-155-01 – CPPB. Work of author MS was supported by R03-CA171809. Author JH conducted the data analysis and worked with senior author SMS and author MS in the drafting of the manuscript. Author MS also contributed to the analytic consultation and interpretation. Author SK aided senior author SMS with the study design and provided a critical review of the manuscript. Author GD aided with data interpretation and provided a critical review of the manuscript. All authors contributed to the manuscript revisions and approved the final version. The authors would additionally like to thank Bryan Juan, study coordinator, and Rhoda Castillo and Misty Wilcox, research assistants, for their help with participant recruitment, data collection, and study management.
Footnotes
Students enrolled in the study were encouraged to test their blood glucose concentrations within 5 minutes of every eating event; however, they were not required to do so. They were instructed to record all eating events, but could refrain from measuring their blood glucose levels if, for any reason, they were uncomfortable or unable to complete the test. This was done to minimize missing eating event data as these data were more. Furthermore, the research staff did not disclose the purpose of collecting these data and, beyond explaining what the test measured, no other potentially leading information (e.g., possible associations between high/low blood glucose concentrations and hunger) was shared with the study participants. The adequate mitigation of any detrimental influence of collecting pre-prandial blood glucose data on our findings is evidenced by consistent associations between PS and PH when including or excluding data collected at these event-contingent assessment points.
Conflict of interest: The authors declare no conflict of interest.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology and Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. doi:10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Almiron-Roig E, Green H, Virgili R, Aeschlimann JM, Moser M, Erkner A. Validation of a new hand-held electronic appetite rating system against the pen and paper method. Appetite. 2009;53:465–468. doi: 10.1016/j.appet.2009.09.014. doi:10.1016/j.appet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- American Psychological Association . Stress in America™. Our Health at Risk. Mind/Body Health. For a Healthy Mind and Body, Talk to a Psychologist. Washington, DC: 2012. [Google Scholar]
- Berteus Forslund H, Torgerson JS, Sjostrom L, Lindroos AK. Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. International Journal of Obesity. 2005;29:711–719. doi: 10.1038/sj.ijo.0802950. doi:10.1038/sj.ijo.0802950. [DOI] [PubMed] [Google Scholar]
- Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. American Journal of Epidemiology. 2009;170:181–192. doi: 10.1093/aje/kwp104. doi:10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change. Can mood measures in diary studies detect change reliably? Personality and Social Psychology Bulletin. 2006;32:917–929. doi: 10.1177/0146167206287721. doi:10.1177/0146167206287721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JM. Heritability of diurnal changes in food intake in free-living humans. Nutrition (Burbank, Los Angeles County, Calif.) 2001;17:713–720. doi: 10.1016/s0899-9007(01)00611-6. [DOI] [PubMed] [Google Scholar]
- de Castro JM. The time of day of food intake influences overall intake in humans. The Journal of Nutrition. 2004;134:104–111. doi: 10.1093/jn/134.1.104. [DOI] [PubMed] [Google Scholar]
- de Castro JM. When, how much and what foods are eaten are related to total daily food intake. British Journal of Nutrition. 2009;102:1228–1237. doi: 10.1017/S0007114509371640. doi:10.1017/S0007114509371640. [DOI] [PubMed] [Google Scholar]
- de Castro JM, Bellisle F, Feunekes GIJ, Dalix AM, DeGraaf C. Culture and meal patterns. A comparison of the food intake of free-living American, Dutch, and French students. Nutrition Research (New York, N.Y.) 1997;17:807–829. [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women. A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Gellman MD, Turner JR. Encyclopedia of behavioral medicine. Springer; New York: 2013. [Google Scholar]
- Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Leblanc V, Provencher V, Begin C, Gagnon-Girouard MP, Corneau L, Tremblay A, et al. Associations between eating patterns, dietary intakes and eating behaviors in premenopausal overweight women. Eating Behaviors. 2012;13:162–165. doi: 10.1016/j.eatbeh.2011.12.002. doi:10.1016/j.eatbeh.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Mattes R, Hollis J, Hayes D, Stunkard AJ. Appetite. Measurement and manipulation misgivings. Journal of the American Dietetic Association. 2005;105:S87–S97. doi: 10.1016/j.jada.2005.02.029. doi:10.1016/j.jada.2005.02.029. [DOI] [PubMed] [Google Scholar]
- McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, et al. Ten putative contributors to the obesity epidemic. Critical Reviews in Food Science and Nutrition. 2009;49:868–913. doi: 10.1080/10408390903372599. doi:10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, O’Connor DB, Conner M. Daily hassles and eating behaviour. The role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. doi:10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychology. 2008;27:S20–S31. doi: 10.1037/0278-6133.27.1.S20. doi:10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA: The Journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. doi:10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H. Anticipation of a psychosocial stressor differentially influences ghrelin, cortisol and food intake among emotional and non-emotional eaters. Appetite. 2014;74:35–43. doi: 10.1016/j.appet.2013.11.018. doi:10.1016/j.appet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Sarker MR, Franks S, Caffrey J. Direction of post-prandial ghrelin response associated with cortisol response, perceived stress and anxiety, and self-reported coping and hunger in obese women. Behavioural Brain Research. 2013;257:197–200. doi: 10.1016/j.bbr.2013.09.046. doi:10.1016/j.bbr.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Schembre SM, Geller KS. Psychometric properties and construct validity of the Weight-Related Eating Questionnaire in a diverse population. Obesity. 2011;19:2336–2344. doi: 10.1038/oby.2011.96. doi:10.1038/oby.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schembre SM, Yuen J. Project TwEATs. A feasibility study testing the use of automated text messaging to monitor appetite ratings in a free-living population. Appetite. 2011;56:465–468. doi: 10.1016/j.appet.2011.01.014. doi:10.1016/j.appet.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. doi:10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyko M, Naab P, Shiffman S, Li R. Modeling complexity of EMA data. Time-varying lagged effects of negative affect on smoking urges for subgroups of nicotine addiction. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2013:S144–S150. doi: 10.1093/ntr/ntt109. doi:10.1093/ntr/ntt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyko MP, Lanza ST, Tan XM, Li RZ, Shiffman S. Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges. Differences between successful quitters and relapsers. Prevention Science. 2012;13:288–299. doi: 10.1007/s11121-011-0264-z. doi:10.1007/s11121-011-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Brownell KD. The stress-eating paradox. Multiple daily measurements in adult males and females. Psychology and Health. 1994;9:425–436. [Google Scholar]
- Stratton RJ, Stubbs RJ, Hughes D, King N, Blundell JE, Elia M. Comparison of the traditional paper visual analogue scale questionnaire with an Apple Newton electronic appetite rating system (EARS) in free living subjects feeding ad libitum. European Journal of Clinical Nutrition. 1998;52:737–741. doi: 10.1038/sj.ejcn.1600636. [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, et al. The use of visual analogue scales to assess motivation to eat in human subjects. A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. The British Journal of Nutrition. 2000;84:405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- Tan XM, Shiyko MP, Li RZ, Li YL, Dierker L. A time-varying effect model for intensive longitudinal data. Psychological Methods. 2012;17:61–77. doi: 10.1037/a0025814. doi:10.1037/A0025814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tan X, Li R, Wagner A. TVEM (time-varying effect model) SAS macro suite users’ guide (Version 2.1.0) The Methodology Center; Penn State: University Park: 2012. [Google Scholar]
- Yannakoulia M, Melistas L, Solomou E, Yiannakouris N. Association of eating frequency with body fatness in pre- and postmenopausal women. Obesity. 2007;15:100–106. doi: 10.1038/oby.2007.503. doi:10.1038/oby.2007.503. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW, Mitchell CJ, True S. Independent effects of palatability and within-meal pauses on intake and appetite ratings in human volunteers. Appetite. 1997;29:61–76. doi: 10.1006/appe.1997.0092. [DOI] [PubMed] [Google Scholar]