Abstract
Previous neuroimaging studies of youth with bipolar disorder(BD) have identified abnormalities in emotion regulation circuitry. Using data from the Longitudinal Assessment of Manic Symptoms Cohort (a clinical sample recruited for behavioral and emotional dysregulation), we examined the impact of BD and medication on activation in these regions. Functional neuroimaging data were obtained from 15 youth with BD who currently were unmedicated with a mood stabilizer or antipsychotic (U-BD), 19 youth with medicated BD (M-BD), a non-bipolar clinical sample with high rates of disruptive behavioral disorders (non-BD, n=59), and 29 healthy controls (HC) while they were shown task-irrelevant morphing emotional faces and shapes. Whole brain analysis was used to identify clusters that showed differential activation to emotion vs. shapes across group. To assess pair-wise comparisons and potential confounders, mean activation data were extracted only from clusters within regions previously implicated in emotion regulation (including amygdala and ventral prefrontal regions). A cluster in the right inferior frontal gyrus (IFG) showed group differences to emotion vs. shapes (159 voxels, corrected p<.05). Within this cluster, U-BD youth showed decreased activation relative to HC (p=.007) and non-BD (p=.004) youth. M-BD also showed decreased activation in this cluster relative to HC and non-BD youth, but these differences were attenuated. Results were specific to negative emotions, and not found with happy faces. IFG findings were not explained by other medications (e.g. stimulants) or diagnoses. Compared to both HC and a non-BD sample, U-BD is associated with abnormally decreased right IFG activation to negative emotions.
Keywords: bipolar disorder, functional MRI (fMRI), psychopharmacology, neuroimaging, emotion regulation
Objectives of the Study and Background
Deficits in emotion regulation are central to the clinical diagnosis of bipolar disorder (BD). The underlying neurobiology of these deficits is hypothesized to involve abnormally increased activation of limbic regions coupled with decreased activation of regulatory prefrontal circuitry(Chen et al., 2011; Womer et al., 2009). Supporting this hypothesis, BD in youth has been associated with abnormalities in amygdala and prefrontal activation during emotion processing tasks. Specifically, several groups have found amygdala hyperactivation in youth with BD during emotional processing(Garrett et al., 2012; Kim et al., 2012; Pavuluri et al., 2007; Rich et al., 2006). Abnormalities have also been found in regions including ventrolateral prefrontal cortex(VLPFC), orbitofrontal cortex(OFC), and anterior cingulate cortex(ACC) during emotion processing, though the direction of these abnormalities differs according to task and study population(Brotman et al., 2013; Dickstein et al., 2007; Ladouceur et al., 2011; Pavuluri et al., 2009). Limited studies with multiple patient groups have shown findings to be specific to BD, and not simply a marker of psychopathology(Passarotti et al., 2010b; Thomas et al., 2012). While medications seem to have a normalizing effect (reducing differences between activation in youth with BD versus healthy controls), few studies compare medicated and non-medicated youth(Hafeman et al., 2012; Singh & Chang, 2012).
The current study examined the effects of diagnosis and psychotropic medication on neural activation during an emotional processing task in a subset of the Longitudinal Assessment of Manic Symptoms(LAMS) cohort, behaviorally and emotionally dysregulated youth who have been followed longitudinally(Findling et al., 2010; Horwitz et al., 2010). Participants in the parent cohort study(n=707) were recruited from 9 clinical settings at four sites(Pittsburgh, Cincinnati, Cleveland, and Columbus), preferentially selected based on an elevated score on a screen for deficits in behavior, emotion and energy regulation(Parent General Behavioral Inventory-10, PGBI-10)(Youngstrom et al., 2008). Follow-up is ongoing for over 80% of these participants, with biannual assessments of clinical symptoms, diagnoses and functional impairments. The aim of the LAMS study is to describe abnormalities in behavioral and emotional regulation and arousal over time, and ultimately to predict progression of functional impairment and disorder. As part of this aim, functional neuroimaging data were collected on a subset of youth at three sites(Pittsburgh, Cincinnati, and Cleveland) while engaging in a number of tasks, including implicit emotion processing.
Our analyses capitalized on several key attributes of this cohort. The sample was large enough to include a number of youth with BD who were currently not medicated with an antipsychotic, antidepressant or mood stabilizing drug, thus permitting comparisons between youth with unmedicated BD(U-BD), medicated bipolar youth(M-BD), and healthy controls(HC). Because recruitment was not based on diagnosis, LAMS included youth who did not meet criteria for BD(non-BD). This unique comparison group allowed us to assess the specificity of neuroimaging findings for BD, and to test whether such results could be explained by co-morbidity.
The current study tested the following hypotheses: (1) youth would activate the amygdala and prefrontal regions (as measured by BOLD signal) in response to emotional stimuli (vs. morphing shapes); (2) these activation patterns would differ across groups defined by diagnosis and medication status; and (3) differences would be most prominent in U-BD (versus HC), attenuated (but still present) in M-BD, and even less robust in non-BD. Specifically, we hypothesized that amygdala activation would be elevated in bipolar youth (U-BD>M-BD>non-BD≥HC), while prefrontal activation would show the opposite pattern (U-BD<M-BD<non-BD≤HC). We also assessed the degree that abnormalities differed across specific emotional stimuli, and the extent to which neuroimaging findings were related to mood state.
Materials and Methods
Participants
A subset of the initial LAMS cohort(n=123) was recruited to participate in the neuroimaging component of the LAMS follow-up study. Additionally, 32 age- and gender-matched HC were scanned for comparison. Informed consent was obtained from parents or guardians after the nature of the study had been fully explained, and youth provided written informed assent. Participants received monetary compensation and a framed structural brain image. This investigation was carried out in accordance with the latest version of the Declaration of Helsinki, and the study design was approved by the appropriate Institutional Review Boards.
Exclusion criteria included pregnancy, inability to participate in scan (claustrophobia, metal objects in the body), positive urine drug and/or salivary alcohol screen on scan day, alcohol/substance abuse in the past three months, severe systemic medical illness, neurological disorders, history of head trauma with loss of consciousness, IQ<70(Weschler, 1999), visual disturbance (<20/40 Snellen visual acuity), inability to complete questionnaires in English, history of physical/sexual abuse, or autistic spectrum disorders/developmental delays.
Data from 30 LAMS youth and three HC were excluded due to data loss and/or excessive head movement (>4mm, as used in previous studies studies(Bebko et al., 2013)), yielding usable scans from 93 LAMS participants and 29 healthy controls. Compared to included youth, those excluded were younger (p=.03) and had lower IQs (p=.0001); they were more likely to have disruptive behavior disorders (DBD) (p=.03) and unmedicated ADHD (p=.004), but did not differ according to group (U-BD, M-BD, N-BD, and HC) (p=.15). (Table S1).
Assessment
Baseline assessments gathered demographic data including age, sex, IQ and parents' education. The Family History Screen(Weissman et al., 2000) tracked 15 psychiatric disorders in biological parents, and included two questions to assess for lifetime parental history of mania. Diagnoses were determined at baseline and every six months using the Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Life Version with WASH-U mood supplement (K-SADS-PL-W)(Kaufman et al., 1997). During these biannual assessments, parents also completed the PGBI-10M. The PGBI-10M score nearest the scanning session (within 6 months) was used. PGBI-10M scores were very stable over the year immediately preceding scan day(Bebko et al., 2013).
On scanning day, the youth and a parent/guardian completed the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale(KMRS) (Axelson et al., 2003) and Depression Rating Scale(KDRS)(Kaufman et al., 1997), to assess for hypomanic/manic and depressive symptom severity, respectively. Interviewers determined summary scores based on all available information. Additional rating scales were administered, including the Screen for Childhood Anxiety Related Disorders(SCARED) and the Moods and Feelings Questionnaire(MFQ). Psychotropic medications taken within the past 24 hours were also recorded.
Participants were categorized into four groups: U-BD (n=15), M-BD (n=19), non-BD (n=59), and HC (n=29). The mean age of the participants at time of scan was 13.6(±2.1) years, and 57% were male. Groups did not differ significantly according to age, gender, IQ, or socioeconomic status; however, sites differed on rates of HC recruitment(Tables 1 and S3). Approximately 1/3 of the clinical sample had DBD, including oppositional defiant disorder, conduct disorder, and DBD NOS, and 2/3 of the clinical sample was diagnosed with ADHD (Tables 1 and S2). Forty-seven percent (28/59) of the non-BD youth had a current or previous diagnosis of depressive disorder. Most bipolar youth were diagnosed with BD-I (21/34); the remainder fit criteria for BD-NOS, according to previously described criteria (Birmaher et al., 2006). We included youth with BD-NOS due to previous work indicating similarities in demographics, clinical features, and family history across the bipolar spectrum (Axelson et al., 2006; Hafeman et al., 2013).
Table 1.
Basic demographic characteristics of the study population.
| U-BD (n=15) |
M-BD (n=19) |
Non-BD (n=59) |
HC (n=29) |
p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 14.4 ± 2.1 | 13.6 ± 2.1 | 13.8 ± 1.9 | 13.1 ± 2.4 | .17 |
| Gender (% male) | 7/15 (47%) | 13/19 (68%) | 37/59 (54%) | 13/29 (44%) | .24 |
| IQ | 100 ± 17 | 106 ± 13 | 104 ± 17 | 107 ± 12 | .54 |
| Other Diagnoses | |||||
| DBD | 4/15 (27%) | 5/19 (26%) | 23/59 (39%) | 0 0%) | .002 |
| ADHD | 6/15 (40%) | 10/19 (52%) | 43/59 (73%) | 0 (0%) | <.0001 |
| Medication | |||||
| Mood Stabilizers | 0/15 (0%) | 9/19 (79%) | 0/59 (5%) | 0 (0%) | <.0001 |
| Antipsychotics | 0/15 (0%) | 16/19 (68%) | 6/59 (7%) | 0 (0%) | <.0001 |
| Antidepressants | 0/15 (0%) | 6/19 (32%) | 7/59 (12%) | 0 (0%) | .003 |
| Stimulants | 4/15 (27%) | 10/19 (53%) | 26/59 (44%) | 0 (0%) | <.0001 |
| Non-stimulant | 2/15 (13%) | 3/19 (16%) | 2/59 (3%) | 0 (0%) | .06 |
DBD = disruptive behavioral disorders; ADHD = attention-deficit hyperactivity disorder
All M-BD youth were on an antipsychotic and/or mood stabilizer medication (lithium or antiepileptic agent); six were also on an antidepressant medication, and 10 were on a stimulant (Table 1). By definition, the U-BD youth were not medicated with mood-altering medication; however, six of the 15 youth took medication for ADHD (non-stimulant (n=2) or stimulant (n=4)). Most non-BD youth were also on at least one psychotropic medication.
Groups differed significantly on measures of depressive and manic symptoms at time of scan (PGBI-10M, KMRS, KDRS, and MFQ), but not anxiety (Table S3). U-BD vs. M-BD youth had higher KDRS scores (p=.001; Table S4).
Dynamic Faces Paradigm
A block-design emotional dynamic faces task evaluated implicit processing of emotional stimuli. Participants watched a series of faces that morphed from neutral to full expression of emotion (happy, sad, fearful, or angry) in 1 second. During control blocks, a luminance-equated shape morphed into a larger shape. Participants identified the foreground color, rendering the emotion task-irrelevant. The subject was shown three blocks for each of the four emotions (12 stimuli per block), and six control blocks (6 stimuli per block), pseudo-randomized so that a single emotion block was not repeated sequentially. This task robustly activates the amygdala and prefrontal emotion processing circuitry in adults(Herringa et al., 2013).
Neuroimaging Analysis
Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM8 http://www.fil.ion.ucl.ac.uk/spm). We used a whole brain analytic approach to assess task activation, which we define as the difference in BOLD signal between emotion blocks and control (shape) blocks (Hypothesis 1); we next assessed differences in task activation between groups (Hypothesis 2). Our a priori regions of interest were the amygdala and prefrontal regions previously implicated in emotion regulation(Phillips et al., 2008; Strakowski et al., 2012): ACC, OFC(BA 11), and VLPFC(BA 47). Thus additional analyses (Hypothesis 3 and Supplemental) were only conducted for clusters with peak voxels in these regions.
Hypothesis 1 - Activation of Amygdala and Prefrontal Regions
Using SPM8, we assessed whole brain activation patterns to emotions vs. shapes across the entire study population (n=122), using a 1-sample t-test and collapsing across group. To adjust for multiple comparisons, we reported only clusters that were Family Wise Error(FWE)-corrected p<.05, at both voxel- and cluster-wise levels.
Hypothesis 2 – Differential activation of these regions across group
We constructed multiple regression models in SPM8 to assess differences in activation to emotional faces vs. shapes between groups. Using a whole brain analysis, we identified voxels that showed significant (p<.01) between-group differences in both the unadjusted and covariate-adjusted (site, gender, age, and IQ) models. This conservative approach facilitated the identification of voxels that were robust to potential confounding factors. To correct for multiple comparisons, we used a cluster-wise threshold determined by Monte Carlo simulations implemented in AlphaSim, to maintain an alpha of .05; clusters were only considered significant if they were larger than the determined threshold(≥146 voxels). This validated technique accounts for spatial correlations between BOLD signal changes in neighboring voxels(Ward, 2002).
Hypothesis 3 – Specific Pair-Wise Comparisons
To determine which pair-wise comparisons explained the group differences observed above, mean BOLD signal change parameter estimates were extracted from significant cluster(s) (generated from Hypothesis 2) with peak voxels in the hypothesized regions. A general linear model (PROC GLM in SAS 9.2) was used to further assess pair-wise contrasts (e.g. M-BD vs. HC), adjusting for site, age, gender, and IQ. Because we only extracted from significant clusters, reported p-values are not spatially corrected for multiple comparisons; however, we used the Tukey test to correct for six pair-wise comparisons across four groups. The impact of parental history of mania and parental education on mean cluster activation was determined by entering these variables separately into the group-adjusted emotion vs. shapes model in SAS 9.2.
Further Analyses
One strength of the LAMS cohort is the heterogeneity of the youth, but this also leads to multiple alternative explanations for the observed results. To address several possible confounds, including co-morbidity, stimulant medication, type of bipolar diagnosis, task performance, and clinical state, we conducted the following analyses in SAS 9.2 on extracted regions from Hypothesis #2. First, we entered each variable into a multivariable model, adjusting for group and demographics, to establish whether (1) the variable significantly predicted cluster activation and (2) group was still significant after adjustment. Second, when possible, we assessed the impact of group in a subset of the population not affected by the potential confound, to determine whether results were driven by this variable. To address the impact of task performance, we re-ran models (in SAS 9.2) on the subset of youth with accuracy >80%.
To determine individual emotion effects, emotion-specific activation data were extracted from the significant cluster(s) identified in Hypothesis 2. General linear models were then constructed in SAS 9.2 to assess between-group differences for each emotion. Results of these supplemental analyses are discussed briefly in the text, and detailed results are given in the Supplemental Appendix.
A number of measures were utilized to address biases that may arise in multisite neuroimaging studies. As recommended by the Biomedical Informatics Research Network (BIRN; http://www.nbirn.net), a BIRN phantom was utilized monthly at all three sites to ensure longitudinal scanner signal stability. In addition, scan site was used as a covariate in the SPM8 analysis (Hypothesis 2) and the GLM in SAS 9.2 (Hypothesis 3). We also conducted sensitivity analyses to determine whether observed patterns of between-group differences in activity were driven by a particular site, by sequentially removing each site and rerunning primary analyses in SAS 9.2.
Results
Hypothesis 1
As predicted, the task (emotions vs. shapes) diffusely activated multiple brain regions, most prominently the right fusiform gyrus, bilateral amygdala, and bilateral inferior frontal gyrus (IFG) (FWE-corrected p<.05) (Figure 1, Table 2). Significant activation was also observed in bilateral middle and superior temporal regions, as well as the left parahippocampal gyrus.
Figure 1.
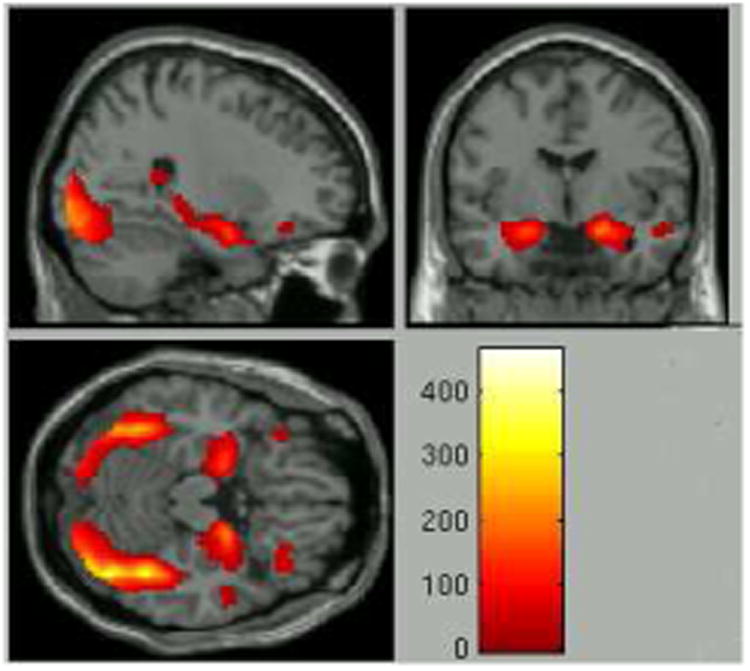
Task activation patterns to all emotions vs. shapes across the entire study sample (whole brain, FWE-corrected p<.05). Z-scores are labeled.
Table 2.
Regions of activation (whole brain, FWE-corrected p<.05). Regions k>50 listed below.
| Region | Peak Coordinates(MNI) | FWE-corrected p | t | k |
|---|---|---|---|---|
| Fusiform gyrus(right) | 40 -48 -22 | <.001 | 21.57 | 8048 |
| Amygdala(right) | 18 -4 -18 | <.001 | 14.39 | 1356 |
| Amygdala(left) | -22 -4 -18 | <.001 | 12.43 | 739 |
| IFG/BA 46(right) | 52 28 18 | <.001 | 8.46 | 836 |
| IFG/BA 47(right) | 34 32 16 | <.001 | 8.05 | 148 |
| Middle temporal gyrus(left) | -54 -60 6 | <.001 | 7.82 | 160 |
| Parahippocampal gyrus(left) | -16 -32 -4 | <.001 | 7.67 | 167 |
| Superior temporal gyrus(right) | 48 -16 -10 | <.001 | 6.79 | 169 |
| IFG/BA 47(left) | -38 26 -16 | <.001 | 6.47 | 61 |
| Superior temporal gyrus(left) | -46 16 26 | <.001 | 6.22 | 220 |
Hypothesis 2
Significant group differences to emotions vs. shape were found in the right IFG (peak voxel in the VLPFC), right cuneus, right middle cingulate cortex, and left insula (voxel-wise p<.01, corrected p<.05) (Table 3). These clusters were robust to confounding, meaning that voxels were significant in both the unadjusted and covariate-adjusted models. Since the peak voxel of the IFG cluster was within an a priori region of interest (VLPFC), we extracted mean activation data for each participant from this cluster for further analyses (Figure 2a).
Table 3.
Whole brain results, found in both unadjusted and covariate-adjusted models. Based on ANOVA of group differences (across emotions vs. shapes), with voxel wise threshold p<.01, corrected to p<.05 using AlphaSim threshold(146 voxels)
| Region | Peak Coordinates(MNI) | F | k |
|---|---|---|---|
| Inferior Frontal Gyrus(right) | 38 16 -16 | 6.35 | 159 |
| Cuneus(right) | 14 -84 14 | 8.18 | 441 |
| Insula(left) | -46 -24 18 | 6.76 | 155 |
| Middle cingulate gyrus(right) | 2 -36 38 | 6.48 | 162 |
Figure 2.
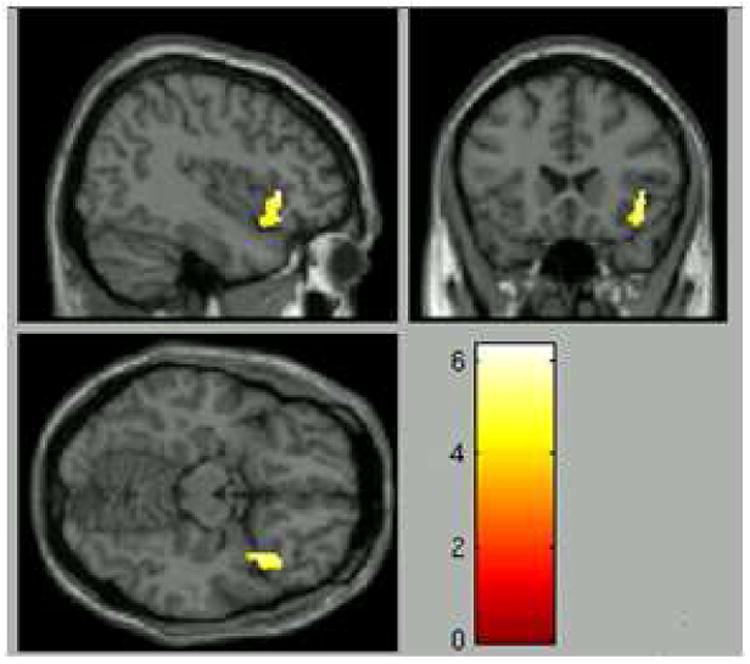
a: Cluster showing differential activation across groups with peak voxel in the a priori region of interest (voxelwise p<.01, corrected p<.05).
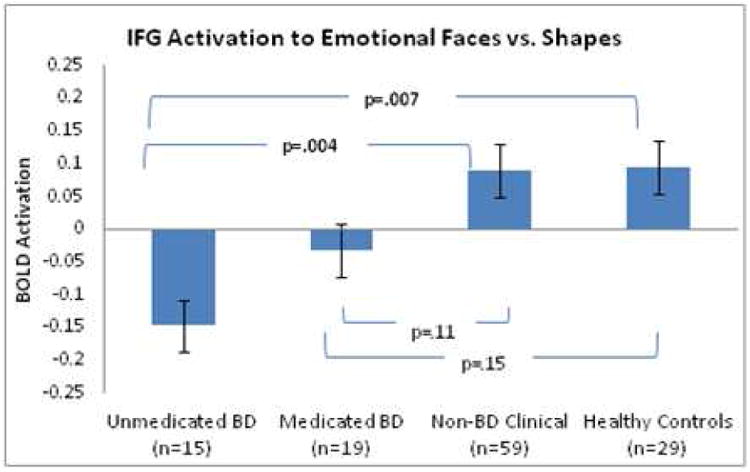
b: Mean activation values across group for the extracted region(159 voxels) for emotions vs. shapes. While both HC and non-BD youth showed significant activation in response to emotions vs. shapes, U-BD showed deactivation; p-values are corrected for multiple comparisons using the Tukey-Kramer method.
Hypothesis 3
While both the HC and non-BD youth showed mean activation in the extracted right IFG cluster during emotions vs. shapes (p<.05), the U-BD group showed deactivation in response to emotional stimuli (p<.05). Relative to both HC and non-BD youth, the U-BD showed decreased mean activation which was significant after correcting for multiple comparisons (corrected p<.01). As predicted, activation in the M-BD youth was intermediate between U-BD and non-BD. No significant differences were observed between HC and non-BD groups. Covariate-adjusted results are shown in Figure 2b. Group effects remained significant after further adjustment for parental education and parental history of mania.
Further Analyses
Group effects remained significant following sequential adjustment for co-morbidity (ADHD, DBD, depression, and anxiety), stimulant usage, and type of bipolar disorder; additionally, none of these variables independently predicted cluster activation. Results from Hypothesis 3 were replicated in (1) the subgroup without co-morbidity and (2) the subgroup of youth not on a stimulant medication, indicating that these factors did not drive observed findings. Additionally, results from Hypothesis 3 were replicated in youth with BD-I (excluding BD-NOS), indicating that sub-syndromal BD was not driving observed results. A sensitivity analyses to determine whether results were driven by a particular site indicated that group differences remained significant in each two-site subgroup (all p<.05).
Effect of Individual Emotions
Supplementary analysis assessing the activation to each emotion indicated that there were group effects to fearful (F=4.08, p=.009), angry (F=2.48, p=.06), and sad faces (F=6.06, p=.0007), but not happy faces (F=0.72, p=.54). Thus results for emotions vs. shapes appear to be driven by neural responses to negative emotions (Figure S1).
Effect of clinical state
To determine whether the abnormality in the extracted IFG cluster correlated with current mood state, we sequentially entered both log-transformed clinician-derived (KMRS and KDRS) and self-report scales (MFQ and SCARED) into the Hypothesis 3 emotions vs. shapes model in SAS 9.2. Adjustment for these variables did not significantly impact the effect of group, indicating that between-group differences were not explained by clinical state. However, KDRS scores were positively correlated with IFG cluster activation (t=2.52, p=.01). While KMRS and PGBI-10 scores did not predict activation in the entire population, both were negatively correlated with IFG cluster activation in the bipolar sample (KMRS: t=-2.23, p=.03, PGBI-10: t=-2.55, p=.02). Thus exploratory results indicate that deficits in positive affect regulation were associated with decreased right IFG activation (in the bipolar sample), while depressive symptoms were associated with increased activation. Other scales (MFQ, SCARED) did not significantly predict cluster activation, and adjustment for these variables did not appreciably change the effect of group.
Effect of Task Performance
There were a significant number of participants with accuracy less than 80% on this task (n=49); these youth were more likely to be in the clinical sample (vs. HC) (p<.0001), although bipolar diagnosis (and medication status) did not significantly affect performance (Table S3). Much of the poor performance was due to missed trials. Three of the participants did not have any response data recorded, and n=7 had no response data for the majority of the trials (likely due to an error in ePrime). Further analyses determined that accuracy, reaction time, or response rate did not significantly impact IFG cluster activation, and adjustment for these variables did not appreciably change the effect of group. To ensure that results were not attributable to youth with poor accuracy, results from Hypothesis 3 were replicated in the sub-population of youth (n=75) who had accuracy >80% (Supplemental Appendix).
Discussion
We found deactivation of the right IFG (peak voxel in the VLPFC) during processing of emotional faces versus shapes in youth with U-BD, as compared to activation in HC and non-BD youth. Qualitatively similar differences were observed in youth with M-BD versus HC and non-BD youth, but these differences were attenuated. Observed IFG activation was negatively correlated with PGBI-10 and KMRS scores, and positively correlated with KDRS scores, pointing to a possible relationship between IFG deactivation and manic state. Given the role of the VLPFC in emotion regulation(Phillips et al., 2008), these results support the hypothesis that youth with BD show abnormalities in emotion regulation circuitry.
The VLPFC is emerging as a region that, given its role in emotion regulation, is potentially important for the pathophysiology of BD(Foland-Ross et al., 2012; Strakowski et al., 2011). Two recent meta-analyses found that BD in adults was associated with decreased VLPFC activation relative to healthy controls(Chen et al., 2011) and adults with major depressive disorder(Delvecchio et al., 2012). Additionally, decreased VLPFC activation correlated with manic symptomatology(Chen et al., 2011), and normalized with treatment in youth with BD during a response inhibition task(Pavuluri et al., 2010).
Current findings build on this previous work in several important ways. Our results extend conclusions of previous meta-analyses from predominately adult populations to a sample of bipolar youth, indicating that the VLPFC might have an important role in the early pathophysiology of BD. The findings that this abnormality is correlated with manic symptoms and attenuated by medication indicates that VLPFC activation during this emotion processing task might be an important marker of treatment and/or clinical state; this builds on work by Pavuluri et al. (2010) that showed a similar finding during a response inhibition task (albeit in the left VLPFC). In addition, our sample provides the unique opportunity to compare bipolar youth to a non-BD clinical sample; this allows us to conclude that our observed results are specific to BD, and not simply attributable to co-morbidity of the sample. Of note, previous work has also implicated the VLPFC in response inhibition(Aron et al., 2004), and has shown decreased VLPFC activation in youth with ADHD, which is ameliorated by stimulant medication(Rubia et al., 2011). In our sample, diagnosis of ADHD and stimulant medication did not predict VLPFC activation, likely due to the nature of the task (emotional faces vs. response inhibition).
Contrary to expectations, while our task led to robust activation of the amygdala across groups, no between-group difference was observed in the amygdala. One possible explanation for this is the likely attenuation of amygdala BOLD signal during each block via repetition suppression (associated with repeated exposures to the same face stimuli), which might have obscured between-group differences(Phillips et al., 2001). Thus it is possible that BD youth initially had a greater magnitude of amygdala activation, but due to attenuation within and across blocks, these differences were not observed. While most studies have shown abnormalities in amygdala activation in bipolar youth during emotion processing, there are certainly exceptions(Brotman et al., 2010; Passarotti et al., 2010a). A recent study found that BD is associated with a decreased ability of the amygdala to modulate in response to increasing intensity of emotional faces(Deveney et al., 2014). Given the dynamic nature of the current task, it is possible that amygdala modulation differed across groups, while mean signal did not.
Differences across groups were driven by the hemodynamic response to negative emotions, and were not evident in response to happy faces vs. shapes. This is possibly due to the greater salience of the negative emotions (relative to happy faces) in the task. Previous work also indicates that the VLPFC is particularly responsive to negative stimuli(Viinikainen et al., 2010), and involved in reappraisal of negative emotions(Wager et al., 2008). Additional regions of differential activation were observed in the right cuneus, right middle cingulate gyrus, and left insula. These findings are consistent with an analysis from a subset of this study population that focused on more posterior regions, and found that BD youth (relative to HC) showed decreased activation in primary visual cortex(Perlman et al., 2013).
This study had a number of strengths, facilitating these novel analyses. First, the large sample size, recruited selectively for deficits in behavioral and emotional regulation, included enough youths with U-BD and M-BD to assess the impact of both diagnosis and medication. Second, the non-BD clinical sample allowed us to test whether the IFG finding was specific to BD, or a general marker for psychopathology in youth. Third, the sample is well-characterized, both at time of scan and historically. Multiple diagnostic interviews have been conducted, improving diagnostic quality and covariate assessment. Thus potential confounders of the relationship between group and IFG activation, such as demographics, parental history of mania, and additional diagnoses, could be adequately tested.
This study also has a number of limitations. First, the U-BD youth were not currently medicated for BD, but they were not necessarily medication naïve, and several were on medication for ADHD. However, results were not driven by this potential confound: when youth on stimulants were excluded, the IFG activation differences between U-BD and HC were even more pronounced. Second, this multisite study used three different scanners, leading to increased noise. Results were not driven by a particular site, and adjustment for site did not appreciably change findings. Although site effects might have obscured more subtle findings, they were not responsible for the primary observed results. Third, task accuracy rates were fairly low: ≈2/3 of the sample had accuracy >80%. Because youth were exposed to the same emotional stimuli regardless of task accuracy, we did not exclude those with accuracy <80% from initial analysis. However, we used the conservative approach of re-running analyses after excluding these youth and results remained robust. Also, adjustment for accuracy and reaction time did not appreciably alter findings. Fourth, a significant proportion of youth were excluded due to excessive movement (33/155). While these youth differed significantly in terms of IQ and proportion with unmedicated ADHD, they did not differ according to group; thus it is unlikely that exclusion due to motion appreciably impacted our findings. Fifth, observational studies comparing unmedicated and medicated youth must be interpreted with some caution, since medications are not randomly assigned, and medication is likely correlated with severity of disorder. However, this confound would not explain the current pattern of results, since the most pronounced abnormalities are found in the unmedicated BD youth (who would likely be less severe than there medicated counterparts). Sixth, the sample size in the BD groups was fairly modest, which might have limited our power to appreciate certain between-group differences; additionally, this limitation made it impossible to assess the effects of individual medications on observed results.
In conclusion, BD was associated with abnormal IFG deactivation in response to negative emotional stimuli. The finding was ameliorated by medication and not found in a non-bipolar clinical sample. Research increasingly implicates the deactivation of the VLPFC in BD in adults, particularly in manic states. VLPFC deactivation to negative emotional stimuli might represent a potential biomarker to predict the development and trajectory of BD. Further work will assess longitudinal relationships between VLPFC activation and symptomatology in youth.
Supplementary Material
Highlights.
Bipolar youth show abnormal right IFG deactivation in response to emotional faces.
IFG deactivation was most prominent in the unmedicated bipolar youth.
IFG deactivation was not found in youth with non-bipolar psychopathology.
Findings are not attributable to task performance or mood state.
Acknowledgments
We would like to acknowledge Satish Iyengar for statistical consultation on this manuscript; Henry Chase for comments on earlier versions of this manuscript; and all of our participants and families, without whom this work would not be possible.
Funding Source: This research was funded by the LAMS 2 grant (5R01MH073953-08). Dr. Hafeman is supported by an MD PhD Supplemental Grant (3R01MH073953-08S1).
Footnotes
Contributors: Drs. Birmaher, Axelson, Horwitz, Arnold, Fristad, Frazier, Youngstrom, Kowatch, Findling, Phillips, and Ms. Gill were instrumental in designing and implementing the parent LAMS study. Drs. Phillips, Travis, Diwadkar, Sunshine, Holland, and Ms. Bonar were involved in the design and implementation of the neuroimaging portion of the LAMS study. Drs. Hafeman, Phillips, and Drevets were involved in designing the analytic strategy for the current study. Drs. Hafeman, Bebko, Bertocci, Perlman, and Fournier were involved in the analysis of the neuroimaging data. Dr. Hafeman wrote the initial draft of the manuscript. Critical feedback was provided by all authors, and all authors contributed to and have approved the final manuscript.
Conflict of Interest: Dr. Arnold received research funding from CureMark, Forest, Lilly, and Shire, advisory board honoraria from Biomarin, Novartis, Noven, Roche, Seaside Therapeutics, and Shire, consulting fees from Tris Pharma, and travel support from Noven. Dr. Frazier received federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from the Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Shire Development, Bristol-Myers Squibb, National Institutes of Health, and the Brain and Behavior Research Foundation. Dr. Findling has received research support, acted as a consultant and/or served on a speaker's bureau for Alexza Pharmaceuticals, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, Clinsys, Cognition Group, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm, Lilly, Lundbeck, Merck, NIH, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Rhodes Pharmaceuticals, Roche, Sage, Seaside Pharmaceuticals, Shire, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. Dr. Drevets is a full-time employee of Janssen Pharmaceuticals Research & Development, of Johnson & Johnson, Inc. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–48. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–70. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Bebko G, Bertocci MA, Fournier JC, Hinze AK, Bonar L, Almeida JR, Perlman SB, Versace A, Schirda C, Travis M, Gill MK, Demeter C, Diwadkar VA, Ciuffetelli G, Rodriguez E, Olino T, Forbes E, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML. Parsing Dimensional vs Diagnostic Category-Related Patterns of Reward Circuitry Function in Behaviorally and Emotionally Dysregulated Youth in the Longitudinal Assessment of Manic Symptoms Study. JAMA Psychiatry. 2013;71:71–80. doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–83. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–9. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Tseng WL, Olsavsky AK, Fromm SJ, Muhrer EJ, Rutenberg JG, Deveney CM, Adleman NE, Zarate CA, Pine DS, Leibenluft E. Fronto-limbic-striatal dysfunction in pediatric and adult patients with bipolar disorder: impact of face emotion and attentional demands. Psychol Med. 2013:1–13. doi: 10.1017/S003329171300202X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, Hietala J, Lawrie SM, Martinot JL, McIntosh AM, Meisenzahl E, Frangou S. Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–13. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Deveney CM, Brotman MA, Thomas LA, Hinton KE, Muhrer EM, Reynolds RC, Adleman NE, Zarate CA, Jr, Pine DS, Leibenluft E. Neural response during explicit and implicit face processing varies developmentally in bipolar disorder. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007;9:679–92. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, Horwitz SM. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry. 2010;71:1664–72. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, Torrisi S, Penfold C, Madsen SK, Thompson PM, Altshuler LL. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59:738–44. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Reiss AL, Howe ME, Kelley RG, Singh MK, Adleman NE, Karchemskiy A, Chang KD. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:821–31. doi: 10.1016/j.jaac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D, Axelson D, Demeter C, Findling RL, Fristad MA, Kowatch RA, Youngstrom EA, Horwitz SM, Arnold LE, Frazier TW, Ryan N, Gill MK, Hauser-Harrington JC, Depew J, Rowles BM, Birmaher B. Phenomenology of bipolar disorder not otherwise specified in youth: a comparison of clinical characteristics across the spectrum of manic symptoms. Bipolar Disord. 2013;15:240–52. doi: 10.1111/bdi.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol Med. 2013;43:1533–42. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA, Frazier TW, Findling RL. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. J Clin Psychiatry. 2010;71:1511–7. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim P, Thomas LA, Rosen BH, Moscicki AM, Brotman MA, Zarate CA, Jr, Blair RJ, Pine DS, Leibenluft E. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. Am J Psychiatry. 2012;169:642–9. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, Birmaher B, Phillips ML. Differential Patterns of Abnormal Activity and Connectivity in the Amygdala-Prefrontal Circuitry in Bipolar-I and Bipolar-NOS Youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–1289. e2. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2010a;16:106–17. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010b;49:1064–80. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O′Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–67. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–19. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010;71:1526–34. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Fournier JC, Bebko G, Bertocci MA, Hinze AK, Bonar L, Almeida JR, Versace A, Schirda C, Travis M, Gill MK, Demeter C, Diwadkar VA, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML. Emotional face processing in pediatric bipolar disorder: evidence for functional impairments in the fusiform gyrus. J Am Acad Child Adolesc Psychiatry. 2013;52:1314–1325. e3. doi: 10.1016/j.jaac.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ. Time courses of left and right amygdalar responses to fearful facial expressions. Hum Brain Mapp. 2001;12:193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–5. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Smith AB, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011;36:1575–86. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD. The neural effects of psychotropic medications in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2012;21:753–71. doi: 10.1016/j.chc.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–25. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee JH, Welge JA, DelBello MP, Fleck DE, Adler CM. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–8. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, Brotman MA, Muhrer EJ, Rosen BH, Bones BL, Reynolds RC, Deveney CM, Pine DS, Leibenluft E. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Arch Gen Psychiatry. 2012;69:1257–66. doi: 10.1001/archgenpsychiatry.2012.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viinikainen M, Jaaskelainen IP, Alexandrov Y, Balk MH, Autti T, Sams M. Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Hum Brain Mapp. 2010;31:1030–40. doi: 10.1002/hbm.20915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. AlphaSim. National Institute of Mental Health; Bethesda, MD: 2002. [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–82. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Weschler D. Weschler abbreviated scale of intelligence (WASI) Psychological Corporation; London: 1999. [Google Scholar]
- Womer FY, Kalmar JH, Wang F, Blumberg HP. A Ventral Prefrontal-Amygdala Neural System in Bipolar Disorder: A View from Neuroimaging Research. Acta Neuropsychiatr. 2009;21:228–238. [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a 10-item mania scale from the Parent General Behavior Inventory for children and adolescents. J Clin Psychiatry. 2008;69:831–9. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


