Summary
We demonstrate enhanced transgenesis in mice by intracytoplasmic injection of envelope-free lentivirus. Envelope-free lentivirus carrying the green fluorescent protein (GFP) gene under the control of the ubiquitin promoter (LVU-GFP) was microinjected into the cytoplasm of mouse zygotes prior to embryo transfer. Ninety-seven percent (31/32) of the adult mice were confirmed transgenic by PCR and Southern blot analysis; all founder mice express GFP when tail snips were examined by fluorescent microscopy prior to genomic DNA extraction. Transgene insertion numbers ranging from 1 to 32 were revealed by Southern blot analysis. Germline transmission was confirmed by the presence of transgene in F1 offspring. As expected, a lower transgenic rate (2.2%; 1/46) resulted when envelope-free LVU-GFP was microinjected into the perivitelline space (PVS) because cell recognition followed by membrane fusion between the viral envelope and the target cell is prerequisite for successful infection by envelope viruses. Here we demonstrate the competence of envelope-free lentivirus in establishing stable gene integration by germline transgenesis in mice at high efficiency, by intracytoplasmic viral injection (INVI) of envelope-free lentivirus into mouse zygotes.
Keywords: transgenesis, intracytoplasmic injection, bald lentivirus, pseudotype lentivirus, blastomere gene transfer
INTRODUCTION
Transgenic technology has facilitated the translation of basic research to clinical and industrial applications, resulting in significant impact on biomedicine and pharmaceutical development. Recent advancement in retroviral and lentiviral vector systems has led gene transfer technology into a new era of gene therapy and transgenic animal modeling. Despite known limitations of such vectors including size limitation for transgene (~10 kb) and gene silencing, these vectors are considered to be among the best choices for gene therapy and the creation of transgenic animals because of their high gene transfer efficiency (Chan, 2004). Transgenic animals have been successfully generated by the injection of vesicular stomatitis virus glycoprotein (VSVG) pseudo-typed retrovirus and lentivirus into the PVS of oocytes (cattle, pigs, and monkeys), zygotes (cattle, mice, and rats), and early preimplantation embryos (cattle, mice, rat) (Cabot et al., 2001; Chan et al., 1998, 2001; Lois et al., 2002). However, the competence of envelope-free lentivirus has not been demonstrated by stable integration and long-term expression of the transgene by the generation of transgenic animals. Although transfection of envelope-free retroviruses using lipofectamine reagent has been described in tissue culture (Abe et al., 1998; Sharma et al., 1997), genomic integration and bioactivity in vivo has not been demonstrated. Additionally, the high gene transfer rate of VSVG pseudotyped retrovirus and lentivirus is in part due to the stability of VSVG protein with its fusogenic characteristic that result in non-cell type specificity and high titer by ultracentrifugation. Moreover, when microinjected into the PVS or co-cultured with the target cells, lentiviruses without envelope are either incapable of achieving gene transfer or at low gene transfer rate (0–2.2%). These observations suggest the critical role of envelope protein in efficient viral infection through natural route.
RESULTS AND DISCUSSION
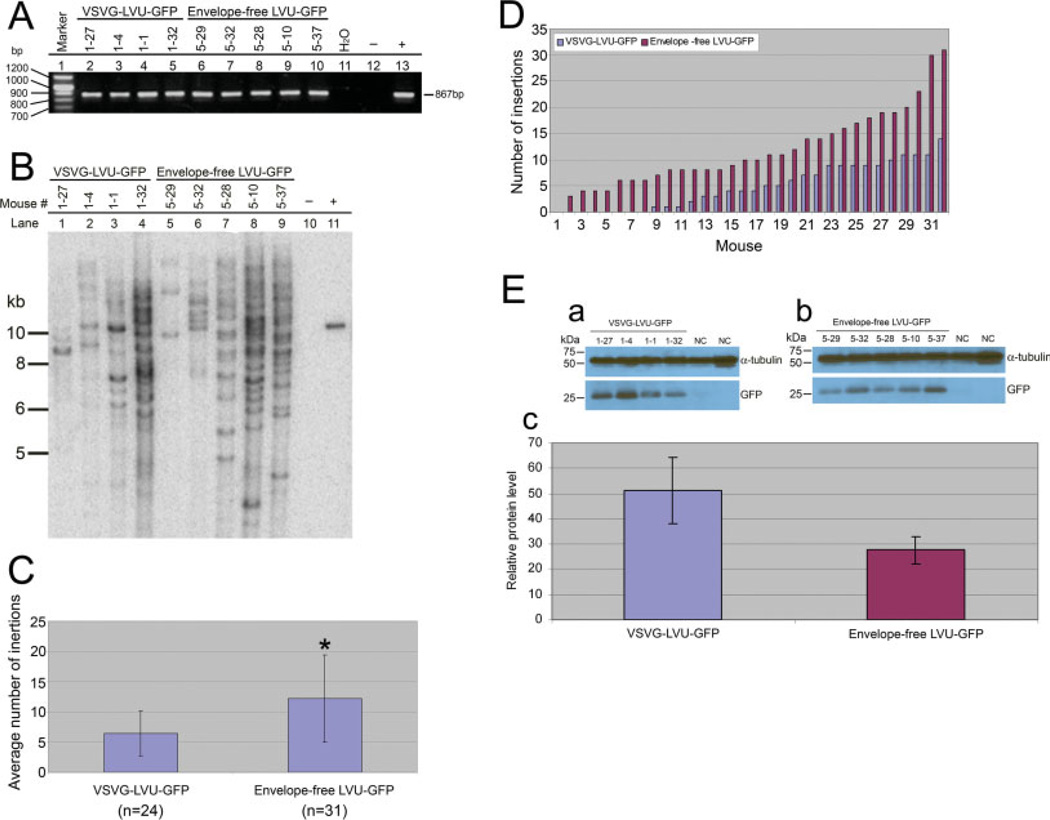
We have shown that envelope-free lentivirus is highly efficient in the generation of transgenic mice. Thirty-two adult mice were generated by intracytoplasmic injection of envelope-free LVU-GFP into mouse zygotes (Table 1). Thirty-one of those mice (97%; 31/32) were confirmed transgenic by PCR and Southern blot analysis (Fig. 1A,B; results of representative founder mice). GFP expression was observed in preimplantation embryos (Fig. 2A), E13 embryos (Fig. 2B-a), as well as at birth (Fig. 2B-b). Intra-cytoplasmic injection of VSVG-LVU-GFP produced a total of 32 adult mice. Among these adult mice, 24 out of 32 (75%) were confirmed transgenic by PCR and Southern blot analysis (Table 1; Fig. 1A,B; results of representative founder mice).
Table 1.
Gene Transfer Efficiency of Transgenic Mice Generated Using VSVG-LVU-GFP and Envelope-Free LVU-GFP
| No. of embryo injected |
No. of ET |
No. of adult mice |
Transgenesis (%) | |
|---|---|---|---|---|
| Intracytoplasmic injection of envelope-free LVU-GFP | 203 | 163 | 32 | 31 (97)a |
| Intracytoplasmic injection of VSVG-LVU-GFP | 132 | 95 | 32 | 24 (75)b |
| Perivitelline space injection of envelope-free LVU-GFP | 90 | 90 | 46 | 1 (2.2)c |
| Perivitelline space injection of VSVG-LVU-GFP | 151 | 97 | 37 | 17 (46)d |
Values in the same column with different superscript differ significantly (P < 0.05).
ET, embryo transfer.
FIG. 1.
Transgenesis of founder mice was confirmed by PCR and Southern blot analysis. Data of representative groups of founder mice based on their insertion number was shown in (A) and (B). Distribution of the number of insertion was determined by the number of band appeared in the Southern blot and the average number of insertion was determined by the total number of insertion of each treatment group divided by the total number of transgenic founder mice (C) and (D). The expression of the GFP was determined by Western analysis of the same representative groups (E). (A) PCR analysis: On the basis of the size of PCR product, four and five founder mice derived by intracyto-plasmic injection of VSVG-LVU-GFP (Lane 2–5) and envelope-free LVU-GFP (Lane 6–10) were confirmed transgenic and used for expression analysis; sizes of the amplicons (867 bp) were comparable to the positive control plasmid DNA (Lane 13). (B) Southern blot analysis of founder mice: Founder mice that carry low (<5; Lanes 1 and 5), medium (between 5 and 20; Lanes 2, 3, 4, 6, and 7) and high (>20; Lanes 8 and 9) numbers of insertion sites were selected. Lanes 1–4 were derived from VSVG-LVU-GFP. Lanes 5–9 were derived from envelope-free LVUGFP. Lane 10 is nontransgenic mouse and Lane 11 is pLVU-GFP positive control. The top panel is the identification number for each animal and the bottom panel is the number of each lane. Genomic DNA was digested with EcoRI, which cut once within the pLVU-GFP. The probe was the PCR product of the pLVU-GFP, which was gel purified and labeled with P32 by Rediprime labeling kit by Amersham. (C) Comparison of insertion number between transgenic founder mice derived from VSVG-LVU-GFP and envelope-free LVU-GFP. Average number of insertions was calculated by the total number of insertions of all founder mice and divided by the number of founder mice in the group, which is 24 in VSVG-LVU-GFP group and 31 in envelope-free LVU-GFP group. A much higher number of insertions was found in founder mice derived from envelope-free LVU-GFP compared to VSVG-LVU-GFP (P < 0.05). Statistical analysis was performed by student t-test. (D) Distribution of transgene insertion number in founder mice derived by intracytoplasmic injection of VSVG-LVU-GFP (n = 32 including eight non-transgenic F0 mice; blue) and envelope-free LVU-GFP (n = 32 including one nontransgenic F0 mouse; red) into zygote. Thirty-two adult mice from each group were arranged in an ascending order of insertion number. Mice with no insertion are nontransgenic mice. Founder mice derived from envelope-free LVU-GFP were mostly carrying an estimate of 6–20 insertion sites compared to 1–10 for VSVG-LVU-GFP. (E) Western analysis of the founder mice: Founder mice were selected based on low, medium, and high numbers of insertion sites. A total number of insertions in each group are 29 and 79 for VSVG-LVU-GFP and envelope-free LVU-GFP, respectively. Protein levels were determined by Western blot normalized with α-tubulin and presented as the average of each group (Fig. 1E–a and 1E-b). There is no difference in protein level (Fig. 1E–c; P > 0.05) when comparing between VSVG-LVU-GFP and envelope-free LVU-GFP derived transgenic founder mice. Statistical analysis was performed by student t-test.
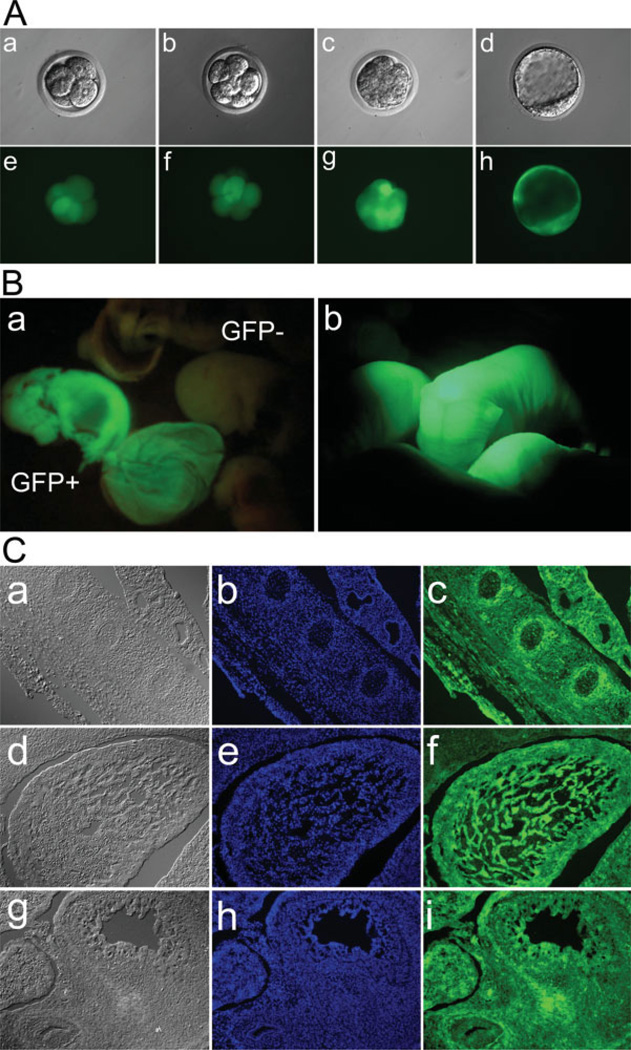
FIG. 2.
Expression of GFP in preimplantation mouse embryos, E13 embryos, Day 1 newborn pups, and tissue sections of E13 embryos produced by intracytoplasmic injection of envelope-free LVU-GFP into zygotes. (A) Transgenic mouse embryos expressing GFP were generated by intracytoplasmic injection of envelope-free LVU-GFP into zygotes. Low GFP expression was observed at the 2-cell stage (data not shown), which gradually increased at 4-cell stage and maintained at high level at blastocyst stage (e–h). Gradual increase of fluorescent intensity during in vitro development suggests stable integration and continuous expression of the GFP transgene. Panels a–d are brightfield images of 4-cell, 8-cell, morula, and blastocyststage mouse embryo. Panels e–h are fluorescent images of corresponding images in a–d. (B). Epi-fluorescent images of E13 mouse embryo (a) and Day 1 newborn pups (b). Nontransgenic embryo (GFP−; right) and GFP expressing embryo (GFP+; left) are shown in (a). GFP expression was found in the whole body of newborn pups (b). (C) GFP expression in different tissues of E13 mouse embryos derived from intracytoplasmic injection of envelope-free LVU-GFP into zygotes. Embryos were frozen-sectioned at 15 µm intervals. All tissues examined expressed GFP when observed under an epifluorescent microscope. a–c: spine; d–f: lung; g–i: intestine. a, d, and g: transmission light image; b, e, and h: Hoechst DNA staining (Blue); c, f, and i: epifluorescent image of GFP (Green).
In the transgenic founder mice created by intracytoplasmic injection of envelope-free LVU-GFP into zygotes, Southern blot analysis revealed multiple transgene insertions (ranging from 1–32; mean = 12 ± 7; n = 31; Fig. 1B,C). Lower number of insertions (1–15; mean = 6 ± 3; n = 24; P < 0.05; Fig. 1B,C) were found in founder mice created by intracytoplasmic injection of VSVG-LVU-GFP into zygotes. This suggests that more integration events are achieved with microinjection of envelope-free viruses into the cytoplasm than microinjected with VSVG-LVU-GFP. In fact, the number of insertions in envelope-free LVU-GFP derived founders is higher than expected base on the estimated titer of the virus. We speculate the titer of envelop-free LVU-GFP was underestimated because precise infection efficiency could not be determined by traditional titering method, titer of envelope-free lentivirus was determined by lipofectamine transfection of envelope-free viral particles in 293FT cells instead of natural infection because of the absence of envelope.
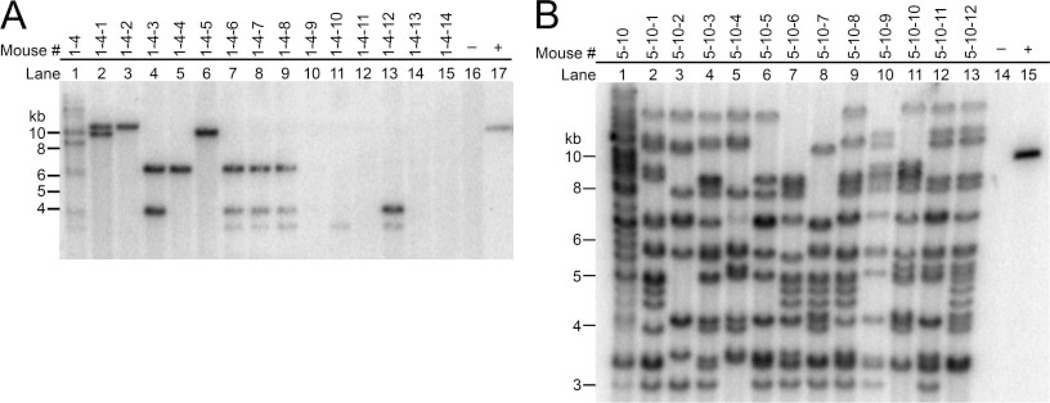
Germline transmission was confirmed in founder mice generated by cytoplasmic injection of VSVG-LVU-GFP and envelope-free LVU-GFP. Germline transmission was achieved in all tested founders (Fig. 3A,B). To determine chimerism, E13 embryos were recovered for analysis. Sections of various tissues were prepared for epifluorescent microscopy to determine the expression patterns of GFP (Fig. 2C). GFP expression was observed in all examined tissues, chimeric GFP expression pattern was not found.
FIG. 3.
Southern blot analysis was used to determine the germline transgenesis of the founder mice. Southern blot of F1 offspring: The transgene segregation pattern of F1 progeny from transgenic founder mice derived from VSVG-LVU-GFP (A) and envelope-free LVU-GFP (B). (A) Lane 1: transgenic founder 1–4; Lanes 2-to-15: F-1 progeny of transgenic founder 1–4; Lane 16: nontransgenic mouse DNA; and Lane 17: pLVU-GFP (positive control). (B) Lane 1: transgenic founder 5–10; Lanes 2-to-13: F-1 progeny of transgenic founder 5–10; Lane 14: nontransgenic mouse DNA and Lane 15: pLVU-GFP (positive control).
Although all founder mice express GFP regardless to the method of lentivirus delivery, whether the expression level of GFP is related to the number of provirus has not been reported. Representative group was identified from VSVG-LVU-GFP and envelope-free LVU-GFP derived founder mice. The two representative groups (compose of low, median and high insertion number; Figure 1B) as shown in the Southern blot analysis has a total of 29 vs. 79 insertions or proviruses respectively. Although the number of insertion is different between VSVS-LVU-GFP and envelope-free LVU-GFP derived founder mice (Fig. 1C,D; P < 0.05), there is no difference in GFP expression level between the two representative groups based on Western blot analysis (Fig. 1E; P > 0.05). This suggests that transgene expression is not related to the number of insertions or the number of the proviruses. However, transgenic rate is related to the method of virus delivery and the presence of the envelope protein (Table 1). One possible explanation is the presence of the VSVG, which is typically removed when membrane fusion occurs after recognition between the target cell and the viral envelope. Therefore, intracytoplasmic injection of VSVG-LVU-GFP may require an additional step to release the viral pre-integration complex from the envelope before nucleus entry and genomic integration. Because envelope-free viruses are not enclosed in an envelope, the pre-integration complex is ready for entering the nucleus, followed by integration. Thus, the absence of envelope and bypassing of cell recognition and membrane-fusion steps by intracytoplasmic injection ensures high gene transfer efficiency.
Virtually all lentiviral gene transfers are pursued using viruses pseudotyped with viral protein from the other viral strains such as VSV. Although similar transgenic efficiency has been reported by our group and others using VSVG-pseudotyped retrovirus and lentivirus (Cabot et al., 2001; Chan et al., 1998, 2001; Lois et al., 2002), this is the first report on creating germline transgenic animals using lentivirus without envelope. Additionally, this is the first report on successful gene transfer by intracytoplasmic injection of VSVG-lentivirus and envelope-free lentivirus into fertilized mammalian zygotes. The transgenic rates of PVS injection and intracytoplasmic injection of VSVG-LVU-GFP are 46% (17/37) and 75% (24/32) respectively, which are lower than that of intracytoplasmic injection of envelope-free LVU-GFP (97%; 31/32; P < 0.05) (Table 1). However, no detectable gene transfer event was observed when envelope-free LVU-GFP were co-cultured with the 293FT cells, although a very low gene transfer rate (2.1%; Table 1) resulted when they were microinjected into the PVS of zygotes. One possible explanation of the low gene transfer rate would be the leakage of envelope-free viral particles into cytoplasm, caused by damage of cytoplasmic membrane during microinjection of viral solution at the PVS. Therefore, we speculate that the low gene transfer rate in PVS microinjection could be resulted from cytoplasmic delivery instead. In fact, the supernatant recovered for the production of high titer lentivirus by ultracentrifugation is a mixture of viral particles with or without envelope. Thus, the high titer VSVG-lentivirus solution is composed of a mixture of VSVG- and envelope-free lentivirus. Our results are consistent with the notion that concentrated viral solution is a mixture of virus with or without envelope. Thus the high gene transfer rate with intracytoplasmic injection of VSVG-LVU-GFP (75%) compared to that of PVS microinjection (46%) could be the result of envelope-free LVU-GFP.
Although high gene transfer rate was achieved by cytoplasmic injection of envelop-free lentiviruses, it is an invasive procedure and adverse effect on developmental rate was observed. Besides the damage that may be caused by the mechanical intrusion of the cytoplasmic membrane, concentrated envelope-free lentiviral solution is composed of a mixture of concentrated ingredients which could have adverse consequence on cellular function. As a result, a reduced developmental rate is expected. By reducing the unknown ingredients in the concentrated envelope-free lentiviruses, an improved purity of envelope-free lentiviral solution may enhance the developmental rate.
Here we report a novel method for achieving high gene transfer efficiency in mouse zygotes. Because of the high gene transfer rate, the newly developed method could be used to achieve stable integration in a single selected cell such as blastomere of early preimplantation embryos. Together with the currently available reporter genes, transgenesis in selected blastomere is possible for long term monitoring and study of gene function at pre- and post-embryonic stage. We have also demonstrated that viral envelope is not required for germline transgenesis. This method can also be potentially applied in other envelope viruses such as adenovirus with comparable efficiency.
MATERIALS AND METHODS
Generation of VSVG-LVU-GFP and Envelope-Free LVU-GFP
A self-inactivated lentiviral vector expressing the GFP gene under the control of ubiquitin promoter was used in this study (Gift from C. Lois). VSVG-LVU-GFP and envelope-free LVU-GFP were generated by transfection of the 293FT packaging cells (Invitrogen). To produce VSVG-LVU-GFP, viral vector was co-transfected with plasmid pΔ8.9 and pVSVG. For envelope-free LVU-GFP, viral vector was co-transfected with pΔ8.9 without pVSVG. Supernatant was collected and concentrated by ultracentrifugation to ~1 × 109 infectious units/ml.
Determining the Titer of VSVG-LVU-GFP and Envelope-Free LVU-GFP
For VSVG-LVU-GFP: The concentration of infectious vector particles (titer) was determined by the expression of GFP. 2.5 × 105 293FT cells were plated in a 6-well plate and used as target cells. The following day, target cells were co-cultured with a serially diluted VSVG-LVUGFP in the presence of 8 µg/ml of polybrene. For envelope-free LVU-GFP: Concentrated envelope-free LVU-GFP were suspended in 50 µl of serum-free optiMEM medium (Invitrogen) containing 4 µl of a stock solution (1 mg/ml) of lipofectamine (Invitrogen). The mixture was incubated at room temperature for 15 min and then added to sub-confluent monolayer of 293FT cells in a 4-well dished. Titer was determined by multiplying the number of GFP positive colonies by the dilution factor and presented by colony forming units (cfu)/ml.
Preparation of Mouse Zygotes for Cytoplasmic Injection and PVS-Injection
Six- to eight-week female ICR mice were superovulated by an intraperitoneal injection of 7.5 IU pregnant mares’ serum gonadotropin (PMSG). Mouse zygotes were recovered from superovulated ICR females after 18 h of 7.5 IU human chorionic gonadotropin (hCG).injection and mating with ICR male mice. The zygotes were cultured in human tubal fluid medium until virus injection was performed. Cytoplasmic injection of lentivirus was performed using a PIEZO device (Primetech, Japan) with a micropipet size of approximately 5 µm inner diameter. For perivitelline space injection, a micropipette of 1–2 µm (inner diameter) was used without the PIEZO device. About 10 picoliters of the concentrated virus was injected into the cytoplasm and about 100–200 picoliters was injected into the PVS. A 2 × 2 experimental design was used to determine the efficiency of VSVG-LVU-GFP and LVU-GFP by injection into the PVS and cytoplasm of zygotes. Mouse zygotes were then cultured in vitro or implanted into the oviducts of pseudopregnant ICR females, and carried to term.
Monitoring GFP Expression in Pre-Implantation Embryo and Pups
Live embryos and pups were placed on an Olympus BX51 epifluorescent microscope equipped with GFP filter and high numerical aperture objectives. Images were captured and analyzed by Qimaging system (Qimaging). Embryos at various embryonic stages (2, 4, 8, morula and blastocyst) were monitored. The number of GFP positive cells was recorded and chimeric rate was determined.
Visualization of GFP Expression in Section of Post-Implantation Embryos
E13 fetuses and Day 1 newborns were dissected from the uterus and removed from maternal tissues. The embryos were washed thoroughly in cold PBS to remove cellular debris and blood. The embryos were then fixed in 4% fresh paraformaldehyde at 4°C over night. The embryos were then washed and perfused in gradual increase of sucrose solution (5, 10, 15, 30%) at 4°C. Embryos were then embedded in OCT for cryosection. A section of 15 µm was cut and examined under fluorescent microscope to identify cell types expressing GFP. Embryo sections were examined by Olympus BX51 microscope and images were analyzed by MetaMorph software (Universal Imaging).
PCR Analysis
For the junction of ubiquitin promoter and GFP gene, the primer set ubiquitin 3′ forward primer: GAGGCGTCAGTTTCTTTGGTC and GFP 5′ reverse primer: CTG-CTGCCCGACAACCACTA yielded an 867-bp amplicon after amplification of pLVU-GFP. Genomic DNA (100 ng) from mouse tail samples were submitted for PCR at 94°C for 5 min, 94°C for 30 s, 63°C for 30 s, and 72°C for 50 s for 35 times, followed by 72°C for 7 min.
Southern Blot Analysis and Determination of Integration Numbers
Genomic DNA was digested with a restriction enzyme that was cut only once within the transgene with an additional cut at the upstream or downstream of the integration site. Therefore, the number of band showing at the blot indicated the number of integration sites in the genome. After digestion using a restriction enzyme, EcoRI, genomic DNA was separated by electrophoresis on a 0.8% agarose gel and transferred to Hybond-N+ nylon membranes (Amersham). There is one EcoRI restriction site in the pLVU-GFP, thus a unique genomic DNA fragment that resulted from the digestion of EcoRI within the transgene and another site at the upstream or downstream region of the insertion site in the genomic DNA was resulted. The blot was hybridized with a (32P)-labeled fragment (junction of 3′ ubiquitin promoter region and 5′ EGFP). Genomic DNA from nontransgenic mice was used as negative controls.
Western Blot Analysis
The expression level of the GFP was determined by Western blot analysis. Total protein was extracted from the liver and concentration was determined by Bradford assay (Bio-Rad). Equal amount (20 µg) of protein extract was boiled prior to loading into polyacrylamide gel. After separation of proteins by electrophoresis, proteins were transferred onto a PVDF membrane (Millipore Immobilon-P, Millipore) using Bio-Rad’s transblot followed by blocking, incubation with primary antibody specifically against GFP, incubation with secondary antibody, and detection using an Amersham ECL kit (Amersham). The expression of GFP was quantified using a densitometer and compared. The quantity of GFP is normalized to endogenous α-tubulin level.
Statistical Analysis
We used Student’s t-test for integration sites and protein analysis, and Chi-square analysis for determining transgenic efficiency. Student t-test was performed with Excel 2003 and Chi-square analysis was done with Statistical Analysis System (SAS).
ACKNOWLEDGMENTS
We thank for Dr. Lloyd Lam for critical readings of the manuscript. We also thank Dr. Carlos Lois for providing lentiviral vector backbone. The procedures were approved by the Emory University Animal Care and Biosafety Committees.
Contract grant sponsor: Yerkes National Primate Research Center base; Contract grant number: RR-00165; Contract grant sponsors: National Center of Research Resources at NIH, Alzheimer Research Consortium.
LITERATURE CITED
- Abe A, Chen ST, Miyanohara A, Friedmann T. In vitro cellfree conversion of noninfectious Moloney retrovirus particles to an infectious form by the addition of the vesicular stomatitis virus surrogate envelope G protein. J Virol. 1998;72:6356–6361. doi: 10.1128/jvi.72.8.6356-6361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot RA, Kühholzer B, Chan AWS, Lai L, Park KW, Chong KY, Schatten G, Murphy CN, Abeydeera LR, Day BN, Prather RS. Transgenic pigs produced using in vitro matured oocytes infected with a retroviral vector. Animal Biotechnol. 2001;12:205–214. doi: 10.1081/ABIO-100108347. [DOI] [PubMed] [Google Scholar]
- Chan AWS. Transgenic nonhuman primates for neurodegenerative diseases. Reprod Biol Endocrinol. 2004;2:39. doi: 10.1186/1477-7827-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AWS, Chong KY, Martinovich C, Simerly C, Schatten G. Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science. 2001;291:309–312. doi: 10.1126/science.291.5502.309. [DOI] [PubMed] [Google Scholar]
- Chan AWS, Homan EJ, Ballou LU, Burns JC, Bremel RD. Transgenic cattle produced by reverse-transcribed gene transfer in oocytes. Proc Natl Acad Sci USA. 1998;95:14028–14033. doi: 10.1073/pnas.95.24.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by Lentivirus vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Sharma S, Murai F, Miyanohara A, Friedmann T. Noninfectious virus-like particles produced by Moloney murine leukemia virusbased retrovirus packaging cells deficient in viral envelope become infectious in the presence of lipofection reagents. Proc Natl Acad Sci USA. 1997;94:10803–10808. doi: 10.1073/pnas.94.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]