The constitutive characters of plants can be structural or biochemical and play an important role in their defense against pathogens. We found that Citrus flower buds smaller than 8 mm long have constitutive structural and biochemical barriers to Colletotrichum spp. infection. In addition, this is the first time that osmophores are reported in Citrus sinensis. Our study shows that natural terpenes of Citrus flowers present a potential use for chemical control of Colletotrichum spp.
Keywords: Antimicrobial activity, calcium oxalate crystals, citrus essential oils, Citrus sinensis, Colletotrichum acutatum, osmophores, plant anatomy, volatile organic compounds
Abstract
The constitutive characters of plants can be structural or biochemical and play an important role in their defence against pathogens. Citrus postbloom fruit drop (PFD) caused by Colletotrichum spp. is one of the most important fungal diseases of citrus. The pathogen infects the flowers, leading to premature fruit drop and reducing citrus production. However, flower buds smaller than 8 mm long are usually not infected by Colletotrichum spp. Thus, this study investigated whether there are constitutive mechanisms in flower buds related to Colletotrichum spp. infection. We studied flower buds that were 2, 3, 4, 8, 12 and 15 mm long and petals, after anthesis, of sweet orange ‘Valência’ using light and scanning electron microscopy and histochemistry. We evaluated the effect of volatile organic compounds (VOCs) in flowers (R-limonene and linalool) on the in vitro growth of Colletotrichum acutatum. We found that the arrangement of the epidermal papillae in the petal primordia, the occurrence of prismatic crystals and the distribution of oil glands are the main differences between buds smaller than 8 mm and buds 8–15 mm long. Osmophores at the tips of petals produced and accumulated phenols, terpenes and lipophilic compounds. Flower buds smaller than 8 mm long have constitutive structural and biochemical barriers to Colletotrichum spp. infection. In addition, this is the first time that osmophores have been reported in citrus. Our study shows that natural terpenes of Citrus flowers inhibit the fungal growth in vitro, highlighting the potential use of terpenes for the chemical control of PFD in citrus.
Introduction
Plant species are attacked by a wide range of pathogens and herbivores, which can alter host survival, growth and reproduction (Wittstock and Gershenzon 2002; Agrios 2005). Plants defend themselves against these enemies using a combination of structural characters and biochemical reactions, which can be either constitutive or induced by attack (Wittstock and Gershenzon 2002; Agrios 2005; Hanley et al. 2007). Constitutive structural traits important to pathogen resistance include cell walls with different compositions, waxy epidermal cuticles, cuticles thickness, trichomes, idioblasts, sclereids and crystalliferous cells (Hudgins et al. 2003; Smith et al. 2006; Freeman and Beattie 2008; Koch and Ensikat 2008).
Citrus postbloom fruit drop (PFD) is a disease caused by Colletotrichum acutatum (Brown et al. 1996) and C. gloeosporioides (Lima et al. 2011; McGovern et al. 2012), both from phylum Ascomycota. The disease causes serious economic production losses of sweet oranges and is considered a limiting factor for producing countries (Timmer et al. 1994). In Brazil, losses caused by PFD can reach 80 % when the flowering coincides with periods of heavy rainfall in areas without the disease control (De Goes et al. 2008; Silva-Junior et al. 2014).
These pathogens can infect flower buds that are still closed (Fagan 1979), although most symptoms are observed during anthesis (Denham and Waller 1981). In the petals, the pathogen penetrates intra- and intercellularly, and also through the stomata. After invasion, acervuli (asexual fruiting bodies) are observed on both sides of the petals (Marques et al. 2013). Typical symptoms are orange-brown lesions (Fagan 1979). The lesions bearing salmon pink acervuli expand rapidly in favourable weather and soon involve all the petal tissues. This stage of the disease is often called blossom blight. The blighted petals remain firmly attached to the basal disc and become hard, dry and reddish brown (Denham and Waller 1981). In the stigma, small peach-brown to dark-brown necrotic spots are formed (Lin et al. 2001), but the pathogen does not penetrate through the epidermal cells of the stigma. A protective lipophilic layer rich in phenolic compounds is formed under the necrotic area, and crystals of calcium oxalate (CaOx) are produced where the pathogen is present (Marques et al. 2013). After infection, hormonal changes occur (Li et al. 2003; Lahey et al. 2004) leading to premature fruit drop and calyx retention for long periods (Timmer et al. 1994).
The developmental stages of flower buds are directly related to the infection caused by C. acutatum and efficiency of PFD control (Fagan 1979; Roberto and Borges 2001). Flower buds smaller than 8 mm are considered resistant to infection (Fagan 1979) and as the flower bud size increases, the buds become more susceptible to the infection. Thus, preventive applications of fungicides are recommended when most petals emerge above the calyx (Silva-Junior et al. 2014).
Citrus plants produce volatile organic compounds (VOCs) as secondary metabolites that play an important role in interaction routes with microorganisms. In citrus plants, the quantity of VOCs is variable and depends on the organ age and type. In the pericarp of green fruits, the oil is composed of β-pinene, sabinene and linalool; however, in ripe fruits, R-limonene and linalool become the most prevalent oils. In petals, the oils include terpenes R-limonene, myrcene, sabinine, linalool, terpineol and others (Attaway et al. 1966). To date, there is no evidence of the site where the synthesis and/or emission of volatiles occur on citrus petals. In several families, the sites where the synthesis occurs are called osmophores or scent glands (Vogel 1990; Dudareva and Pichersky 2000; Effemert et al. 2006). Osmophores have been reported only once for the Rutaceae (Bussell et al. 1995). Volatile organic compounds are produced naturally in plants and may be associated with constitutive biochemical defence mechanisms. They can also be extracted and used as an alternative to conventional chemical control, as they present fewer environmental hazards (Wightwick et al. 2010).
Floral buds smaller than 8 mm are considered resistant to pathogen infection (Fagan 1979). However, the mechanisms involved in this resistance have never been determined. We hypothesized that floral buds smaller than 8 mm have constitutive structural and chemical barriers that are associated with the resistance of those buds. This study aims to describe the anatomical structure of flower buds at different developmental stages to identify the presence of constitutive defences to infection caused by C. acutatum. We also evaluated the effect of the most common VOCs in flowers on the in vitro growth of C. acutatum, to verify their contribution to bud resistance.
Methods
Samples
Five-year-old potted sweet orange plants [Citrus sinensis var. Valência] were grown in 5-L plastic pots and maintained in a greenhouse at the Fund for Citrus Plant Protection (FUNDECITRUS), located in the municipality of Araraquara, São Paulo State, Brazil. Flowering was induced by pruning and water restriction. Small flower buds with 2, 3 and 4 mm, flower buds with expanded corolla (8, 12 and 15 mm), and petals after anthesis were collected for anatomical analyses.
Light microscopy and histochemistry
The flower buds and the petals were collected, longitudinally sectioned and fixed in Karnovsky fixative (Karnovsky 1965; modified by phosphate buffer pH 7.2) for 48 h. During this phase, the samples were placed in a vacuum pump to remove air bubbles formed in the tissues. The samples were then dehydrated in a graded ethanol series and embedded in Leica historesin® (Heraeus Kulzer, Hanau, Germany). Flower buds were also fixed in formalin-ferrous sulfate solution to detect phenolic compounds (Johansen 1940; Jensen 1962). The infiltration time of the flower buds was 1 month or more, depending on the developmental stage of the buds. The blocks were sliced in a Leica RM 2045 rotary microtome to produce 5–7-μm thick sections. The sections were mounted on glass slides and stained with 1 % Toluidine Blue (Sakai 1973) for histological analyses. The slides were mounted in synthetic resin Entellan® (Merck, Darmstadt, Germany).
For histochemical analysis, Sudan black B was used to detect lipophilic compounds (Pearse 1968), 10 % ferric chloride was used to detect phenolic substances (Johansen 1940) and Ruthenium red for pectic compounds (Chamberlain 1932). After staining, the slides were embedded in the synthetic resin Entellan®. Calcium oxalate crystals were visualized under polarized light and their chemical nature was confirmed by analyzing their solubility in 1 % sulfuric acid (Johansen 1940). To confirm the presence of osmophores on the petal primordia and petal apex, samples were collected and immediately cross-sectioned on a sliding microtome Leica MS 2000R. To identify and characterize the osmophores' secretions the following histochemical techniques were used: rhodamine B (Jin et al. 2011) for sugar esters; Neutral Red (0.01 % in aqueous solution pH 8.0) to detect secretory activity (Dudareva and Pichersky 2000; Effemert et al. 2006); Nadi reagent for terpenoids (David and Carde 1964); Nile blue sulfate for acidic and neutral lipids (Cain 1947); and 10 % ferric chloride for phenolic compounds (Johansen 1940). Images from slides were captured digitally through a Leica DMLB microscope with a video camera attached to a PC, using Leica IM50 image analysis software.
Scanning electron microscopy
Ten samples of each developmental stage of the flower buds and the petals were fixed in a Karnovsky solution adjusted to pH 7.2 using phosphate buffer (Karnovsky 1965, modified), dehydrated using a series of graded ethyl alcohols (10, 30, 50, 70, 90 and 100 %), critical point-dried using CO2 (Horridge and Tamm 1969), mounted on aluminium stubs using double-sided carbon tape and coated with a 30–40 nm gold film. Images were captured using a LEO VP 435 scanning electron microscope at an accelerating voltage of 20 kV.
Effect of volatile organic compounds on the mycelial growth of C. acutatum
Volatile organic compounds of plants found in petals of sweet orange ‘Valência’ were tested in vitro for antifungal activity against C. acutatum. Two compounds were selected according to the study of Attaway et al. (1966): the R-limonene, 97 %, Sigma-Aldrich and linalool, 97 %, Sigma-Aldrich. R-limonene is one of the main components of essential citrus oils, the group of monoterpene hydrocarbons. Linalool is also found in essential oils from different citrus organs and belongs to the chemical group of monoterpene alcohols. Glass slides with doses of 1, 10, 100 and 1000 µg mL−1 of active ingredient of each compound were placed on one side of the polystyrene plate. On the other side of the plate, the culture medium BDA was added and a disc of mycelium of 0.5 cm diameter of C. acutatum (isolate Ca142 of Fundecitrus collection) was added to the medium. Plates containing water blades with and without the compounds served as control. The plates were sealed with Parafilm and maintained in growth chambers at 25 °C. After 7 days, the diameter of the colony was measured. Growth inhibition was calculated and the values were used to estimate the EC50 (effective concentration to reduce growth by 50 %) and minimal inhibitory concentration (MIC). The experiment was performed in triplicate with three replications; each plate was considered one repetition.
Results
Structural and histochemical analyses
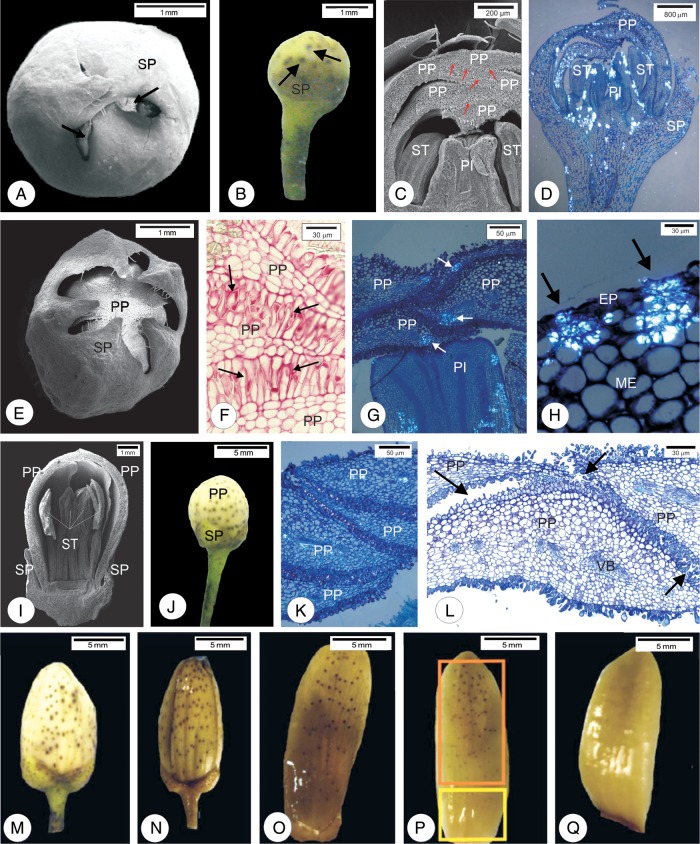
In flower buds 2 mm long, the apexes of the five sepal primordia overlap (Fig. 1A and B), protecting the other flower whorls (Fig. 1C). In flower buds 3–4 mm long, sepal primordia separated to accommodate expansion of the underlying corolla (Fig. 1E). After fixation with ferrous sulfate in formalin, the sepal primordia have oil glands high in phenolic compounds (Figs 1B and 2A). Petal tips overlap and distal papillate regions press together, especially in 2, 3 and 4 mm buds (Fig. 1C, F and G), relative to the stages of 8–12 mm long (Fig. 1K and L).
Figure 1.
Scanning electron micrographs (A, C, E, I), photomicrographs under non-polarized light (F, L) and polarized light (D, G, H, K) and photographs (B, M–Q) of flower buds of sweet orange (C. sinensis ‘Valência’). (A–D) Flower buds 2 mm long. (A) Note the small exposure of the petal primordia (arrow). (B) Oil gland of the sepal primordia (arrows). (C) Apex of the petal primordia show compact pattern of intermixing (arrows). (D) Overview of the bud under polarized light. Note crystals distributed in the primordia. (E–H) Flower bud 4 mm long. (E) Note larger exposure of the corolla. (F) Arrangement in the apexes of the papillary cells of the petal primordia (arrows) after staining with ruthenium red. (G) Crystals grouped in the apexes of the petal primordia. (H) Crystals inside the cells surrounding the substomatal chambers of petal primordia. (I–K) Flower buds 8 mm long. (I and J) Note the exposure of the corolla. (K) Absence of crystals in the mesophyll cells of the petals. (L and M) Flower buds 12 mm long. (L) Detail of the apex of the flower bud where the loose arrangement of papillary cells is observed (arrows). (M–Q) Fixation with ferrous sulfate in formalin. (N) Flower buds 15 mm long. (O) Flower bud after anthesis. (P) Abaxial face of petal. Note the smaller number of oil glands along the petal base (yellow rectangle) when compared to the apex (orange rectangle). (Q) Note the absence of oil glands on the adaxial face of the petal. EP, epidermis; ST, stamens; ME, mesophyll; PI, pistil; PP, petal primordia; SP, sepal primordia; VB, vascular bundle.
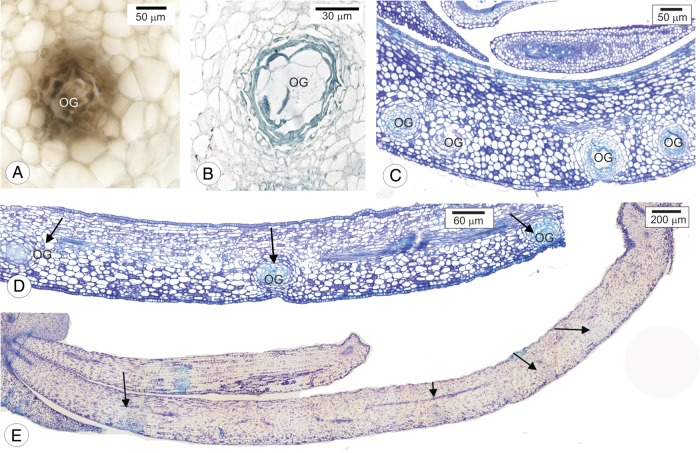
Figure 2.
Cross-sections of oil glands in petal primordia of sweet orange (Citrus sinensis ‘Valência’). (A) Oil gland cells reacting positively to ferrous sulfate in formalin. (B) Oil gland cells stained with Sudan Black B. (C–E) Distribution of oil glands (arrows) in flower buds 4, 8 and 12 mm long. OG, oil gland.
Under polarized light, buds shorter than 8 mm have CaOx crystals in the mesophyll, either dispersed (Fig. 1D) or grouped (Fig. 1G). This grouping occurs near the apexes of the petal primordia. The crystals are also observed inside the cells surrounding substomatal chambers (Fig. 1H).
In the buds 8 mm long (Fig. 1I and J) or longer (Fig. 1M and N), petal primordia are exposed, distal papillate regions begin to separate (Fig. 1K and L) and few CaOx crystals are seen (Fig. 1K).
Flower buds up to 8 mm long have uniform distribution of oil glands (Fig. 1J). However, as the flower buds develop, oil glands nearer the petal base spread, whereas oil glands at petal tips remain clustered (Fig. 1M and N). This pattern continues in subsequent stages of flower development (Fig. 1O and P). The flower buds expose only the abaxial face where oil glands are present. Anthesis exposes the adaxial petal surface, which is free of oil glands (Fig. 1Q).
Oil glands contain phenolic (Fig. 2A) and lipophilic (Fig. 2B) compounds. In flower buds 4 mm long, glands are concentrated near each other (Fig. 2C), but become more spread out as petals enlarge in 8 mm- (Fig. 2D) and 12-mm-long buds (Fig. 2E). The distribution pattern of glands in buds 12 mm long is uneven, as most glands are observed near the apex of the petal primordia (Fig. 2E).
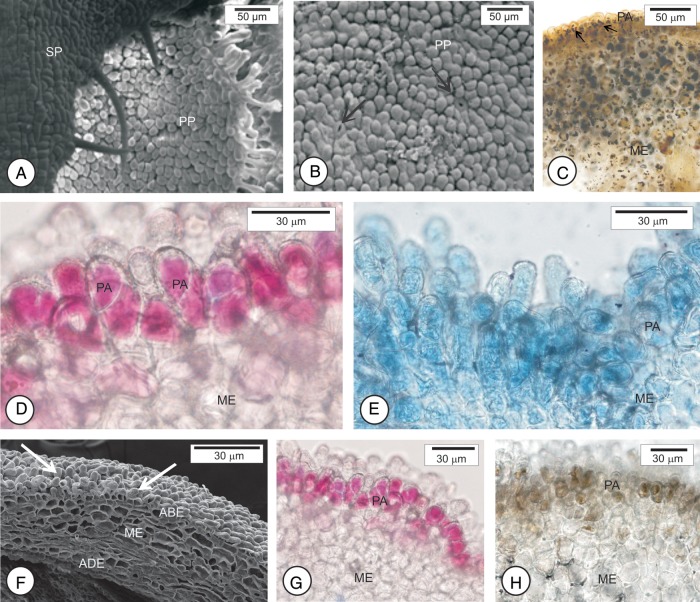
The region of exposed corolla in flower buds 2–4 mm long is composed of papillary cells (Fig. 3A and B). In flower buds 2 mm long, starch accumulates in cells of the mesophyll and papillae (Fig. 3C). In the other stages analyzed, in buds 3, 4, 8 and 12 mm long (Fig. 3F), papillary cells react positively to the neutral red dye (Fig. 3D and G), to Nile blue sulfate (Fig. 3E) and to ferric chloride (Fig. 3H). These papillae also have a positive reaction to the NADI reagent, indicating the presence of terpenes (not shown).
Figure 3.
Scanning electron micrographs (A, B, F) and photomicrographs (C–E, G, H) of osmophores in different developmental stages of flower buds of sweet orange (C. sinensis ‘Valência’). (A–C) Flower buds 3 mm long. The region exposed is composed of papillary cells among which the stomata occur (arrow in B). (C) Note starch accumulation in the papillary cells. (D and E) Buds 8 mm long. Papillary cells stained with neutral red dye (D) and with Nile blue sulfate (E). (F–H) buds 12 mm long. (F) Papillary cells (arrows) that react positively to neutral red dye (G) and ferric chloride (H). ABE, abaxial epidermis; ADE, adaxial epidermis; ME, mesophyll; PA, papillae; PP, petal primordia; SP, sepal primordia.
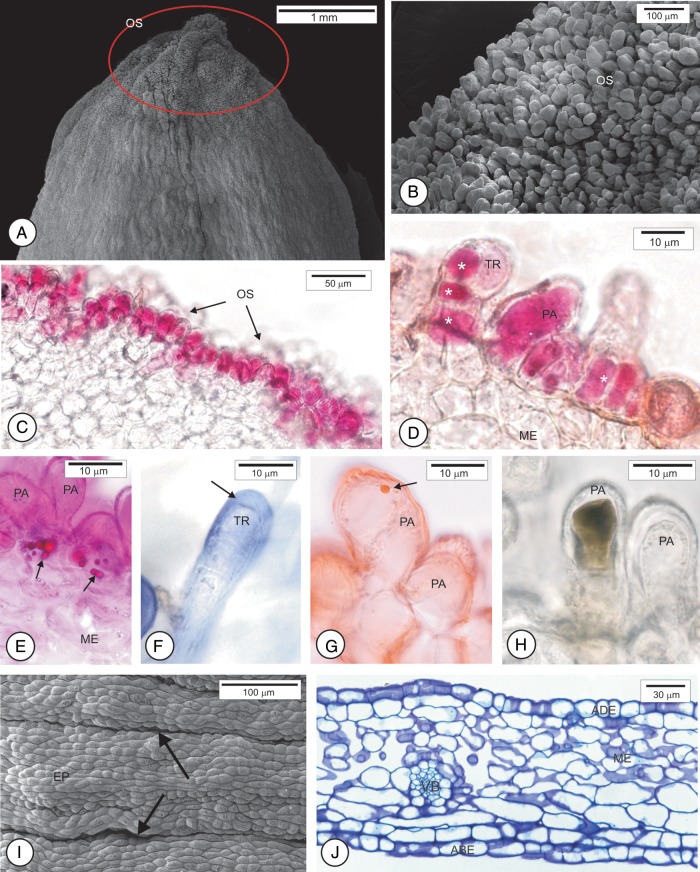
The petals from the fully open flowers show distinct epidermal features at the apex, medium and base. At the apex (Fig. 4A), cells are papillary (Fig. 4B) among which some uniseriate trichomes occur (Fig. 4C). The histochemical tests allowed us to confirm that the petal apex is an osmophore. Epidermal cells stained with neutral red dye (Fig. 4C and D), show sugar esters (Fig. 4E), and react positively to the NADI reagent, indicating the presence of terpenes (Fig. 4F) and lipid drops (Fig. 4G). Some cells react positively to ferric chloride indicating the presence of phenolic compounds (Fig. 4H). The surface of the medium region shows depressions and protrusions and the papillae are less prominent than at the apex and are covered with striated cuticle (Fig. 4I). At the petal base, epidermal cells are tabular (Fig. 4J) and the cuticle shows no stretch marks.
Figure 4.
Scanning electron micrographs (A, B, H) and photomicrographs (C–G, I) of healthy petal of sweet orange (C. sinensis ‘Valência’). (A) Overview of the petal with the apex delimited by the circle. (B and C) Detail of the osmophore (OS) in the region delimited in A. (C and D) Papillae and trichome with contents stained with neutral red dye (*). (E) Sugar esters (arrows) evidenced by Rhodamine B. (F) Positive reaction to the NADI reagent at the edge of the trichome (arrow). (G) Lipid droplet stained with Sudan IV. (H) Phenolic content evidenced by ferric chloride. (I) Overview of medium region of the petal where depressions (arrows) and protrusions are observed. (J) Cross-section at the petal base. ABE, abaxial epidermis; ADE, adaxial epidermis; VB, vascular bundle; ME, mesophyll; PA, papillae; TR, trichome; EP, epidermis.
The mesophyll is homogeneous and composed of parenchyma braciform cells (Fig. 4J). In the mesophyll, oil glands are turned only to the abaxial surface (Fig. 2C–E). The vascular bundles are collateral (Fig. 4J). The stomata occur on both faces.
Effect of VOCs on the mycelial growth of C. acutatum
The monoterpene alcohol linalool showed higher inhibition of the mycelial growth of C. acutatum than monoterpene hydrocarbon R-limonene (Fig. 5). Linalool reduced the mycelial growth of C. acutatum by 30 % at 100 µg mL−1, against 15 % inhibition of R-limonene at the same dose. At 1000 µg mL−1, linalool inhibited mycelial growth 100 %, while R-limonene remained <20 % inhibition. At lower concentration of some repetitions of R-limonene, small stimulation of the growth of the C. acutatum was observed. The EC50 and MIC for the linalool are between 100 and 1000 µL L−1; however, for the limonene, the EC50 is greater than 1000 µg mL−1 (Fig. 5).
Figure 5.
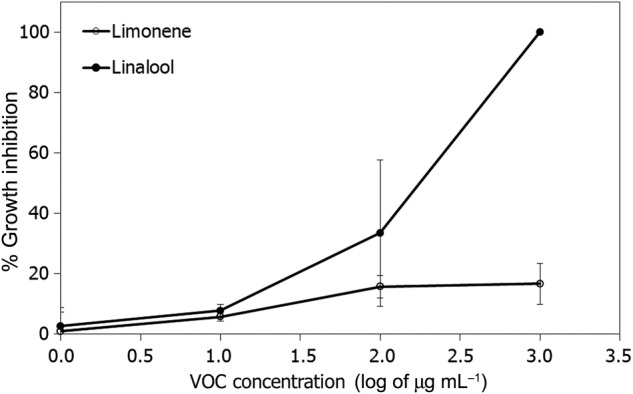
Inhibition of mycelial growth of C. acutatum at different concentrations of R-limonene and linalool. Dots represent the average of three experiments and three replicates for each rate.
Discussion
The anatomical analyses of the flower buds show structural characteristics that may explain the lower incidence of infection in buds smaller than 8 mm. The compact arrangement of papillae in the petal primordia in young flower buds could be considered a structural barrier to infection caused by C. acutatum. On the other hand, buds longer than 8 mm show loose arrangement, thus offering less resistance to the infection caused by this pathogen. These results corroborate Fagan (1979) in Belize, where flower rot symptoms were expressed only in flower buds during the expansion stage of the corolla and only in those buds longer than 8 mm. The author states that flower buds smaller than 8 mm may be more resistant to infection; however, susceptibility increases dramatically as the flower buds age, remaining high until anthesis.
The lower infection rate of flower buds smaller than 8 mm caused by C. acutatum can also be associated with the presence of CaOx crystals in the petal primordia. According to Ceita et al. (2007), crystal degradation produces H2O2 in specific points in the tissue and plays an important role in programmed cell death, acting effectively in pathogen control. The application of Ca in large quantities leads to the formation of intracellular CaOx crystals (Choi et al. 2000; Faheed et al. 2013). There is a positive correlation between the Ca concentration provided to the plant and the number of crystals formed (Zindler-Frank et al. 2001), as well as with their length and width (Faheed et al. 2013). Therefore, C. sinensis plants receiving high doses of Ca possibly show a large number of CaOx crystals in flower buds and, consequently, are more resistant to infection caused by C. acutatum. Studies on nutrition based on different Ca sources could be conducted to further investigate this possibility.
The occurrence of osmophores at the petal apexes, consisting of papillary cells and glandular trichomes, has not yet been documented for C. sinensis. These osmophores are morphologically similar to those described in some Orchidaceae (Pridgeon and Stern 1985; Pansarin et al. 2009; Melo et al. 2010). In smaller flower buds, cells in the mesophyll of petal primordia accumulate starch in the early developmental stages of the osmophores. This accumulation may supply carbon for later production of VOCs (Pridgeon and Stern 1983; Stern et al. 1987; Effemert et al. 2006). According to Knudsen et al. (1993) the VOCs released by osmophores in 60 families of plants are: hydrocarbons, esters, ethers, aldehydes, ketones, terpenes, benzenoids, phenylpropanoids, isoprenoids and nitrogen- and sulfur-containing compounds. Among the terpenes, the mono- and sesquiterpenes stand out (Dudareva and Pichersky 2000). The osmophores of C. sinensis produce and accumulate phenols, terpenes and lipophilic compounds in their cells (Fig. 4). These substances have a recognized role as antifungal agents (Caccioni et al. 1998; Lattanzio et al. 2006). In addition to their ecological function (Proctor et al. 1996), the compounds accumulated by osmophore cells may act as a constitutive chemical barrier. Further studies need to be conducted to identify the compounds secreted by C. sinensis osmophores.
In flower buds smaller than 8 mm, oil glands are nearer to each other when compared with larger flower buds. The low incidence of lesions in flower buds smaller than 8 mm could possibly be linked to the concentrated oil glands, which may defend the tissues against pathogen attack. Essential oils of C. sinensis represent a constitutive defence against pathogens (Caccioni et al. 1998). Some terpenes in citrus oil glands have been shown to inhibit bacteria (Dabbah et al. 1970) and fungi (Moleyar and Narasimham 1986; Caccioni et al. 1998; Sharma and Tripathi 2008; Viuda-Martos et al. 2008).
Linalool is expressed in petals and in green and ripe fruit, while limonene appears mostly in ripe fruit. Linalool showed high antifungal activity against C. acutatum at doses higher than 100 µg mL−1; conversely, limonene showed low antifungal activity at 1000 µg mL−1. Linalool is usually the predominant VOC in flowers of different citrus species, mainly in sweet orange. However, there is a wide variation in concentration of this compound, from 22 to 52 % depending on the cultivar and the developmental stage of the flower (Alissandrakis et al. 2003; Jabalpurwala et al. 2009; Azam et al. 2013). In tangerines, limonene and myrcene did not inhibit in vitro germination of conidia of Alternaria alternata at the dose of 130 µg mL−1. However, linalool showed an antifungal effect, inhibiting >97 % of the germination of conidia by >97 % at a similar dose (Yamasaki et al. 2007). In addition, limonene showed a stimulating effect on the germination of Penicillium digitatum and P. italicum, causal agents of mould in citrus fruits, (Droby et al. 2008). The presence of alcoholic terpenes such as linalool in oil glands on petals of the Valência orange may be linked to the resistance of flower buds smaller than 8 mm. Nevertheless, our results suggest that the presence of limonene in flowers of C. sinensis is not associated with the mechanisms of resistance to infection caused by C. acutatum. Transformed orange plants that accumulated a smaller amount of limonene in the fruit peel have a higher resistance to fungus P. digitatum and bacterium Xanthomonas citri subsp. citri, causal agent of citrus canker (Rodriguez et al. 2013).
The high susceptibility of the fully open flower to infection caused by C. acutatum may be related to structural features of the petal flower after opening that promote exposure of the adaxial face of the petal (which has no oil glands). In addition, this surface is composed of depressions and protrusions, which may facilitate the deposition of conidia and the formation of appressoria that occur preferentially along the anticline walls of epidermal cells (Marques et al. 2013). Petals could be more vulnerable to fungal infection because of softened cell walls (due to auxin-induced cell expansion). Ultrastructural studies of the parenchyma cell wall may contribute to better understanding of the vulnerability of the parenchyma cell to fungal infection.
Conclusions
We conclude that the difference in response to infection caused by the fungus C. acutatum in flower buds of C. sinensis at different developmental stages may be linked to constitutive structural and biochemical barriers. The presence of CaOx crystals, the pattern of papilla and the distribution, density and composition of citrus glands should be taken into consideration in further studies on disease control, once these constitutive natural defences are more evident in flower buds smaller than 8 mm in relation to flower buds longer than 8 mm, susceptible to the disease. For instance, spraying fungicides to control the disease can be delayed until the flower buds are longer than 8 mm. In addition, this study showed for the first time the site where synthesis and emission of volatiles occur in citrus petals. The site is characterized as an osmophore that produces and accumulates phenol, terpenes and lipophilic compounds. In addition, we demonstrate that alcoholic compounds such as monoterpenes present in citrus flowers have antifungal action against C. acutatum and may be associated with constitutive biochemical defences. These compounds have the potential to be used in further studies on chemical control of the pathogen or on genetic transformation and their overexpression in tissues.
Sources of Funding
This work was supported by São Paulo Research Foundation (FAPESP—projects 2008/541764, 2009/00425-6).
Contributions by the Authors
J.P.R.M. conceived of the project, planned the research, carried out the techniques and drafted the manuscript. L.A. and M.B.S. discussed the phytopathological aspects and contributed with the critical review of the manuscript. G.J.S.-J. carried out the in vitro experiments of antifungal effect of VOCs. B.A.-d.G. oversaw the research, conceived of the project, made the critical histological analysis and participated of the manuscript writing. All authors read and approved the final manuscript.
Conflicts of Interest Statement
None declared.
Acknowledgements
The authors thank Fund for Citrus Plant Protection (FUNDECITRUS) for the use of laboratories and greenhouses, Ms R.G.P. for the technical assistance and NAP/MEPA, ESALQ/USP for microscopy facilities.
Literature Cited
- Agrios GN. Plant pathology. 5th edn. London: Elsevier; 2005. [Google Scholar]
- Alissandrakis A, Daferera D, Tarantilis PA, Polissiou M, Harizanis PC. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chemistry. 2003;82:575–582. doi: 10.1016/S0308-8146(03)00013-X. [DOI] [Google Scholar]
- Attaway JA, Pieringer AP, Barabas LJ. The origin of citrus flavor components-iv. the terpenes of “valencia” orange leaf, peel, and blossom oils. Phytochemistry. 1966;5:1273–1279. doi: 10.1016/S0031-9422(00)86123-3. [DOI] [Google Scholar]
- Azam M, Song M, Fan F, Zhang B, Xu Y, Xu C, Chen K. Comparative analysis of flower volatiles from nine Citrus at three blooming stages. International Journal of Molecular Sciences. 2013;14:22346–22367. doi: 10.3390/ijms141122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AE, Sreenivasaprasad K, Timmer LW. Molecular characterization of slow-growing orange and key lime anthracnose strains of Colletotrichum from citrus as C. acutatum. Phytopathology. 1996;86:523–527. doi: 10.1094/Phyto-86-523. [DOI] [Google Scholar]
- Bussell BM, Considine JA, Spadek ZE. Flower and volatile oil ontogenyin Boronia megastigma. Annals of Botany. 1995;76:457. doi: 10.1006/anbo.1995.1120. [DOI] [Google Scholar]
- Caccioni DRL, Guizzardi M, Biondi DM, Renda A, Ruberto G. Relationship between volatile components of citrus fruit, essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. International Journal of Food Microbiology. 1998;43:73–79. doi: 10.1016/S0168-1605(98)00099-3. [DOI] [PubMed] [Google Scholar]
- Cain AJ. The use of Nile Blue in the examination of lipids. Quarterly Journal of Microscopical Science. 1947;88:383–392. [Google Scholar]
- Ceita GO, Macedo JNA, Santos TB, Alemanno L, da Silva Gesteira A, Micheli F, Mariano AC, Gramacho KP, da Costa Silva D, Meinhardt L, Mazzafera P, Pereira GAG, de Mattos Cascardo JC. Involvement of calcium oxalate degradation during programmed cell death in Theobroma cacao tissues triggered by the hemibiotrophic fungus Moniliophthora perniciosa. Plant Science. 2007;173:106–117. doi: 10.1016/j.plantsci.2007.04.006. [DOI] [Google Scholar]
- Chamberlain CJ. Methods in plant histology. Chicago: Chicago Press; 1932. [Google Scholar]
- Choi E, Harada E, Tsuboi H, Morita Y, Kusano H, Sano H. Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta. 2000;213:45–50. doi: 10.1007/s004250000487. [DOI] [PubMed] [Google Scholar]
- Dabbah R, Edwards VM, Moats WA. Antimicrobial action of some citrus fruit oils on selected food-borne bacteria. Applied Microbiology. 1970;19:27–31. doi: 10.1128/am.19.1.27-31.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Carde JP. Coloration différentielle dês inclusions lipidique et terpeniques dês pseudophylles du Pin maritime au moyen du reactif Nadi. Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences. 1964;258:1338–1340. [Google Scholar]
- De Goes A, Garrido RBO, Reis RF, Baldassari RB, Soares MA. Evaluation of fungicide applications to sweet orange at different flowering stages for control of postbloom fruit drop caused by Colletotrichum acutatum. Crop Protection. 2008;27:71–76. doi: 10.1016/j.cropro.2007.04.007. [DOI] [Google Scholar]
- Denham TG, Waller JM. Some epidemiological aspects of post-bloom fruit drop disease (Colletotrichum gloeosporioides) in citrus. Annals of Applied Biology. 1981;98:65–77. doi: 10.1111/j.1744-7348.1981.tb00423.x. [DOI] [Google Scholar]
- Droby S, Eick A, Macarisin D, Cohen L, Rafael G, Stange R, McColum G, Dudai N, Nasser A, Wisniewski M, Shapira R. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biology and Technology. 2008;49:386–396. doi: 10.1016/j.postharvbio.2008.01.016. [DOI] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiology. 2000;122:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effemert U, Buss D, Rohrbeck D, Piechulla B. Localization of the synthesis and emission of scent compounds within the flower. In: Dudareva N, Pichersky E, editors. Biology of floral scent. Boca Raton, FL: CRC Press; 2006. pp. 105–124. [Google Scholar]
- Fagan HJ. Postbloom fruit drop of citrus, a new disease of citrus associated with a form of Colletotrichum gloeosporioides. Annals of Applied Biology. 1979;91:13–20. doi: 10.1111/j.1744-7348.1979.tb07408.x. [DOI] [Google Scholar]
- Faheed F, Mazen A, Abd Elmohsen S. Physiological and ultrastructural studies on calcium oxalate crystal formation in some plants. Turkish Journal of Botany. 2013;37:139–152. [Google Scholar]
- Freeman B, Beattie GA. An overview of plant defenses against pathogens and herbivores. The Plant Health Instructor. 2008 doi: 10.1094/PHI-I-2008-0226-01. [DOI] [Google Scholar]
- Hanley ME, Lamontb BB, Fairbanksb MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics. 2007;8:157–178. doi: 10.1016/j.ppees.2007.01.001. [DOI] [Google Scholar]
- Horridge GA, Tamm SL. Critical point drying for scanning electron microscopy study of ciliar motion. Science. 1969;13:871–818. doi: 10.1126/science.163.3869.817. [DOI] [PubMed] [Google Scholar]
- Hudgins JW, Krekling T, Franceschi VR. Distribution of calcium oxalate crystals in the secondary phloem of conifers: a constitutive defense mechanism. New Phytologist. 2003;159:677–690. doi: 10.1046/j.1469-8137.2003.00839.x. [DOI] [PubMed] [Google Scholar]
- Jabalpurwala FA, Smoot JM, Rouseff RL. A comparison of citrus blossom volatiles. Phytochemistry. 2009;70:1428–1434. doi: 10.1016/j.phytochem.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Jensen WA. Botanical histochemistry. San Francisco: W. H. Freeman; 1962. [Google Scholar]
- Jin S, Kanagaraj A, Verma D, Lange T, Daniell H. Release of hormones from conjugates: chloroplast expression of β-glucosidase results in elevated phytohormone levels associated with significant increase in biomass and protection from aphids or whiteflies conferred by sucrose esters. Plant Physiology. 2011;155:222–235. doi: 10.1104/pp.110.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill Book Company; 1940. [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. Journal of Cell Biology. 1965;27:137–138. [Google Scholar]
- Knudsen JT, Tollsten L, Bergström LG. Floral scents—a checklist of volatile compounds isolated by head-space technique. Phytochemistry. 1993;33:253–280. doi: 10.1016/0031-9422(93)85502-I. [DOI] [Google Scholar]
- Koch K, Ensikat HJ. The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron. 2008;39:759–772. doi: 10.1016/j.micron.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Lahey KA, Yuan R, Burns JK, Ueng PP, Timmer LW, Chung KR. Induction of phytohormones and differential gene expression in citrus flowers infected by the fungus Colletotrichum acutatum. Molecular Plant-Microbe Interactions. 2004;17:1394–1401. doi: 10.1094/MPMI.2004.17.12.1394. [DOI] [PubMed] [Google Scholar]
- Lattanzio V, Lattanzio VMT, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F, editor. Phytochemistry: advances in research. Trivandrum: Research Signpost; 2006. pp. 23–67. [Google Scholar]
- Li W, Yuan R, Burns JK, Ueng PP, Timmer LW, Chung KR. Genes for hormone biosynthesis and regulation are highly expressed in citrus flowers infected with fungus Colletotrichum acutatum, the causal agent of postbloom fruit drop. Journal of the American Society for Horticultural Science. 2003;128:578–583. [Google Scholar]
- Lima WG, Spósito MB, Amorim L, Gonçalves FP, De Filho PAM. Colletotrichum gloeosporioides, a new causal agent of citrus post-bloom fruit drop. European Journal of Plant Pathology. 2011;131:157–165. doi: 10.1007/s10658-011-9795-1. [DOI] [Google Scholar]
- Lin YJ, Stover E, Sonoda R, Rosskopf E. Stigma and style necrosis is associated with postbloom fruit drop disease in citrus following artificial inoculation. Hortscience. 2001;36:1138. [Google Scholar]
- Marques JPR, Amorin L, Spósito MB, Appezzato-da-Glória B. Histopathology of postbloom fruit drop caused by Colletotrichum acutatum in citrus flowers. European Journal of Plant Pathology. 2013;135:783–790. doi: 10.1007/s10658-012-0120-4. [DOI] [Google Scholar]
- McGovern RJ, Seijo TE, Hendricks K, Roberts PD. New report of Colletotrichum gloeosporioides causing postbloom fruit drop on citrus in Bermuda. Canadian Journal of Plant Pathology. 2012;34:187–194. doi: 10.1080/07060661.2012.670137. [DOI] [Google Scholar]
- Melo MC, Borba EL, Paiva EAS. Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae) Plant Systematics and Evolution. 2010;286:141–151. doi: 10.1007/s00606-010-0294-1. [DOI] [Google Scholar]
- Moleyar V, Narasimham P. Antifungal activity of some essential oil components. Food Microbiology. 1986;3:331–336. doi: 10.1016/0740-0020(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Pansarin LM, Castro MM, Sazima M. Osmophore and elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and their relation to pollination. Botanical Journal of the Linnean Society. 2009;159:408–415. doi: 10.1016/0740-0020(86)90017-1. [DOI] [Google Scholar]
- Pearse AGE. Histochemistry, theoretical and applied. 3rd edn. Vol. 1. London: Churchill Press; 1968. [Google Scholar]
- Pridgeon AM, Stern WL. Ultrastructure of osmophores in Restrepia (Orchidacea) American Journal of Botany. 1983;70:1233–1243. doi: 10.2307/2443293. [DOI] [Google Scholar]
- Pridgeon AM, Stern WL. Osmophores of Scaphosepalum (Orchidaceae) Botanical Gazette. 1985;146:115–123. doi: 10.1086/337505. [DOI] [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. London: Harper Collins; 1996. [Google Scholar]
- Roberto SR, Borges AV. Efeito do estágio do desenvolvimento das flores e aplicação de fungicidas no controle da podridão floral dos citros. Revista Brasileira de Fruticultura. 2001;23:306–309. doi: 10.1590/S0100-29452001000200021. [DOI] [Google Scholar]
- Rodriguez A, Alquézar B, Peña L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytologist. 2013;197:36–48. doi: 10.1111/j.1469-8137.2012.04382.x. [DOI] [PubMed] [Google Scholar]
- Sakai WS. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technology. 1973;48:247–249. doi: 10.3109/10520297309116632. [DOI] [PubMed] [Google Scholar]
- Sharma N, Tripathi A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiological Research. 2008;16:337–344. doi: 10.1016/j.micres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Silva-Junior GJ, Spósito MB, Marin DR, Amorim L. Efficacy and timing of application of fungicides for control of citrus Postbloom fruit drop. Crop Protection. 2014;59:51–56. doi: 10.1016/j.cropro.2014.01.012. [DOI] [Google Scholar]
- Smith JA, Blanchette RA, Burnes TA, Gillman JH, David AJ. Epicuticular wax and white pine blister rust resistance in resistant and susceptible selections of eastern white pine (Pinus strobus) Phytopathology. 2006;96:171–177. doi: 10.1094/PHYTO-96-0171. [DOI] [PubMed] [Google Scholar]
- Stern WL, Curry KJ, Pridgeon AM. Osmophores, floral features, and systematics of Stanhopea (Orchidaceae) American Journal of Botany. 1987;74:1323–1331. doi: 10.2307/2444310. [DOI] [Google Scholar]
- Timmer LW, Agostini JP, Zitko SE, Zulfiqar M. Postbloom fruit drop, an increasingly prevalent disease of citrus in the Americas. Plant Disease. 1994;78:329–334. doi: 10.1094/PD-78-0329. [DOI] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008;19:1130–1138. doi: 10.1016/j.foodcont.2007.12.003. [DOI] [Google Scholar]
- Vogel S. The role of scent glands in pollination. Washington: Smithsonian Institution; 1990. [Google Scholar]
- Wightwick A, Walters R, Allinson G, Reichman S, Menzies N. Environmental risks of fungicides used in horticultural production systems. In: Carisse O, editor. Fungicides. 2010. [DOI] [Google Scholar]
- Wittstock U, Gershenzon J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology. 2002;5:300–307. doi: 10.1016/S1369-5266(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Kunoh H, Yamamoto H, Akimitsu K. Biological roles of monoterpene volatiles derived from rough lemon (Citrus jambhiri Lush) in citrus defense. Journal of General Plant Pathology. 2007;73:168–179. doi: 10.1007/s10327-007-0013-0. [DOI] [Google Scholar]
- Zindler-Frank E, Honow R, Hesse A. Calcium and oxalate content of the leaves of Phaseolus vulgaris at different calcium supply in relation to calcium oxalate crystal formation. Journal of Plant Physiology. 2001;158:139–144. doi: 10.1078/0176-1617-00045. [DOI] [Google Scholar]