Abstract
Combination antiretroviral therapy in pregnant women with human immunodeficiency virus has dramatically decreased maternal-to-child transmission. Highly treatment-experienced pregnant patients have limited effective treatment options due to past toxicities and viral resistance. We present 8 pregnancies in 7 perinatally infected women successfully treated with salvage regimens containing darunavir, etravirine, raltegravir, or enfuvirtide.
Keywords: darunavir, enfuvirtide, etravirine, pregnancy, raltegravir
Antiretroviral (ARV) treatment during pregnancy is routinely used to control maternal human immunodeficiency virus (HIV) disease as well as to prevent maternal-to-child transmission (MTCT). Epidemiologic and clinical trials have shown that women who receive effective ARVs that reduce HIV RNA to <1000 copies/mL or to undetectable levels have very low rates of MTCT [1, 2]. Current guidelines recommend use of antepartum combination ARVs to all pregnant women with HIV regardless of viral load (VL) or CD4 count. Standard regimens are similar regardless of pregnancy status and typically include 2 nucleoside reverse transcriptase inhibitors (NRTIs) with either a nonnucleoside reverse transcriptase inhibitor (NNRTI) or 1 or more protease inhibitors (PIs). Because of extensive experience with lamivudine and zidovudine (ZDV) in pregnancy, this combination remains the currently recommended NRTI backbone in pregnancy. Nevirapine is the preferred NNRTI for pregnant women, and Lopinavir/ritonavir or atazanavir are the preferred PIs [3].
According to the most recent New York State HIV/AIDS Surveillance Annual Report, as of December 2010, there were more than 5500 reported and confirmed cases of children and adolescents with HIV/acquired immunodeficiency syndrome (AIDS) age ≤24 years living in New York [4]. With widespread availability of combination ARV therapy (cART), these patients are living longer and many are approaching or are already of reproductive age. As a group, they have unique treatment issues, particularly in the face of pregnancy. Those perinatally infected are often treatment-experienced and harbor multidrug-resistant (MDR) virus. Standard regimens may provide inadequate virologic control (VL <1000 copies/mL), thus practitioners must consider “salvage” combinations in these challenging cases. With the ongoing development of novel ARVs, new options are available, including ritonavir-boosted darunavir (PI), etravirine (NNRTI), raltegravir (integrase inhibitor), and enfuvirtide (fusion inhibitor). Insufficient data exist to provide guidance for obstetricians faced with prescribing these regimens during pregnancy or for pediatricians following infants exposed to these drugs in utero.
METHODS
We conducted a retrospective chart review of all HIV-infected pregnant patients seen in the Mount Sinai Obstetrics-Infectious Disease Clinic from January 2004 through January 2012 who received salvage regimens. Patients were considered on “salvage” if given the following ARVs during pregnancy for >7 consecutive days: darunavir, etravirine, raltegravir, or enfuvirtide. Salvage was chosen, after virologic failure with standard cART, by a multidisciplinary team, including obstetricians and infectious diseases specialists, based on resistance testing (Table 1), safety, and tolerability. Side effects and adherence were discussed at each visit. Patients were encouraged to participate in directly observed therapy (DOT) on either an inpatient or outpatient basis. The study was approved by the Mount Sinai School of Medicine Institutional Review Board.
Table 1.
Summary of Pregnancies Before and After Salvage Regimens
| Patient | Age During Pregnancy (years) Race/Ethnicity | Reverse Transcriptase Mutations | Protease Inhibitor Mutations | Salvage Regimen | CD4 Prior to Salvage, (cells/mm3 [%]) | CD4 at Delivery, (cells/mm3 [%]) | Peak VL During Pregnancy (copies/mL) | VL at Delivery (copies/mL) | Mode of Delivery | Gestational Age (weeks) | Birth Weight (grams) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 18 Hispanic/ Hispanic |
None | M36I L63P |
TDF/FTC ETR DRV RTV |
129 (25) | 174 (30) | 27 690 | <20 | Spontaneous vaginal | 37 | 2614 |
| B | 20 Hispanic/ Hispanic |
None | L63P L90M V77I I13V |
TDF/FTC DRV RTV RAL |
486 (30) | 486 (30) | 7811 | 57 | C/S Failure to progress | 41 | 3110 |
| C | 20 Black/Not Hispanic |
K103S G190A |
I13V L63P |
ZDV TDF/ FTC DRV RTV RAL |
8 (2) | 5 (2) | 141 332 | 191 | Elective C/S | 39 | 2240 (SGA) |
| D | 21 Black/Not Hispanic |
M41L M184V T215 A/D/N Y181C |
L63P A71T D30N V77I N88D I13V |
ABC TDF 3TC ETR RAL |
66 (8) | 65 (6) | 66 171 | 79 | Emergency C/S | 35 | 2110 (SGA) |
| E (1) | 22 Black/Not Hispanic |
a | a | ABC TDF 3TC DRV RTV ENF |
56 (5) | 81 (4) | 74 754 | 639 | Elective C/S | 35b | 2300 |
| E (2) | 23 Black/Not Hispanic |
a | a | ZDV TDF 3TC DRV RTV RAL |
22 (2) | 32 (2) | 60 192 | 54 178 | Repeat C/S | 38b | 2460 (SGA) |
| F | 24 Black/Not Hispanic |
None | L10I E35P I13V I62V G16E M36I L63T |
TDF/FTC DRV RTV RAL |
198 (19) | 198 (19) | 45 224 | 50 | Repeat C/S | 37 | 2520 (SGA) |
| G | 21 Black/Not Hispanic |
K70R D67E |
V77I | TDF/FTC ATV RTV RAL |
422 (19) | 444 (28) | 9992 | 521 | Primary elective C/S | 37 c | 2830 |
Abbreviations: ABC, abacavir; 3TC, lamivudine; ATV, atazanavir; C/S, C-section; DRV, darunavir; ENF, enfuvirtide; ETR, etravirine; FTC, emtricitabine; RAL, raltegravir; RTV, ritonavir; SGA, small for
gestational age; TDF, tenofovir; VL, viral load.
aInformation unavailable.
bNeonate received ZDV, 3TC, LPV/RTV.
cNeonate received ZDV, NVP.
RESULTS
Eight pregnancies in 7 HIV-infected women on salvage were identified (Table 1). All women acquired HIV perinatally, representing 50% of perinatally infected women receiving care in clinic during that time. Those with childhood records available (3 of 7) had documentation of at least 1 year of ZDV monotherapy, and 2 of 7 had documented ZDV resistance. There were no cases of hepatitis B or C coinfection. All subjects became anemic (hemoglobin, ≤10.5 g/dL) by the second trimester (range, 6.0–10.0). No patients had elevated transaminases (aspartate transaminase, >50 U/L; alanine transaminase, >53 U/L) or bilirubin (total, >1.2 mg/dL; direct, >0.8 mg/dL) or required discontinuation of salvage regimens due to toxicity or intolerance. Viral load at delivery was <1000 copies/mL in 7 of 8 patients. Mean VL (calculated as the average of all VLs measured during pregnancy) was decreased in 7 of 8 pregnancies, compared with the year prior, but 6 months postpartum, was increased in all cases except 1 (Figure 1).
Figure 1.
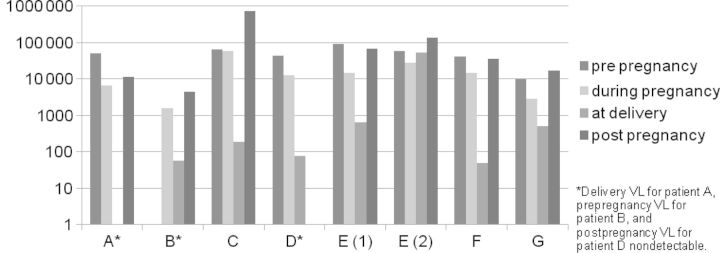
Mean viral loads before, during, and after pregnancy and at delivery (log scale). Abbreviation: VL, viral load.
Median gestational age of infants was 37 weeks (range, 35–41). Two deliveries were premature at 35 weeks, 1 due to preterm labor and the other after a nonreactive fetal nonstress test. There were no cases of preterm premature rupture of membranes, preeclampsia, or gestational diabetes. Although only 1 patient had a VL >1000 at delivery, 7 of 8 deliveries were via C-section (C/S). One was a primary, elective C/S for a wheelchair bound patient with severe scoliosis and respiratory compromise. All women received intrapartum intravenous ZDV.
Median birth weight was 2490 grams (range, 2110–3110). Four of eight babies were small for gestational age (SGA) [5], but weight and height growth velocity in the first 6 months of life was equivalent to or surpassed normal rates (700 grams/month and 2 cm/month) for all babies except one who was premature. Appropriate neurological and motor milestones were met by all babies with this information recorded at 2 weeks (5 of 7), 2 months (5 of 7), 4 months (3 of 7), and 6 months (7 of 7).
One infant died within 9 hours of delivery. By autopsy report, underlying cause of death was prematurity, complicated by marked hyaline membrane disease and pneumothorax. The remaining newborns went to the well-baby nursery. They underwent HIV polymerase chain reaction testing at birth, 2 weeks, 1 month, and 4 months, and they were all HIV-uninfected.
Four of seven babies (including those whose mothers had ZDV-resistant virus) received standard 6-week courses of ZDV for chemoprophylaxis. Babies born to mothers unable to achieve virologic control at least 2 weeks prior to delivery received combination therapy.
DISCUSSION
We report overall encouraging results using salvage regimens containing darunavir, etravirine, raltegravir, or enfuvirtide in pregnancy. Regimens were well-tolerated with no reported cases of maternal toxicities requiring dose interruption or discontinuation. We observed a higher than expected number of SGA infants as well as a neonatal demise. However, given the small numbers of patients and varying regimens used, it is difficult to infer causality. Overall, there were no safety concerns in the infants. All were HIV-uninfected and met basic growth and neurodevelopmental milestones by 6 months. No congenital anomalies were noted, but salvage regimens were initiated after the first trimester, the period of major organogenesis. Despite high peak VLs for some prior to salvage, all infants tested were HIV-uninfected. Other case reports using these ARVs in highly treatment-experienced patients had similar findings–minimal adverse events in mothers (most notable, liver dysfunction, spontaneously resolved) and no major congenital anomalies in the exposed infants, but this series is the largest to date [6, 7].
Selection of cART in this population of perinatally infected pregnant women is challenging. Prior exposure to multiple ARVs, MDR, complicated psychosocial issues, and poor adherence results in complex treatment considerations. Guidelines for use of ARVs in pregnancy exist [3], but the management of treatment-experienced HIV-infected patients during pregnancy must be addressed on a case-by-case basis. Genotypic and phenotypic resistance patterns should be considered as well as tolerability and ease of administration. Of note, although not all patients showed laboratory evidence of extensive multiclass resistance, given their past ARV exposures, we suspect others may have had mutations not found in the current medical records. Insufficient information is available from clinical trials to assist in the selection of salvage regimens. Studies from the last decade have shown minimal increased risk of preterm birth in pregnant women receiving PI-based cART; however, given the clear benefits for both mothers' health and prevention of MTCT, PIs are not contraindicated in pregnancy [3]. Reported experience with integrase and fusion inhibitors in pregnancy has been limited.
Placental passage of drug may aid in the prevention of MTCT, but it may also contribute to fetal toxicity. It has been demonstrated for darunavir [8], etravirine [9], and raltegravir [10], but there is debate regarding placental transfer of enfuvirtide.
Results from the Antiretroviral Pregnancy Registry, which collects and evaluates data on the outcomes of pregnancy exposures to ARVs, do not show increased risk of overall birth defects compared with population-based surveillance systems or when comparing rates after first-trimester ARV exposure to those with exposure later in pregnancy, but, as a voluntary reporting system, these results may be subject to reporting bias [11]. The SMARTT arm of the Pediatric HIV/AIDS Cohort Study (PHACS) is currently ongoing and aims to estimate the incidence of conditions and diagnoses potentially related to in utero exposure to ARVs or exposure in the first 2 months of life among children born of HIV-infected mothers. Another recent study of over 1500 HIV-exposed infants and uninfected infants exposed to ARVs and to trimethoprimin-sulfamethoxazole in utero and in early infancy demonstrated that serious adverse events related to hemoglobin, absolute neutrophil count, total lymphocyte count, and glucose were experienced by fewer than 10% of infants in the first 6 months of life [12]. Although these results are encouraging, it is clear that further pharmacokinetic, efficacy, and safety data are needed for these medications in pregnancy as well as for exposed infants.
Although this is a small series, it is the largest published to date using these salvage medications in perinatally infected pregnant women who are highly treatment-experienced with limited treatment options. Through successful use of salvage and a multidisciplinary team approach, including DOT, we were able to achieve virologic control in 7 of 8 pregnancies and though there was a single infant death, the remaining babies were all HIV-uninfected without congenital anomalies. Although mean VLs were decreased in 7 of 8 pregnancies, compared with the year prior, a full log decrease was only achieved in 1 pregnancy. Six months postpartum, VLs were detectable in all but 1 case due to maternal nonadherence with cART after delivery, highlighting the long-term challenges of caring for this population.
Acknowledgments
We thank all patients and staff at the Mount Sinai Obstetrics-Infectious Disease Clinic.
Financial support. J. J. received salary support from the National Institute of Child Health and Human Development 1K23HD070760-01A1 during the preparation of this manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335:1621–9. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 3.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at: http://aidsinfo.nih.gov/guidelines . Accessed August 2, 2012. [Google Scholar]
- 4.NYS Department of Health. New York State HIV/AIDS Surveillance Annual Report For Cases Diagnosed Through December 2010. New York: August 2012. [Google Scholar]
- 5.Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 6.Furco A, Gosrani B, Nicholas S, et al. Successful use of darunavir, etravirine, enfuvirtide and tenofovir/emtricitabine in pregnant woman with multiclass HIV resistance. AIDS. 2009;23:434–5. doi: 10.1097/QAD.0b013e32832027d6. [DOI] [PubMed] [Google Scholar]
- 7.Taylor N, Touzeau V, Geit M, et al. Raltegravir in pregnancy: a case series presentation. Int J STD AIDS. 2011;22:358–60. doi: 10.1258/ijsa.2011.010469. [DOI] [PubMed] [Google Scholar]
- 8.Ivanovic J, Bellagamba R, Nicastri E, et al. Use of darunavir/ritonavir once daily in treatment-naive pregnant woman: pharmacokinetics, compartmental exposure, efficacy and safety. AIDS. 2010;24:1083–4. doi: 10.1097/QAD.0b013e32833653b2. [DOI] [PubMed] [Google Scholar]
- 9.Izurieta P, Kakuda TN, Feys C, Witek J. Safety and pharmacokinetics of etravirine in pregnant HIV-1-infected women. HIV Med. 2011;12:257–8. doi: 10.1111/j.1468-1293.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 10.McKeown DA, Rosenvinge M, Donaghy S, et al. High neonatal concentrations of raltegravir following transplacental transfer in HIV-1 positive pregnant women. AIDS. 2010;24:2416–8. doi: 10.1097/QAD.0b013e32833d8a50. [DOI] [PubMed] [Google Scholar]
- 11.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 January 2012. Available at: www.APRegistry.com . Accessed August 2, 2012. [Google Scholar]
- 12.Read JS, Huo Y, Patel K, et al. Laboratory abnormalities among HIV-exposed, uninfected infants: IMPAACT Protocol P1025. J Ped Infect Dis. 2012;1:92–102. doi: 10.1093/jpids/pis036. [DOI] [PMC free article] [PubMed] [Google Scholar]


