Abstract
Protein kinase D (PKD) is an evolutionarily conserved protein kinase family with structural, enzymological, and regulatory properties different from the PKC family members. Signaling through PKD is induced by a remarkable number of stimuli, including G protein-coupled receptor agonists and polypeptide growth factors. PKD1, the most studied member of the family, is increasingly implicated in the regulation of a complex array of fundamental biological processes, including signal transduction, cell proliferation and differentiation, membrane trafficking, secretion, immune regulation, cardiac hypertrophy and contraction, angiogenesis and cancer. PKD mediates such diverse array of normal and abnormal biological functions via dynamic changes in its spatial and temporal localization, combined with its distinct substrate specificity. Studies on PKD thus far indicate a striking diversity of both its signal generation and distribution and its potential for complex regulatory interactions with multiple downstream pathways, often regulating the sub-cellular localization of its targets.
Introduction: regulation of PKD signaling
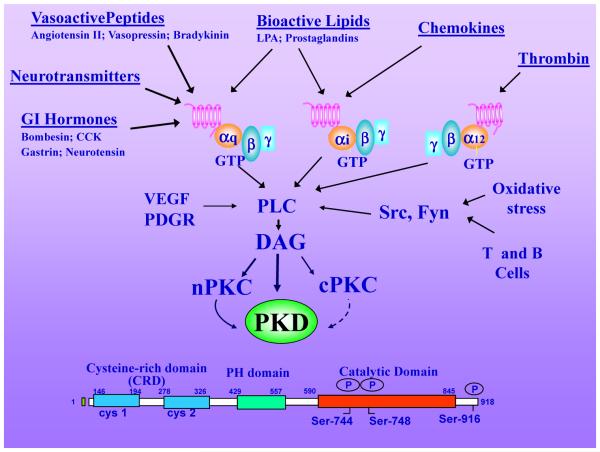
A large number of external signals involved in inter-cell communication, including hormones, neurotransmitters, growth and developmental factors, cytokines and bioactive lipids bind to receptors that promote the stimulation of the isoforms of the phospholipase C (PLC)1 family. PLCs, catalyze the hydrolysis of phosphatidylinositol 4,5-biphosphate to produce two second messengers: Ins (1,4,5)P3, which triggers the release of Ca2+ from internal stores and DAG, which elicits cellular responses through classic (α, β, γ) and novel (δ, ε, η, θ) isoforms of PKC [reviewed in (78)]. The mechanisms by which PKC-mediated signals are propagated to critical downstream targets remain incompletely understood. Protein kinase D1 (PKD1)1, the founding and most studied member of a new protein kinase family within the CAMK group (comprising PKD1, PKD2 and PKD3) and separate from the PKCs [reviewed in (78)], are attracting intense attention. PKD1 (96), formerly called PKCμ (41), has been extensively studied in vitro, with regards to identifying the functions of its domains and the effect of cell signaling on its activity and sub-cellular localization. In unstimulated cells, PKD1 is in a state of low catalytic (kinase) activity maintained by autoinhibition mediated by the N-terminal domain, a region containing a repeat of cysteine-rich zinc finger-like motifs and a pleckstrin homology domain [(33, 34, 78, 101); mouse PKD1 domains in Fig. 1]. PKD1 could be stimulated by phosphatidylserine micelles containing either DAG or phorbol esters in cell-free preparations (97). These early studies implied that PKD1 represents a novel component of the signal transduction pathways initiated by DAG in target cells.
Figure 1. PKDs activation by multiple stimuli.
Hormones, growth factors, neurotransmitters, chemokines, bioactive lipids, proteases and oxidative stress induce PLC-mediated hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) to produce DAG at the plasma membrane, which in turn mediates the translocation of inactive PKDs from the cytosol to that cellular compartment. DAG also recruits, and simultaneously activates, novel PKCs to the plasma membrane which mediate transphosphorylation of PKD1 on Ser744 (in mouse PKD1). DAG and PKC-mediated transphosphorylation of PKD act synergistically to promote PKD catalytic activation and autophosphorylation on Ser748. The modular structure of PKD (mouse PKD1) is illustrated as an example of the PKD family. PKD1 is the most studied member of the family and its knockout induces embryonic lethality. Further details are provided in the text.
Subsequent studies, aimed to define PKD1 regulation within intact cells, elucidated a mechanism of PKD1 activation distinct from the direct stimulation of enzyme activity by DAG/phorbol ester plus phospholipids obtained in vitro. Specifically, cell treatment with phorbol esters or DAG analogues induced a dramatic inter-conversion of PKD1 from an inactive to an active form, as shown by in vitro kinase assays performed in the absence of lipid co-activators. In all these cases, PKD1 activation was potently blocked by prior treatment with PKC inhibitors that did not directly inhibit PKD1 catalytic activity (116), suggesting that rapid PKD1 activation within intact cells is mediated through PKCs. Indeed, cotransfection of PKD1 with active mutant forms of “novel” PKCs (PKCs δ, ε, η, θ), resulted in robust PKD1 activation in the absence of cell stimulation. Studies with multiple receptor agonists (see Fig.1), PKD1 mutants and purified PKD1 led to a model that envisages phosphorylation of PKD1 on the activation loop residues Ser744 and Ser748 (residues #corresponding to the mouse PKD1, Fig. 1) as a direct “on/off” switch for catalytic activity [reviewed in (78)]. More recent studies demonstrated that the rapid PKC-dependent PKD1 activation is followed by a sustained, PKC-independent, phase of catalytic activation and phosphorylation induced by stimulation with Gq-coupled receptor agonists, including bombesin and vasopressin (38, 82). These studies also revealed that PKD1 provides an example of a protein kinase in which each residue of the activation loop is regulated by different upstream mechanisms, namely transphosphorylation for Ser744 and autophosphorylation for Ser748 (38, 82). Thus, PKD1 is a point of convergence and integration of multiple stimuli that is rapidly activated through PKCs and persistently, through PKC-dependent or PKC-independent pathways.
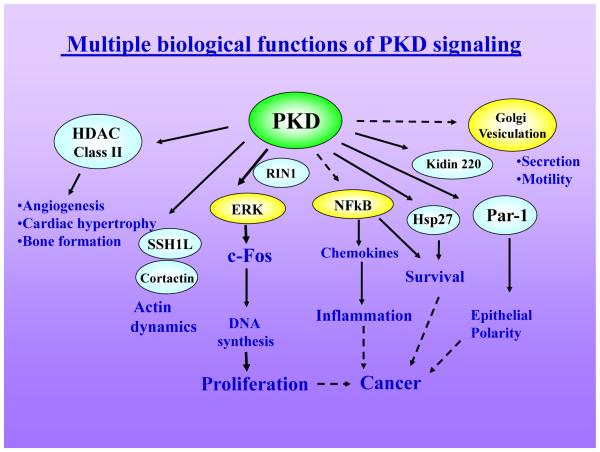
Additional studies showed that PKD family members undergo rapid redistributions in response to cell stimulation. PKD1 and PKD2 translocate from the cytosol to DAG-containing microenvironments in the plasma membrane (75) followed by PKC-dependent reverse translocation of PKD from the plasma membrane to the cytosol and subsequent accumulation in the nucleus (74). In contrast, PKD3 is continuously shuttling between the cytoplasm and the nucleus (73). Pools of PKD family members also localize at the Golgi complex (53, 108) and mitochondria (12). In addition, PKD1 and PKD2 contain short PDZ-binding motifs in their C-termini; VSIL in PKD1 and ISVL in PKD2. Recently, the Na+/H+ exchanger regulatory factor 1 (NHERF-1), a PDZ domain-containing protein, has been shown to interact with these sequences of PKD1 and PKD2, implying that PKD can form complexes with NHERF-1 (46). Thus, PKD can regulate targets in a variety of sub-cellular locations and consequently control multiple cellular activities. Accordingly, PKD has been implicated in the regulation of a remarkable array of fundamental biological processes including cell proliferation, polarity, migration and differentiation, membrane trafficking, pain transmission, inflammation, angiogenesis, cardiac contractility and hypertrophy and cancer. The regulation of PKD multi-site phosphorylation, translocation and catalytic activity has been reviewed previously (78). This article focuses on the function of PKD signaling in the regulation of multiple biological processes (Fig. 2) and highlights novel concepts emerging from these recent studies (Fig. 3).
Figure 2. PKD signaling regulates multiple normal and abnormal biological processes.
Active PKD phosphorylates a variety of cellular targets at specific sites thereby regulating its sub-cellular localization (as in Fig. 3) or activity (see Table 1 for examples of substrates and of the consensus sequence phosphorylated by PKD). Solid lines indicate direct phosphorylation of substrates (in light blue). Broken lines represent processes in which PKD is implicated but the sequence of molecular events has not been elucidated. Further details are in the text.
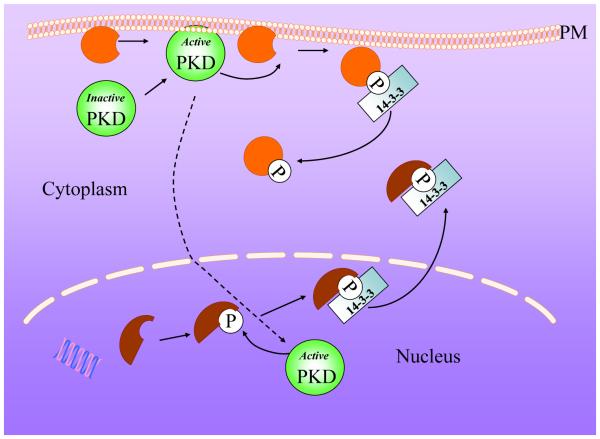
Figure 3. Schematic representation of the mechanism by which PKD modulates intracellular localization of its substrates.
In many cases, the phosphorylation of PKD substrates induces binding of 14-3-3 proteins that sequester them to the cytosol, thereby preventing them from acting at the plasma membrane (e.g. RIN1, Par-1, DLC1) or at the nucleus (e.g. HDACS 5 and HDACS 7). An emerging theme is that PKD signaling regulates cell function by altering the sub-cellular localization of its substrates.
PKD signaling in cell proliferation and differentiation
Fibroblast cell proliferation and the PKD/RIN1 axis
In fibroblasts, PKD1 can be activated by multiple growth-promoting GPCR agonists (Fig. 1) that act through Gq, G12 and Gi (112-114, 117), suggesting that PKD functions in mediating mitogenic signaling (78). Indeed, overexpression of either PKD1 or PKD2 potentiated DNA synthesis and cell proliferation induced by Gq-coupled receptor agonists in Swiss 3T3 fibroblasts (82-84, 115). Reciprocally, depletion of PKD1 by transfection of these cells with siRNAs targeting PKD1 markedly attenuated GPCR-induced mitogenesis (82). These results indicate that PKD1 catalytic activation plays a critical and selective role in GPCR mitogenic signaling.
A key pathway involved in mitogenic signaling induced by GPCRs is the extracellular-regulated protein kinase (ERK) cascade (77). The duration and intensity of MEK/ERK/RSK activation are of critical importance for determining specific biological outcomes, including proliferation and differentiation. The potentiating effect of PKD on GPCR-induced cell proliferation has been linked to its ability to increase the duration of the MEK/ERK/RSK pathway leading to accumulation of immediate gene products, (e.g. c-Fos) that stimulate cell cycle progression (82, 83).
Although the targets of PKD responsible for the transmission of its mitogenic signal have not been fully identified, putative substrates are beginning to emerge. A number of scaffolding proteins and endogenous inhibitors have been implicated in the regulation of the intensity and duration of the ERK pathway. Modeling the activation of the ERK pathway indicates that scaffolds regulate its intensity whereas inhibitors modulate its duration (17). The activity and localization of scaffolding and inhibitory proteins are regulated by phosphorylation thereby offering new mechanisms for controlling the MEK/ERK/RSK pathway. PKD1 has been shown to phosphorylate Ras and Rab Interactor 1 (RIN1, see Table 1 for the canonical sequence surrounding the PKD1 phosphorylation site), a multidomain protein that in its unphosphorylated form inhibits Ras/Raf interaction and thereby prevents ERK activation (103). The phosphorylation of RIN1 at Ser351 by PKD induces binding of 14-3-3 proteins that restricts RIN1 to the cytosol thereby abrogating its ability of blocking Ras/Raf-1 interaction at the plasma membrane [(103) and Fig. 3]. Interestingly, the sustained phase of ERK activation induced by Gq-coupled receptor agonists in PKD-overexpressing 3T3 cells requires epidermal growth factor receptor (EGFR) tyrosine kinase activity (82). It is conceivable that GPCR-induced EGFR transactivation is necessary for the generation of Ras-GTP (77), the partner of Raf. PKD activation facilitates Ras/Raf interaction by phophorylating RIN1, which is then sequestered in the cytosol in a complex with 14-3-3 proteins (Fig. 3). The Sprouty (Spry) proteins also act as inhibitors of the Ras/ERK pathway downstream of receptor tyrosine kinases. Interestingly, Spry2 has been shown to interact with PKCδ and prevent PKD activation (10), providing another link in the pathways connecting PKD with ERK activation.
TABLE 1.
Identified substrates of the PKD family
| PKD SUBSTRATE | RESIDUE | TARGET SEQUENCE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 | −3 | 0 | ||||||||||
| CERT | (human) | S132 | S | L | R | R | H | G | S | M | V | S |
| Cortactin | (human) | S298 | K | L | A | K | H | E | S | Q | Q | D |
| CREB | (human) | S133 | I | L | S | R | R | P | S | Y | R | K |
| DLC-1 | (human) | S327 | P | V | T | R | T | R | S | L | S | A |
| HDAC5 | (human) | S259 | P | L | R | K | T | A | S | E | P | N |
| HDAC5 | (human) | S498 | P | L | S | R | T | Q | S | S | P | L |
| HDAC7 | (human) | S155 | P | L | R | K | T | V | S | E | P | N |
| HDAC7 | (human) | S358 | P | L | S | R | T | R | S | E | P | L |
| HDAC7 | (human) | S486 | P | L | S | R | A | Q | S | S | P | A |
| HPK1 | (human) | S171 | T | L | A | R | R | L | S | F | I | G |
| HSP27 | (human) | S82 | A | L | S | R | Q | L | S | S | G | V |
| Kidins22 | (rat) | S918 | T | I | T | R | Q | M | S | F | D | L |
| MYBPC3 | (human) | S304 | S | L | L | K | K | S | S | F | R | T |
| Par-1 | (human) | S400 | K | V | Q | R | S | V | S | A | N | P |
| Rin1 | (human) | S351 | P | L | L | R | S | M | S | A | A | F |
| SSH-1 | (human) | S978 | P | L | K | R | S | H | S | L | A | K |
| TNNI | (rat) | S24 | P | V | R | R | R | S | S | A | N | Y |
| TLR5 | (human) | S805 | Q | L | M | K | H | Q | S | I | R | G |
| TRPV1 | (rat) | S116 | R | L | Y | D | R | R | S | I | F | D |
Note that in most cases, PKD phosphorylates a serine surrounded by a sequence characterized by L/V/I at position −5 and R/K at position −3. Less strict requirements are seen at other positions.
The PKD/ERK/DNA synthesis signaling module is not confined to fibroblasts but operates in a variety of cell types, including cancer cells harboring Ras mutations. For example, the GPCR agonist neurotensin induces PKC-dependent PKD activation (25) and acts as potent growth factor for pancreatic cancer PANC-1 cells (44). PKD1 overexpression in these cells increases DNA synthesis, cell proliferation and anchorage-independent proliferation and potentiates neurotensin-stimulated DNA synthesis, at least in part, via prolongation of ERK signaling (45). Pancreatic cancer cells express RIN1 and neurotensin promotes complex formation between RIN1 and 14-3-3 proteins via PKD in these cells (Sinnett-Smith, Guha and Rozengurt, unpublished results). Additional examples, discussed below, show that the PKD/ERK/DNA synthesis module operates in other cell types, including epithelial and endothelial cells, and will emphasize other mechanisms by which PKD can participate in cell regulation.
Epidermal keratinocyte cell proliferation and reversible differentiation
Regulation of epidermal cell growth and differentiation has been extensively studied in primary cultures of mouse keratinocytes through modulation of extracellular Ca2+ levels. PKD is prominently expressed in proliferating primary keratinocytes and down-regulated during differentiation of these cells, suggesting that PKD plays a pro-mitogenic and/or anti-differentiation role in these cells (20). Differentiated keratinocytes, generated by culturing in medium containing millimolar Ca2+, maintain regenerative potential. Lowering Ca2+ (to micromolar) stimulates reinitiation of DNA synthesis and cell proliferation in primary cultures of mouse keratinocytes (39). PKD1 has been identified as a major regulator of this proliferative response through sustained activation of the ERK pathway (39). Interestingly, PKD1 activation was mediated by a PKC-independent pathway involving autophosphorylation at Ser748, similar to the mechanism of PKD1 activation recently elucidated in other cell types (38, 82). These results substantiate a role of PKD in epidermal regeneration and wound healing.
The PKD/HDAC axis and endothelial cell proliferation
Vascular endothelial growth factors (VEGFs) and their endothelial tyrosine kinase receptors play a central role in the regulation of blood and lymphatic vessel development and are essential for normal and abnormal angiogenesis. PKD signaling is necessary for VEGF expression by tumor cells (2, 66) and ERK activation, gene expression and DNA synthesis in VEGF-stimulated endothelial cells (105).
Acetylation/deacetylation of histones is a fundamental mechanism for the control of gene expression. Class II histone deacetylases (i.e. HDACs 4, 5, 7 and 9) regulate chromatin structure by interacting with various transcription factors to repress their activity. PKD phosphorylates specific residues in class II HDACS (see Table 1) leading to their association with 14-3-3 proteins in endothelial cells and other cell types (as illustrated schematically in Fig. 3). Sequestration of HDACs in the cytoplasm via 14-3-3 complex formation relieves target genes in the nucleus from HDAC repressive actions, thereby facilitating gene expression.
HDAC7 has been implicated in the regulation of endothelial cells morphology, migration, and capacity to form capillary tube-like structures in vitro (62). VEGF induced exit of HDAC7 from the nucleus through PKD-mediated phosphorylation of serines (Table 1) that complex with 14-3-3, promoting nuclear export of HDAC7 and activation of VEGF-responsive genes in endothelial cells (27, 62). Expression of a signal-resistant HDAC7 mutant protein in these cells inhibited proliferation and migration in response to VEGF (102).
HDAC5 has also been identified as a negative regulator of angiogenesis (95). In addition to HDAC7, VEGF stimulates PKD-dependent phosphorylation of HDAC5 on Ser259/498 (Table 1), which leads to HDAC5 nuclear exclusion and transcriptional activation (28, 102). Collectively, these studies imply that the complex program of gene expression and migration triggered by VEGF in endothelial cells leading to angiogenesis is orchestrated, at least partly, by PKD-mediated phosphorylation of both HADC5 and HDAC7, leading to their nuclear extrusion in these cells. Accordingly, PKD has emerged as an attractive target for anti-angiogenic therapy.
Osteoblast differentiation and the PKD/HDAC/Runx axis
Bone morphogenetic proteins (BMPs) are multi-functional growth factors that belong to the transforming growth factor beta (TGFβ) superfamily. They contribute to the formation of bone and connective tissues by inducing the differentiation of mesenchymal cells into bone-forming cells. Recent studies demonstrated that BMP-2 induces PKD activation through a PKC-independent pathway during osteoblast lineage progression (51) and that PKD is required for the effects of BMP-2 on osteoblast differentiation (8). BMP-2 induces export of HDAC7 from the nucleus in mesenchymal cells that is associated with increased HDAC7 phosphorylation and 14-3-3 binding (40). HDAC7 represses the activity of Runx2, a master transcriptional regulator of skeletal biology. PKD induces HDAC7 nuclear export and thereby alleviates repression of Runx2-mediated transcription (40). Although other pathways may be involved, these results establish a mechanism by which BMP-2 signaling regulates Runx2 activity via PKD-dependent inhibition of HDAC7 transcriptional repression.
PKD function in the regulation of cell vesicle trafficking, secretion and polarity.
In addition to being located at the plasma membrane, cytoplasm and nucleus, a pool of PKD1 and PKD2 is situated at the Golgi complex where it regulates the budding of secretory vesicles from the trans-Golgi network (53, 108). Inactivation of PKD (e.g. by expression of kinase-deficient mutants of PKD) blocks fission of trans-Golgi network (TGN) transport carriers, inducing the appearance of long tubules filled with cargo. At the TGN, active PKD1 and PKD2 phosphorylate Golgi-localized substrates (23, 30, 65), including phosphatidylinositol 4-kinase IIIb (PI4KIIIb), a key player required for fission of TGN-to-plasma membrane carriers (30). PI4KIIIb is recruited to the TGN membrane by the small GTPase ARF, and activated by PKD-mediated phosphorylation to generate PI(4)P, which then recruits the machinery that is required for carrier fission (24). The precise signal that stimulates PKD activation at the Golgi remains unclear. Recent reports proposed intracellular Ca2+ released from internal stores in response to Gq-coupled receptor agonists (47) or direct translocation of βγ subunits from the plasma membrane to the Golgi (35, 79).
In agreement with a role in regulating Golgi function, PKD has been implicated in secretion. For example, PKD stimulates secretion of neurotensin from the human endocrine BON cells via the PKD protein substrate Kidins220, [kinase D-interacting substrate of 220 kDa (6, 32) and Table 1] (52). PKD also plays a critical role in regulating angiotensin II-induced cortisol and aldosterone secretion from H295R cells, a human adrenocortical cell line (76). Recent studies revealed a novel p38δ-PKD pathway that regulates insulin secretion and survival of pancreatic β cells, suggesting a critical role for PKD in the development of diabetes mellitus (94).
In addition to secretion, the regulation of Golgi cargo to the plasma membrane has been implicated in fibroblast locomotion, localized Rac1-dependent leading edge activity (72) and integrin recruitment to newly formed focal adhesions (106). DLC1 (deleted in liver cancer), a negative regulator of Rho, is a tumor suppressor gene deleted almost as frequently as p53 in common cancers, including breast, colon, and lung. Activation of PKC/PKD induced association of DLC1 with 14-3-3 proteins (80) via phosphorylation of Ser327 and Ser431 (Table 1). In turn, Rho activation leads to PKD activation (113). While these studies imply a role of PKD in cell motility, other studies showed that PKD regulates the phosphorylation of cofilin (18, 71) and cortactin (19), leading to reduced cell motility. Although PKD contributes to cell motility and actin dynamics, its role appears complex, depending on cell context and exposure to specific stimuli.
Neuronal and Epithelial Cell Polarity.
Establishing and maintaining cellular polarity is of fundamental importance for the functions of a variety of cell types, including neuronal and epithelial cells. Early neurons develop initial polarity by mechanisms analogous to those used by migrating cells. In line with this notion, PKDs has been shown to play a role in neuronal protein trafficking. In these cells PKD1 and PKD2 regulate TGN-derived sorting of dendritic proteins and axon formation and hence have a role in establishing neuronal polarity (4, 109). PKD has been also implicated in the maintenance of dendritic arborization (14).
In polarized epithelial cells, PKD1 and PKD2, but not PKD3, specifically regulate the production of TGN carriers destined to the basolateral membrane rather than to the apical membrane and consequently, PKD family members may play an important role in the generation of epithelial polarity (108). Another major mechanism involved in establishing cell polarity is mediated by the evolutionary conserved PAR (partitioning-defective) genes, including Par-1. Treatment of cells with phorbol-12-myristate-13-acetate (PMA) induced PKD-mediated phosphorylation of Par-1 on a residue (Ser400; Table1) that promotes Par-1 binding to 14-3-3, thereby promoting its dissociation from lateral plasma membrane and inhibiting its activity [(104); Fig. 3). These results suggest that PKD may play a role in regulating cell polarity via phosphorylation of Par-1. Additional experiments using physiological stimuli rather than PMA are necessary to substantiate this important hypothesis. Interestingly, PKD isoforms regulate learning and behavior in Caenorhabditis elegans integrating external information in neuronal and intestinal epithelial cells (22).
PKD signaling and regulation of immune function
A prominent PKC/PKD axis has been demonstrated in B and T lymphocytes (78). PKD is cytosolic in unstimulated T cells (56), but it rapidly polarizes to the immunological synapse in response to antigen/antigen presenting cells. PKD repositioning is driven by the accumulation of DAG at the immunological synapse (87). As in other cell types cells, PKD in lymphocytes phosphorylates and regulates class II HDACs (15, 57, 70). PKD family has also been implicated in regulating the activity of β1 integrins in T cells via Rap1 (59) and IL-2 promoter in response to TCR stimulation (36).
The activation of PKD by antigen receptors is a sustained response associated with changes in PKD intracellular location. The function of PKD at these different locations has been probed in an in vivo model using active PKD mutants targeted to either the plasma membrane or the cytosol of pre-T cells of transgenic mice (55). Studies of these mice have shown that PKD can substitute for the pre-T cell receptor and induce both proliferation and differentiation of T cell progenitors in the thymus. Moreover, cellular localization of PKD within a thymocyte is critical; membrane-targeted and cytosolic PKD control different facets of pre-T cell differentiation (55). Subsequent studies probed the Rho requirements for the actions of constitutively active PKD mutants localized at the plasma membrane or the cytosol in pre-T cells of transgenic mice. Membrane-localized PKD regulation of pre- T cell differentiation was shown to be Rho-dependent, but the actions of cytosol-localized PKD were not (63). These studies demonstrated that the link between PKD and Rho is determined by the cellular location of PKD in T lymphocytes.
Toll-like receptors (TLRs) have been identified as primary innate immune receptors. TLRs distinguish between different patterns of pathogens and activate a rapid innate immune response. Recent results implicated PKD in TLR 2, 5 and 9 function in different cell types (37, 69). Specifically, PKD is a downstream target in TLR9 signaling in macrophages (69) and TLR2 in mouse bone marrow-derived mast cells (64). The TLR-interacting protein myeloid differentiation factor 88 (MyD88) has been suggested to play a role in TLR-induced PKD activation (68). In turn, PKD-mediated phosphorylation of TLR5 on Ser805 (Table 1) appears necessary for TLR5 response to its ligand, flagellin. TLR5 phosphorylation contributes to p38MAPK activation and production of inflammatory cytokines in epithelial cells (37). Although the precise role of PKD in TLR function remains incompletely understood, these studies provide evidence indicating that PKD plays a role in the regulation of the innate immune response mediated by this class of pattern recognition receptors.
PKD signaling in disease: inflammatory responses, cardiac hypertrophy and cancer PKD, inflammation and oxidative stress
NF-κB is a key transcription factor that is activated by multiple receptors and regulates the expression of a wide variety of proteins that control innate and adaptive immunity. A number of studies indicate that PKD is a mediator of NF-κB induction in a variety of cells exposed to GPCR agonists or oxidative stress (9, 60, 86, 89, 90, 92). In view of the increasing recognition of the interplay between inflammation and cancer development, a possible role of PKD in linking these processes is of importance. However, the precise molecular mechanisms remain incompletely understood.
Stimulation of human colonic epithelial NCM460 cells with the GPCR agonist and bioactive lipid lysophosphatidic acid (LPA) led to a rapid and striking activation of PKD2, the major isoform of the PKD family expressed by these cells (9). LPA stimulated the production of interleukin 8 (IL-8), a potent pro-inflammatory chemokine, and stimulated NF-κB activation. PKD2 gene silencing dramatically reduced LPA-stimulated NF-κB promoter activity and IL-8 production. These results imply that PKD2 mediates LPA-stimulated IL-8 secretion in NCM460 cells through a NF-κB-dependent pathway. PKD2 has also been implicated in mediating NF-κB activation by Bcr-Abl in myeloid leukemia cells (60). Prostaglandins (e.g. PGE2) produced through COX-2 play a critical role in colon cancer development and colonic myofibroblasts are major contributors to their generation. Recent results demonstrated that knockdown of PKD1 in these cells prevented the synergistic increase in COX-2 expression induced by the pro-inflammatory mediators bradykinin and tumor necrosis factor (TNF)-α (110). Thus, these novel results raise the attractive possibility that PKD plays a critical role in mediating COX-2 expression in response to potent pro-inflammatory mediators in human colonic myofibroblasts.
NF-κB also plays a critical role in inflammatory and cell death responses during acute pancreatitis. The PKC isoforms PKCδ and ε are key regulators of NF-κB activation induced by cholecystokinin-8 (CCK-8), an agonist that induces pancreatitis when administered to rodents at supra-maximal doses. PKD was shown to function downstream of PKCδ and PKCε in pancreatic acinar cells stimulated by CCK-8. Specifically, PKD was necessary for NF-κB activation induced by these GPCR agonists in pancreatic cells (111). These results identify PKD as a novel element in the signaling pathways mediating NF-κB activation in acute pancreatitis. PKD has been also identified as one of the critical factors n the development of hypersensitivity pneumonitis caused by microbial agents (43). Inhibition of PKD1 activation could be an effective way to control acute inflammatory conditions in diverse organs.
Since the original finding that oxidative stress induces PKD activation, partly via PKC-mediated activation loop phosphorylation, and partly through Src-mediated PKD tyrosine phosphorylation (100), a number of reports confirmed that PKD is a sensor of oxidative stress (16, 86, 89-92, 94, 99). Oxidative stress induces PKD1 activation loop phosphorylation on Ser744 and Ser748 leading to catalytic activation (16). A number of studies have shown that PKD1 opposes the apoptotic effects of oxidative stress in a variety of cells (81, 85, 86, 88, 91, 94).
A recent study using pancreatic β cells, demonstrated that stress signals markedly induced TNFAIP3/A20, a zinc finger–containing, immediate early-response gene with potent antiapoptotic and anti-inflammatory functions (50). In fact, A20 is an early NF-κB-responsive gene that encodes a ubiquitin-editing protein that is involved in the negative feedback regulation of NF-κB signaling (11). Interestingly, other studies demonstrated that PKD induces A20 promoter activity (54). It is plausible that PKD initiates not only an inflammatory response via NF-κB but also stimulates expression of the antiapoptotic and anti-inflammatory A20, as a feedback mechanism that protect cells subjected to stress signals, including oxidative stress.
PKD has also been implicated in pain transmission, a typical response during inflammation. The vanilloid receptor type 1 (VR1 or TRPV1) is a vanilloid-gated, nonselective cation channel that belongs to the transient receptor potential (TRP) channel superfamily which is regulated by phosphorylation by several protein kinases, including PKCε and PKD1 (Table 1). Thus, PKD1 is a modulator of VR1 activity which contributes to allodynia and hyperalgesia development.
Cardiac hypertrophy and contraction
Several years after its identification, PKD family members were shown to be expressed and regulated in ventricular myocytes (31). Treatment of these cells with either PMA or an alpha1-adrenergic receptor (AR) agonist induced rapid PKD activation through PKC (31). Subsequent studies demonstrated that PKD is implicated as a mediator of cardiac hypertrophy, a condition associated with elevated risk for the development of heart failure, and clarified the mechanism by which PKD exerts such profound influence in the heart [see (1) for review].
As mentioned above, PKD directly phosphorylates class II HDAC5, an enzyme that suppresses cardiac hypertrophy (98). PKD-mediated phosphorylation of HDCA5 neutralizes its ability to suppress cardiac hypertrophy by triggering nuclear export (93, 98). The A-Kinase Anchoring Protein (AKAP)-Lbc, which is upregulated in hypertrophic cardiomyocytes, has been proposed to couple PKD activation with the phosphorylation-dependent nuclear export of HDAC5 (7). In turn, other studies demonstrated that augmented myocardial PKD activity induces cardiac troponin I (TNNI) phosphorylation at Ser24 (Table 1) and cardiac myosin binding protein C (MYBPC3) phosphorylation at Ser304 (Table 1) which reduces myofilament Ca2+ sensitivity and increases cross-bridge cycle rate, implying that altered PKD activity impacts on contractile function (3, 13). Mice with cardiac-specific deletion of PKD1 were viable and showed diminished hypertrophy, fibrosis, and fetal gene activation as well as improved cardiac function in response to pressure overload or chronic adrenergic or angiotensin II signaling (21).
The cAMP-response element-binding protein (CREB) is phosphorylated on Ser133 by several upstream kinases. PKD has been identified as a CREB-Ser133 kinase (Table 1) that contributes to cardiac remodeling (67). Collectively, these studies indicate that PKD transduces stress stimuli involved in pathological cardiac remodeling and suggest that it could be a novel target in heart disease.
Cancer
Given the widespread role of PKDs in signal transduction, migration, secretion, gene expression, differentiation and proliferation, it is not surprising that PKD signaling has been implicated in a variety of cancer cells, including those originated from the pancreas, liver, gastrointestinal tract, breast, prostate and lung. Recently, two review articles have discussed apparently contrasting roles of the PKD family in cancerous cells from different tissues (26, 49). Consequently, the role of PKD in cancer is not revisited here in detail. However, it is worth noting that in addition of directly promoting cancer cell proliferation, PKD has been implicated in regulating several aspects of the tumor microenvironment, including angiogenesis, inflammation and COX-2 induction. Furthermore, a recent study identified a recurrent mutation of PRKD1 in human breast and colon cancers (42). Consequently, PKD is a potential target for therapy, at least in certain cancers from some tissues, including pancreas and prostate [(26, 45, 49, 107) but see (5) for a different view].
Concluding Remarks
A great deal of progress has been made in understanding the regulatory mechanisms of activation and sub-cellular localization of PKDs and the role of novel PKCs in mediating rapid PKD family phosphorylation at the activation loop. As in other phosphorylation cascades, inducible activation loop phosphorylation provides a mechanism of signal integration and amplification. Interestingly, new results uncovered that the regulation of the activation loop phosphorylation of PKD is more complex than previously thought, with the participation of different mechanism at different times, especially in cells stimulated by Gq-coupled receptor agonists (82).
Recent advances demonstrate an important role of the PKDs in an array of fundamental biological processes, including cell proliferation, motility, polarity, MAP kinase pathways, cardiac hypertrophy, inflammation and cancer (Fig. 2). The involvement of PKDs in mediating such a diverse array of normal and abnormal biological activities in different subcellular compartments is likely to depend on the dynamic changes in their spatial and temporal localization, combined with its distinct substrate specificity (see Table 1). It seems that a variety of biological responses attributed originally to PKCs are in fact executed by PKDs. Selective PKD inhibitors (29, 48, 61) and animal models using PKD transgenics or tissue specific knockout are emerging and will serve to further clarify the function(s) of PKD isoforms in vivo. In this context, it is important to point out that global knockout of PKD1 induces embryonic lethality in mice, with incomplete penetrance (21). Mice deficient in PKD1 enzymatic activity, via homozygous expression of PKD1(S744A/S748A) “knockin” alleles, also induced embryonic lethality (58). These results demonstrated the importance of the phosphorylation of the activation loop residues Ser744 and Ser748 in the regulation of PKD1 in vivo.
In view of the multifunctional roles of PKD, the search for physiological substrates is gathering pace and already a considerable number of interesting molecules have been identified as PKD targets (examples in Table 1). As developed in this article, an emerging theme is that PKD modulates multiple aspects of cell function by altering the sub-cellular localization of its substrates, either interfering with their membrane or nuclear localization, as shown schematically in Fig. 3.
In conclusion, studies on PKD thus far indicate a remarkable diversity of both its signal generation and distribution and its potential for complex regulatory interactions with multiple downstream pathways. It is increasingly apparent that the members of the PKD subfamily are key players in the regulation of cell signaling, organization, migration, inflammation and normal and abnormal cell proliferation. PKD emerges as a valuable target for development of novel therapeutic approaches in common diseases, including cardiac hypertrophy and cancer.
Acknowledgments
The help of Mr. James Sinnett-Smith in the preparation of this article is greatly appreciated. Studies from the laboratory of ER are supported in part by National Institutes of Health Grants R01-DK 55003, R0-1DK56930, R21CA137292 and P30-DK41301. ER holds the Hirshberg Chair of Pancreatic Cancer Research.
Footnotes
- PLC
- phospholipase C
- DAG
- diacylglycerol
- PKC
- protein kinase C
- PKD
- protein kinase D
- PKD2
- protein kinase D2
- PKD3
- protein kinase D3
- CRD
- cysteine-rich domain
- PH
- pleckstrin homology
- GPCR
- G protein-coupled receptor
- CaMKs
- Ca2+/calmodulin-dependent protein kinases
- PDBu
- phorbol 12,13-dibutyrate
- PMA
- phorbol-12-myristate-13-acetate
- siRNA
- small interfering RNA
REFERENCES
- 1.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: emerging roles in health and disease. Circ Res. 2008;102:157–163. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- 2.Azoitei N, Pusapati GV, Kleger A, MÃüller P, Köfer R, Genze F, Wagner M, van Lint J, Carmeliet P, Adler G, Seufferlein T. Protein kinase D2 is a crucial regulator of tumour cellǎ£“endothelial cell communication in gastrointestinal tumours. Gut. 2010;59:1316. doi: 10.1136/gut.2009.206813. [DOI] [PubMed] [Google Scholar]
- 3.Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct Sarcomeric Substrates Are Responsible for Protein Kinase D-mediated Regulation of Cardiac Myofilament Ca2+ Sensitivity and Cross-bridge Cycling. J Biol Chem. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisbal M, Conde C, Donoso M, Bollati F, Sesma J, Quiroga S, Diaz Anel A, Malhotra V, Marzolo MP, Caceres A. Protein kinase d regulates trafficking of dendritic membrane proteins in developing neurons. J Neurosci. 2008;28:9297–9308. doi: 10.1523/JNEUROSCI.1879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas MHU, Du C, Zhang C, Straubhaar J, Languino LR, Balaji KC. Protein Kinase D1 Inhibits Cell Proliferation through Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Secretion in Prostate Cancer. Cancer Res. 2010;70:2095–2104. doi: 10.1158/0008-5472.CAN-09-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera-Poch N, Sanchez-Ruiloba L, Rodriguez-Martinez M, Iglesias T. Lipid raft disruption triggers protein kinase C and Src-dependent protein kinase D activation and Kidins220 phosphorylation in neuronal cells. J Biol Chem. 2004;279:28592–28602. doi: 10.1074/jbc.M312242200. [DOI] [PubMed] [Google Scholar]
- 7.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celil AB, Campbell PG. BMP-2 and Insulin-like Growth Factor-I Mediate Osterix (Osx) Expression in Human Mesenchymal Stem Cells via the MAPK and Protein Kinase D Signaling Pathways. J Biol Chem. 2005;280:31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- 9.Chiu TT, Leung WY, Moyer MP, Strieter RM, Rozengurt E. Protein kinase D2 mediates lysophosphatidic acid-induced interleukin 8 production in nontransformed human colonic epithelial cells through NF-{kappa}B. Am J Physiol Cell Physiol. 2007;292:C767–777. doi: 10.1152/ajpcell.00308.2006. [DOI] [PubMed] [Google Scholar]
- 10.Chow SY, Yu CY, Guy GR. Sprouty2 Interacts with Protein Kinase CÎ′ and Disrupts Phosphorylation of Protein Kinase D1. J Biol Chem. 2009;284:19623–19636. doi: 10.1074/jbc.M109.021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coornaert B, Carpentier I, Beyaert R. A20: Central Gatekeeper in Inflammation and Immunity. J Biol Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowell CF, Doppler H, Yan IK, Hausser A, Umezawa Y, Storz P. Mitochondrial diacylglycerol initiates protein-kinase-D1-mediated ROS signaling. J Cell Sci. 2009;122:919–928. doi: 10.1242/jcs.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, Mayr M, Kentish JC, Avkiran M. Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ Res. 2007;100:864–873. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 14.Czondor K, Ellwanger K, Fuchs YF, Lutz S, Gulyas M, Mansuy IM, Hausser A, Pfizenmaier K, Schlett K. Protein Kinase D Controls the Integrity of Golgi Apparatus and the Maintenance of Dendritic Arborization in Hippocampal Neurons. Mol Biol Cell. 2009;20:2108–2120. doi: 10.1091/mbc.E08-09-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dequiedt F, Van Lint J, Lecomte E, Van Duppen V, Seufferlein T, Vandenheede JR, Wattiez R, Kettmann R. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J Exp Med. 2005;201:793–804. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doppler H, Storz P. A Novel Tyrosine Phosphorylation Site in Protein Kinase D Contributes to Oxidative Stress-mediated Activation. J Biol Chem. 2007;282:31873–31881. doi: 10.1074/jbc.M703584200. [DOI] [PubMed] [Google Scholar]
- 17.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 18.Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11:545–556. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eiseler T, Hausser A, De Kimpe L, Van Lint J, Pfizenmaier K. Protein kinase D controls actin polymerization and cell motility through phosphorylation of cortactin. J Biol Chem. 2010;285:18672–18683. doi: 10.1074/jbc.M109.093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernest Dodd M, Ristich VL, Ray S, Lober RM, Bollag WB. Regulation of Protein Kinase D During Differentiation and Proliferation of Primary Mouse Keratinocytes. J Investig Dermatol. 2005;125:294–306. doi: 10.1111/j.0022-202X.2005.23780.x. [DOI] [PubMed] [Google Scholar]
- 21.Fielitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci U S A. 2008;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y, Ren M, Feng H, Chen L, Altun ZF, Rubin CS. Neuronal and Intestinal Protein Kinase D Isoforms Mediate Na+ (Salt Taste)-Induced Learning. Science Signaling. 2009;2:ra42. doi: 10.1126/scisignal.2000224. [DOI] [PubMed] [Google Scholar]
- 23.Fugmann T, Hausser A, SchÃüffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase Dǎ£“mediated phosphorylation of the ceramide transfer protein. J Cell Biol. 2007;178:15–22. doi: 10.1083/jcb.200612017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghanekar Y, Lowe M. Signalling for secretion. Nat Cell Biol. 2005;7:851–853. doi: 10.1038/ncb0905-851. [DOI] [PubMed] [Google Scholar]
- 25.Guha S, Rey O, Rozengurt E. Neurotensin Induces Protein Kinase C-dependent Protein Kinase D Activation and DNA Synthesis in Human Pancreatic Carcinoma Cell Line PANC-1. Cancer Res. 2002;62:1632–1640. [PubMed] [Google Scholar]
- 26.Guha S, Tanasanvimon S, Sinnett-Smith J, Rozengurt E. Role of protein kinase D signaling in pancreatic cancer. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.07.002. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha CH, Jhun BS, Kao H-Y, Jin Z-G. VEGF Stimulates HDAC7 Phosphorylation and Cytoplasmic Accumulation Modulating Matrix Metalloproteinase Expression and Angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1782–1788. doi: 10.1161/ATVBAHA.108.172528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin Z-G. Protein Kinase D-dependent Phosphorylation and Nuclear Export of Histone Deacetylase 5 Mediates Vascular Endothelial Growth Factor-induced Gene Expression and Angiogenesis. J Biol Chem. 2008;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, Kelland L, Jamieson S, Sutherland R, Raynham T, Charles M, Bagherazadeh A, Foxton C, Boakes A, Farooq M, Maru D, Diagaradjane P, Matsuo Y, Sinnett-Smith J, Gelovani J, Krishnan S, Aggarwal BB, Rozengurt E, Ireson CR, Guha S. A Novel Small-Molecule Inhibitor of Protein Kinase D Blocks Pancreatic Cancer Growth In vitro and In vivo. Mol Cancer Ther. 2010;9:1136–1146. doi: 10.1158/1535-7163.MCT-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase III[beta] at the Golgi complex. Nat Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haworth RS, Goss MW, Rozengurt E, Avkiran M. Expression and activity of protein kinase D/protein kinase C mu in myocardium: Evidence for alpha(1)-adrenergic receptor- and protein kinase C-mediated regulation. J Mol Cell Cardiol. 2000;32:1013–1023. doi: 10.1006/jmcc.2000.1143. [DOI] [PubMed] [Google Scholar]
- 32.Iglesias T, Cabrera-Poch N, Mitchell MP, Naven TJ, Rozengurt E, Schiavo G. Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J Biol Chem. 2000;275:40048–40056. doi: 10.1074/jbc.M005261200. [DOI] [PubMed] [Google Scholar]
- 33.Iglesias T, Rozengurt E. Protein kinase D activation by deletion of its cysteine-rich motifs. FEBS Lett. 1999;454:53–56. doi: 10.1016/s0014-5793(99)00772-3. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias T, Rozengurt E. Protein kinase D activation by mutations within its pleckstrin homology domain. J Biol Chem. 1998;273:410–416. doi: 10.1074/jbc.273.1.410. [DOI] [PubMed] [Google Scholar]
- 35.Irannejad R, Wedegaertner PB. Regulation of Constitutive Cargo Transport from the trans-Golgi Network to Plasma Membrane by Golgi-localized G Protein βγ Subunits. J Biol Chem. 2010;285:32393–32404. doi: 10.1074/jbc.M110.154963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irie A, Harada K, Tsukamoto H, Kim JR, Araki N, Nishimura Y. Protein kinase D2 contributes to either IL-2 promoter regulation or induction of cell death upon TCR stimulation depending on its activity in Jurkat cells. Int Immunol. 2006;18:1737–1747. doi: 10.1093/intimm/dxl108. [DOI] [PubMed] [Google Scholar]
- 37.Ivison SM, Graham NR, Bernales CQ, Kifayet A, Ng N, Shobab LA, Steiner TS. Protein Kinase D Interaction with TLR5 Is Required for Inflammatory Signaling in Response to Bacterial Flagellin. J Immunol. 2007;178:5735–5743. doi: 10.4049/jimmunol.178.9.5735. [DOI] [PubMed] [Google Scholar]
- 38.Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser(744) and Ser(748) phosphorylation. J Biol Chem. 2008;283:12877–12887. doi: 10.1074/jbc.M800442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jadali A, Ghazizadeh S. Protein Kinase D Is Implicated in the Reversible Commitment to Differentiation in Primary Cultures of Mouse Keratinocytes. J Biol Chem. 2010;285:23387–23397. doi: 10.1074/jbc.M110.105619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen ED, Gopalakrishnan R, Westendorf JJ. Bone Morphogenic Protein 2 Activates Protein Kinase D to Regulate Histone Deacetylase 7 Localization and Repression of Runx2. J Biol Chem. 2009;284:2225–2234. doi: 10.1074/jbc.M800586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–6148. [PubMed] [Google Scholar]
- 42.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, Moorhead M, Chaudhuri S, Tomsho LP, Peters BA, Pujara K, Cordes S, Davis DP, Carlton VEH, Yuan W, Li L, Wang W, Eigenbrot C, Kaminker JS, Eberhard DA, Waring P, Schuster SC, Modrusan Z, Zhang Z, Stokoe D, de Sauvage FJ, Faham M, Seshagiri S. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y-I, Park J-E, Brand DD, Fitzpatrick EA, Yi A-K. Protein Kinase D1 Is Essential for the Proinflammatory Response Induced by Hypersensitivity Pneumonitis-Causing Thermophilic Actinomycetes Saccharopolyspora rectivirgula. J Immunol. 2010;184:3145–3156. doi: 10.4049/jimmunol.0903718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kisfalvi K, Guha S, Rozengurt E. Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol. 2005;202:880–890. doi: 10.1002/jcp.20187. [DOI] [PubMed] [Google Scholar]
- 45.Kisfalvi K, Hurd C, Guha S, Rozengurt E. Induced overexpression of protein kinase D1 stimulates mitogenic signaling in human pancreatic carcinoma PANC-1 cells. J Cell Physiol. 2010;223:309–316. doi: 10.1002/jcp.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunkel MT, Garcia EL, Kajimoto T, Hall RA, Newton AC. The Protein Scaffold NHERF-1 Controls the Amplitude and Duration of Localized Protein Kinase D Activity. J Biol Chem. 2009;284:24653. doi: 10.1074/jbc.M109.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunkel MT, Newton AC. Calcium Transduces Plasma Membrane Receptor Signals to Produce Diacylglycerol at Golgi Membranes. J Biol Chem. 285:22748–22752. doi: 10.1074/jbc.C110.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaValle C, Bravo-Altamirano K, Giridhar K, Chen J, Sharlow E, Lazo J, Wipf P, Wang QJ. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol. 2010;10:5. doi: 10.1186/1472-6769-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaValle CR, George KM, Sharlow ER, Lazo JS, Wipf P, Wang QJ. Protein kinase D as a potential new target for cancer therapy. Biochim Biophys Acta - Reviews on Cancer. 2010 doi: 10.1016/j.bbcan.2010.05.003. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to Regulate TNF-Induced NF-kappa B and Cell Death Responses in A20-Deficient Mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemonnier J, Ghayor C, Guicheux J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Chen LA, Townsend CM, Jr., Evers BM. PKD1, PKD2, and Their Substrate Kidins220 Regulate Neurotensin Secretion in the BON Human Endocrine Cell Line. J Biol Chem. 2008;283:2614–2621. doi: 10.1074/jbc.M707513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 54.Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, McShea A, Grey ST. Nuclear Factor-{kappa}B Regulates {beta}-Cell Death: A Critical Role for A20 in {beta}-Cell Protection. Diabetes. 2006;55:2491–2501. doi: 10.2337/db06-0142. [DOI] [PubMed] [Google Scholar]
- 55.Marklund U, Lightfoot K, Cantrell D. Intracellular location and cell context-dependent function of protein kinase D. Immunity. 2003;19:491–501. doi: 10.1016/s1074-7613(03)00260-7. [DOI] [PubMed] [Google Scholar]
- 56.Matthews SA, Iglesias T, Rozengurt E, Cantrell D. Spatial and temporal regulation of protein kinase D (PKD) EMBO J. 2000;19:2935–2945. doi: 10.1093/emboj/19.12.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, Scharenberg AM. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol. 2006;26:1569–1577. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthews SA, Navarro MN, Sinclair LV, Emslie E, Feijoo-Carnero C, Cantrell DA. Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem J. 2010;432:153–163. doi: 10.1042/BJ20101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medeiros RB, Dickey DM, Chung H, Quale AC, Nagarajan LR, Billadeau DD, Shimizu Y. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity. 2005;23:213–226. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Mihailovic T, Marx M, Auer A, Van Lint J, Schmid M, Weber C, Seufferlein T. Protein Kinase D2 Mediates Activation of Nuclear Factor {kappa}B by Bcr-Abl in Bcr-Abl+ Human Myeloid Leukemia Cells. Cancer Res. 2004;64:8939–8944. doi: 10.1158/0008-5472.CAN-04-0981. [DOI] [PubMed] [Google Scholar]
- 61.Monovich L, Vega RB, Meredith E, Miranda K, Rao C, Capparelli M, Lemon DD, Phan D, Koch KA, Chapo JA, Hood DB, McKinsey TA. A novel kinase inhibitor establishes a predominant role for protein kinase D as a cardiac class IIa histone deacetylase kinase. FEBS Lett. 2010;584:631–637. doi: 10.1016/j.febslet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 62.Mottet D, Bellahcene A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V. Histone Deacetylase 7 Silencing Alters Endothelial Cell Migration, a Key Step in Angiogenesis. Circ Res. 2007;101:1237–1246. doi: 10.1161/CIRCRESAHA.107.149377. [DOI] [PubMed] [Google Scholar]
- 63.Mullin MJ, Lightfoot K, Marklund U, Cantrell DA. Differential Requirement for RhoA GTPase Depending on the Cellular Localization of Protein Kinase D. J Biol Chem. 2006;281:25089–25096. doi: 10.1074/jbc.M603591200. [DOI] [PubMed] [Google Scholar]
- 64.Murphy TR, Legere HJ, III, Katz HR. Activation of Protein Kinase D1 in Mast Cells in Response to Innate, Adaptive, and Growth Factor Signals. J Immunol. 2007;179:7876–7882. doi: 10.4049/jimmunol.179.11.7876. [DOI] [PubMed] [Google Scholar]
- 65.Nhek S, Ngo M, Yang X, Ng MM, Field SJ, Asara JM, Ridgway ND, Toker A. Regulation of oxysterol-binding protein golgi localization through protein kinase D-mediated phosphorylation. Mol Biol Cell. 2010;21:2327–2337. doi: 10.1091/mbc.E10-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ochi N, Tanasanvimon S, Matsuo Y, Tong Z, Sung B, Aggarwal BB, Sinnett-Smith J, Rozengurt E, Guha S. Protein kinase D1 promotes anchorage-independent growth, invasion, and angiogenesis by human pancreatic cancer cells. J Cell Physiol. 2010 doi: 10.1002/jcp.22421. (In press) [DOI] [PubMed] [Google Scholar]
- 67.Ozgen N, Obreztchikova M, Guo J, Elouardighi H, Dorn GW, II, Wilson BA, Steinberg SF. Protein Kinase D Links Gq-coupled Receptors to cAMP Response Element-binding Protein (CREB)-Ser133 Phosphorylation in the Heart. J Biol Chem. 2008;283:17009–17019. doi: 10.1074/jbc.M709851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park J-E, Kim Y-I, Yi A-K. Protein Kinase D1 Is Essential for MyD88-Dependent TLR Signaling Pathway. J Immunol. 2009;182:6316–6327. doi: 10.4049/jimmunol.0804239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park J-E, Kim Y-I, Yi A-K. Protein Kinase D1: A New Component in TLR9 Signaling. J Immunol. 2008;181:2044–2055. doi: 10.4049/jimmunol.181.3.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parra M, Kasler H, McKinsey TA, Olson EN, Verdin E. Protein Kinase D1 Phosphorylates HDAC7 and Induces Its Nuclear Export after T-cell Receptor Activation. J Biol Chem. 2005;280:13762–13770. doi: 10.1074/jbc.M413396200. [DOI] [PubMed] [Google Scholar]
- 71.Peterburs P, Heering J, Link G, Pfizenmaier K, Olayioye MA, Hausser A. Protein Kinase D Regulates Cell Migration by Direct Phosphorylation of the Cofilin Phosphatase Slingshot 1 Like. Cancer Res. 2009;69:5634–5638. doi: 10.1158/0008-5472.CAN-09-0718. [DOI] [PubMed] [Google Scholar]
- 72.Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Rey O, Papazyan R, Waldron RT, Young SH, Lippincott-Schwartz J, Jacamo R, Rozengurt E. The nuclear import of protein kinase D3 requires its catalytic activity. J Biol Chem. 2006;281:5149–5157. doi: 10.1074/jbc.M508014200. [DOI] [PubMed] [Google Scholar]
- 74.Rey O, Sinnett-Smith J, Zhukova E, Rozengurt E. Regulated nucleocytoplasmic transport of protein kinase D in response to G protein-coupled receptor activation. J Biol Chem. 2001;276:49228–49235. doi: 10.1074/jbc.M109395200. [DOI] [PubMed] [Google Scholar]
- 75.Rey O, Young SH, Cantrell D, Rozengurt E. Rapid Protein Kinase D Translocation in Response to G Protein-coupled Receptor Activation. Dependence on Protein Kinase C. J Biol Chem. 2001;276:32616–32626. doi: 10.1074/jbc.M101649200. [DOI] [PubMed] [Google Scholar]
- 76.Romero DG, Welsh BL, Gomez-Sanchez EP, Yanes LL, Rilli S, Gomez-Sanchez CE. Angiotensin II-Mediated Protein Kinase D Activation Stimulates Aldosterone and Cortisol Secretion in H295R Human Adrenocortical Cells. Endocrinology. 2006;147:6046–6055. doi: 10.1210/en.2006-0794. [DOI] [PubMed] [Google Scholar]
- 77.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 78.Rozengurt E, Rey O, Waldron RT. Protein Kinase D Signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 79.Saini DK, Karunarathne WKA, Angaswamy N, Saini D, Cho J-H, Kalyanaraman V, Gautam N. Regulation of Golgi structure and secretion by receptor-induced G protein beta-gama complex translocation. Proc Natl Acad Sci U S A. 2010;107:11417–11422. doi: 10.1073/pnas.1003042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scholz R-P, Regner J, Theil A, Erlmann P, Holeiter G, Jahne R, Schmid S, Hausser A, Olayioye MA. DLC1 interacts with 14-3-3 proteins to inhibit RhoGAP activity and block nucleocytoplasmic shuttling. J Cell Sci. 2009;122:92–102. doi: 10.1242/jcs.036251. [DOI] [PubMed] [Google Scholar]
- 81.Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007;22(Suppl 1):S45–48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- 82.Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, Waldron RT, Rozengurt E. Protein Kinase D Mediates Mitogenic Signaling by Gq-coupled Receptors through Protein Kinase C-independent Regulation of Activation Loop Ser744 and Ser748 Phosphorylation. J Biol Chem. 2009;284:13434–13445. doi: 10.1074/jbc.M806554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in swiss 3T3 cells. J Biol Chem. 2004;279:16883–16893. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- 84.Sinnett-Smith J, Zhukova E, Rey O, Rozengurt E. Protein kinase D2 potentiates MEK/ERK/RSK signaling, c-Fos accumulation and DNA synthesis induced by bombesin in Swiss 3T3 cells. J Cell Physiol. 2007;211:781–790. doi: 10.1002/jcp.20984. [DOI] [PubMed] [Google Scholar]
- 85.Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-{delta} pathway activation. Am J Physiol Cell Physiol. 2006;290:C1469–1476. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song J, Li J, Qiao J, Jain S, Mark Evers B, Chung DH. PKD prevents H2O2-induced apoptosis via NF-[kappa]B and p38 MAPK in RIE-1 cells. Biochem Biophys Res Commun. 2009;378:610. doi: 10.1016/j.bbrc.2008.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Storz P. Mitochondrial ROS - radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Storz P, Doppler H, Toker A. Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor kappaB. Mol Pharmacol. 2004;66:870–879. doi: 10.1124/mol.104.000687. [DOI] [PubMed] [Google Scholar]
- 90.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Storz P, Doppler H, Toker A. Protein Kinase D Mediates Mitochondrion-to-Nucleus Signaling and Detoxification from Mitochondrial Reactive Oxygen Species. Mol Cell Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sucharov CC, Langer S, Bristow M, Leinwand L. Shuttling of HDAC5 in H9C2 cells regulates YY1 function through CaMKIV/PKD and PP2A. Am J Physiol Cell Physiol. 2006;291:C1029–1037. doi: 10.1152/ajpcell.00059.2006. [DOI] [PubMed] [Google Scholar]
- 94.Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, Ramracheya R, Caille D, Jiang H, Platt KA, Meda P, Aebersold R, Rorsman P, Ricci R. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urbich C, Rossig L, Kaluza D, Potente M, Boeckel J-N, Knau A, Diehl F, Geng J-G, Hofmann W-K, Zeiher AM, Dimmeler S. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113:5669–5679. doi: 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- 96.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Lint JV, Sinnett-Smith J, Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J Biol Chem. 1995;270:1455–1461. doi: 10.1074/jbc.270.3.1455. [DOI] [PubMed] [Google Scholar]
- 98.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem. 2004;279:27482–27493. doi: 10.1074/jbc.M402875200. [DOI] [PubMed] [Google Scholar]
- 100.Waldron RT, Rozengurt E. Oxidative stress induces protein kinase D activation in intact cells - Involvement of Src and dependence on protein kinase C. J Biol Chem. 2000;275:17114–17121. doi: 10.1074/jbc.M908959199. [DOI] [PubMed] [Google Scholar]
- 101.Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem. 2003;278:154–163. doi: 10.1074/jbc.M208075200. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–7743. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Waldron RT, Dhaka A, Patel A, Riley MM, Rozengurt E, Colicelli J. The RAS Effector RIN1 Directly Competes with RAF and Is Regulated by 14-3-3 Proteins. Mol Cell Biol. 2002;22:916–926. doi: 10.1128/MCB.22.3.916-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watkins JL, Lewandowski KT, Meek SE, Storz P, Toker A, Piwnica-Worms H. Phosphorylation of the Par-1 polarity kinase by protein kinase D regulates 14-3-3 binding and membrane association. Proc Natl Acad Sci U S A. 2008;105:18378–18383. doi: 10.1073/pnas.0809661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong C, Jin Z-G. Protein Kinase C-dependent Protein Kinase D Activation Modulates ERK Signal Pathway and Endothelial Cell Proliferation by Vascular Endothelial Growth Factor. J Biol Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmicro promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 108.Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin DM, Huang YH, Zhu YB, Wang Y. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J Neurosci. 2008;28:8832–8843. doi: 10.1523/JNEUROSCI.1291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoo J, Chung C, Slice L, Sinnett-Smith J, Rozengurt E. Protein kinase D mediates synergistic expression of COX-2 induced by TNF-{alpha} and bradykinin in human colonic myofibroblasts. Am J Physiol Cell Physiol. 2009;297:C1576–1587. doi: 10.1152/ajpcell.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, Pandol SJ. Protein kinase D1 mediates NF-kappaB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1190–1201. doi: 10.1152/ajpgi.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuan J, Slice LW, Gu J, Rozengurt E. Cooperation of Gq, Gi, and G12/13 in protein kinase D activation and phosphorylation induced by lysophosphatidic acid. J Biol Chem. 2003;278:4882–4891. doi: 10.1074/jbc.M211175200. [DOI] [PubMed] [Google Scholar]
- 113.Yuan J, Slice LW, Rozengurt E. Activation of Protein Kinase D by Signaling through Rho and the alpha Subunit of the Heterotrimeric G Protein G13. J Biol Chem. 2001;276:38619–38627. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- 114.Yuan JZ, Slice L, Walsh JH, Rozengurt E. Activation of protein kinase D by signaling through the alpha subunit of the heterotrimeric G protein G(q) J Biol Chem. 2000;275:2157–2164. doi: 10.1074/jbc.275.3.2157. [DOI] [PubMed] [Google Scholar]
- 115.Zhukova E, Sinnett-Smith J, Rozengurt E. Protein Kinase D Potentiates DNA Synthesis and Cell Proliferation Induced by Bombesin, Vasopressin, or Phorbol Esters in Swiss 3T3 Cells. J Biol Chem. 2001;276:40298–40305. doi: 10.1074/jbc.M106512200. [DOI] [PubMed] [Google Scholar]
- 116.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 1996;15:6220–6230. [PMC free article] [PubMed] [Google Scholar]
- 117.Zugaza JL, Waldron RT, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, endothelin, bradykinin, and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. J Biol Chem. 1997;272:23952–23960. doi: 10.1074/jbc.272.38.23952. [DOI] [PubMed] [Google Scholar]