Abstract
Outflow tract (OFT) malformation accounts for ∼30% of human congenital heart defects and manifests frequently in TBX1 haplo-insufficiency associated DiGeorge (22q11.2 deletion) syndrome. OFT myocardium originates from second heart field (SHF) progenitors in the pharyngeal and splanchnic mesoderm (SpM), but how these progenitors are deployed to the OFT is unclear. We find that SHF progenitors in the SpM gradually gain epithelial character and are deployed to the OFT as a cohesive sheet. Wnt5a, a non-canonical Wnt, is expressed specifically in the caudal SpM and may regulate oriented cell intercalation to incorporate SHF progenitors into an epithelial-like sheet, thereby generating the pushing force to deploy SHF cells rostrally into the OFT. Using enhancer trap and Cre transgenes, our lineage tracing experiments show that in Wnt5a null mice, SHF progenitors are trapped in the SpM and fail to be deployed to the OFT efficiently, resulting in a reduction in the inferior OFT myocardial wall and its derivative, subpulmonary myocardium. Concomitantly, the superior OFT and subaortic myocardium are expanded. Finally, in chick embryos, blocking the Wnt5a function in the caudal SpM perturbs polarized elongation of SHF progenitors, and compromises their deployment to the OFT. Collectively, our results highlight a critical role for Wnt5a in deploying SHF progenitors from the SpM to the OFT. Given that Wnt5a is a putative transcriptional target of Tbx1, and the similar reduction of subpulmonary myocardium in Tbx1 mutant mice, our results suggest that perturbing Wnt5a-mediated SHF deployment may be an important pathogenic mechanism contributing to OFT malformations in DiGeorge syndrome.
Introduction
Malformation of the outflow tract (OFT), which gives rise to the myocardium at the base of the ascending aorta and pulmonary artery, occurs in approximately one-third of all congenital heart defects observed in humans (1) and is a frequent symptom in complex genetic disorders such as the TBX1 haploinsufficiency associated DiGeorge (22q11.2 deletion) syndrome (DGS) (2). Therefore, understanding the developmental mechanisms involved in OFT formation is essential for designing diagnostic and therapeutic approaches for OFT-related cardiac defects in humans.
The OFT is initially present as a single vessel between the aortic sac and the right ventricle, and the myocardium in the OFT arises from the recruitment of mesodermal progenitors located in an extra-cardiac region known as the second heart field (SHF). The SHF extends from the rostral pharyngeal mesoderm to the caudal splanchnic mesoderm (SpM), and was identified by the expression of several genes and the contribution of cells expressing these genes to the heart (3–12). Additional mouse genetic analyses have demonstrated that SHF progenitors in the pharyngeal and SpM are prefigured to give rise to distinct myocardial populations that occupy initially the superior and inferior wall of the OFT, and later the base of the aorta and the pulmonary artery, respectively (13–16).
Cardiac progenitor cells in the SHF are maintained in a finely balanced state of proliferation and differentiation and are progressively deployed to the OFT to bring about its elongation. Maximal OFT elongation is essential to complete cardiac looping, allowing the OFT to align properly over the inter-ventricular septum. Consequently, upon cardiac neural crest (CNC) cell invasion, the OFT is divided into the aorta and the pulmonary artery that can establish their appropriate connections with the left and right ventricles (4,6). Disrupting any of the early events during OFT development can perturb its septation and/or remodeling, resulting in a spectrum of OFT defects such as persistent truncus arteriosus (PTA), a septation defect or various forms of alignment/remodeling defects including double outlet right ventricle (DORV), overriding aorta and transposition of the great arteries (4,6,17–19). While numerous studies have defined the signaling and transcriptional network involved in regulating SHF proliferation and differentiation (20–30), the cellular and molecular mechanisms underlying SHF deployment are largely unknown.
Here, we present genetic and experimental evidence that the presumptive planar cell polarity (PCP) ligand, Wnt5a, is critically required for SHF deployment. The PCP pathway, a branch of the β-catenin independent non-canonical Wnt signaling pathway, is an evolutionarily conserved mechanism that regulates cellular polarity and directional tissue morphogenesis during convergence and extension (CE). PCP signaling in vertebrates is postulated to initiate through the interaction of non-canonical Wnt ligands, such as Wnt5a and Wnt11, with specific transmembrane receptors including Frizzled (Fz) and Ror2. The signaling is then transduced through a set of core PCP proteins such as Vangl2 and Disheveled (Dvl), and context-specific effectors such as Daam1 (31). In Xenopus and zebrafish, PCP signaling has been shown to play a critical role in CE-mediated axial elongation by regulating medio-laterally oriented intercalation and directional migration of mesodermal cells (32–35). In mice and humans, disruption of non-canonical Wnt/PCP signaling has been linked to disruption of epithelial cell polarity (36–39), failure of neural tube closure (38,40–45) and skeletal defects (46–52), underscoring the significance of PCP signaling in mammalian development and human diseases.
Loss of function mutations in several non-canonical Wnt/core PCP genes also result in a spectrum of OFT malformations in mice, from DORV to PTA (53–56). Our recent studies revealed that Dvl-mediated PCP signaling is required specifically in the SHF lineage for OFT morphogenesis (57). Furthermore, we found that both Wnt5a and core PCP genes Dvl1/2 and Vangl2 were required for early OFT lengthening, and that Wn5a genetically interacted with core PCP genes during OFT lengthening, suggesting a critical role of Wnt5a-initiated PCP signaling for efficient contribution of SHF cells to the OFT. Finally, Wnt5a transcripts and Dvl2 were strongly co-expressed in SHF progenitors in the caudal SpM. SHF progenitors in the caudal SpM of Dvl1−/−; Dvl2−/− and Wnt5a−/−mutants had no cell proliferation or apoptosis defects, but lacked the normal protrusive morphology and displayed defective actin organization and filopodia formation. Unlike wild-type cells, which were organized into a cohesive sheet, Wnt5a and Dvl1/2 mutant cells aggregated into compact clusters. These observations lead us to propose a novel model in which Wnt5a, acting through PCP, induces oriented cell intercalation to continuously recruit SHF progenitors into a cohesive sheet at the caudal end, thereby providing the driving force to deploy SHF cells rostrally into the OFT (57).
Interestingly, transcriptional activation of Wnt5a in the caudal SpM has recently been shown to require Tbx1, the candidate gene in DGS (58). DGS is the most common genomic micro-deletion syndrome in humans and is associated with haploinsufficiency of the TBX1 locus (11,59–63). OFT malformations occur at high rate and in a highly variable fashion in DGS patients who harbor a similar 3 Mb deletion, suggesting that genetic variations in other loci can significantly influence the OFT phenotype induced by the deletion (2,64). Tbx1 deletion in mice recapitulates the OFT defects in DGS patients, and previous studies indicate that Tbx1 sustains proliferation and suppresses differentiation of SHF progenitors, possibly through multiple Fgf genes (11,65–67). In contrast, a more recent study reveals that the loss of Tbx1 in mice also specifically diminishes Wnt5a expression in SHF progenitors in the caudal SpM (58). In conjunction with our prior studies (57), this finding implies that Tbx1 may have an additional role in promoting Wnt5a-mediated morphogenetic processes in the SHF, and that genetic variations in WNT5A and/or its downstream PCP components may act as genetic modifiers for OFT abnormalities in DGS.
Herein, we use mouse genetics and experimental manipulation in chick to understand how Wnt5a contributes to OFT development and demonstrate a critical role of Wnt5a in regulating polarized cell behavior in SHF progenitors to promote their efficient deployment to the OFT. Our results may provide novel insight into the etiology, diagnosis and therapeutic approaches for cardiac defects in DGS.
Results
SHF cells in the SpM gain epithelial character and are deployed to the OFT as a cohesive sheet
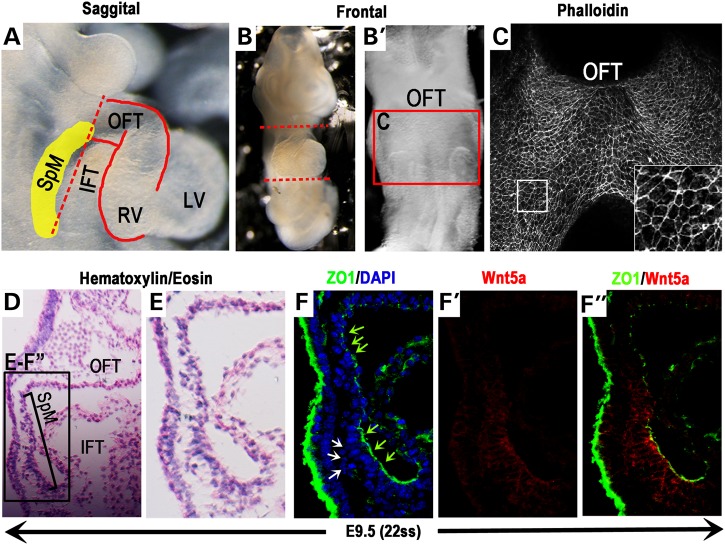
To gain insight into the morphogenetic processes that could drive the deployment of SHF progenitors to the OFT, we first investigated their organization in the SpM (yellow shaded area in Fig. 1A). On the ventral view, confocal scan of whole-mount phalloidin-stained E9.5 mouse SpM reveals that SHF cells in this region are organized as an epithelial-like sheet, in which individual cells display polygonal morphology and are tightly packed together with enriched F-actin around their apical cortex (Fig. 1B and C).
Figure 1.
SHF cells in the SpM gain epithelial character and display caudally restricted Wnt5a expression. To analyze the cellular character of SHF cells located in the SpM (yellow shaded area in A), hearts of E9.5 mouse embryos (∼22 somite stage) were removed along the dotted lines (A & B). The SpM region behind the heart tube (red box in B′) was stained with phalloidin and imaged ventrally (C). Inset in C shows magnified view of the boxed area. (D) H&E stained sagittal section of E9.5 embryo and enlarged view (black box in D) shown in (E). Immunostaining of adjacent sections shows that SHF progenitors in this region are organized as an epithelial-like sheet with tight junction marker ZO1 localized at their apical cell–cell junctions (F, green arrows). At the very caudal end of the SpM nearing the IFT, however, ZO1 expression is missing in groups of loosely packed, multi-layered SHF cells behind this epithelial sheet (white arrows in F). (F′ & F″) Co-immunostaining with an anti-Wnt5a antibody shows that Wnt5a protein is distributed in a highly restricted fashion in the caudal SpM. IFT, inflow tract; OFT, outflow tract; SpM, splanchnic mesoderm.
Given that SHF cells arise from the mesodermal lineage, their epithelial-like organization in the SpM is intriguing. To further examine this finding, we performed immunostaining for Zonula Occludens 1 (ZO1), a protein involved in tight junction formation in epithelial tissues (68,69). In E9.5 sagittal sections (Fig. 1D), ZO1 is localized specifically at the apical cell–cell junctions in the SpM between the OFT and IFT (inflow tract) (green arrows in Fig. 1F), indicating that SHF progenitors in this region are indeed organized as an epithelial-like sheet with characteristic tight junction formation. Interestingly, however, at the caudal end of the SpM nearing the IFT, ZO1 expression is missing in groups of loosely packed, multi-layered, mesenchymal-like SHF cells behind this epithelial sheet (white arrows in Fig. 1F). The fact that these ZO1 negative SHF cells are only found behind the epithelial sheet in the caudal SpM region suggests to us that they may undergo a mesenchymal-to-epithelial like transition to become incorporated into an epithelial-like sheet, and subsequently up-regulate ZO1 expression.
Our studies in mice are also consistent with previous histological studies in chick embryos, which have described SHF cells in the rostral SpM as ‘a pseudo-stratified columnar layer of epithelium’ (70). Together, these data on cellular organization support the idea that SHF cells in the SpM are deployed to the OFT not as individual, actively migrating cells, but instead as a cohesive, epithelial-like sheet.
The non-canonical Wnt ligand Wnt5a is distributed specifically in SHF cells in the caudal SpM
Interestingly, our immunostaining reveals that the presumptive PCP ligand, Wnt5a, is distributed specifically in the caudal SpM (Fig. 1F′) where loosely packed, ZO1 negative SHF progenitors are found, but not in the rostral SpM where SHF cells are present as a single epithelial-like sheet (Fig. 1F′). This caudally restricted expression of Wnt5a protein closely mimics that of Wnt5a mRNA (57), and suggests to us that Wnt5a may act in an auto- or paracrine fashion to promote CE-like, oriented intercalation of the loosely packed multi-layered SHF cells into a cohesive epithelial sheet in the caudal SpM. We hypothesize that such continuous intercalation event may generate the driving force to push the SpM rostrally into the OFT to promote OFT elongation. This model also provides a parsimonious explanation for how SHF cells can be deployed from the SpM to the OFT as a cohesive, epithelial sheet.
SHF progenitors are trapped in the SpM of Wnt5a−/− mutants
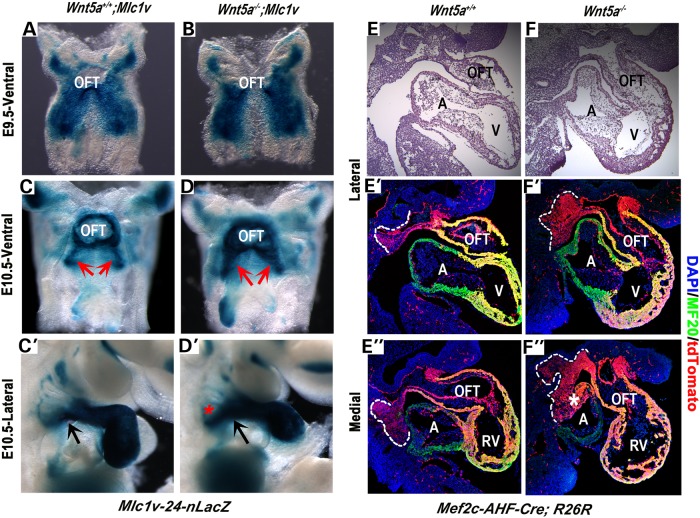
Based on the above model, we predicted that abolishing Wnt5a function would perturb the caudal intercalation of SHF progenitors and subsequently prevent the efficient deployment of SHF cells from the SpM to the OFT. To test this hypothesis, we employed the SHF-specific enhancer trap transgene Mlc1v-nLacZ-24 (Mlc1v) to monitor SHF cell deployment. Mlc1v harbors a nuclear LacZ (nLacZ) reporter gene integrated at the Fgf10 locus (5). In Mlc1v embryos, LacZ expression is found initially in SHF progenitors in the SpM at E8.5 and E9.5 (5; Fig. 2A). At E10.5, however, LacZ expressing cells are no longer present in the SpM, but instead are found in the SHF derived OFT and right ventricle (5; Fig. 2C and C′), indicating that SHF progenitors from the SpM have been recruited to the OFT (5). Therefore, by examining the spatio-temporal pattern of LacZ expression in Mlc1v, we could assess how efficiently SHF cells could be deployed out of the SpM in embryos lacking Wnt5a.
Figure 2.
SHF progenitors are trapped in the SpM of Wnt5a−/− mutants. (A and B) X-gal staining of E9.5 (22–24 somite stage) wild-type and Wnt5a−/− embryos carrying the Mlc1v enhancer trap transgene reveals that SHF progenitors in the SpM are present in a similar bilateral pattern along the dorsal pericardial wall. (C–D′) At E10.5 (35–36 somite stage), X-gal staining shows increased accumulation of Mlc1v-expressing SHF cells in the SpM of Wnt5a−/− mutants on both ventral (compare LacZ expression region indicated by arrows between C and D) and lateral views (compare C′ and D′; arrows indicate the point of connection of the OFT to the body wall; asterisk in D′ indicates expanded LacZ expression area in the SpM). (E–F″) Lineage tracing with Mef2c-AHF-Cre and the R26R-tdTomato Cre reporter reveals an increase of tdTomato-positive SHF cells (red) are retained in the SpM of Wnt5a−/−mutants at E10.5 (35–36 somite stage). (E and F) H&E stained sagittal sections. (E′–F′) Fluorescent images of adjacent sections. Red signals represent Mef2c-Cre lineage expressing tdTomato [outlined with white-dotted lines in the lateral (E′ and F′) and medial (E″ and F″) portion of the SpM]; green signals represent MF20-postive myocardial cells in the heart. White asterisk in F″ denotes auto fluorescence caused by aberrant blood pooling in the atrium of Wnt5a−/− mutant. A, atrium; OFT, outflow tract; RV, right ventricle.
At E9.5, LacZ expressing SHF progenitors are present in the SpM as bilateral streams in both control and Wnt5a−/−; Mlc1v embryos with no apparent differences (Fig. 2A and B), indicating that SHF specification is not perturbed in Wnt5a null mutants. By E10.5, after SHF progenitors have been recruited into the OFT, very few LacZ expressing cells can be detected in the SpM of control embryos (Fig. 2C and C′). In contrast, significantly increased X-gal staining is observed in the SpM of Wnt5a−/−;Mlc1v embryos, suggesting aberrant accumulation of SHF progenitors in the SpM (Fig. 2D and D′). Interestingly, prior analyses of Mlc1v expression in Tbx1−/− mutants have demonstrated altered accumulation of SHF progenitors in the SpM (71), consistent with the idea that Tbx1 activates Wnt5a expression to promote SHF deployment.
To ascertain that the increased accumulation of LacZ positive SHF cells in the SpM of Wnt5a−/−; Mlc1v mice was due to compromised deployment rather than altered expression of Mlc1v, we performed additional genetic lineage analyses using the SHF-specific Cre transgene Mef2c-AHF-Cre (9). Mef2c-AHF-Cre is known to be expressed in the SpM that harbors SHF progenitors (9). When crossed with Cre reporters such as R26R-tdTomato (72), all the Mef2c-AHF-Cre expressing cells and their descendents can be permanently marked and visualized by tdTomato expression. In E10.5 control embryos, sagittal sections reveal that while the entire SHF-derived OFT and right ventricle are tdTomato positive, only a small number of tdTomato expressing SHF progenitors are present in the SpM (outlined with white dotted lines in Fig. 2E′ and E″), indicating that the majority of the SHF cells have been deployed to the OFT. In contrast, in Wnt5a−/−mutants, a greater number of tdTomato expressing SHF cells are present in the SpM when compared with the controls (compare the area outlined by white-dotted lines in Fig. 2F′ and F″ to that in Fig. 2E′ and E″).
As a third approach to investigate whether loss of Wnt5a results in increased accumulation of SHF cells in the SpM, we assessed the expression of SHF progenitor marker Islet1 (3,29). Immunostaining of sagittal sections revealed a significant increase of Islet1 positive cells in the SpM of E10.5 Wnt5a−/−mutants (Supplementary Material, Fig. S1).
Collectively, our analyses with transgene expression, lineage tracing and the SHF marker Islet1 consistently demonstrate an abnormal accretion of SHF progenitors in the SpM of Wnt5a−/− embryos. Importantly, this abnormality cannot be attributed to increased cell survival or turnover since both apoptosis and proliferation are unaltered in the SpM of Wnt5a−/− embryos (57; data not shown). Therefore, these results are more consistent the idea that Wnt5a is required for efficient deployment of SHF progenitors from the SpM to the OFT.
The SpM derived inferior OFT myocardium and subpulmonary myocardium are reduced in Wnt5a−/−mutants
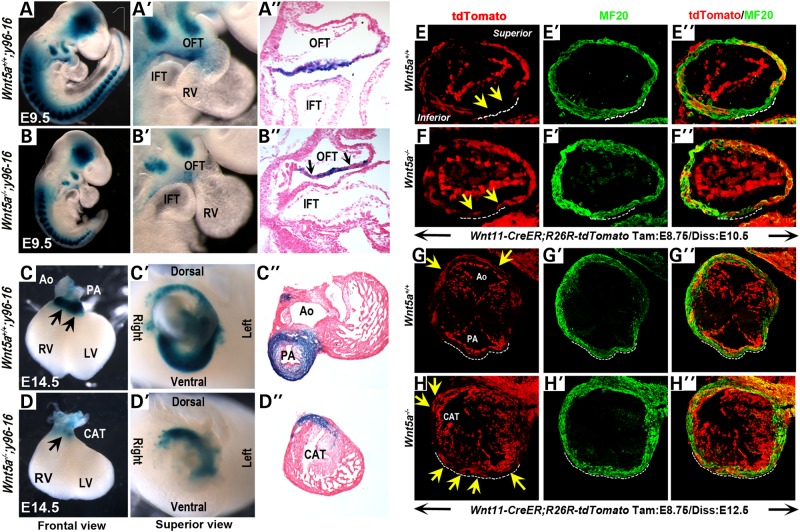
To further establish a role of Wnt5a in the deployment of SHF cells, we directly assessed how loss of Wnt5a might affect the contribution of SHF progenitors in the SpM to the OFT. Analyses of the y96-myf5–16-nLacZ (y96-16) enhancer trap line have revealed that a sub-population of SHF progenitors in the SpM give rise specifically to the inferior myocardial wall of the OFT. In embryos carrying y96-16, which harbors an nLacZ cassette inserted close to the Semaphorin 3c (Sema3c) locus, LacZ expressing cells are observed initially at E9.5 in the inferior OFT myocardium, contiguous with the SpM (14; Fig. 3A–A″). Subsequently, upon OFT rotation and morphogenesis, LacZ positive cells are found to occupy the subpulmonary myocardium at E12.5–14.5 (14; Fig. 3C–C″). Therefore, by analyzing y96-16 transgene expression in Wnt5a−/− embryos at different stages, we can assess specifically the contribution of the SHF progenitors from the SpM to the OFT.
Figure 3.
Wnt5a−/− mutants display reduced inferior OFT myocardium and ectopically extended superior OFT myocardium. X-gal staining and histological sections of E9.5 (22–24 somite stage) Wnt5a+/+;y96-16 embryos show that y96-16 expressing cells are normally present as a contiguous layer in the inferior OFT myocardial wall (A–A″). In contrast, in Wnt5a−/− mutants y96-16 expressing cells are significantly reduced in number (B–B″) and present as small patches intermingled with LacZ negative cells (black arrows, B″). At E14.5, LacZ positive cells are present mainly in the subpulmonary myocardium of Wnt5a+/+;y96-16 embryos (arrows, frontal view in C; superior view in C′; and section analysis in C″). In Wnt5a−/−;y96-16 hearts, however, few LacZ expressing cells are observed in the ventral region at the base of the OFT (arrow, frontal view in D; superior view in D′; and section analysis in D″), and only a few LacZ expressing cells are present in the dorsal region of the OFT (D′, D″). (E–H″) Lineage tracing with the Wnt11-CreER BAC transgene and R26R-tdTomato Cre reporter. When Cre is activated by a single administration of tamoxifen at E8.75, control littermates harvested at E10.5 (35–36 somite stage) show tdTomato labeled cells (red) mainly present in the superior and lateral wall of the OFT myocardium (in addition to the entire endocardium; myocardial cells are identified by MF20 immunostaining), and excluded from the inferior myocardial wall of the OFT (yellow arrows and white dotted lines in E-E″). Subsequently at E12.5, these tdTomato-labeled cells occupy the dorsal subaortic myocardium (arrows in G) and are absent from the ventral subpulmonary myocardium (white-dotted line in G-G″). In Wnt5a−/− mutant littermates, however, a few tdTomato expressing cells are ectopically present in the inferior OFT myocardium at E10.5 (arrows in F) and in the myocardium around the entire base of the OFT at E12.5 (arrows in H). In all embryos in (E)–(H), tdTomato expression is also observed in endocardial cells, which are negative for the myocardial marker MF20 (green). OFT, outflow tract; IFT, inflow tract; RV, right ventricle; LV, left ventricle; Ao, aorta; PA, pulmonary artery; CAT, common arterial trunk.
We found significant reduction of LacZ expressing cells in the OFT of Wnt5a−/−; y96-16 mutants by E9.5 (compare Fig. 3A–A′ to Fig. 3B–B′). On sagittal sections, the LacZ expressing cells in control embryos are present in a continuous layer in the inferior myocardial wall of the OFT (Fig. 3A″). In Wnt5a−/−; y96-16 mutants, however, there are fewer LacZ expressing cells in the inferior OFT, and they are not present in a continuous layer but instead are in small patches intermingled with LacZ negative cells (black arrows, Fig. 3B″). This observation supports the idea that the deployment of SHF progenitors from the SpM is reduced in Wnt5a−/− mutants, resulting in fewer y96-16 expressing SHF cells contributing to the inferior wall of the OFT.
To examine the developmental consequence of this initial reduction in the inferior OFT myocardial population, we performed X-gal staining on E14.5 hearts. On frontal (Fig. 3C) and superior (Fig. 3C′) views, control hearts show predominant LacZ expression around the base of the pulmonary artery, and transverse sections reveal that the LacZ expressing cells mainly occupy the subpulmonary myocardium (Fig. 3C″). In contrast, in Wnt5a−/−; y96-16 mutant hearts, LacZ expression is strikingly absent from the base of the OFT on frontal views (Fig. 3D). Superior views and sections of Wnt5a−/−; y96-16 mutant hearts reveal that while a few LacZ expressing cells are present in the dorsal and lateral regions of the OFT, they are completely absent from the ventral region (Fig. 3D′ and D″). This pattern of X-gal staining in Wnt5a−/−; y96-16 is remarkably similar to that in Tbx1−/−; y96-16 mutants (Supplementary Material, Fig. S2; 14), indicating that Wnt5a−/− mutants closely phenocopy the OFT morphogenesis defect in Tbx1−/− mutants and implying that similar pathogenic processes may underlie the OFT defects in the two mutants.
The superior OFT myocardium extends ectopically into the inferior OFT myocardial wall in Wnt5a−/−mutants
Studies using different enhancer trap lines and Cre transgenes have shown that the superior and inferior myocardial walls of E9.5 OFT are predetermined to give rise to the subaortic and the subpulmonary myocardium, respectively (13,15). Since the inferior OFT and the subpulmonary myocardium were significantly reduced and mal-positioned in Wnt5a−/− hearts, we next sought to determine whether the superior OFT and the subaortic myocardial regions were also affected in these mutants. To this end, we performed lineage tracing with a Wnt11-CreER BAC transgenic line (manuscript in press, TS&JW) which expresses tamoxifen-inducible Cre under the Wnt11 promoter.
We crossed Wnt11-CreER transgenic mice with R26R-tdTomato Cre reporter mice and induced Cre activity by a single administration of tamoxifen at E8.75. When control littermates from tamoxifen-injected pregnant dams are harvested at E10.5, tdTomato expressing cells are observed mainly in the superior and lateral wall of the OFT myocardium (in addition to the entire endocardium) (Fig. 3E–E″), and are specifically excluded from the inferior myocardial wall of the OFT (outlined with white-dotted lines in Fig. 3E–E″). Subsequently, when these embryos are harvested at E12.5, tdTomato expressing cells are largely present around dorsal subaortic myocardium (yellow arrows in Fig. 3G), but distinctly absent from the ventral subpulmonary myocardium (outlined with white-dotted lines in Fig. 3G–G″). Therefore, the contribution of Wnt11-CreER lineage to the OFT is complementary to that of the y96-16 lineage (Fig. 3A–D; 13).
In contrast, in E10.5 Wnt5a−/−; Wnt11-CreER;R26R-tdTomato mutant littermates derived from E8.75 tamoxifen-induced dams, some tdTomato expressing cells are present ectopically in the inferior OFT myocardial wall (arrows in Fig. 3F–F″). Further, at E12.5, Wnt5a−/−; Wnt11-CreER; R26R-tdTomato mutant littermates display abnormal tdTomato expression in both the dorsal and ventral myocardium around the common arterial trunk (arrows in Fig. 3H–H″). These data suggest that in Wnt5a−/− mutants, owing to a reduced SHF contribution to form the inferior OFT (Fig. 3B′ and D′), the myocardial cells that normally occupy only the superior OFT extend ectopically to populate the inferior region, leading to an OFT that no longer possesses a distinct subpulmonary myocardial character.
Interestingly, analyses with the Myf5-nLacZ-A17-T55 (T55) enhancer trap line, which displays a specific contribution to the superior OFT myocardium similar to our Wnt11-CreER, reveal that in Tbx1−/−mutant hearts the superior OFT myocardium is also extended ectopically and populates the presumptive inferior region of the OFT (14). In light of current our current data, it seems very likely that the conotruncal abnormalities in Wnt5a−/− and Tbx1−/− mutant may originate from similar developmental defects in OFT morphogenesis.
SHF progenitors in the caudal SpM are deployed to the inferior OFT in a Wnt5a-dependent fashion in the chick
Our mouse genetic studies with the four different enhancer trap and Cre transgenes consistently demonstrate that a subpopulation of SHF progenitors are trapped in the SpM and fail to be deployed to the OFT efficiently in Wnt5a−/− mutants. However, since these assays all rely on transgene or Cre reporter expression to label and trace cell lineages, we wished to confirm these findings using a different labeling method. To this end, we extended our studies to chick embryos in which a small cohort of cells can be physically labeled with the vital dye DiI and traced in ex-ovo cultures (73). Secondly, while the strong and restricted expression of Wnt5a in the caudal SpM implies that it acts in an auto- or paracrine fashion in this region to promote SHF deployment, our analyses in Wnt5a null mice cannot exclude the possibility that Wnt5a expressed elsewhere may be causing the deployment defect. Given that chick embryos also display Wnt5a expression in the SpM (56), we used a function-blocking antibody (74,75) to locally inhibit Wnt5a activity and assess its role within SHF cells in the SpM.
SHF progenitors in the caudal SpM of HH14 chick embryos are deployed into the OFT
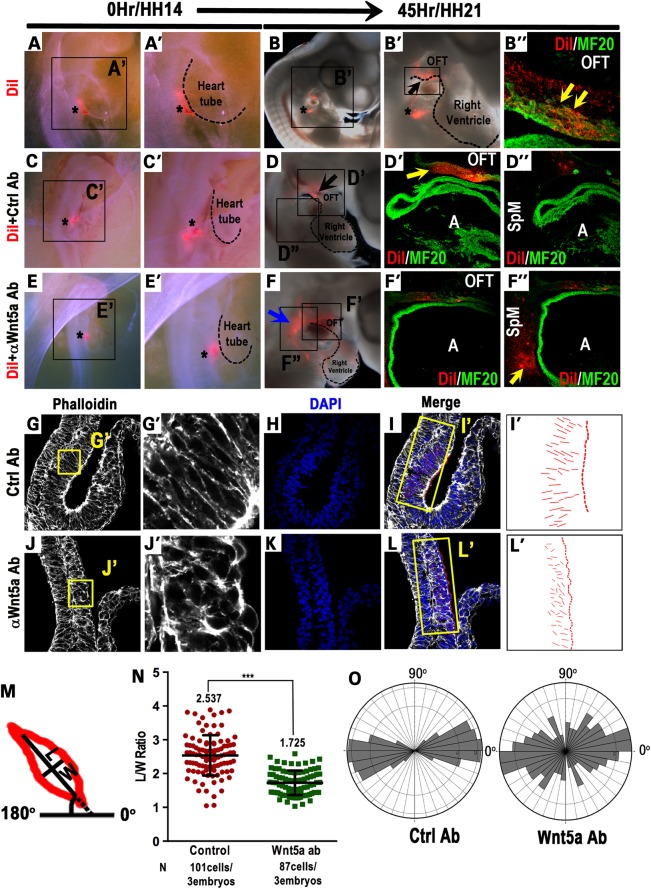
We labeled SHF progenitors in the caudal SpM of HH14 chick embryos (equivalent to ∼E9.0 in mice) with the fluorescent lipophilic dye, DiI (asterisk in Fig. 4A and A′), and cultured the embryos in a shell-less culture system (76) for 45 h to reach HH21. Epi-fluorescence examination of the embryos reveals intense DiI labeling in the inferior region of the OFT (arrows in Fig. 4B and B′) and sagittal sections show that DiI-labeled cells are present as a continuous cohort in the inferior OFT myocardial wall and co-express myocardial marker MF20 (yellow arrow in Fig. 4B″). To our knowledge, this result is the first to directly demonstrate that by HH14, SHF progenitors in the caudal SpM are deployed directionally as a cohort to give rise to the inferior OFT myocardium.
Figure 4.
Chick SHF progenitors in the caudal SpM are deployed to the inferior OFT in a Wnt5a-dependent fashion. DiI was injected into the caudal SpM of HH14 chick embryos (asterisk in A and A′). After culturing for 45 h to HH21, DiI-labeled cells were observed in the inferior OFT region (B and B′). Sagittal sections confirmed that DiI-labeled cells (red) were present in the inferior OFT myocardial wall and co-expressed myocardial marker MF20 (green, yellow arrows in B″). Asterisks in B and B′ denote the injection site that occasionally retains some DiI labeling. (C–F″) HH14 chick embryos were co-injected with a mixture of control rat IgG and DiI (asterisk in C and C′), or anti-Wnt5a IgG and DiI (asterisk in E and E′), and harvested and analyzed at HH21. In control-injected embryos, DiI-labeled cells (red) were observed in the inferior OFT myocardium (D and D′), but were absent from the SpM region (D″). In anti-Wnt5a IgG-injected embryos, however, a large number of DiI-labeled cells were retained in SpM region behind the heart (blue arrow in F, yellow arrow in F″), and only few DiI-labeled cells were present in the OFT (F′). (G–O) HH14 embryos in which control or anti-Wnt5a IgG were injected into the caudal SpM were harvested 15 h later to assess cellular morphology in the SpM. Sagittal sectioning and phalloidin staining showed that in anti-Wnt5a IgG-injected embryos, SHF cells in the caudal SpM displayed diminished and disorganized actin polymerization (compare G′ and J′). In control-injected embryos, SHF cells in the SpM are largely elongated perpendicular to the plane of the SpM and along the dorsal-ventral (D–V) axis (G and G′); whereas in anti-Wnt5a IgG-injected embryos, SHF cells in the SpM appeared to be more rounded and randomly oriented (J and J′). To quantify cell polarity, lines were drawn along the long axis of each cell (I and L) and represented in (I′) and (L′). Length to width ratios (LWR) and the angularity of each SHF cell in the SpM were calculated as depicted (M). (N) Measurement of the LWR in SHF cells in control or Wnt5a-injected embryos. (O) Rose diagrams depicted that control SHF cells were largely aligned perpendicular to the plane of the SpM (90°) and along the D–V axis of the embryo (0°), whereas in anti-Wnt5a IgG-injected embryos, SHF cells displayed randomized orientation. A, atrium; HH, Hamburger–Hamilton stages; LWR, length-to-width ratio; OFT, outflow tract; SpM, splanchnic mesoderm.
Blocking Wnt5a function in the caudal SpM perturbs SHF cell deployment
To determine whether Wnt5a is required in the caudal SpM for SHF cell deployment, we co-injected a function blocking rat anti-Wnt5a antibody (74,75) together with DiI into the caudal SpM at HH14 (Fig. 4C–F). As summarized in Table 1, our results show that in control antibody (Rat IgG) injected embryos (asterisk in Fig. 4C and C′) cultured to HH21, DiI-labeled cells are present mainly in the inferior OFT myocardial wall (10–16 embryos, Table 1; whole mount in Fig. 4D; sections in Fig. 4D′) but not in the SpM (Fig. 4D″), indicating that the deployment of chick SHF cells from the SpM to the OFT is not perturbed by rat IgG per se. In contrast, in anti-Wnt5a antibody-injected embryos (asterisk in Fig. 4E and E′), the majority of the DiI-labeled cells are retained in the SpM behind the heart (14 of 19 embryos, Table 1; blue arrow in Fig. 4F) at HH21. Section analyses confirm that only few labeled cells reach the inferior OFT (Fig. 4F′), and the vast majority of DiI-labeled cells are trapped in the SpM (yellow arrow in Fig. 4F″). These results provide compelling evidence that Wnt5a activity within SHF progenitors in the SpM contributes significantly to their deployment to the OFT.
Table 1.
Blocking Wnt5a function in SHF progenitors in the caudal SpM compromises their deployment to the OFT
| Injection solution | Embryos injected at HH14 | Embryos with DiI label at HH21 |
Embryos without DiI label at HH21 | ||
|---|---|---|---|---|---|
| In OFT (%) | In OFT + SpM (%) | In SpM (%) | |||
| DiI + Rat IgG | 16 | 10 (71%) | 2 (14.3%) | 2 (14.3%) | 2 |
| DiI + anti-Wnt5a IgG | 19 | 3 (17.6%) | 5 (29.4%) | 9 (53%) | 2 |
Wnt5a function in the caudal SpM is required for SHF progenitors to undergo polarized elongation
To understand how Wnt5a function in the SpM may promote SHF cell deployment, we injected control or anti-Wnt5a antibody into the caudal SpM at HH14 and harvested the embryos 15 h later. Phalloidin staining of sagittal sections reveals that in anti-Wnt5a IgG-injected chick embryos, SHF cells in the SpM display reduced and disorganized actin polymerization (Fig. 4G′ and J′), reminiscent of the defects observed in SHF cells in Wnt5a−/− mouse mutants (57). Furthermore, phalloidin staining reveals that in control antibody treated embryos, SHF progenitors in the SpM are elongated in a polarized fashion, resembling cells undergoing medio-laterally oriented intercalation during CE (34,77). Their mean length-to-width ratio (LWR) is 2.537 ± 0.6 (n = 101 cells/3 embryos), with 73% of the SHF progenitors orienting their long axes within a ±20° arc perpendicular to the plane of the SpM (90°) and along the D-V axis of the embryo (0°) (Fig. 4I–O). In contrast, in anti-Wnt5a antibody treated embryos, the SHF progenitors are less elongated with a significantly reduced LWR of 1.725 ± 0.4 (n = 87cells/3 embryos) (P < 0.0001). Moreover, their orientation becomes randomized with only 52% of the cells aligning their long axis within the ±20° arc (Fig. 4L, L′, N, O).
To determine whether similar polarity defects also occur in mice lacking Wnt5a, we examined SHF cells in the caudal SpM where endogenous Wnt5a is expressed (Fig. 1F′ and F′). Indeed, we found that whereas wild-type SHF cells displayed a mean LWR of 1.823 and preferentially aligned their long axes perpendicular to the plane of the SpM, Wnt5a−/− SHF cells displayed less elongated morphology with a significantly reduced LWR of 1.287 and more randomized orientation (Supplementary Material, Fig. S3).
Together, our chick and mouse studies suggest that Wnt5a function is required in the caudal SpM for SHF progenitors to acquire polarized elongation, a pre-requisite for oriented cell intercalation that may provide the driving force for deploying SHF cells from the SpM to the OFT.
Discussion
The role of Wnt5a in SHF deployment
The discovery of the SHF has profoundly changed our view on how the heart forms and how congenital heart defects arise in humans. Extensive studies over the last 15 years have uncovered the mechanisms that govern SHF progenitor specification, maintenance and differentiation (4,17,78,79). However, how these progenitors are actually deployed to their target destination, the OFT and the right ventricle, has for the most part not been described. We have demonstrated previously that Dvl-mediated PCP signaling is required in the SHF lineage for OFT elongation, and proposed a model in which a Wnt5a→ Dvl signaling cascade acts within SHF progenitors to regulate their oriented intercalation, thereby generating the pushing force to promote the deployment of SHF cells from the SpM to the OFT (57).
In this study, we focused on the presumptive PCP ligand Wnt5a to test this model. Using enhancer trap lines and Cre-mediated lineage tracing, our mouse studies show that the loss of Wnt5a causes: (i) increased accumulation of a subpopulation of SHF progenitors in the SpM; (ii) reduced contribution of SpM-derived SHF cells to the inferior OFT and subpulmonary myocardium; and (iii) aberrant extension of superior OFT myocardial cells to the inferior region, resulting in an OFT that possesses primarily subaortic fate but lacks distinct subpulmonary myocardial identity. These defects collectively provide the genetic proof that Wnt5a is required for deploying SHF progenitors from the SpM to the inferior OFT myocardial wall. Alternatively, it is possible that these defects could be caused in part by alterations in SHF cell proliferation, apoptosis or specification. However, we consider these alternate explanations less likely because our previous studies have revealed no change in the rate of cell proliferation or apoptosis in the SpM of Wnt5a−/− mutants (57). Our current analyses with the Mlc1v enhancer trap line, a marker of SHF progenitors (5,71), also demonstrate no defects in SHF specification in Wnt5a−/− mutants at E9.5 (Fig. 2B), and prior studies have concluded that SHF markers Fgf10, Fgf8 and Tbx1 are expressed normally in Wnt5a−/− mutants at E9.5 (56). Therefore, we reason that the primary role of Wnt5a is to regulate SHF progenitor cell deployment rather than specification or expansion. This view is also consistent with the fact that in other vertebrate model organisms, Wnt5a activates non-canonical Wnt/PCP signaling to regulate morphogenetic cell movement, but not cell survival, proliferation or specification (80,81).
Our current studies also provide clues on how Wnt5a may promote SHF deployment. Our investigation of the cellular organization indicates that SHF progenitors in the caudal SpM initially are a loosely packed, multi-layered structure devoid of ZO1 expression (Fig. 1; 57). But as they move rostrally towards the OFT, they are converted to a single layered epithelial-like structure with strong ZO1 and F-actin distribution at apical cell–cell junctions. Therefore, SHF progenitors in the SpM are deployed into the OFT as a cohesive, epithelial-like sheet rather than as individual, actively migrating cells. Given the cellular organization and our current understanding of PCP signaling and tissue morphogenesis, Wnt5a may regulate three possible cellular behaviors to promote SHF deployment. First, as we proposed above, Wnt5a regulates oriented cell intercalation in the caudal SpM to convert multiple layered SHF progenitors to a single layered epithelial-like sheet, thereby generating the pushing force required to deploy the SpM rostrally into the OFT. Secondly, since PCP signaling is known to regulate cell division planes (82,83), Wnt5a may orient SHF progenitors within the epithelial-like sheet to divide along the rostral–caudal axis of the SpM, thereby extending the SpM rostrally into the OFT. Thirdly, given the recent finding that PCP signaling may also regulate epithelial morphogenesis by generating multicellular rosettes that form and resolve in a directional fashion (84–86), it is likely that Wnt5a may promote polarized SpM elongation towards the OFT through a rosette-based mechanism.
Our data provide most support for the first cellular mechanism in which Wnt5a may promote oriented cell intercalation. First, we find that both the Wnt5a protein (Fig. 1F′ and F′) and transcript (56,57,87) are expressed in a highly restricted fashion in the caudal SpM where SHF progenitors are still a multi-layered structure, but their expression terminates in the rostral SpM, where SHF progenitors have been converted to a single layered epithelial-like sheet. Secondly, in Wnt5a−/− mice, SHF cells in the caudal SpM lack the normal protrusive morphology and display diminished actin polymerization, and form aberrant, compact clusters (57). Thirdly, in chick embryos, blocking Wnt5a function in SHF cells in the caudal SpM prevents them from undergoing polarized elongation known to be required for oriented cell intercalation, and subsequently compromises their efficient deployment to the OFT (Fig. 4). Together, our data are most consistent with Wnt5a acting in an auto- or paracrine fashion to regulate oriented, CE-like cell intercalation in the caudal SpM. Our data, however, do not exclude the other two possibilities that Wnt5a may also regulate oriented cell division or a rosette-based mechanism to promote SpM morphogenesis. Addressing these two cellular behaviors, however, will require sophisticated live imaging that is currently difficult to perform in the SpM due to its close proximity to the rapidly beating heart.
Impaired SHF deployment in Wnt5a mutants affects OFT morphogenesis
Understanding and characterizing the deployment defects also provide significant insights into how the severe OFT malformation arises in Wnt5a−/− mice. The OFT malformation in Wnt5a−/− mice was originally described as PTA, and was attributed to a reduced migration of the CNC into the OFT and consequently, a failure to septate the OFT (56). Our previous genetic analysis of Dishevelled1/2 (57) and a recent report on core PCP genes Vangl1/2 (88), however, have both demonstrated that PCP signaling is not required for neural crest cell migration in the mouse. Therefore, the OFT septation defect in Wnt5a−/− mice may not arise directly from a deficiency in Wnt5a-guided CNC migration as previously proposed (56), but instead from secondary events following loss of Wnt5a.
In light of our current finding that in Wnt5a−/− mutants, the inferior OFT myocardium, marked by the y96-16 enhancer trap transgene, is diminished due to a specific impairment of SHF deployment from the SpM to the OFT, there are two explanations for how the OFT malformations may arise. First, the y96-16 transgene is integrated on chromosome 5 close to Sema3c, which encodes a secreted ligand for the PlexinA2 receptor to guide CNC migration and OFT septation (14,89,90). The severe loss of y96-16 expressing cells in Wnt5a−/− mutants (Fig. 3) implies a significant reduction in the myocardial cells capable of expressing Sema3c, resulting in decreased CNC migration and faulty OFT septation. Secondly, an increasing body of literature has revealed that the superior and the inferior wall of the OFT myocardium are molecularly distinct and pre-determined to contribute specifically to the subaortic and subpulmonary myocardium, respectively (13–16). Therefore, in Wnt5a−/− mutants, a severe reduction in the y96-16 lineage, which initially occupies the inferior OFT myocardium and subsequently the subpulmonary myocardium, is predicted to result in atresia or stenosis of the pulmonary trunk (18). Conversely, our analyses with the Wnt11-CreER transgene show that the remaining OFT myocardium possesses mainly a subaortic identity. Together, these results raise the possibility that the primary OFT malformation in Wnt5a−/− mutants manifests as atretic (absent) pulmonary trunk rather than PTA. These two possibilities are not mutually exclusive but rather are both plausible consequences secondary to the SHF deployment defect in Wnt5a−/− mutants. It is most likely that a combination of the two events leads to the OFT morphogenesis defects in Wnt5a−/− mutants.
Perturbation of Wnt5a-mediated SHF deployment as a pathogenic mechanism contributing to OFT malformation in DiGeorge syndrome
Our finding that Wnt5a acting within SHF progenitors in the caudal SpM to promote their deployment may also reveal a novel pathogenic mechanism in DGS. Interestingly, a recent mouse study demonstrates that transcription of Wnt5a in the caudal SpM, but not the heart per se, requires Tbx1 (58), the candidate gene in DGS (61–63). Moreover, cell culture experiments suggest that Tbx1 may activate Wnt5a transcription by binding to the TBEs (T-Box binding elements) and recruiting the BAF chromatin remodeling complex to the Wnt5a genomic region (58). Therefore, an important function of Tbx1 is likely to be promoting SHF deployment through activating Wnt5a transcription, and disruption of Wnt5a-mediated SHF deployment may contribute significantly to the pathogenesis of OFT malformations in DGS.
Our enhancer trap and Cre transgene-based lineage studies in Wnt5a−/− mice provide strong supporting evidence for this idea. Our analyses show increased accumulation of Mlc1v-positive SHF progenitors in the SpM of Wnt5a−/− mice (Fig. 2), similar to the prior studies of Mlc1v expression in Tbx1−/− mutants (71). Furthermore, the diminished inferior OFT/subpulmonary myocardium in Wnt5a−/− mutants, revealed by analyzing the y96-16 lineage (Fig. 3), is remarkably similar to that in Tbx1−/−mutants (Supplementary Material, Fig. S2; 14). Finally, our studies with the Wnt11-CreER transgene demonstrate an ectopic extension of the superior OFT and subaortic myocardium in Wnt5a−/− mutants (Fig. 3), and a similar defect has also been identified in Tbx1−/−mutant through analyzing the T55 enhancer trap line (14). Collectively, these results indicate that the SHF and OFT morphogenesis defects in Tbx1−/− mutants can be closely recapitulated by the loss of Wnt5a, consistent with the idea that disrupting Wnt5a-mediated SHF deployment may be an important pathogenic mechanism contributing to OFT defects in Tbx1−/− mutants.
Previous studies have highlighted the critical role of Tbx1 in regulating Fgf genes to sustain proliferation and suppress differentiation of SHF progenitors (11,65–67). In support of this, loss of Fgf8 in mice can recapitulate the spectrum of developmental defects, especially the OFT malformations, observed in DGS patients and Tbx1 mutant mice (66). Restoring Fgf signaling in the Tbx1 expression domain, however, is not sufficient to rescue the OFT defects in Tbx1 null mutants (91), suggesting that Tbx1 has additional important functions besides regulating Fgf to control SHF cell proliferation and differentiation. Activating Wnt5a expression to promote SHF deployment may account for one of the additional functions of Tbx1. Furthermore, given the finding that Fgf signaling can also promote the dynamics of cell movement (92), and that Fgf and Wnt5a can act together to regulate polarized morphogenesis in the limb (48), it is also possible that Tbx1 activates both Fgf and Wnt5a expression to regulate SHF deployment. In either case, it will be interesting to test in the future whether simultaneously restoring both Fgf and Wnt5a signaling can suppress the OFT defects in Tbx1 null mutants.
Cardiovascular abnormalities are the most prominent and morbid feature of DGS, which is characterized by haploinsufficiency of the chromosome 22q11.2 region harboring the TBX1 locus (11,59–63). In DGS patients with the similar 3 Mb deletion, however, OFT malformations manifest in a highly variable fashion, supporting the idea that genetic variations in other loci can significantly influence the phenotypic outcome of OFT malformations induced by the deletion (2,64). Given our current finding that Wnt5a signaling is critical in promoting SHF deployment and may contribute significantly to the pathogenesis of OFT malformations in Tbx1 mutants, it will be especially interesting to determine whether genetic variations at WNT5A or its presumptive downstream genes in the PCP pathway may modify the penetrance and/or phenotypic outcome of cardiovascular defects in DGS patients. Conversely, future development of approaches to activate Wnt5a signaling may provide us with new therapeutic avenues for the OFT malformations in DGS.
Materials and Methods
Mouse strains and genotyping
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). Wnt5a and Tbx1 mutant mice and Rosa26-tdTomato (B6;129S6-Gt(ROSA)26Sortm9(CAG−tdTomato)Hze/J) (R26R-tdTomato) Cre reporter mice (Jackson Laboratory) were genotyped as described (61,72,87,93). The Mef2c-AHF-Cre transgene and Mlc1v-24-nLacZ (Mlc1v) and y96-myf5–16-nLacZ (y96-16) enhancer trap lines have been described previously (5,9,94). Wnt11-CreER BAC transgenic mice were generated (manuscript in press—T.S. and J.W.) and genotyped by PCR using primers CreA1 (CCG GGC TGC CAC GAC CAA) and CreA2 (GGC GCG GCA ACA CCA TTT TT). All strains were maintained in a C57B6/SJL/FvB mixed background. Animal care and use were approved by the Animal Care and Use Committee of the University of Alabama at Birmingham.
Tamoxifen administration, embryo collection, imaging and X-gal staining
Wnt5a+/− mice were crossed with either Wnt5a+/−;Mlc1v or Wnt5a+/−; y96-16 mice to obtain control and mutant Wnt5a−/−;Mlc1v or Wnt5a−/−; y96-16 embryos, respectively. Wnt5a+/−;Wnt11-CreER mice were crossed with Wnt5a+/−; R26R-tdTomato mice to obtain Wnt5a−/−; Wnt11-CreER;R26R-tdTomato embryos. Wnt5a+/+ or Wnt5a+/− littermate embryos were used as controls. The morning of the plug was designated as embryonic day (E) 0.5; embryos were collected at various stages between E9.5 and E14.5 and their yolk sacs were retained for genotyping. Wnt11-CreER transgene bearing pregnant dams were singly gavaged with tamoxifen (Sigma, MO, USA; T-5648, dissolved to 10 mg/ml in corn oil) at 2 mg/40 g body weight. Embryos were fixed in 4% paraformaldehyde (PFA) at 4°C and subsequently stored in PBS before proceeding with cryo-embedding and sectioning using a standard protocol (57). X-gal staining was performed as previously described (95). X-gal stained embryos were post-fixed, cryo-embedded and sectioned. Sections were counter-stained with eosin and mounted in Vectashield mounting medium. Whole-mount and X-gal stained embryo and section images were captured using a Leica MZ16FA fluorescence stereomicroscope equipped with a multi-fluorescent filter set and a DFC490 CCD camera.
Chick embryo culture and injections
Fertilized chicken eggs (Charles River Avian Services, CT, USA) were incubated in a forced draft incubator at 39°C and 80% humidity. Shell-less cultures were set up at HH14 as previously described (76). The caudal SpM of HH14 embryos was injected with either DiI (1,1′-dioctadecyl-3,3,3′-tetramethylindocarbocyanine perchlorate, Molecular Probes, Inc.) alone, with 50–70 nl of DiI + Rat IgG (2 mg/ml, R&D Systems, MN, USA) or with DiI + anti-Wnt5a antibody (2 mg/ml, R&D Systems). Injected embryos were cultured for 45 h and harvested at HH21. Harvested embryos were fixed overnight in 4% PFA at 4°C and were subsequently washed and stored in PBS. Fixed embryos were scored for the presence of the DiI label in either the OFT or the SpM. Embryos were imaged with a Leica MZ16FA fluorescence stereomicroscope and were processed for cryo-embedding and sectioning. For analyzing the short-term effect of anti-Wnt5a antibody injection on cellular morphology and architecture, embryos were injected with Rat IgG or anti-Wnt5a neutralizing antibody (74,75) caudally into the SpM. Rat IgG/anti-Wnt5a antibody was mixed with Fast Green dye (Sigma) to visualize site of injection. These embryos were harvested 15 h post-injection, cryo-embedded and sectioned. Phalloidin staining was performed and the LWR and angles of alignment for SpM cells were measured as depicted in Figure 4M using Adobe Photoshop CS5. Statistical analyses were performed using Graphpad Prism 6.0. Angular measurements were plotted using Rose.NET (Todd Thompson, http://mypage.iu.edu/~tthomps/programs/home.htm).
Fluorescent immuno-staining
Cryosections were fixed with cold 4%PFA for 5 min, washed with PbTX (0.1% Tween in PBS) and incubated in blocking solution (PbTX+1%BSA) followed by incubation with primary antibodies overnight at 4°C. Subsequently, sections were washed with PbTx and incubated with appropriate secondary antibodies for 1 h at room temperature. Sections were then washed with PbTx and mounted in Vectashield mounting medium containing DAPI (Vector Labs, CA, USA). Primary antibodies used were mouse anti-MF20 (1:15, DSHB, Iowa), Rat anti-Wnt5a (1:100, R&D Systems) and Phalloidin-FITC (1:250, Sigma). Alexa Fluor 647-conjugated donkey anti-mouse IgG (1:500, Invitrogen, CA, USA) and DyLight 488-conjugated donkey anti-mouse IgG (1:250, Jackson ImmunoResearach, PA, USA). All fluorescent confocal images were acquired with an Olympus FV1000 Laser Confocal Scanning microscope and were subsequently analyzed using the FV10-ASW software. Images were compiled and linearly adjusted for brightness, contrast and color balance using Adobe Photoshop CS5.
Supplementary Material
Funding
This work was supported by grants from the National Institute of Health (R01 HL109130) and American Heart Association (14GRNT20380467) to J.W.; Fondation pour la Recherche Médicale (DEQ20110421300) to R.G.K.; the George and Jean Brumley, Jr. Neonatal-Perinatal Research Institute of Duke University, AHA (BGIA7370008) and The Hartwell Foundation to MRH; and an AHA pre-doctoral fellowship (12PRE12060081) to T.S.
Supplementary Material
Acknowledgement
We thank Dr Brenda Rongish for helpful discussions during the course of this study.
Conflict of Interest statement. None declared.
References
- 1.Bruneau B.G. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- 2.Momma K. Cardiovascular anomalies associated with chromosome 22q11.2 deletion syndrome. Am. J. Cardiol. 2010;105:1617–1624. doi: 10.1016/j.amjcard.2010.01.333. [DOI] [PubMed] [Google Scholar]
- 3.Cai C.L., Liang X., Shi Y., Chu P.H., Pfaff S.L., Chen J., Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyer L.A., Kirby M.L. The role of secondary heart field in cardiac development. Dev. Biol. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly R.G., Brown N.A., Buckingham M.E. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 6.Li P., Pashmforoush M., Sucov H.M. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev. Cell. 2010;18:480–485. doi: 10.1016/j.devcel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Q., Zhou B., Pu W.T. Reassessment of Isl1 and Nkx2–5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev. Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mjaatvedt C.H., Nakaoka T., Moreno-Rodriguez R., Norris R.A., Kern M.J., Eisenberg C.A., Turner D., Markwald R.R. The outflow tract of the heart is recruited from a novel heart-forming field. Dev. Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 9.Verzi M.P., McCulley D.J., De Val S., Dodou E., Black B.L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 10.Waldo K.L., Kumiski D.H., Wallis K.T., Stadt H.A., Hutson M.R., Platt D.H., Kirby M.L. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 11.Xu H., Morishima M., Wylie J.N., Schwartz R.J., Bruneau B.G., Lindsay E.A., Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- 12.Liao J., Aggarwal V.S., Nowotschin S., Bondarev A., Lipner S., Morrow B.E. Identification of downstream genetic pathways of Tbx1 in the second heart field. Dev. Biol. 2008;316:524–537. doi: 10.1016/j.ydbio.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajolle F., Zaffran S., Meilhac S.M., Dandonneau M., Chang T., Kelly R.G., Buckingham M.E. Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field. Dev. Biol. 2008;313:25–34. doi: 10.1016/j.ydbio.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Theveniau-Ruissy M., Dandonneau M., Mesbah K., Ghez O., Mattei M.G., Miquerol L., Kelly R.G. The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circ. Res. 2008;103:142–148. doi: 10.1161/CIRCRESAHA.108.172189. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand N., Roux M., Ryckebusch L., Niederreither K., Dolle P., Moon A., Capecchi M., Zaffran S. Hox genes define distinct progenitor sub-domains within the second heart field. Dev. Biol. 2011;353:266–274. doi: 10.1016/j.ydbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochais F., Dandonneau M., Mesbah K., Jarry T., Mattei M.G., Kelly R.G. Hes1 is expressed in the second heart field and is required for outflow tract development. PLoS ONE. 2009;4:e6267. doi: 10.1371/journal.pone.0006267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black B.L. Transcriptional pathways in second heart field development. Semin. Cell Dev. Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirby M.L. Pulmonary atresia or persistent truncus arteriosus: is it important to make the distinction and how do we do it? Circ. Res. 2008;103:337–339. doi: 10.1161/CIRCRESAHA.108.174862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCulley D.J., Kang J.O., Martin J.F., Black B.L. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev. Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ai D., Fu X., Wang J., Lu M.F., Chen L., Baldini A., Klein W.H., Martin J.F. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc. Natl Acad. Sci. USA. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen E.D., Wang Z., Lepore J.J., Lu M.M., Taketo M.M., Epstein D.J., Morrisey E.E. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J. Clin. Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L., Cui L., Zhou W., Dufort D., Zhang X., Cai C.L., Bu L., Yang L., Martin J., Kemler R., et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc. Natl Acad. Sci. USA. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manisastry S.M., Han M., Linask K.K. Early temporal-specific responses and differential sensitivity to lithium and Wnt-3A exposure during heart development. Dev. Dyn. 2006;235:2160–2174. doi: 10.1002/dvdy.20878. [DOI] [PubMed] [Google Scholar]
- 24.Park E.J., Watanabe Y., Smyth G., Miyagawa-Tomita S., Meyers E., Klingensmith J., Camenisch T., Buckingham M., Moon A.M. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135:3599–3610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilagan R., Abu-Issa R., Brown D., Yang Y.P., Jiao K., Schwartz R.J., Klingensmith J., Meyers E.N. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Lin Y., Zhang Y., Lan Y., Lin C., Moon A.M., Schwartz R.J., Martin J.F., Wang F. Frs2alpha-deficiency in cardiac progenitors disrupts a subset of FGF signals required for outflow tract morphogenesis. Development. 2008;135:3611–3622. doi: 10.1242/dev.025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyer L.A., Kirby M.L. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev. Biol. 2009;330:305–317. doi: 10.1016/j.ydbio.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goddeeris M.M., Schwartz R., Klingensmith J., Meyers E.N. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- 29.High F.A., Jain R., Stoller J.Z., Antonucci N.B., Lu M.M., Loomes K.M., Kaestner K.H., Pear W.S., Epstein J.A. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J. Clin. Invest. 2009;119:1986–1996. doi: 10.1172/JCI38922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon C., Qian L., Cheng P., Nigam V., Arnold J., Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat. Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habas R., Kato Y., He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 32.Heisenberg C.P., Tada M., Rauch G.J., Saude L., Concha M.L., Geisler R., Stemple D.L., Smith J.C., Wilson S.W. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 33.Jessen J.R., Topczewski J., Bingham S., Sepich D.S., Marlow F., Chandrasekhar A., Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 35.Yin C., Kiskowski M., Pouille P.A., Farge E., Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J. Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Mark S., Zhang X., Qian D., Yoo S.J., Radde-Gallwitz K., Zhang Y., Lin X., Collazo A., Wynshaw-Boris A., et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K.P., Dai X., Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Guo N., Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devenport D., Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Hamblet N.S., Mark S., Dickinson M.E., Brinkman B.C., Segil N., Fraser S.E., Chen P., Wallingford J.B., Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kibar Z., Vogan K.J., Groulx N., Justice M.J., Underhill D.A., Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 42.Murdoch J.N., Doudney K., Paternotte C., Copp A.J., Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 43.Kibar Z., Torban E., McDearmid J.R., Reynolds A., Berghout J., Mathieu M., Kirillova I., De Marco P., Merello E., Hayes J.M., et al. Mutations in VANGL1 associated with neural-tube defects. N. Engl J. Med. 2007;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 44.De Marco P., Merello E., Consales A., Piatelli G., Cama A., Kibar Z., Capra V. Genetic analysis of disheveled 2 and disheveled 3 in human neural tube defects. J. Mol. Neurosci. 2013;49:582–588. doi: 10.1007/s12031-012-9871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Marco P., Merello E., Rossi A., Piatelli G., Cama A., Kibar Z., Capra V. FZD6 is a novel gene for human neural tube defects. Hum. Mutat. 2012;33:384–390. doi: 10.1002/humu.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B., Sinha T., Jiao K., Serra R., Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum. Mol. Genet. 2011;20:271–285. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao B., Song H., Bishop K., Elliot G., Garrett L., English M.A., Andre P., Robinson J., Sood R., Minami Y., et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gros J., Hu J.K., Vinegoni C., Feruglio P.F., Weissleder R., Tabin C.J. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr. Biol. 2010;20:1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Person A.D., Beiraghi S., Sieben C.M., Hermanson S., Neumann A.N., Robu M.E., Schleiffarth J.R., Billington C.J., Jr, van Bokhoven H., Hoogeboom J.M., et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev. Dyn. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afzal A.R., Rajab A., Fenske C.D., Oldridge M., Elanko N., Ternes-Pereira E., Tuysuz B., Murday V.A., Patton M.A., Wilkie A.O., et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat. Genet. 2000;25:419–422. doi: 10.1038/78107. [DOI] [PubMed] [Google Scholar]
- 51.DeChiara T.M., Kimble R.B., Poueymirou W.T., Rojas J., Masiakowski P., Valenzuela D.M., Yancopoulos G.D. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat. Genet. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 52.Oldridge M., Fortuna A.M., Maringa M., Propping P., Mansour S., Pollitt C., DeChiara T.M., Kimble R.B., Valenzuela D.M., Yancopoulos G.D., et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat. Genet. 2000;24:275–278. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- 53.Hamblet N.S., Lijam N., Ruiz-Lozano P., Wang J., Yang Y., Luo Z., Mei L., Chien K.R., Sussman D.J., Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 54.Nassar H., Paul M., Ahmad I., Berkovits D., Bettan M., Collon P., Dababneh S., Ghelberg S., Greene J.P., Heger A., et al. Stellar (n,gamma) cross section of 62Ni. Phys. Rev. Lett. 2005;94:092504. doi: 10.1103/PhysRevLett.94.092504. [DOI] [PubMed] [Google Scholar]
- 55.Phillips H.M., Murdoch J.N., Chaudhry B., Copp A.J., Henderson D.J. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ. Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 56.Schleiffarth J.R., Person A.D., Martinsen B.J., Sukovich D.J., Neumann A., Baker C.V., Lohr J.L., Cornfield D.N., Ekker S.C., Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr. Res. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- 57.Sinha T., Wang B., Evans S., Wynshaw-Boris A., Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Dev. Biol. 2012;370:135–144. doi: 10.1016/j.ydbio.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L., Fulcoli F.G., Ferrentino R., Martucciello S., Illingworth E.A., Baldini A. Transcriptional control in cardiac progenitors: Tbx1 interacts with the BAF chromatin remodeling complex and regulates Wnt5a. PLoS Genet. 2012;8:e1002571. doi: 10.1371/journal.pgen.1002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scambler P.J. The 22q11 deletion syndromes. Hum. Mol. Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- 60.Scambler P.J., Kelly D., Lindsay E., Williamson R., Goldberg R., Shprintzen R., Wilson D.I., Goodship J.A., Cross I.E., Burn J. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- 61.Jerome L.A., Papaioannou V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 62.Liao J., Kochilas L., Nowotschin S., Arnold J.S., Aggarwal V.S., Epstein J.A., Brown M.C., Adams J., Morrow B.E. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- 63.Merscher S., Funke B., Epstein J.A., Heyer J., Puech A., Lu M.M., Xavier R.J., Demay M.B., Russell R.G., Factor S., et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 64.Aggarwal V.S., Morrow B.E. Genetic modifiers of the physical malformations in velo-cardio-facial syndrome/DiGeorge syndrome. Dev. Disabil. Res. Rev. 2008;14:19–25. doi: 10.1002/ddrr.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Fulcoli F.G., Tang S., Baldini A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ. Res. 2009;105:842–851. doi: 10.1161/CIRCRESAHA.109.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frank D.U., Fotheringham L.K., Brewer J.A., Muglia L.J., Tristani-Firouzi M., Capecchi M.R., Moon A.M. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aggarwal V.S., Liao J., Bondarev A., Schimmang T., Lewandoski M., Locker J., Shanske A., Campione M., Morrow B.E. Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum. Mol. Genet. 2006;15:3219–3228. doi: 10.1093/hmg/ddl399. [DOI] [PubMed] [Google Scholar]
- 68.Umeda K., Matsui T., Nakayama M., Furuse K., Sasaki H., Furuse M., Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 69.Stevenson B.R., Siliciano J.D., Mooseker M.S., Goodenough D.A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waldo K.L., Hutson M.R., Ward C.C., Zdanowicz M., Stadt H.A., Kumiski D., Abu-Issa R., Kirby M.L. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Kelly R.G., Papaioannou V.E. Visualization of outflow tract development in the absence of Tbx1 using an FgF10 enhancer trap transgene. Dev. Dyn. 2007;236:821–828. doi: 10.1002/dvdy.21063. [DOI] [PubMed] [Google Scholar]
- 72.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ezin M., Fraser S. Time-lapse imaging of the early avian embryo. Methods Cell Biol. 2008;87:211–236. doi: 10.1016/S0091-679X(08)00211-2. [DOI] [PubMed] [Google Scholar]
- 74.Bodmer D., Levine-Wilkinson S., Richmond A., Hirsh S., Kuruvilla R. Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. J. Neurosci. 2009;29:7569–7581. doi: 10.1523/JNEUROSCI.1445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blakely B.D., Bye C.R., Fernando C.V., Horne M.K., Macheda M.L., Stacker S.A., Arenas E., Parish C.L. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS ONE. 2011;6:e18373. doi: 10.1371/journal.pone.0018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yelbuz T.M., Waldo K.L., Kumiski D.H., Stadt H.A., Wolfe R.R., Leatherbury L., Kirby M.L. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.cir.0000023044.44974.8a. [DOI] [PubMed] [Google Scholar]
- 77.Wallingford J.B., Rowning B.A., Vogeli K.M., Rothbacher U., Fraser S.E., Harland R.M. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 78.Vincent S.D., Buckingham M.E. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr. Top. Dev. Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 79.Evans S.M., Yelon D., Conlon F.L., Kirby M.L. Myocardial lineage development. Circ. Res. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kilian B., Mansukoski H., Barbosa F.C., Ulrich F., Tada M., Heisenberg C.P. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 81.Wallingford J.B., Vogeli K.M., Harland R.M. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int. J. Dev. Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- 82.Gong Y., Mo C., Fraser S.E. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 83.Saburi S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S.E., Harrison R., Mount R., McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 84.Blankenship J.T., Backovic S.T., Sanny J.S., Weitz O., Zallen J.A. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Lienkamp S.S., Liu K., Karner C.M., Carroll T.J., Ronneberger O., Wallingford J.B., Walz G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura T., Honda H., Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi T.P., Bradley A., McMahon A.P., Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 88.Pryor S.E., Massa V., Savery D., Andre P., Yang Y., Greene N.D., Copp A.J. Vangl-dependent planar cell polarity signalling is not required for neural crest migration in mammals. Development. 2014;141:3153–3158. doi: 10.1242/dev.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown C.B., Feiner L., Lu M.M., Li J., Ma X., Webber A.L., Jia L., Raper J.A., Epstein J.A. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- 90.Feiner L., Webber A.L., Brown C.B., Lu M.M., Jia L., Feinstein P., Mombaerts P., Epstein J.A., Raper J.A. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- 91.Vitelli F., Lania G., Huynh T., Baldini A. Partial rescue of the Tbx1 mutant heart phenotype by Fgf8: genetic evidence of impaired tissue response to Fgf8. J. Mol. Cell Cardiol. 2010;49:836–840. doi: 10.1016/j.yjmcc.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benazeraf B., Francois P., Baker R.E., Denans N., Little C.D., Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 94.Bajolle F., Zaffran S., Kelly R.G., Hadchouel J., Bonnet D., Brown N.A., Buckingham M.E. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ. Res. 2006;98:421–428. doi: 10.1161/01.RES.0000202800.85341.6e. [DOI] [PubMed] [Google Scholar]
- 95.Kelly R., Alonso S., Tajbakhsh S., Cossu G., Buckingham M. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J. Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.