Abstract
BACKGROUND
Heparin-induced thrombocytopenia (HIT) develops as a result of platelet (PLT) activation by anti-platelet factor 4 (PF4)/heparin complex antibodies. Despite repeated exposure to heparin, patients undergoing chronic intermittent hemodialysis (HD) rarely develop HIT. We investigated the possibility that HD decreases/removes PF4 from PLT surfaces and/or plasma, thereby disfavoring immune complex formation as a mechanism of protection against HIT.
MATERIALS AND METHODS
We enrolled 20 patients undergoing chronic HD at the Penn Presbyterian Medical Center. Blood samples were drawn before, during and after treatment in the presence and absence of heparin. PF4, PF4/heparin antibody, heparin, and P-selectin levels were measured.
RESULTS
No patients demonstrated clinical symptoms of HIT. PLT surface PF4 levels decreased and plasma PF4 levels increased concurrently with increase in plasma heparin concentration. In the absence of heparin, PLT surface and plasma PF4 levels were unchanged. Anti-PF4/heparin antibodies, which were non-functional by the serotonin release assay, were detectable in 8 patients. PLT surface P-selectin levels did not change during treatment.
CONCLUSIONS
Removal of PLT surface and/or plasma PF4 as a mechanism of protection against HIT in patients undergoing HD is not supported by the results of our study, although the transient decrease in PLT surface PF4 in the presence of large amounts of heparin remains a candidate mechanism. The small sample size, single type of dialyzer membrane, and early sampling time points may have led to the inability to detect changes in PF4 levels. Future studies should explore other potential protective mechanisms.
Keywords: Hemodialysis, HIT, PF4, heparin, platelet, mechanism
Heparin-induced thrombocytopenia (HIT) is a transient, prothrombotic, autoimmune disorder mediated by antibodies that recognize ultralarge complexes (ULCs) of platelet factor 4 (PF4) and heparin. Our laboratory has shown that unfractionated heparin and tetrameric PF4 form ultralarge complexes (ULC; >670kDa) only over a narrow molar ratio of heparin to PF4 of approximately 1:1.[1] These ULCs are thought to be central to HIT pathogenesis. Changes in the molar ratio of heparin to PF4 by as little as 40% reduce ULC formation and increase/favor formation of smaller, less pathogenic complexes.[1, 2] The antigenicity of the complex depends on the molar ratio of the reactants as well as on the length, chemical composition, and structure of the GAG itself.[3]
PF4 (CXCL4) is a small molecule (70 amino acids) positively charged CXC chemokine that is released in high concentrations from PLT α-granules upon PLT activation.[4] Basal PF4 concentration in the plasma is very low (<1nM) while the normal serum content is more than a thousand-fold higher (1–2.5 µM).[5, 6] PF4 monomers polymerize to form non-covalently linked tetramers with a molecular weight of approximately 32,000 Da at physiologic pH and ionic strength.
Hemodialysis (HD) removes waste products such as creatinine and urea as well as free water by diffusion of solutes across a semipermeable membrane and by a countercurrent flow mechanism whereby the dialysate flows in the opposite direction to blood flow in the extracorporeal circuit. Solute removal can be characterized as high-efficiency or low efficiency based on the ability to remove small solutes such as urea or high-flux or low-flux based on the ability to remove large solutes, such as β2-microglobulin (~12,000 Da, negatively charged).[7, 8] Due to the larger pore size of high-flux dialyzers, it is possible that PF4 monomers (8,000 Da) and dimers (16,000 Da) may be removed during dialysis. Removal of PF4 during HD could shift the equilibrium to favor dissociation of PF4 tetramers.
HD commonly utilizes heparin as an anticoagulant. As much as 6000U of heparin may be administered during each HD session.[9] Yamamoto and coworkers[10] reported that in patients newly initiated on HD in Japan, the incidence of HIT was 3.9% (6/154). The mean duration to the development of HIT after the initiation of HD was 18 days. However, in patients undergoing chronic intermittent HD for 3 months or longer (mean period of dialysis, 70.3 ± 74 months; median period of dialysis, 41 months), HIT is rare (0.6%) despite repeated heparin exposure.[9, 11, 12]
Several mechanisms may be responsible for the low rates of HIT in patients undergoing chronic intermittent HD. Two possible mechanisms are the repeated systemic exposure to large amounts of heparin or removal of PF4 during HD, either of which may alter the heparin to PF4 ratio in a manner that disfavors the formation of ULCs.[1] PF4 is removed during low-density lipoprotein (LDL) apheresis by dextran sulfate removal[13] and represents a possible protective mechanism from HIT in that patient population. It is currently unknown if PF4 is removed by HD. We therefore investigated the ability of HD to decrease PF4 in the plasma as a potential protective mechanism against HIT.
MATERIALS AND METHODS
Study population and design
The study population included 20 consecutive patients receiving HD treatment in the DaVita outpatient dialysis clinic at Penn Presbyterian Medical Center. All patients were greater than 18 years old with end-stage renal disease due to hypertension, diabetes mellitus, or lupus nephritis. This study was approved by the University of Pennsylvania Institutional Review Board and written informed consent was obtained from the study participants. Inclusion criteria included age greater than or equal to 18 years; receiving outpatient chronic (≥ 3 months) thrice weekly HD for end-stage renal disease of any etiology; HD access via arteriovenous fistula, graft or central venous catheter; HD using the Polyflux Revaclear capillary dialyzer (Gambro, Lakewood, CO); and HD with heparin. Exclusion criteria included the inability to provide informed consent, a history of HIT, and current pregnancy.
This study occurred over two separate patient visits. In the first visit, HD was carried out according to standard procedures. A commercially available dialysis system (Phoenix, Gambro, Lakewood, CO) using the Polyflux Revaclear capillary dialyzer was used. Heparin dosing varied for each patient according to actual body weight. Patients less than 60 kg received a loading dose of 1000 U, patients weighing 60 to 90 kg received a loading dose of 1500 U, and patients weighing greater than 90 kg received a loading dose of 2000 U. All patients received a maintenance heparin dose of 500 U/hour. Heparin was discontinued 60 min prior to the end of the HD treatment if the patient had an arterio-venous fistula (AVF) or arterio-venous graft (AVG) as HD access. If the patient had a central venous catheter (CVC) as HD access, heparin was discontinued 30 min prior to the end of the HD treatment. Approximately 10 mL of whole blood was drawn into two citrated tubes (Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ) immediately before (pretreatment) and after the completion of HD (posttreatment) from the arterial port. Twenty minutes after starting HD, 10 mL of whole blood was also drawn proximal to the dialyzer from the arterial port (20 min predialyzer) and distal to the dialyzer from the venous port (20 min postdialyzer). At the second study visit, the standard HD procedure was modified by withholding heparin for the first 20 minutes. Whole blood (10 mL) was drawn into citrated tubes from the arterial port (No heparin) after 20 minutes. Heparin was then administered as regularly prescribed. After 20 minutes of HD with heparin, another whole blood sample was drawn from the arterial port (Heparin). All samples were kept at room temperature (RT) before processing to prevent PLT activation and were processed within 15 minutes of collection.
PLT-poor plasma preparation
Whole blood samples were centrifuged twice at 2000×g for 10 minutes in a swinging bucket rotor. The supernatant was then transferred to a fresh 15 mL conical tube. Small aliquots were made and all tubes were frozen at −80°C until analysis.
PF4 measurement
PF4 was quantified using a PF4 enzyme-linked immunosorbent assay (ELISA) kit (Zymutest, Hyphen BioMed, Neuville-sur-Oise, France) according to the manufacturer’s instructions with slight modifications. Briefly, 200 µL of PF4 standard (supplied by the manufacturer) or diluted patient samples were introduced into each well in duplicate and incubated for one hour at RT. Patient samples were diluted as follows to ensure that all measurements were in assay range: pretreatment and posttreatment samples, 1:10 to 1:20; and precolumn and postcolumn samples, 1:5 to 1:20. Each well was then washed five times with 300 µL wash solution. Two- hundred microliters of conjugate (anti-PF4 polyclonal antibody coupled with peroxidase) was added into each well and incubated for one hour at RT. After the wells were washed five times, substrate (TMB/H2O2) was added and incubated in each well for two minutes at RT. The reaction was stopped with 50 µL of 0.45 mol/L sulfuric acid and the absorbance at 450 nm was measured.
Anti-PF4/heparin antibody measurement
Anti-PF4/heparin antibodies were measured using the polyspecific PF4 enhanced kit (GTI Diagnostics, Waukesha, WI) according to manufacturer’s instructions. Briefly, 300 µL of working wash solution was added to each well and incubated for 10 minutes at RT. Fifty microliters of diluted (1:50) positive control, negative control and patient sample were introduced into each well in duplicate and incubated for 45 minutes at 37°C. The wells were then washed four times with 300 µL working wash solution. Fifty microliters of diluted conjugate (goat anti-human immunoglobulin [IgG/A/M] conjugated to alkaline phosphatase) was added to each well and incubated for 45 minutes at 37°C. The wells were washed four times and incubated with 100 µL of substrate (p-nitrophenyl phosphate) in the dark for 30 minutes at room RT. The reaction was stopped by adding 100 µL of 3 mol/L sodium hydroxide stopping solution, and the absorbance (optical density, OD) at 405 nm was measured.
Serotonin release assay
The serotonin release assay was performed as previously described [14]. Briefly, platelet rich plasma (PRP) from healthy donors was incubated with 0.5 µl carbon-14 labeled 5-hydroxytryptamine creatinine sulfate (GE Life Sciences, Piscataway, NJ) per milliliter of PRP for 20 minutes at 37°C. Serotonin uptake was inhibited by adding 1 mmole/ml imipramine (Sigma-Aldrich, St. Louis, MO) to the PRP.
Sera from subjects with a positive PF4/heparin antibody ELISA were tested for heparin dependent serotonin release. Negative and positive controls contained sera from patients previously known to have negative or positive serotonin release, respectively. Five different concentrations of heparin (0.05 units/ml, 0.1 units/ml, 0.5 units/ml, 1 unit/ml and 2 units/ml) (Abbott Laboratories, Abbott Park, IL) were studied. In addition, a positive ADP control was performed (in the absence of heparin). The percent release was calculated for all conditions as previously described [14]. The normal range is 0–5%. A serotonin release >10% in the presence of 0.5–2 U/ml heparin and ≤5% in the absence of heparin was considered positive.
Flow cytometric measurement of PLT surface antigens
Two microliters of whole blood collected in citrate anticoagulant were incubated with phycoerythrin-labeled anti-P-selectin, peridinin chlorophyll protein-cyanine dye 5.5-labeled anti-CD41a, and Alexa fluor 488 labeled anti-PF4 antibodies for 30 minutes at RT. The cells were then fixed with 1% paraformaldehyde solution (Cytofix, BD, Franklin Lakes, NJ) for 30 minutes at 4°C. Flow cytometric analysis of PLT surface antigens was performed on a flow cytometer (LSRFortessa, BD) and the data were analyzed using its accompanying software (FACSDiva, BD) and FlowJo version 7.6.1. The geometric mean fluorescence intensity of PF4 and P-selectin were compared between samples.
Heparin anti-Xa chromogenic assay
Heparin activity in plasma samples was measured using a heparin anti-Xa chromogenic assay (Diagnostica Stago, Asnières-sur-Seine, France) and a coagulation analyzer (Coag-A-Mate II, bioMerieux, Marcy l'Etoile, France) according to the manufacturer’s instructions. Briefly, plasma was diluted 1:9 in FXa buffer and warmed to 37°C. Heparin antithrombin III reagent was then added after which FXa was added to the reaction. The reaction was allowed to proceed for 60 seconds at 37°C. Heparin substrate was then added and the absorbance change was monitored for 30 seconds.
Statistical analysis
Data were plotted and statistical analyses were performed using computer software (GraphPad Prism 5, GraphPad Software Inc, La Jolla, CA; STATA, StataCorp LP, College Station, TX). ANOVA was used to determine if differences among repeated-measures variable samples were present and the Bonferroni multiple comparison test was used to determine which pairs were significantly different. A p value of less than 0.05 was considered significant.
RESULTS
Twenty patients were enrolled in the study, 15 of whom also participated in the second study visit (Table 1). The mean age was 62 years. The majority of patients were African Americans (19/20), reflecting the demographic make-up of the patient population in the dialysis clinic. Patients had end-stage renal disease due to hypertension (n=14), diabetes mellitus (n=14) and/or lupus nephritis (n=1). Dialysis parameters and type of access are further described in Table 1. None of the patients developed clinical manifestations of HIT during the course of the study.
Table 1.
Demographic and baseline characteristics of patients
| Characteristics | Number |
|---|---|
| N | 20 |
| Gender | |
| Male | 9 (45%) |
| Female | 11 (55%) |
| Age (years, mean) | 62 |
| Race | |
| African American | 19 (95%) |
| Caucasian | 1 (5%) |
| Cause of End Stage Renal Disease** | |
| Hypertension | 14 |
| Diabetes mellitus | 14 |
| Lupus nephritis | 1 |
| Heparin dose (U, mean) | 2563 |
| Blood flow rate (mL/min, mean) | 433 |
| Length of Hemodialysis Treatment (min, mean) | 229 |
| Duration on Hemodialysis (months, mean) | 41 |
| Type of access* | |
| AVG | 8 |
| AVF | 11 |
| CVC | 1 |
AVG = arterio-venous graft, AVF = arterio-venous fistula, CVC = central venous catheter
Patients could have more than one cause.
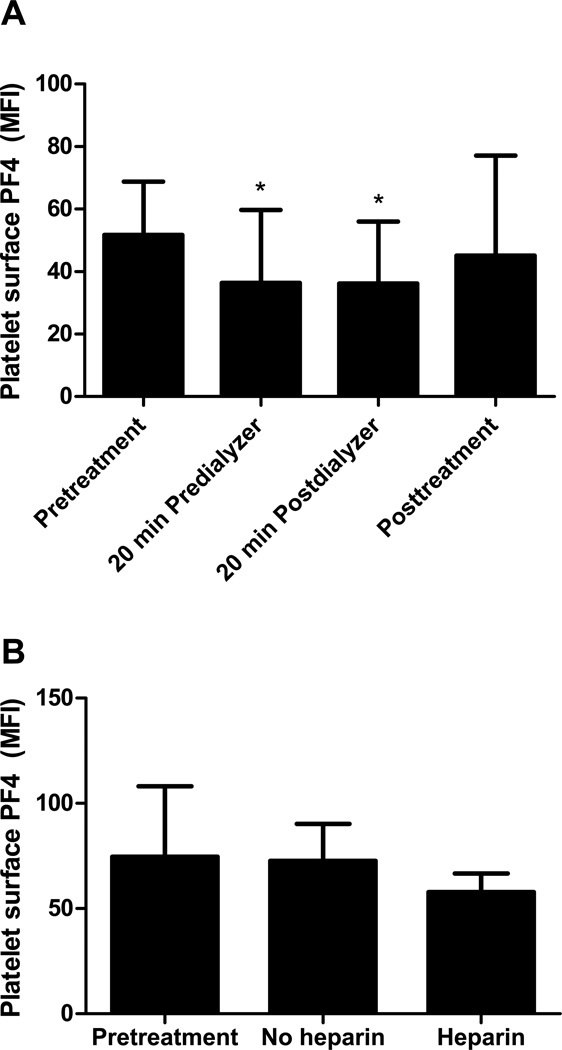
PLT surface PF4 levels decreased during treatment (Fig. 1A). Twenty minutes after HD was initiated, PLT surface PF4 levels proximal and distal to the dialyzer were significantly lower compared to the pretreatment level (n=20; pretreatment vs. 20 min predialyzer and 20 min postdialyzer mean fluorescent intensity [MFI], 51.71 ± 17.04 vs. 36.38 ± 23.34 and 36.17 ± 19.83, p≤0.001 for both comparisons). There was no significant difference in PF4 levels on PLT surfaces proximal (20 min predialyzer) and distal (20 min postdialyzer) to the dialyzer after 20 minutes of HD. The posttreatment PLT surface PF4 level (45.11 ± 32.05) was intermediate between and not significantly different from the pretreatment or 20 min levels. In the absence of heparin, pretreatment PLT surface PF4 levels did not differ from the 20 min predialyzer levels (n=15; pretreatment vs no heparin MFI, 74.63 ± 33.51 vs 72.72 ± 17.60, p=0.81; Fig. 1B). Upon the addition of heparin, PLT surface PF4 levels decreased compared to pretreatment levels, although this did not reach statistical significance with samples from 15 patients (heparin MFI, 57.85 ± 8.85, p=0.1; Fig. 1B).
Figure 1. PLT surface PF4 decreased in the presence of heparin.
PLT surface PF4 levels were measured by flow cytometry as MFI. (A) In the presence of heparin, PLT surface PF4 levels 20 minutes after the start of dialysis proximal and distal to the dialyzer decreased significantly compared to the pretreatment level. Data are the mean ± SD. N=20. *p≤0.001 (B) In the absence of heparin, PLT surface PF4 levels remained unchanged from pretreatment levels. Upon the addition of heparin, PLT surface PF4 levels decreased. Data are the mean ± SD. N=15.
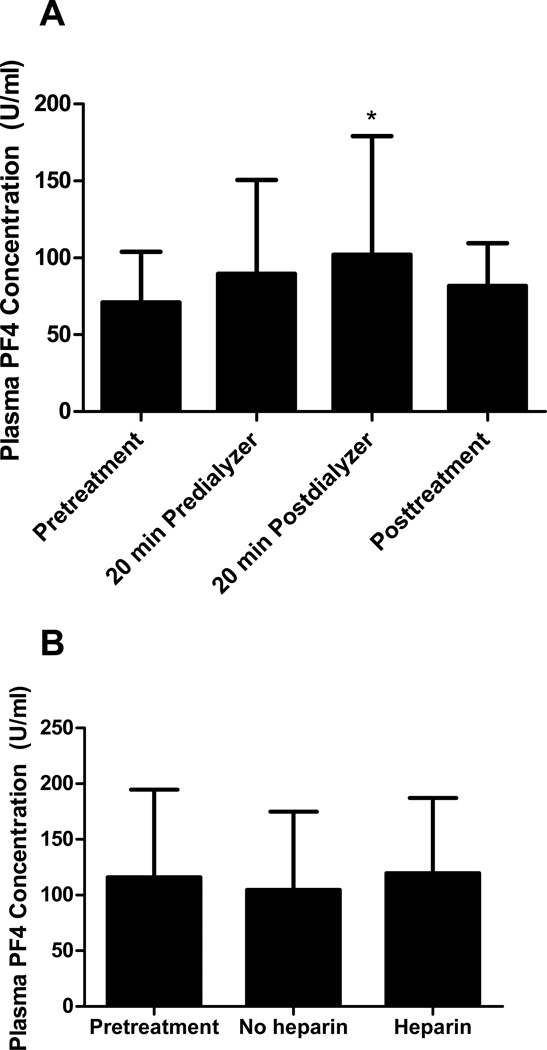
PF4 levels in the plasma from peripheral blood drawn distal to the dialyzer 20 minutes after the start of HD were significantly higher compared to pretreatment levels (n=20; pretreatment vs. 20 min postdialyzer, 70.89 ± 32.94 U/mL vs. 102 ± 77.14 U/mL, p<0.05; Fig. 2A). There was no significant difference between PF4 levels in the plasma proximal and distal to the dialyzer after 20 minutes of HD. The posttreatment plasma PF4 level was not significantly different from the pretreatment, 20 min predialyzer or 20 min postdialyzer levels (n=20; 20 min predialyzer, 89.58 ±60.95 U/mL; posttreatment, 81.81 ± 27.68 U/mL; Fig. 2A). In the absence of heparin, plasma PF4 levels after 20 minutes of HD were similar to pretreatment levels (n=15; pretreatment, 115.9 ± 78.78 U/mL; no heparin, 104.8 ± 70.06 U/mL; Figure 2B). Twenty minutes after the addition of heparin in this subset of patients, predialyzer plasma PF4 levels were not significantly different from pretreatment levels (n=15; heparin, 119.6 ± 67.55; Figure 2B), consistent with the results obtained for the overall study population (Figure 2A). These findings indicate that PLT surface PF4 may decrease during HD as a result of binding to heparin in the plasma.
Figure 2. Plasma PF4 increased in the presence of heparin.
Plasma PF4 levels were measured using the Zymutest PF4 ELISA kit. (A) Plasma PF4 levels 20 minutes after the start of dialysis distal to the dialyzer increased significantly compared to the pretreatment level. Data are the mean ± SD. N=20. *p<0.05. (B) In the absence of heparin, plasma PF4 levels remained unchanged from pretreatment levels. The addition of heparin did not increase predialyzer plasma PF4 levels. Data are the mean ± SD. N=15.
The heparin concentration in the plasma increased significantly during treatment and decreased to near pretreatment levels by the end of treatment, as expected. The heparin concentrations in the plasma 20 minutes after the start of HD proximal and distal to the dialyzer were significantly higher than pretreatment concentrations (data not shown). The heparin concentration in the plasma distal to the dialyzer (20 min postdialyzer) was significantly higher compared to the heparin concentration in the plasma proximal to the dialyzer and posttreatment. These findings are consistent with heparin administration practices during HD. Heparin infusion into the blood as it enters the dialyzerleads to a higher heparin concentration in the postdialyzer blood compared to the predialyzer blood. Discontinuation of heparin 30 to 60 minutes before the end of HD leads to a lower posttreatment heparin concentration due to its clearance from the circulation.
Eight of the 20 patients demonstrated the presence of anti-PF4/heparin antibodies in at least one blood sample according to the manufacturer’s cutoff for positivity (optical density [OD] ≥0.4) in the anti-PF4/heparin antibody ELISA. Antibody levels remained unchanged during HD (data not shown). None of these antibodies were functional as determined by the serotonin release assay. These results indicate that clinically significant anti-PF4/heparin antibodies were not generated during chronic intermittent HD treatment and that HD treatment did not remove anti-PF4/heparin antibodies. The latter finding is consistent with the inability of dialyzers to allow the passage of large molecules such as immunoglobulins through the membrane.
PLT surface P-selectin was measured as a marker of PLT activation. There were no significant changes in P-selectin levels on the surface of PLTs or platelet size during and after HD treatment as determined by flow cytometry (data not shown). The presence or absence of heparin did not lead to significant changes in PLT surface P-selectin levels (data not shown). When PLTs from a subset of patients (n=12) were incubated with TRAP, a significant increase in PLT surface P-selectin levels was observed (data not shown). These data suggest that HD treatment with the Polyflux Revaclear capillary dialyzer did not significantly activate functionally intact PLTs.
DISCUSSION
Patients undergoing chronic intermittent HD are repeatedly exposed to heparin and yet HIT rarely develops in this patient population. Anti-PF4/heparin antibodies preferentially bind to ultralarge heparin and PF4 complexes, which form when the molar ratio of the reactants is approximately 1:1. [1] Deviation from this ratio by increasing or decreasing heparin relative to PF4 or vice versa leads to a smaller proportion of ULCs and a larger proportion of smaller complexes which are less antigenic/pathogenic. Processes that introduce a large concentration of heparin such as HD treatment or decrease the concentration of PF4 in the plasma and/or PLT surfaces may provide a rationale for the prevention of HIT.
We previously showed that LDL apheresis via dextran sulfate adsorption (DSA) removes plasma PF4 and reduces the amount of PF4 on the surface of circulating PLTs.[13] We proposed that this reduction in surface PF4 may decrease ULC formation and/or recognition by anti-PF4/heparin antibodies, providing a potential explanation for the near absence of HIT in hypercholesterolemic patients undergoing LDL apheresis. The current study investigated whether plasma PF4 and/or PLT surface PF4 are removed by HD treatment as a potential protective mechanism against HIT in patients undergoing chronic intermittent HD.
In this study, we showed that PLT surface PF4 levels decreased and plasma PF4 levels distal to the dialyzer increased during the first twenty minutes of HD concurrent with the increase in plasma heparin concentration (Fig 1A, 2A). When heparin concentrations decreased due to discontinuation of heparin 30 to 60 minutes prior to the end of HD, plasma PF4 levels decreased and PLT surface PF4 levels increased compared to the 20 min postdialyzer levels, though these changes did not reach statistical significance (Fig 1A, 2A). Furthermore, when dialysis was performed in the absence of heparin, 20 min predialyzer (no heparin) PLT surface and plasma PF4 levels did not differ from pretreatment levels (pretreatment, Fig. 1B and 2B). Collectively, these results suggest that PLT surface PF4 levels decreased during HD due to the presence of heparin. This may be directly as a result of the removal of positively charged PLT surface PF4 molecules by heparin, or indirectly as a result of equilibration of surface PF4 after binding of plasma PF4 to heparin. When heparin concentrations declined at the end of the procedure due to clearance, PF4 molecules were released and bound once again to cell surfaces. Thus, the rarity of HIT in patients undergoing chronic intermittent HD does not appear to be due to removal of PF4 by HD. However, the presence of large amounts of heparin may provide protection via a transient reduction in PLT surface PF4 levels.
PF4/heparin antibodies were detected in at least one blood sample from 8 of the 20 patients in our study. The prevalence of antibodies (40%) in our study is higher compared to prior reports of 3% to 12% in patients undergoing chronic intermittent HD.[10, 15–18] This could be due to a number of factors including the small number of patients in our study, multiple sampling of individual subjects, and/or differences in race/ethnicity, heparin type and dose, duration on HD or medical history. The antibody levels remained unchanged throughout the course of HD,consistent with the inability of the dialyzer membrane to allow passage of large proteins such as immunoglobulins which are approximately 146,000 Da in size. Thus, the potential protective mechanism against the development of HIT in chronically hemodialyzed patients does not appear to be dependent on a reduction of antibody levels during HD.
A randomized double-blind study of trauma patients found that patients undergoing major surgery had a higher risk of developing PF4/heparin antibodies and HIT compared to patients undergoing minor surgery when exposed to an identical heparin regimen.[19] It has been speculated that differences in the degree of inflammation and platelet activation between minor and major trauma patients may account for this difference. Based on the findings of this study, another potential explanation for the lower incidence of HIT in patients undergoing chronic intermittent HD compared to those starting HD is that patients on chronic HD are not acutely ill and have less baseline inflammation and platelet activation. Patients starting HD, on the other hand, are often acutely ill and may have a higher degree of platelet activation at baseline which could explain the increased incidence of HIT in this population.[10]
Platelet count changes in association with hemodialysis were not specifically measured in this study. No significant change in PLT activation or size was detected in these patients during and after HD treatment in the presence and absence of heparin (data not shown). The degree of platelet activation during HD is partly dependent on the type of dialyzer membrane used. The membranes used in contemporary times and in our study are synthetic and are more biocompatible than the cellulose or cellulose-derived membranes used in the 1970s and 1980s that were known to activate complement and platelets and sequester leukocytes. Some synthetic membranes such as polysulfone have been found to lead to greater platelet activation compared to other synthetic membranes like cuprammonium rayon.[20] The Poracton membrane of the Polyflux Revaclear capillary dialyzer specifically used in this study was designed to reduce thrombogenicity and sequestration of proteins and cells.
In summary, our findings do not support the hypothesis that HD using the Polyflux Revaclear capillary dialyzer protects against the development of HIT by removing plasma and/or PLT surface PF4, although the transient lowering of PLT surface PF4 by heparin remains a candidate mechanism. Limitations in our study design, including a small sample size, single type of dialyzer membrane, and early sampling time points may have led to the inability to detect changes in plasma and/or PLT surface PF4 levels. As studies unravel more details about the pathogenesis of HIT, other targets may be identified that are removed or affected by chronic intermittent HD that could explain the low incidence of HIT in this population. An understanding of the mechanisms of protection of chronic intermittent HD against the development of HIT may be leveraged for the development of novel modalities for the prevention or treatment of HIT. This study argues against the possibility that removal of plasma and/or PLT surface PF4 during chronic intermittent HD protects against the development of HIT. Future studies should explore other potential protective mechanisms.
Abbreviations
- HIT
Heparin-induced thrombocytopenia
- HD
Hemodialysis
- PLT
platelet
- PF4
platelet factor 4
- GAGs
glycosaminoglycans
- ULC
ultralarge complexes
- LDL
low-density lipoprotein
- AVF
arterio-venous fistula
- AVG
arterio-venous graft
- CVC
central venous catheter
- ELISA
enzyme-linked immunosorbent assay
- PRP
platelet rich plasma
- MFI
mean fluorescent intensity
- OD
optical density
- DSA
dextran sulfate adsorption
- SD
standard deviation
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Rauova L, Poncz M, McKenzie SE, Reilly MP, Arepally G, Weisel JW, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105:131–138. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- 2.Horsewood P, Warkentin TE, Hayward CP, Kelton JG. The epitope specificity of heparin-induced thrombocytopenia. Br J Haematol. 1996;95:161–167. doi: 10.1046/j.1365-2141.1996.d01-1876.x. [DOI] [PubMed] [Google Scholar]
- 3.Greinacher A, Alban S, Dummel V, Franz G, Mueller-Eckhardt C. Characterization of the structural requirements for a carbohydrate based anticoagulant with a reduced risk of inducing the immunological type of heparin-associated thrombocytopenia. Thromb Haemost. 1995;74:886–892. [PubMed] [Google Scholar]
- 4.Sachais BS, Rux AH, Cines DB, Yarovoi SV, Garner LI, Watson SP, et al. Rational design and characterization of platelet factor 4 antagonists for the study of heparin-induced thrombocytopenia. Blood. 2012 doi: 10.1182/blood-2012-01-406801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol. 2000;67:471–478. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- 6.Kasper B, Petersen F. Molecular pathways of platelet factor 4/CXCL4 signaling. Eur J Cell Biol. 2011;90:521–526. doi: 10.1016/j.ejcb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Kerr PG, Huang L. Review: membranes for haemodialysis. Nephrology (Carlton) 2010;15:381–385. doi: 10.1111/j.1440-1797.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 8.Ward RA. Do clinical outcomes in chronic hemodialysis depend on the choice of a dialyzer? Semin Dial. 2011;24:65–71. doi: 10.1111/j.1525-139X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 9.Luzzatto G, Bertoli M, Cella G, Fabris F, Zaia B, Girolami A. Platelet count, anti-heparin/platelet factor-4 antibodies and tissue factor pathway inhibitor plasma antigen level in chronic dialysis. Thromb Res. 1998;89:115–122. doi: 10.1016/s0049-3848(97)00301-0. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, Koide M, Matsuo M, Suzuki S, Ohtaka M, Saika S, et al. Heparin-induced thrombocytopenia in hemodialysis patients. Am J Kidney Dis. 1996;28:82–85. doi: 10.1016/s0272-6386(96)90134-1. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T, Kobayashi H, Matsuo M, Wanaka K, Nakamoto H, Matsushima H, et al. Frequency of anti-heparin- PF4 complex antibodies (HIT antibodies) in uremic patients on chronic intermittent hemodialysis. Pathophysiol Haemost Thromb. 2006;35:445–450. doi: 10.1159/000102052. [DOI] [PubMed] [Google Scholar]
- 12.O'Shea SI, Sands JJ, Nudo SA, Ortel TL. Frequency of anti-heparin-platelet factor 4 antibodies in hemodialysis patients and correlation with recurrent vascular access thrombosis. Am J Hematol. 2002;69:72–73. doi: 10.1002/ajh.10032. [DOI] [PubMed] [Google Scholar]
- 13.Tanhehco YC, Rux AH, Sachais BS. Low-density lipoprotein apheresis reduces platelet factor 4 on the surface of platelets: a possible protective mechanism against heparin-induced thrombocytopenia and thrombosis. Transfusion. 2011;51:1022–1029. doi: 10.1111/j.1537-2995.2010.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirschman RJ, Shulman NR. The use of platelet serotonin release as a sensitive method for detecting anti-platelet antibodies and a plasma anti-platelet factor in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 1973;24:793–802. doi: 10.1111/j.1365-2141.1973.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 15.Boon DM, van Vliet HH, Zietse R, Kappers-Klunne MC. The presence of antibodies against a PF4- heparin complex in patients on haemodialysis. Thromb Haemost. 1996;76:480. [PubMed] [Google Scholar]
- 16.Carrier M, Knoll GA, Kovacs MJ, Moore JC, Fergusson D, Rodger MA. The prevalence of antibodies to the platelet factor 4 -heparin complex and association with access thrombosis in patients on chronic hemodialysis. Thromb Res. 2007;120:215–220. doi: 10.1016/j.thromres.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Greinacher A, Zinn S, Wizemann, Birk UW. Heparin-induced antibodies as a risk factor for thromboembolism and haemorrhage in patients undergoing chronic haemodialysis. Lancet. 1996;348:764. doi: 10.1016/s0140-6736(05)65684-x. [DOI] [PubMed] [Google Scholar]