Abstract
Background: Total weight loss induced by energy restriction is highly variable even under tightly controlled conditions. Identifying weight-loss discriminants would provide a valuable weight management tool and insights into body weight regulation.
Objective: This study characterized responsiveness to energy restriction in adults from variables including the plasma metabolome, endocrine and inflammatory markers, clinical indices, body composition, diet, and physical activity.
Methods: Data were derived from a controlled feeding trial investigating the effect of 3–4 dairy product servings in an energy-restricted diet (2092 kJ/d reduction) over 12 wk. Partial least squares regression was used to identify weight-loss discriminants in 67 overweight and obese adults. Linear mixed models were developed to identify discriminant variable differences in high- vs. low-weight–loss responders.
Results: Both pre- and postintervention variables (n = 127) were identified as weight-loss discriminants (root mean squared error of prediction = 1.85 kg; Q2 = 0.43). Compared with low-responders (LR), high-responders (HR) had greater decreases in body weight (LR: 2.7 ± 1.6 kg; HR: 9.4 ± 1.8 kg, P < 0.01), BMI (in kg/m2; LR: 1.0 ± 0.6; HR: 3.3 ± 0.5, P < 0.01), and total fat (LR: 2.2 ± 1.1 kg; HR: 8.0 ± 2.1 kg, P < 0.01). Significant group effects unaffected by the intervention were determined for the respiratory exchange ratio (LR: 0.86 ± 0.05; HR: 0.82 ± 0.03, P < 0.01), moderate physical activity (LR: 127 ± 52 min; HR: 167 ± 68 min, P = 0.02), sedentary activity (LR: 1090 ± 99 min; HR: 1017 ± 110 min, P = 0.02), and plasma stearate [LR: 102,000 ± 21,000 quantifier ion peak height (QIPH); HR: 116,000 ± 24,000 QIPH, P = 0.01].
Conclusions: Overweight and obese individuals highly responsive to energy restriction had accelerated reductions in adiposity, likely supported in part by higher lipid mobilization and combustion. A novel observation was that person-to-person differences in habitual physical activity and magnitude of weight loss were accompanied by unique blood metabolite signatures. This trial was registered at clinicaltrials.gov as NCT00858312.
Keywords: weight loss, metabolomics, obesity, physical activity, statistical modeling, body composition, branched-chain amino acids, respiratory exchange ratio, calorie restriction
Introduction
Weight loss is the physical manifestation of a multifaceted adaptation to negative energy balance, requiring a concert of biological and behavioral factors. Indeed, there are substantial differences in individual responses to energy restriction even in tightly controlled interventions. Although this variability is generally associated with genetic variation (1–4) or dietary compliance (5–7), results have been inconsistent, likely reflecting diversity among studied populations, dietary treatments, and outcome measurements (8). Clearly, differences in metabolic and behavioral factors driving person-to-person variability in weight loss remain to be fully elaborated.
Many behavioral and biological factors have been assessed individually to examine their effect on energy balance and cellular metabolism. For example, physical activity increases energy expenditure and weight loss when combined with energy restriction (9, 10). Resting metabolic rate and resting respiratory exchange ratio (RER)8 are directly related to energy balance and are associated with weight loss and gain (11–13). Other studies have identified cellular (2, 14) and endocrine (15–17) markers that affect energy metabolism. However, few studies have integrated these factors in a unifying model to describe weight-loss variability.
Based on these and other findings, weight loss is likely the result of behavior (volitional or habitual physical activity), as well as person-to-person differences in thermogenesis and mitochondrial fuel partitioning. To better understand factors underlying this complexity, and to unmask connections between these factors, this study combined metabolomic, clinical, body composition, endocrine, inflammatory, dietary, and physical activity data to define a weight-loss “responder” phenotype in overweight and obese adults consuming an energy-restricted, controlled diet. Using multivariate statistical modeling, we identified determinants of weight loss that provide insight into potential mechanisms that could drive individual responsiveness to energy restriction.
Methods
Subjects
Overweight and obese [BMI (in kg/m2): 28–37] men and women were recruited (n = 71) from the Davis and Sacramento, California, communities. Main inclusion criteria included age (women: 20–45 y; men: 20–50 y), habitually low dairy consumption (≤1 serving of dairy/d), and typical calcium intake ≤600 mg/d. Detailed selection criteria and consort diagrams are published elsewhere (18). The availability of paired pre- and postintervention plasma aliquots limited the sample size to 67 subjects for this analysis.
Study design
This study constitutes a secondary analysis of a randomized control trial originally designed to investigate the effect of 3–4 servings of dairy products on weight loss during energy restriction. In-depth details regarding the study design and results pertaining to the primary intervention have been previously reported (18). Participants were studied for a total of 15 wk with all foods provided. Weeks 1–3 of the study were considered a run-in period and were designed to establish each participant’s total energy requirements. Energy intake to maintain body weight was initially estimated from gender-specific prediction equations for overweight individuals adjusted for self-reported physical activity (19, 20). Participants were then provided weighed foods based on these estimates. Food consumption was observed during this period by study personnel. Body weight was monitored daily during the run-in period, and energy intake was adjusted based on increases or decreases in total weight of ±3% for 3 consecutive days. An individual was considered in energy balance when body weight was stable for ≥5 consecutive days. The following 12 wk (study weeks 4–15) were designed as the intervention period. During the intervention period, final energy intake requirements established from the 3-wk run-in period were reduced by 2092 kJ/d (500 kcal/d) and subjects were randomly assigned to either an adequate dairy (3–4 servings of dairy/d) or a low dairy (≤1 serving of dairy/d) group. One dairy serving was equal to a 240 mL serving of milk or yogurt, a 56 g serving of processed cheese, or a 42 g serving of natural cheese. Preintervention data were collected near the end of the run-in period (study week 3) and postintervention data were collected near the end of the intervention period (study week 15), except for physical activity. Physical activity measurements were conducted during study weeks 4 and 14 because of study-related commitments during weeks 3 and 15. All procedures were approved by the Institutional Review Board for the Protection of Human Subjects at the University of California, Davis. All subjects were informed of the study requirements and provided written informed consent before participation.
Dietary intake and compliance
All run-in and intervention diets contained comparable amounts of macronutrients and fiber (fat, carbohydrates, and protein at ∼35%, 49%, and 16% of total energy, respectively, and fiber at 8–10 g/4186 kJ). Participants consumed 2 of the 3 meals/d at the USDA Agricultural Research Service Western Human Nutrition Research Center throughout the run-in period and during weeks 1 and 2 of the intervention period. Between intervention weeks 3 and 9, meals were “packaged to go” and consumed off-site. During these weeks, all food was weighed and measured before distribution and compliance was monitored by returned food containers and food diary records twice weekly. Subjects agreed not to use any dietary supplements during the course of the study. Each individual’s caffeine intake was assessed at baseline and kept constant throughout intervention. A priori compliance determinations included adherence to diet treatment (consumption of low dairy or adequate dairy servings), energy intake within 837 kJ of prescription, and consumption by the adequate dairy group of 95% of the 295 total dairy servings during the intervention. Only individuals who met all compliance criteria in a given week were counted as compliant for the week. Total study compliance was defined as meeting compliance for ≥10 intervention weeks and all participants achieved this criterion.
Body composition measurements
Body weight was measured to the nearest 0.1 kg in light clothing (shoes and jewelry removed). Height was measured with the use of a wall-mounted stadiometer and recorded to the nearest 0.1 cm. BMI was calculated as kilograms per meter squared. Waist circumference was measured with a metal nonstretchable tape measure to the nearest 0.1 cm in the standing position against bare skin with the abdomen relaxed and arms at sides. Total and regional fat measurements were assessed with the use of DXA (GE Lunar, Prodigy model). Daily calibration procedures were performed according to manufacturer instructions. To reduce variance in the data, DXA scans were analyzed by a single operator. Intra-abdominal adipose tissue was measured with the use of computed tomography transabdominal slices (Siemens, Somaton 16 CT Scanner) as previously described (18).
Assessment of physical activity
Participants were instructed to maintain their typical physical activity throughout the study. Physical activity was monitored with the use of an omnidirectional accelerometer (Actical; Philips Electronics). This sensor integrates motion amplitude and frequency, producing an electrical current that increases with intensity of motion that is stored as activity counts (ACs). Sedentary, light, moderate, and vigorous activity are defined as <100 ACs, 100–1535 ACs, 1535–3962 ACs, and >3962 ACs, respectively (21). Participants were instructed to wear the Actical attached to a belt and worn over the left hip, except during bathing or swimming, for 7 d. At the end of the week, participants returned the Actical to the Western Human Nutrition Research Center, where the data were downloaded and analyzed. A day was considered complete if the Actical contained at least 12 h of data. Data are reported as physical activity over a 24 h period averaged over all days worn. Physical activity data were analyzed for sedentary, light, moderate, and vigorous activity and expressed as energy expenditure for each level of activity and time spent at each activity level.
Resting metabolic rate
Subject resting metabolic rate was measured in the morning after a 12 h fast. Before the measurement, subjects were made comfortable in a hospital bed and instructed to rest quietly in a reclined position for 15 min. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured in reclining subjects with the use of an automated metabolic measuring cart (TrueMax 2400 Metabolic Measurement System, ParvoMedics). The RER (VCO2:VO2) was determined from the resting steady-state VCO2 and VO2 values, whereas energy expenditure was calculated with the use of the Weir equation (22) and converted to kilojoules per day as previously described (18).
Blood collection and analytical analyses
Blood was collected by venipuncture after an overnight fast, and serum and plasma were stored at −80°C until analyzed. Clinical assessment of serum total cholesterol, HDL cholesterol, LDL cholesterol, glucose, hematocrit, and hemoglobin were performed at the University of California, Davis, Medical Center. Serum insulin, leptin, IL-1β, IL-6, IL-8, and TNF-α were measured with multiplex technology (Millipore; Bio-Plex, Bio-Rad). Serum C-reactive protein was measured with a chemiluminescence analyzer and high-sensitivity C-reactive protein kit (Immulite, Siemens Diagnostics). Serum 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol were measured by RIA (DiaSorin and Immunodiagnostics Systems, respectively) as previously described (23). As reported previously (24), the following variables were measured by commercial ELISAs: bone-specific alkaline phosphatase (Metra BAP, Quidel); pyridinoline (Metra PYD, Quidel); osteocalcin (Metra Osteocalcin, Quidel); C-terminal telopeptide of type I collagen (CrossLaps, Nordic Bioscience Diagnostics); plasma plasminogen activator inhibitor-1 (Technozym Actibind PAI-1, Technoclone). Heparin plasma zinc was assessed on a Varian Vista inductively coupled plasma atomic emission spectrophotometer.
Plasma metabolite analysis
Plasma samples were extracted and derivatized by silylation/methyloximation before analysis by GC/time-of-flight MS for untargeted metabolomics (25). The resulting data were processed with the use of the BinBase database (26) to match metabolic feature retention indices and mass spectral data against the Fiehn mass spectral library of 1200 authentic metabolite spectra and the 2005 National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health Mass Spectral Library. Metabolites were reported if present within at least 50% of each study design group (27). Metabolite quantifier ion peak heights were normalized to the sum intensities of all known metabolites and used for statistical analyses.
Statistics
All statistical analyses were conducted in R version 3.0.1 (28). Data are presented as means ± SDs in text. α was set at 0.05 for all analyses.
Pre- and postintervention comparisons.
Mann-Whitney U tests were used to compare pre- and postintervention differences in all measurements. This nonparametric test was conducted on untransformed data because of the prevalence of data that were unable to achieve a normal distribution after log transformations. Based on Anderson-Darling tests (29) at α = 0.05, 80% of the data were not normally distributed. False discovery rates (FDR) for Mann-Whitney U tests were assessed with the use of Storey’s q-value (30). A q-value of 0.2 (i.e., 20% false discovery at α = 0.05) was selected as the FDR control level.
Partial least squares regression.
Before analysis, pre- and postintervention data were separated, missing data points were imputed with a singular value decomposition-based method (31, 32), and the resulting data were condensed into a single data frame. Missing data accounted for 0.22% of all data and were never >3.7% for a single variable. Variables that did not have a normal distribution were log transformed.
Principal component analyses were used to identify potential confounders, including measurement time (pre- and postintervention), treatment (low and adequate dairy), gender (male and female), and season of intervention (winter, spring, summer, and fall). Gender-specific clusters were identified in the nonmetabolomics data (e.g., body composition, serum endocrine and inflammation markers, and physical activity) (Supplemental Figure 1A). Therefore, nonmetabolomics variables were gender-adjusted with the use of multiple linear regressions, and subsequent principal component analyses of residuals verified adjustment success (Supplemental Figure 1B). Gender-adjusted residuals were used in further multivariate analyses. The metabolomics data did not require adjustment.
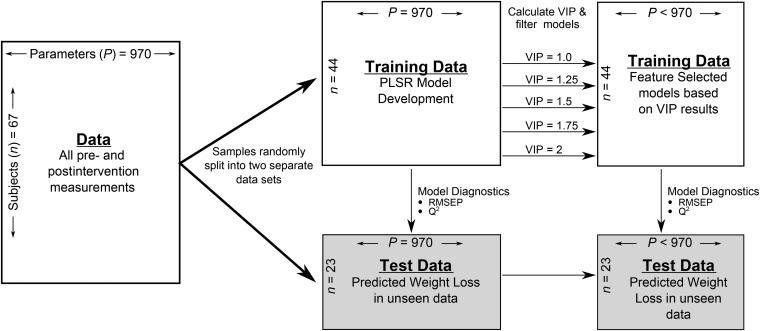
Because of the high dimensionality and collinear nature of the data, partial least squares regression (PLSR) was used to identify variables associated with participant change in weight. We used a development/validation scheme to evaluate PLSR models and selected discriminant variables using a filter approach with the development dataset. A diagram of the model development and validation is presented in Figure 1. In short, data were stratified by week, and one-third of row-wise samples were randomly removed as an independent external validation dataset to assess model performance. The orthogonal scores algorithm was implemented with the use of the R “pls” package (33). Model performance and quality assessment was determined with the use of root mean square error of prediction (RMSEP) and the Q2 statistic. Development data were scaled and centered to unit variance before model development. Validation data were scaled and centered with development scales and centered values.
FIGURE 1.
Schematic of multivariate statistical modeling approach of weight loss in overweight and obese subjects consuming an energy deficit of 2092 kJ/d (500 kcal/d) for 12 wk. PLSR, partial least squares regression; RMSEP, root mean square error of prediction; VIP, variable importance in projection.
Because our objective was to evaluate the simultaneous interactions and influences of multiple physiologic systems on weight loss, all variables were included in an initial PLSR model with weight change as the dependent variable. Variable importance in projection (VIP) scores, a weighted measure of the contribution of each variable according to the variance explained in the dependent variable, were assessed for all variables (34, 35). Subsequent PLSR models were fitted based on selected VIP cutoffs and assessed for model performance. Final model selection minimized complexity (i.e., low number of explanatory variable), while maintaining model performance.
Analysis of high- and low-weight–loss responders.
Total weight loss was assessed as pre-intervention minus postintervention weight loss and was assessed for normal distribution. ANOVA was used to determine if weight loss differed by treatment, season, or gender. Total weight loss was visually assessed for normality with a frequency distribution plot and a Q-Q plot and also tested via the Anderson-Darling test. Study data were then stratified based on weight-loss tertiles with “high-responders” (i.e., individuals with the greatest weight loss) defined as those with weight loss ≥7.2 kg and “low-responders” (i.e., individuals with the least weight loss) defined as those with weight loss <5.2 kg. Individuals who lost between 5.2 and 7.2 kg were considered “responders.” Weight-loss cutoffs were determined to allow equal populations among tertiles (n = 22, 23, and 22 for high-responder, responder, and low-responder tertiles, respectively). Responders were removed from further analyses to highlight the differences between high- and low-responders.
Linear mixed model analysis was used to assess differences in pre- and postintervention time points and high- and low-responder classes with subject identification as a random effect (36). Post hoc analysis of fixed effects used pairwise contrast analysis (37) adjusted by FDR correction (38). Significant interactions were further assessed for pre- and postintervention differences within high- and low-responder classes by linear mixed model analysis.
Variables selected in the final PLSR model and determined to have either a significant group fixed effect or interaction were evaluated with the use of Spearman correlations and hierarchical cluster analysis.
Miscellaneous.
Other R packages essential for manuscript preparation, graphics, and additional analyses in no particular order of importance include reshape2 (39), plyr (40), ggplot2 (41), ggthemes (42), ReporteRs (43), multcompView (44), and Hmisc (45).
Results
Participant and variable characteristics.
The majority of participants were women (71.6%) aged 19–49 y (32.9 ± 9.2 y). A total of 940 pre- and postintervention variables were assessed and all changes from pre- to postintervention are provided in Supplemental Table 1.
Differences in clinical, endocrine, and energy balance variables.
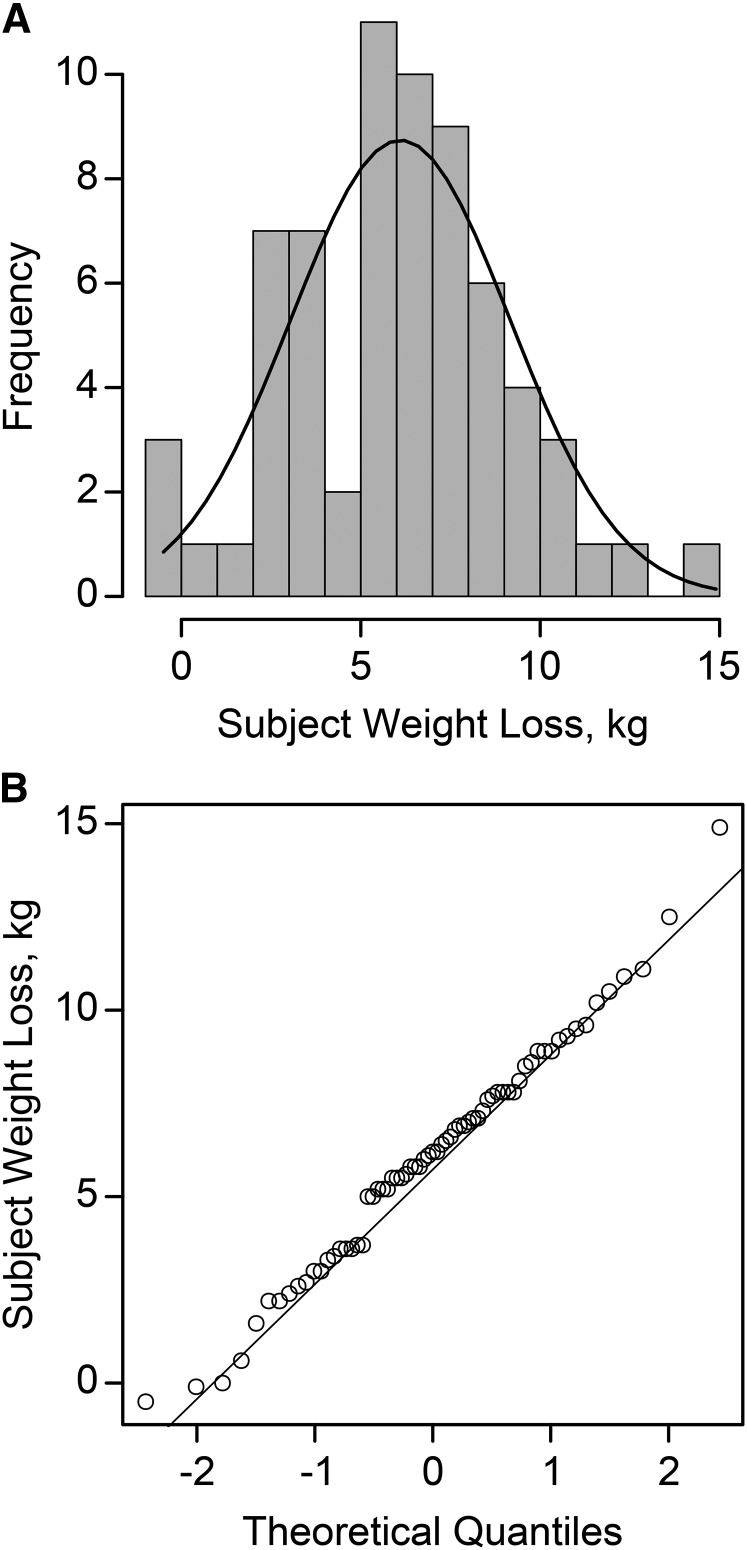
Total weight loss ranged from −0.5 to 14.9 kg and was normally distributed (Figure 2). No differences in weight loss were detected by treatment, season, gender, or initial BMI classification.
FIGURE 2.
Distribution histogram (A) and Q-Q plot (B) of total weight loss in overweight and obese subjects (n = 67) after consuming an energy deficit of 2092 kJ/d (500 kcal/d) for 12 wk. Q, quantile.
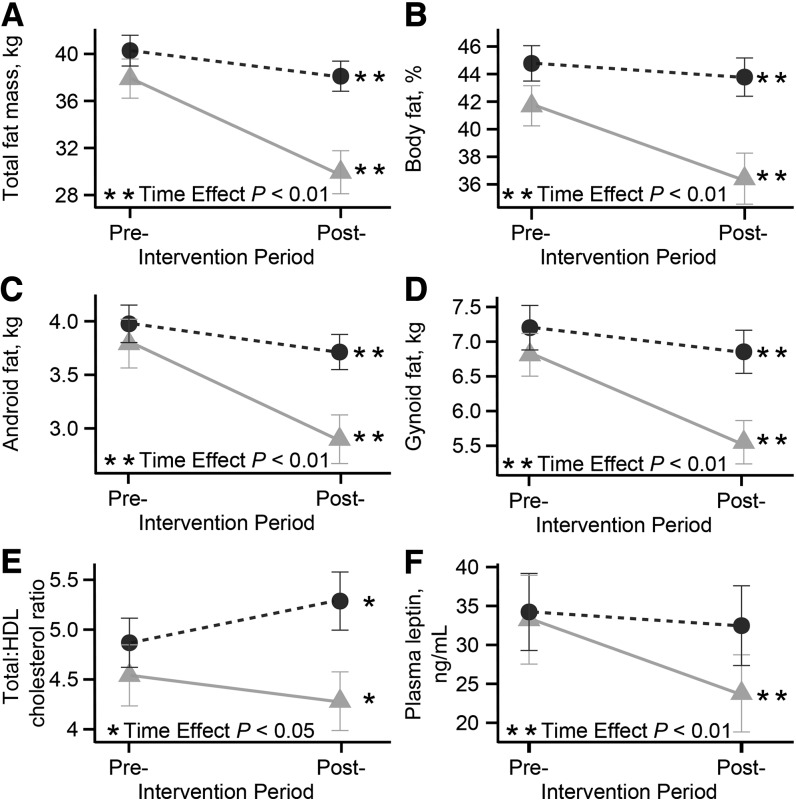
High- and low-responders did not differ by plasma insulin, glucose, TGs, or total cholesterol (Table 1). Compared with low-responders, high-responders had greater decreases over time in total body weight, BMI, total and percentage body fat, gynoid and android fat, and serum leptin concentrations (Table 1 and Figure 3). The total-to-HDL cholesterol ratio decreased in high-responders but increased in low-responders (Table 1 and Figure 3).
TABLE 1.
Clinical, serum endocrine, and energy balance characteristics of overweight and obese subjects responsive or nonresponsive to energy restriction before and after a 12 wk weight-loss intervention1
| High-responder |
Low-responder |
Linear mixed model analysis2 |
|||||
| Variable | Preintervention | Postintervention | Preintervention | Postintervention | Responder Group | Week | Interaction |
| Clinical variables | |||||||
| Total body weight, kg | 91.8 ± 12 | 82.5 ± 12 | 91.4 ± 11 | 88.7 ± 11 | 0.90 | <0.01* | <0.01* |
| BMI, kg/m2 | 32.5 ± 2.9 | 29.2 ± 3.0 | 32.4 ± 2.5 | 31.4 ± 2.3 | 0.89 | <0.01* | <0.01* |
| Total cholesterol, mg/dL | 157 ± 27 | 155 ± 29 | 162 ± 33 | 164 ± 40 | 0.67 | 0.55 | 0.40 |
| HDL cholesterol, mg/dL | 37.7 ± 13 | 38.6 ± 10 | 34.8 ± 9.9 | 32.6 ± 10 | 0.37 | 0.64 | 0.253 |
| LDL cholesterol, mg/dL | 101 ± 24 | 97.4 ± 22 | 108 ± 23 | 112 ± 32 | 0.42 | 0.27 | 0.123 |
| Total:HDL cholesterol ratio | 4.50 ± 1.4 | 4.30 ± 1.4 | 4.90 ± 1.2 | 5.30 ± 1.4 | 0.42 | 0.08 | <0.01* |
| TGs, mg/dL | 91.4 ± 39 | 93.0 ± 60 | 96.1 ± 44 | 100 ± 40 | 0.74 | 0.82 | 0.81 |
| Glucose, mg/dL | 83.5 ± 7.0 | 84.7 ± 7.1 | 82.2 ± 8.4 | 85.7 ± 9.2 | 0.60 | 0.40 | 0.28 |
| Total fat mass, kg | 37.9 ± 7.8 | 29.9 ± 8.6 | 40.3 ± 6.1 | 38.1 ± 6.0 | 0.28 | <0.01* | <0.013* |
| Body fat, % | 41.7 ± 6.8 | 36.4 ± 8.7 | 44.8 ± 6.0 | 43.8 ± 6.5 | 0.16 | <0.01* | <0.013* |
| Android fat, kg | 3.80 ± 1.1 | 2.90 ± 1.1 | 4.00 ± 0.80 | 3.70 ± 0.80 | 0.52 | <0.01* | <0.013* |
| Gynoid fat, kg | 6.80 ± 1.4 | 5.50 ± 1.5 | 7.20 ± 1.5 | 6.80 ± 1.5 | 0.38 | <0.01* | <0.013* |
| Total lean mass, kg | 49.9 ± 9.3 | 49.0 ± 9.7 | 47.1 ± 9.4 | 46.5 ± 9.7 | 0.33 | 0.01 | 0.54 |
| Endocrine variables | |||||||
| Insulin, pmol/L | 38.7 ± 1.0 | 32.1 ± 18 | 46.4 ± 26 | 46.1 ± 22 | 0.26 | 0.06 | 0.20 |
| HOMA-IR | 2.60 ± 1.4 | 2.20 ± 1.3 | 3.00 ± 1.6 | 3.20 ± 1.6 | 0.32 | 0.10 | 0.11 |
| Leptin, μg/L | 33.2 ± 26 | 23.8 ± 0.20 | 34.2 ± 23 | 32.5 ± 24 | 0.89 | <0.01* | 0.013* |
| 25-hydroxycholecalciferol, nmol/L | 36.6 ± 19 | 42.5 ± 18 | 32.3 ± 13 | 36.5 ± 10 | 0.36 | 0.01 | 0.563 |
| 1,25-dihydroxycholecalciferol, pmol/L | 126 ± 22 | 118 ± 32 | 120 ± 27 | 111 ± 26 | 0.51 | 0.19 | 0.883 |
| Energy balance variables | |||||||
| Energy intake, kJ/d | 10,700 ± 1900 | 8620 ± 1900 | 11,100 ± 1700 | 9070 ± 1700 | 0.36 | <0.01* | 0.04 |
| Energy restriction, % | 20.1 ± 3.5 | 24.3 ± 5.8 | 19.1 ± 2.7 | 23.0 ± 4.6 | 0.45 | <0.01* | 0.76 |
| Total PA-EE, kJ/d | 3650 ± 1400a | 3260 ± 1400a,b | 2910 ± 100a,b | 2610 ± 910b | 0.05 | 0.02 | 0.60 |
| Average PA-EE, kJ/min | 2.60 ± 1.2a | 2.30 ± 1.0a,b | 2.10 ± 0.70a,b | 1.80 ± 0.7b | 0.04 | 0.02 | 0.59 |
| Total activity, ACs/d | 22,000 ± 15,000 | 20,100 ± 14,000 | 17,500 ± 8300 | 13,700 ± 6000 | 0.19 | 0.29 | 0.51 |
| Average activity, ACs/min | 160 ± 110 | 144 ± 99 | 124.5 ± 58 | 97.0 ± 43 | 0.16 | 0.25 | 0.56 |
| RER | 0.81 ± 0.03b | 0.82 ± 0.03b | 0.86 ± 0.06a | 0.86 ± 0.05a | <0.01* | 0.79 | 0.943 |
| RMR-EE, kJ/d | 6780 ± 1100 | 7450 ± 1300 | 7280 ± 1500 | 7470 ± 1100 | 0.37 | 0.01* | 0.24 |
Values are means ± SDs, n = 22 per group. High-responder and low-responder represent high- and low-weight–loss tertiles, respectively. Pairwise comparisons of means were conducted on variables with a significant responder group fixed effect. Serum glucose and all serum endocrine measurements were conducted after an overnight fast. Means without a common letter differ with the use of Tukey’s honestly significant difference tests (P < 0.05). AC, activity count; PA-EE, physical activity energy expenditure; RER, respiratory exchange ratio; RMR-EE, resting metabolic rate energy expenditure; VIP, variable importance in projection.
Subject identification was input as a random effect. Statistical significance was set at P ≤ 0.05. *Statistically significant (Padjusted ≤ 0.05) after false discovery rate correction (38).
Featured in partial least squares regression modeling weight loss at a VIP cutoff of 1.5.
FIGURE 3.
Pre- and postintervention changes in selected clinical, adiposity, and endocrine indices including total fat mass (A), body fat (B), android fat (C), gynoid fat (D), total:HDL cholesterol ratio (E), and plasma leptin (F) in high- and low-weight–loss responders. Variables presented in the figure had a significant interaction term in linear mixed model analyses. High-responder (circles) and low-responder (triangles) represent high- and low-weight–loss tertiles, respectively. Serum cholesterol, HDL cholesterol, and leptin were measured after an overnight fast. Values are means ± SEMs, n = 22 for both groups. Post-, postintervention; Pre-, preintervention.
High-responders had a significantly lower RER at both pre- and postintervention than did low-responders, but had no differences in energy intake, percentage restriction of energy intake, or resting energy expenditure (Table 1). Total physical activity energy expenditure was greater in high-responders over the entire study than in low-responders. Both groups had an ∼10% decline in total physical activity after the intervention.
Identification of weight-loss discriminants.
Important discriminants of weight loss were ranked based on VIP scores calculated from the initial PLSR model (Supplemental Table 2). Subsequent PLSR models were fitted based on VIP cutoffs and assessed for model performance (Supplemental Table 3). PLSR models fitted with VIP cutoffs of 1 and 1.25 resulted in models with the lowest RMSEP of weight loss in participants in the validation set (1.62 kg and 1.63 kg for VIP score = 1 and VIP score = 1.25, respectively). However, these models still retained 33% and 23% of all variables measured, respectively. Restricting the VIP cutoff to 1.5 resulted in a PLSR model with a slightly higher RMSEP of 1.81 kg, but retained only 13% of measured variables; this was thus the model with the lowest complexity with performance statistics comparable to the inclusive PLSR model (Supplemental Table 3). This model was chosen as the optimal model.
The optimal PLSR model discriminated total weight loss with 127 variables. Many of the clinical, endocrine, and energy balance variables described above were featured in this model (e.g., total body weight, BMI, adiposity measurements, serum leptin, total-to-HDL cholesterol ratio, and RER). HDL and LDL cholesterol, 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol were also included. Conversely, whereas total physical activity energy expenditure differed between high- and low-responders, they were not featured in the PLSR model. Physical activity variables selected in the PLSR model are provided in Table 2. Based on linear mixed model analyses, only time and percentage time in sedentary and moderate activity were significantly different between high- and low-responders, with high-responders engaging in less sedentary and greater moderate activity (Table 2).
TABLE 2.
Physical activity characteristics of overweight and obese subjects responsive or nonresponsive to energy restriction before and after a 12 wk weight-loss intervention1
| High-responder |
Low-responder |
Linear mixed model analysis2 |
|||||
| Variable | Preintervention | Postintervention | Preintervention | Postintervention | Responder group | Week | Interaction |
| Time sedentary, min/d | 1000 ± 120b | 1030 ± 110a,b | 1080 ± 99a | 1100 ± 100a | 0.02 | 0.10 | 0.46 |
| Time sedentary, % | 71.8 ± 8.7 | 73.2 ± 7.6 | 77.0 ± 6.4 | 77.1 ± 6.4 | 0.02 | 0.21 | 0.39 |
| Sedentary activity, ACs/d | 3660 ± 820 | 3680 ± 930 | 3840 ± 810 | 400 ± 960 | 0.47 | 0.85 | 0.65 |
| Time light, % | 15.8 ± 4.5 | 15.4 ± 3.6 | 13.6 ± 4.0 | 14.1 ± 3.8 | 0.08 | 0.65 | 0.38 |
| Time light, min/d | 219 ± 63 | 215 ± 49 | 189 ± 56 | 199 ± 52 | 0.09 | 0.78 | 0.38 |
| Light activity, ACs/d | 19,700 ± 7000 | 19,200 ± 5400 | 16,600 ± 6200 | 17,800 ± 6000 | 0.12 | 0.76 | 0.32 |
| Light energy expenditure activity, kJ/d | 933 ± 28 | 858 ± 205 | 816 ± 290 | 828 ± 250 | 0.13 | 0.06 | 0.14 |
| Time moderate, % | 12.2 ± 5.1a | 11.9 ± 4.8a,b | 9.3 ± 3.8a,b | 8.8 ± 3.8b | 0.03 | 0.49 | 0.98 |
| Time moderate, min/d | 169 ± 71a | 166 ± 67a,b | 130 ± 52a,b | 123 ± 53b | 0.04 | 0.59 | 0.95 |
| Average moderate activity, ACs/d | 954 ± 320 | 895 ± 270 | 1050 ± 320 | 900 ± 180 | 0.26 | 0.28 | 0.25 |
| Active days, n/wk | 6.9 ± 0.9 | 6.7 ± 0.8 | 6.8 ± 0.5 | 6.2 ± 1 | 0.72 | 0.58 | 0.19 |
Values are means ± SDs, n = 22 per group. Physical activity indices featured in partial least squares regression modeling weight loss at a VIP cutoff of 1.5. Pairwise comparisons of means were conducted on variables with a significant responder group fixed effect. Means without a common letter differ with the use of Tukey’s honestly significant difference tests. AC, activity count; VIP, variable importance in projection.
Subject identification was input as a random effect. Statistical significance was set at P ≤ 0.05. No physical activity indices were found to be significant at α = 0.05 after false discovery rate correction (38).
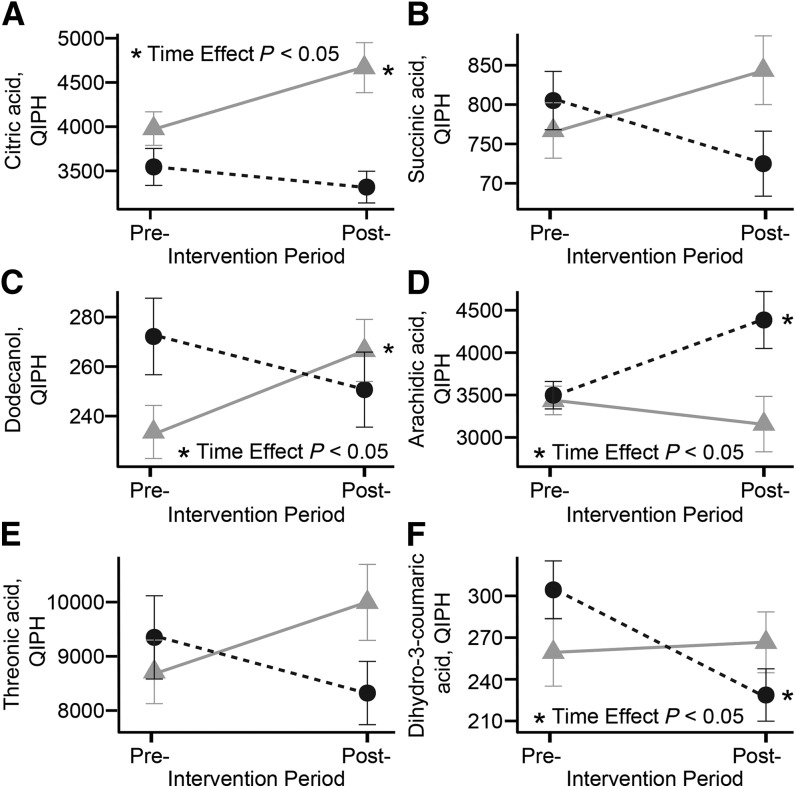
Several metabolites were found to be differentially affected in high- and low-responder groups and retained in the discriminant model. Annotated metabolites featured in the PLSR model are provided in Table 3, whereas nonannotated metabolites are provided in Supplemental Table 4. Plasma stearic (18:0), oleic (18:1n–9), and palmitic (16:0) acids were significantly greater in high- than in low-responders, likely indicative of the enhanced adiposity loss and lower RER. Additionally, the product of leucine catabolism, 2-ketoisocaproic acid, was lower, whereas myo-inositol was greater in high- than in low-responders. Threonic acid, succinic acid, citric acid, dodecanol, and dihydro-3-coumaric acid either increased or were unchanged in high-responders, whereas they generally decreased in low-responders (Figure 4). In contrast, arachidic acid (20:0) was unchanged in high-responders and increased in low-responders.
TABLE 3.
Plasma metabolite characteristics of overweight and obese subjects responsive or nonresponsive to energy restriction before and after a 12 wk weight-loss intervention1
| High-responder |
Low-responder |
Linear mixed model analysis2 |
|||||
| Variable | Preintervention | Postintervention | Preintervention | Postintervention | Responder loss group | Week | Interaction |
| Stearic acid, QIPH | 121,000 ± 22,000a | 111,000 ± 25,000a,b | 102,000 ± 23,000b | 101,000 ± 20,000b | 0.01 | 0.09 | 0.28 |
| Oleic acid, QIPH | 10,700 ± 5200a | 9080 ± 3700a,b | 7970 ± 3000 b | 7390 ± 3300b | 0.02 | 0.14 | 0.50 |
| 2-ketoisocaproic acid, QIPH | 1960 ± 1100b,c | 1880 ± 990c | 3450 ± 2600a,b | 3940 ± 2700a | 0.02 | 0.87 | 0.41 |
| Myo-inositol, QIPH | 13,400 ± 2700a | 13,300 ± 3200a | 11,700 ± 2400a,b | 10,600 ± 1900b | 0.03 | 0.86 | 0.30 |
| Palmitic acid, QIPH | 26,900 ± 5500a | 24,700 ± 5500a,b | 23,100 ± 6000b | 22,300 ± 6400b | 0.04 | 0.16 | 0.51 |
| Citric acid, QIPH | 39,800 ± 8900 | 46,700 ± 13,000 | 35,400 ± 9800 | 33,200 ± 8400 | 0.17 | 0.01 | 0.01 |
| Dihydro-3-coumaric acid, QIPH | 259 ± 110 | 267 ± 100 | 304 ± 98 | 229 ± 88 | 0.15 | 0.78 | 0.02 |
| Dodecanol, QIPH | 234 ± 50 | 266 ± 59 | 272 ± 73 | 251 ± 71 | 0.05 | 0.06 | 0.03 |
| Arachidic acid, QIPH | 3440 ± 790 | 3160 ± 1500 | 3500 ± 760 | 4380 ± 1600 | 0.87 | 0.46 | 0.03 |
| Succinic acid, QIPH | 767 ± 170 | 844 ± 200 | 805 ± 170 | 725 ± 190 | 0.50 | 0.16 | 0.04 |
| Threonic acid, QIPH | 8710 ± 2700 | 9990 ± 3300 | 9350 ± 3600 | 8320 ± 2700 | 0.50 | 0.12 | 0.05 |
| Glycerol-α-phosphate, QIPH | 402 ± 120 | 419 ± 100 | 350 ± 65 | 374 ± 77 | 0.07 | 0.53 | 0.86 |
| Conduritol-β-epoxide, QIPH | 1960 ± 1100 | 1780 ± 1100 | 1470 ± 700 | 1260 ± 590 | 0.07 | 0.44 | 0.94 |
| 4-deoxythreonic acid/4-deoxyerythronic acid, QIPH | 1650 ± 600 | 1830 ± 1000 | 1260 ± 510 | 1420 ± 700 | 0.09 | 0.26 | 0.92 |
| Isoheptadecanoic acid, QIPH | 2310 ± 500 | 2100 ± 440 | 2060 ± 540 | 1910 ± 480 | 0.10 | 0.10 | 0.73 |
| 1-monopalmitin, QIPH | 169 ± 33 | 185 ± 44 | 188 ± 47 | 210 ± 52 | 0.14 | 0.22 | 0.78 |
| Cholesterol, QIPH | 402,000 ± 220,000 | 351,000 ± 180,000 | 308,000 ± 230,000 | 303,000 ± 240,000 | 0.16 | 0.07 | 0.25 |
| 3-hydroxybutanoic acid, QIPH | 12,400 ± 7200 | 18,700 ± 17,000 | 10,800 ± 9500 | 11,500 ± 8200 | 0.64 | 0.03 | 0.17 |
| Uric acid, QIPH | 37,100 ± 15,000 | 33,300 ± 14,000 | 31,400 ± 12,000 | 31,300 ± 15,000 | 0.19 | 0.11 | 0.27 |
| Proline, QIPH | 91,700 ± 37,000 | 85,900 ± 39,000 | 111,000 ± 77,000 | 114,000 ± 41,000 | 0.22 | 0.70 | 0.66 |
| N-acetylmannosamine, QIPH | 320 ± 96 | 336 ± 86 | 351 ± 68 | 349 ± 70 | 0.22 | 0.52 | 0.62 |
| Methionine sulfoxide, QIPH | 5870 ± 2600 | 6440 ± 2600 | 4900 ± 3100 | 4180 ± 2400 | 0.24 | 0.46 | 0.24 |
| Urea, QIPH | 158,000 ± 77,000 | 200,000 ± 100,000 | 198,000 ± 120,000 | 206,000 ± 150,000 | 0.27 | 0.16 | 0.42 |
| Phosphoric acid, QIPH | 91,500 ± 17,000 | 90,700 ± 23,000 | 86,200 ± 25,000 | 94,700 ± 31,000 | 0.48 | 0.90 | 0.33 |
| Behenic acid, QIPH | 468 ± 190 | 471 ± 170 | 588 ± 240 | 673 ± 770 | 0.35 | 0.98 | 0.64 |
| 3-methoxytyrosine, QIPH | 226 ± 49 | 247 ± 46 | 217 ± 53 | 215 ± 80 | 0.62 | 0.22 | 0.35 |
| 5-β-cholestanol, QIPH | 180 ± 92 | 188 ± 71 | 213 ± 200 | 176 ± 130 | 0.41 | 0.81 | 0.36 |
| 5-methoxytryptamine, QIPH | 466 ± 200 | 553 ± 500 | 580 ± 680 | 565 ± 340 | 0.42 | 0.54 | 0.61 |
| β-alanine, QIPH | 944 ± 360 | 833 ± 310 | 927 ± 410 | 893 ± 240 | 0.87 | 0.24 | 0.57 |
| Taurine, QIPH | 3980 ± 1800 | 4110 ± 1800 | 4020 ± 3400 | 3810 ± 2000 | 0.96 | 0.84 | 0.72 |
Values are means ± SDs, n = 22 per group. Annotated metabolites featured in partial least squares regression modeling weight loss at a VIP cutoff of 1.5. Pairwise comparisons of means were conducted on variables with a significant responder group fixed effect. All plasma metabolite measurements were conducted after an overnight fast. Means without a common letter differ with the use of Tukey’s honestly significant difference tests. QIPH, quantifier ion peak height; VIP, variable importance in projection.
Subject identification was input as a random effect. Statistical significance was set at P ≤ 0.05. No metabolites were found to be significant at α = 0.05 after false discovery rate correction (38).
FIGURE 4.
Pre- and postintervention changes in selected plasma metabolites, including citric acid (A), succinic acid (B), dodecanol (C), arachidic acid (D), threonic acid (E), and dihydro-3-coumaric acid (F) in high- and low-weight–loss responders. Metabolites presented in the figure had a significant interaction term in linear mixed model analyses. High-responder (circles) and low-responder (triangles) represent high- and low-weight–loss tertiles, respectively. Plasma metabolites were measured after an overnight fast. Values are means ± SEMs, n = 22 for both groups. Post-, postintervention; Pre-, preintervention; QIPH, quantifier ion peak height.
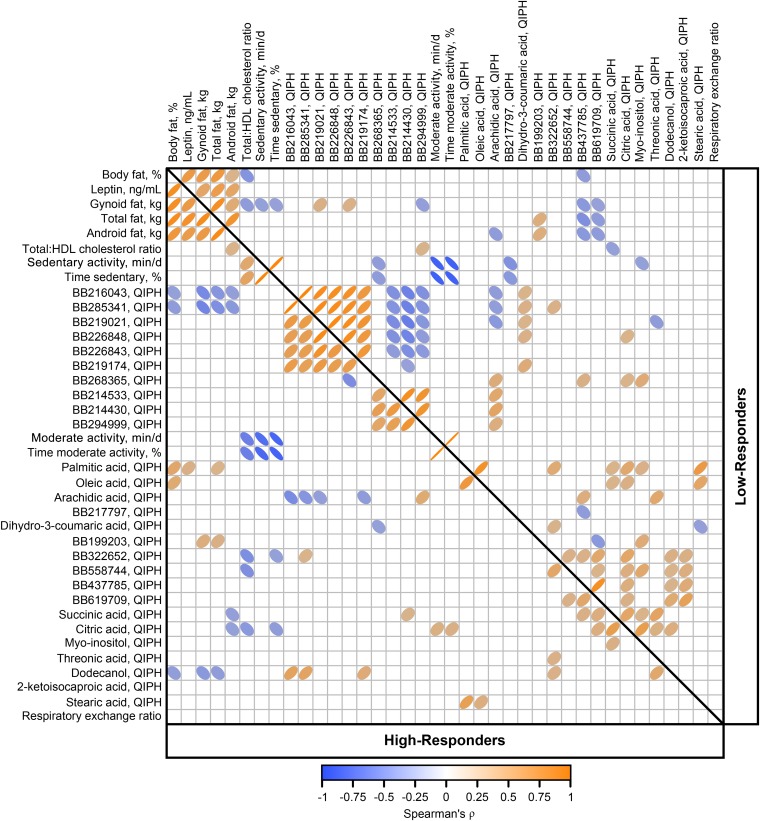
Variables featured in the final model that were significantly different between high- and low-responders were combined into a Spearman correlation matrix to assess factor inter-relations (Figure 5). Body fat indices, physical activity variables, tricarboxylic acids, and many nonannotated metabolites were highly correlated. In high- but not low-responders, oleic and palmitic acids were positively correlated to percentage body fat, whereas palmitic acid was also correlated to total fat and leptin in high-responders. Conversely, oleic and palmitic acids were positively correlated with succinic and citric acids in low- but not high-responders. The total-to-HDL cholesterol ratio was negatively correlated to percentage body fat and gynoid fat in low-responders, but not in high-responders and, although not apparent in low-responders, the ratio was positively correlated to sedentary activity and negatively correlated to moderate activity in high-responders.
FIGURE 5.
Spearman correlation matrix of variables associated with weight-loss responsiveness in overweight and obese men and women (n = 67) consuming an energy deficit of 2092 kJ/d (500 kcal/d) for 12 wk. Variables were selected by partial least squares regression as discriminant of weight loss and found to be significantly different between high- and low-responders in linear mixed model analyses. High-responder and low-responder represent high- and low-weight–loss tertiles (n = 22 for both groups), respectively. Direction or color of ellipses represent positive or negative correlation, respectively. Darker color and thinner ellipses represent strength (Spearman’s ρ) of correlations. Only significant correlations at α = 0.05 are displayed. BB, BinBase identification numbers; QIPH, quantifier ion peak height.
Discussion
An integrative approach allowed us to identify multiple physiologic and behavioral factors associated with higher weight loss in overweight and obese subjects consuming a 2092 kJ/d (500 kcal/d) energy deficit over 12 wk. We observed distinct differences in factors associated with lipid distribution and oxidation in subjects losing the greatest amount of weight as compared with those who lost the least. As expected, subjects with the greatest weight loss had accelerated losses in regional and total adiposity. The observation that high-responders have a lower RER and elevated serum FAs is consistent with other reports of increased FA oxidation in individuals more responsive to dietary energy restriction (2, 14, 46). The difference in RER is particularly interesting because the preintervention RER was measured during the weight maintenance phase of the study, and thus before energy restriction–associated changes in RER.
Individual differences in habitual activity may be a key factor in determining an individual’s response to weight-loss intervention. We suspect that engagement in moderate activity as opposed to sedentary activity was the driving force behind the high-responders’ apparent ability to better mobilize and utilize their lipid reserves compared with low-responders. It is well known that physical activity leads to weight loss with energy restriction (47–49) and is a key treatment option for metabolic syndrome and insulin resistance (50, 51). Our results suggest that physical activity results in a beneficial metabolic phenotype, possibly manifested through improved skeletal muscle lipid oxidation (52).
By leveraging metabolomics, we observed several plasma metabolites that were significantly different between high- and low-responders, indicating that this phenotype has an impact on metabolism beyond lipids. For example, high-responders had lower 2-ketoisocaproic acid abundance than did low-responders. This metabolite is a direct product of leucine catabolism and may suggest higher mitochondrial flux of this amino acid through the rate-limiting branched chain ketoacid dehydrogenase complex in branched-chain amino acid–consuming tissues. Intriguingly, higher plasma concentrations of leucine, other branched-chain amino acids, and branched-chain ketoacids have been correlated with insulin resistance in humans (25, 53, 54) and reduced activity of the branched chain ketoacid dehydrogenase complex associated with the insulin-resistant state may be the mechanism behind these observations (55).
Tricarboxylic acids succinate and citrate were disparately regulated in high- and low-responders and negatively correlated to oleic and palmitic acid in low-responders. Increases in citrate and succinate oxidation have been noted in skeletal muscle biopsies from sedentary obese adults after a 16 wk moderate-exercise intervention (56); thus, these observations may suggest alterations in tricarboxylic acid cycle dynamics related to FA oxidation. Still, it is difficult to conclusively relate plasma changes of carboxylic acids to changes in tissue mitochondria, and a targeted analysis of muscle biopsies would be required to confirm this interpretation.
Although metabolomics allowed us to link biological determinants of weight loss to behavioral and clinical outcomes, it is also accompanied by additional study limitations. The statistical analysis of such large datasets is subject to over-fitting and over-interpretation because of the “small n/large P” paradigm. We have attempted to minimize model over-fitting through data training/test splits. Furthermore, global metabolomic results should be considered to be hypothesis-generating until confirmed with more targeted approaches. Additionally, this study was a secondary study from a tightly controlled feeding study and, although biological variation is likely reduced, we may not have enough power to assess true high-responders from low-responders. Moreover, these subjects were overweight and obese, but still considered metabolically healthy. Therefore, our results may not extend to overweight and obese individuals with metabolic disorders. Lastly, we did not have access to daily physical activity data and report measurements over a 24 h period (including activity associated with sleeping). Therefore, when considering physical activity associated with waking hours, sedentary activity measurements reported herein are overestimated whereas time-adjusted light, moderate, and vigorous physical activity indices are underestimated.
One exciting, yet challenging outcome of the current research was the observation that the majority of determinants of weight loss were metabolites that do not have full structural identification and annotation. Uniform correlations among these metabolites suggest they are either associated with similar metabolic processes or partially derivatized products of a single or highly-related compounds. Additionally, many of these unknown metabolites had strong correlations with body composition markers and other known metabolites, making their identification a priority for future studies.
In summary, we assessed blood metabolomics and clinical chemistry, along with hormonal and inflammatory biomarkers, body composition, and dietary and physical activity measurements to identify a broad range of variables that characterize weight loss in overweight and obese adults consuming a 12 wk energy-restricted diet in a controlled feeding intervention. With the use of multivariate analyses, ∼13% of the measured pre- and postintervention variables could be used to accurately model weight loss. Our findings suggest that overweight and obese individuals highly responsive to energy restriction are characterized by greater losses in total and regional adiposity that are supported by a lower RER and characterized by greater lipid mobilization. Person-to-person differences in habitual moderate physical activity and sedentary behavior are associated with this phenotype. The current research supports the idea that persons who incorporate regular light-to-moderate physical activity into daily activity will benefit most in terms of total weight and adipose reduction achieved by dietary interventions.
Supplementary Material
Acknowledgments
NLK, SHA, and MDVL designed the research; BDP, NLK, OF, SHA, and MDVL conducted the research; NLK, OF, SHA, and MDVL provided essential reagents and materials; BDP and JWN analyzed the data; BDP, NLK, SHA, MDVL, and JWN interpreted the data; BDP wrote the manuscript; and BDP and JWN had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AC, activity count; FDR, false discovery rate; PLSR, partial least squares regression; RER, respiratory exchange ratio; RMSEP, root mean square error of prediction; VCO2, carbon dioxide production; VIP, variable importance in projection; VO2, oxygen consumption.
References
- 1.Adamo KB, Dent R, Langefeld CD, Cox M, Williams K, Carrick KM, Stuart JS, Sundseth SS, Harper ME, McPherson R, et al. . Peroxisome proliferator-activated receptor gamma 2 and acyl-CoA synthetase 5 polymorphisms influence diet response. Obesity (Silver Spring) 2007;15:1068–75. [DOI] [PubMed] [Google Scholar]
- 2.Gerrits MF, Ghosh S, Kavaslar N, Hill B, Tour A, Seifert EL, Beauchamp B, Gorman S, Stuart J, Dent R, et al. . Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J Lipid Res 2010;51:2394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo T, Nakata Y, Katayama Y, Iemitsu M, Maeda S, Okura T, Kim MK, Ohkubo H, Hotta K, Tanaka K. PPARG genotype accounts for part of individual variation in body weight reduction in response to calorie restriction. Obesity (Silver Spring) 2009;17:1924–31. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo T, Nakata Y, Murotake Y, Hotta K, Tanaka K. Effects of FTO genotype on weight loss and metabolic risk factors in response to calorie restriction among Japanese women. Obesity (Silver Spring) 2012;20:1122–6. [DOI] [PubMed] [Google Scholar]
- 5.Williamson DA, Anton SD, Han H, Champagne CM, Allen R, Leblanc E, Ryan DH, Rood J, McManus K, Laranjo N, et al. . Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. J Behav Med 2010;33:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya SD, Elci OU, Sereika SM, Music E, Styn MA, Turk MW, Burke LE. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence 2009;3:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira PJ, Silva MN, Coutinho SR, Palmeira AL, Mata J, Vieira PN, Carraca EV, Santos TC, Sardinha LB. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity (Silver Spring) 2010;18:725–35. [DOI] [PubMed] [Google Scholar]
- 8.Stubbs J, Whybrow S, Teixeira P, Blundell J, Lawton C, Westenhoefer J, Engel D, Shepherd R, McConnon A, Gilbert P, et al. . Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obes Rev 2011;12:688–708. [DOI] [PubMed] [Google Scholar]
- 9.Jakicic JM. The effect of physical activity on body weight. Obesity (Silver Spring) 2009;17: Suppl 3:S34–8. [DOI] [PubMed] [Google Scholar]
- 10.DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. Effect of Physical Activity on Weight Loss, Energy Expenditure, and Energy Intake During Diet Induced Weight Loss. Obesity (Silver Spring) 2014;22:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray GA, Smith SR, DeJonge L, de Souza R, Rood J, Champagne CM, Laranjo N, Carey V, Obarzanek E, Loria CM. Effect of diet composition on energy expenditure during weight loss: the POUNDS LOST Study. Int J Obes (Lond) 2012;36:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis AC, Hyatt TC, Hunter GR, Gower BA. Respiratory quotient predicts fat mass gain in premenopausal women. Obesity (Silver Spring) 2010;18:2255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259:E650–7. [DOI] [PubMed] [Google Scholar]
- 14.Harper ME, Dent R, Monemdjou S, Bezaire V, Van Wyck L, Wells G, Kavaslar GN, Gauthier A, Tesson F, McPherson R. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes 2002;51:2459–66. [DOI] [PubMed] [Google Scholar]
- 15.Verdich C, Toubro S, Buemann B, Holst JJ, Bulow J, Simonsen L, Sondergaard SB, Christensen NJ, Astrup A. Leptin levels are associated with fat oxidation and dietary-induced weight loss in obesity. Obes Res 2001;9:452–61. [DOI] [PubMed] [Google Scholar]
- 16.Reinehr T, Kleber M, de Sousa G, Andler W. Leptin concentrations are a predictor of overweight reduction in a lifestyle intervention. Int J Pediatr Obes 2009;4:215–23. [DOI] [PubMed] [Google Scholar]
- 17.Labayen I, Ortega FB, Ruiz JR, Lasa A, Simón E, Margareto J. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J Clin Endocrinol Metab 2011;96:E996–1000. [DOI] [PubMed] [Google Scholar]
- 18.Van Loan MD, Keim NL, Adams SH, Souza E, Woodhouse LR, Thomas A, Witbracht M, Gertz ER, Piccolo B, Bremer AA, et al. . Dairy foods in a moderate energy restricted diet do not enhance central rat, weight, and intra-abdominal adipose tissue losses nor reduce adipocyte size or inflammatory markers in overweight and obese adults: A controlled feeding study. J Obes 2011;143:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine (US); Panel on Macronutrients. Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press, 2005. [Google Scholar]
- 20.Baecke JA, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–42. [DOI] [PubMed] [Google Scholar]
- 21.Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep 2011;22:15–23. [PubMed] [Google Scholar]
- 22.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo BD, Dolnikowski G, Seyoum E, Thomas AP, Gertz ER, Souza EC, Woodhouse LR, Newman JW, Keim NL, Adams SH, et al. . Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients 2013;5:3352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labouesse MA, Gertz ER, Piccolo BD, Souza EC, Schuster GU, Witbracht MG, Woodhouse LR, Adams SH, Keim NL, Van Loan MD. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone 2014;64:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 2010;5:e15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiehn O, Wohlgemuth G, Scholz M. Setup and annotation of metabolomic experiments by integrating biological and mass spectrometric metadata: Springer, 2005. [Google Scholar]
- 27.Scholz M, Fiehn O. SetupX–a public study design database for metabolomic projects. Pac Symp Biocomput 2007:169–80. [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. 2010. [Google Scholar]
- 29.Gross J, Ligges U.. nortest: Tests for normality. R package version 10–2 2012. [Google Scholar]
- 30.Dabney A, Storey JD, Warnes GR. qvalue: Q-value estimation for false discovery rate control. R package version 1.32.0. [Google Scholar]
- 31.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics 2001;17:520–5. [DOI] [PubMed] [Google Scholar]
- 32.Wong J. imputation: imputation. R package version 2.0.1 [Internet]. c2013 [cited 2015 Jan 9]. Available from: http://CRAN.R-project.org/package=imputation.
- 33.Mevik BH, Wehrens R. The pls package: Principal component and partial least squares regression in R. J Stat Softw 2007;18. [Google Scholar]
- 34.Mehmood T, Liland KH, Snipen L, Saebo S. A review of variable selection methods in Partial Least Squares Regression. Chemometr Intell Lab 2012;118:62–9. [Google Scholar]
- 35.Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab 2001;58:109–30. [Google Scholar]
- 36.Pinheiro J, Bates D, DebRoy S, R Development Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2013;R package version 3.1–111. [Google Scholar]
- 37.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests for random and fixed effects for linear mixedeffects models (lmer objects of lme4 package). R package version 2.0–11 [Internet]. c2014 [cited 2015 Jan 9]. Available from: http://CRAN.R-project.org/package=lmerTest.
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995;57:289–300. [Google Scholar]
- 39.Wickham H. Reshaping data with the reshape package. J Stat Softw 2007;21:1–20. [Google Scholar]
- 40.Wickham H. The split-apply-combine strategy for data analysis. J Stat Softw 2011;40:1–29. [Google Scholar]
- 41.Wickham H. Ggplot2: elegant graphics for data analysis. New York: Springer, 2009. [Google Scholar]
- 42.Arnold JB. ggthemes: Extra themes, scales and geoms for ggplot. R package version 1.7.0 [Internet]. c2014 [cited 2015 Jan 9]. Available from: http://CRAN.R-project.org/package=ggthemes.
- 43.Gohel D. ReporteRs: Microsoft Word, Microsoft Powerpoint and HTML documents generation from R. R package version 0.5.5 [Internet]. c2014 [cited 2015 Jan 9]. Available from: http://CRAN.R-poject.org/package=ReporteRs.
- 44.Graves S, Piepho H-P, Selzer L, Dorai-Raj S. multcompView: Visualizations of Paired Comparisons. R package version 0.1–5 [Internet]. c2012 [cited 2015 Jan 9]. Available from: http://CRAN.R-project.org/package=multcompView.
- 45.Harrell FE., Jr Hmisc: Harrell Miscellaneous. R package version 3.14–4 [Internet]. c2014 [cited 2015 Jan 9]. Available from: http://CRAN.R-project.org/package=Hmisc.
- 46.Keim NL, Barbieri T, Van Loan M. Physiological and biochemical variables associated with body fat loss in overweight women. Int J Obes 1991;15:283–93. [PubMed] [Google Scholar]
- 47.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, Thomas SB, Brown J, McTigue K, Hames KC, et al. . Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010;304:1795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000;133:92–103. [DOI] [PubMed] [Google Scholar]
- 50.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 2003;52:2191–7. [DOI] [PubMed] [Google Scholar]
- 51.Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 2011;111:1554–60. [DOI] [PubMed] [Google Scholar]
- 52.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 2005;115:1699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. . Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK, Fleischman A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatric obesity 2013;8(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2011;2:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 2005;288:E818–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.