Abstract
Background: Low circulating 25-hydroxyvitamin D [25(OH)D] is prevalent in African Americans, but predictors of vitamin D status are understudied compared to Caucasian populations.
Objective: We investigated whether certain environmental and genetic factors are predictors of circulating 25(OH)D in 989 elderly African Americans participating in the Health, Aging, and Body Composition (Health ABC) Study.
Methods: Regression analysis estimated the cross-sectional association of nongenetic (environmental) factors with 25(OH)D. Single nucleotide polymorphisms (SNPs) associated with 25(OH)D in Caucasian genome-wide association studies (GWASs) were analyzed for association with serum 25(OH)D, including analyses of all imputed SNPs in identified genomic regions. Genome-wide complex trait analysis (GCTA) evaluated the association of all (genome-wide) genotyped SNPs with serum 25(OH)D in the Health ABC Study with replication in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort.
Results: Gender, study site, season of blood draw, body mass index, dietary supplement use, dairy and cereal consumption, Healthy Eating Index score, and walking >180 min/wk were associated with 25(OH)D (P < 0.05), jointly explaining 25% of the variation in circulating 25(OH)D. Multivitamin supplement use was the strongest predictor of circulating 25(OH)D, and supplement users had a 6.3-μg/L higher serum 25(OH)D concentration compared with nonusers. Previous GWAS-identified gene regions were not replicated in African Americans, but the nonsynonymous rs7041 SNP in group-specific component (vitamin D binding protein) was close to significance thresholds (P = 0.08), and there was evidence for an interaction between this SNP and use of multivitamin supplements in relation to serum 25(OH)D concentration (P = 0.04). Twenty-three percent (95% CI: 0%, 52%) of the variation in serum 25(OH)D was explained by total genetic variation in a pooled GCTA of 2087 Health ABC Study and MESA African-American participants, but population substructure effects could not be separated from other genetic influences.
Conclusions: Modifiable dietary and lifestyle predictors of serum 25(OH)D were identified in African Americans. GCTA confirms that a proportion of 25(OH)D variability is attributable to genetic variation, but genomic regions associated with the 25(OH)D phenotype identified in prior GWASs of European Americans were not replicated in the Health ABC Study in African Americans.
Keywords: vitamin D, 25-hydroxyvitamin D, supplements, GC, GCTA, rs7041
Introduction
In addition to well-known roles in calcium absorption and skeletal outcomes, vitamin D regulates over 900 genes involved in physiologic functions throughout the body (1, 2). Serum 25-hydroxyvitamin D [25(OH)D]13, the major circulating biomarker of vitamin D status (1), is converted to active vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D] primarily in the kidney, but conversion also occurs in extra-renal tissues throughout the body (3). Serum 25(OH)D is derived from dietary intake (food or supplements) and skin exposure to ultraviolet radiation (1, 4).
Approximately 30% of Americans are at risk of inadequate or deficient serum 25(OH)D, according to a recent NHANES report (5). Both African-American and elderly populations are at high risk of vitamin D inadequacy partly because of a lower capacity for endogenous synthesis of vitamin D from sunlight (4, 6). In the NHANES, 73% of non-Hispanic black adults were at risk of inadequate vitamin D [defined as serum 25(OH)D <20 μg/L] compared with only 21% of non-Hispanic white adults (5).
Although genetic and nongenetic predictors of 25(OH)D have been well described in Caucasian populations (7–14), fewer studies have focused on determinants of 25(OH)D in African Americans. Several modifiable predictors of serum 25(OH)D have been identified in African Americans, including intake of vitamin D–containing foods and supplements, sun exposure, and BMI (10, 15–21). Vitamin D status is heritable, and heritability estimates from twin and family-based studies range from 28% to 80%; although most estimates derive from Caucasian populations (22–25), a study in 42 African-American families reported a heritability coefficient of 28% (17). Genome-wide association studies (GWASs) in Caucasians identified genetic predictors of vitamin D status that explain between 1% and 4% of phenotypic variability (7, 8). In African Americans, only 2 published population-based candidate gene studies, limited by small sample size or male-only study populations, investigated genetic predictors of vitamin D status (26, 27).
To date, to our knowledge no studies have investigated the respective contribution of both genetic and nongenetic predictors to variability in serum 25(OH)D in elderly African Americans. Given that this population is at risk of vitamin D inadequacy, it is important to understand the relative contributions of modifiable and nonmodifiable predictors of serum 25(OH)D. We hypothesized that both environmental and genetic factors contribute to circulating serum 25(OH)D status in an elderly African-American population, and our objective was to estimate the variability explained by genes and environment, respectively.
Methods
Study population.
The primary analyses were in the Health, Aging, and Body Composition (Health ABC) Study, which comprises 3075 participants recruited between April 1997 and June 1998, aged 70–79 y at baseline, and selected as a random sample of whites and all black Medicare-eligible residents of zip codes in and around Memphis, Tennessee, and Pittsburgh, Pennsylvania. Eligibility criteria included the ability to walk 0.25 mile (0.4 km), climb 10 stairs, and perform activities of daily living without difficulty. Additionally, eligible participants were required to be free of life-threatening disease with the intent to stay in the area for ≥3 y (20). The Institutional Review Boards at the University of Memphis and the University of Pittsburgh granted approval to conduct the Health ABC Study, and all participants provided written informed consent. The Cornell University Committee on Human Subjects approved the study reported herein.
The Health ABC Study comprised 1281 African-American participants. For the current study, participants without the 12-mo follow-up exam (n = 46) were excluded because of missing data on both serum 25(OH)D and dietary intake. Additional exclusion criteria include missing a serum 25(OH)D measurement (n = 126), abnormally high serum 25(OH)D [defined as 25(OH)D ≥150 μg/L; n = 1], and end-stage kidney failure (defined as glomerular filtration rate <15; n = 3). A total of 116 participants were missing key dietary data, thus, 989 participants comprised the sample for nongenetic analysis. A total of 980 participants had genotype and serum 25(OH)D data, which comprised the sample for genetic analyses.
Genome-wide analyses were replicated in the Multi-Ethnic Study of Atherosclerosis (MESA), which is a multicenter, prospective cohort study of clinical and subclinical cardiovascular disease. The MESA cohort was comprised of 6815 men and women, aged 45–85 y, and free of clinical cardiovascular disease at the first examination (2000–2002) (28). In this study we included the 1198 African-American participants with both genotype and serum 25(OH)D data from the first examination. Institutional Review Board approval was granted at each study site and written informed consent was obtained from each participant.
Data collection.
In the Health ABC Study, data on gender, education, smoking status, and other covariates were collected from a baseline survey administered by trained interviewers. BMI and physical activity (self-report, minutes spent walking/wk) were obtained from data collected at the 12-mo visit.
Trained interviewers assessed dietary intake at the 12-mo visit using a Block FFQ modified for the Health ABC Study (Block Dietary Data Systems). Nutrient intakes and daily servings of food groups were estimated, and food group information was used to calculate a Healthy Eating Index (HEI) score ranging from 0 to 100 (29). The HEI estimates how well each participant’s diet matches US Dietary Guidelines; the Health ABC Study HEI scores were calculated based on compliance with the 1992 USDA Food Guide Pyramid (29, 30) and were grouped into “good” (HEI score ≥81), “needs improvement” (51–80), and “poor” (<51) (29). Dietary supplement use was also assessed at the 12-mo visit. Further details are provided elsewhere (20, 29).
In the Health ABC Study, serum 25(OH)D was measured in fasting blood samples collected at the 12-mo visit. A 2-step radioimmunoassay kit was used to measure 25(OH)D concentrations (25-hydroxyvitamin D 125I RIA kit; DiaSorin), with an interassay coefficient of variation of 6.78% (20). In the MESA, serum 25(OH)D was assayed by HPLC-tandem mass spectrometry [Waters Xevo TQ mass spectrometer; further details provided elsewhere (31)]. Season of blood draw was defined as winter (December to February), spring (March to May), summer (June to August), and fall (September to November).
The Illumina Human 1M-Duo custom chip was used for genotyping in the Health ABC Study; race-specific genotype imputation was performed with MACH version 1.0.16 using reference panel data from HapMap release 22 Build 36 (32). In the MESA replication cohort, the Affymetrix Genome-Wide Human 6.0 array was used for genotyping; genotypes were defined with use of the Birdseed calling algorithm (33). Race-specific genotype imputation was performed with IMPUTE2, as described elsewhere (34, 35). Studied single nucleotide polymorphisms (SNPs) were required to have minor allele frequency (MAF) >1%, Hardy-Weinberg P values >1 × 10−6, imputation quality scores >0.3 (for analyses using imputed SNP data), and call rates >95%. Principal components of ancestry were previously computed for both the Health ABC Study and MESA (35, 36).
Statistical analysis.
Regression analysis explored associations of demographic, dietary, and environmental predictors hypothesized to contribute to variation in serum 25(OH)D. Predictors associated with log-transformed 25(OH)D at P < 0.05 were further evaluated in multivariate models to determine a final set of variables jointly associated with log-transformed 25(OH)D. All multivariate models were adjusted for age, gender, study site, and season of blood draw.
Normality plots revealed a slightly right-skewed distribution of serum 25(OH)D. However, multivariate model results were equivalent for natural log-transformed and untransformed serum 25(OH)D phenotypes, and thus results for the untransformed serum 25(OH)D outcome are presented for ease of interpretation.
Single SNPs previously associated with 25(OH)D in the GWAS of Caucasians (7, 8) (see Supplemental Methods for details) were tested for replicative associations (considered P < 0.05) with serum 25(OH)D in ordinary least squares linear regression models, and imputed genotypes (also referred to as imputed SNPs) on identified genes were also tested. Models for log-transformed vs. untransformed serum 25(OH)D phenotypes were equivalent, thus, results are presented for the untransformed serum 25(OH)D phenotype for ease of interpretation. SNP by nutrient interactions were tested through the addition of an interaction term to the multivariate model; interaction analyses only tested SNP interactions with variables that were important predictors of serum 25(OH)D because of power limitations. All models estimating genetic-serum 25(OH)D associations were adjusted for age, gender, study site, season of blood draw, and principal components (population substructure).
SAS version 9.3 (SAS Institute) was used for all regression analyses, and all statistical tests were 2-sided.
An analysis to estimate the overall genetic contribution to serum 25(OH)D variability used all genotyped SNPs (genome-wide) with an MAF >1% tested jointly for association with serum 25(OH)D. This analysis used genome-wide complex trait analysis (GCTA) software, which is based on a linear mixed model approach [v. 1.04; for details of the method see Yang et al. (37)] to estimate the variance in serum 25(OH)D explained by additive genetic variation. Ninety-one distantly related Health ABC Study participants (genetic relatedness score >0.05) were removed from the data set before analysis, leaving a total of 889 participants for the GCTA. The GCTA was replicated in 1198 African Americans in the MESA, after excluding 136 distantly related participants. Models in both cohorts were adjusted for age, gender, study site, and season of blood draw, and were run with and without further adjustment for ancestry principal components; the log-transformed serum 25(OH)D phenotype was used for comparability across cohorts. GCTA estimates from the 2 cohorts were meta-analyzed with METAL software (38) with use of an inverse-variance weighted meta-analysis model.
Results
The mean serum 25(OH)D concentration in Health ABC Study African Americans was 20.7 μg/L (Table 1), and the prevalence of risk of 25(OH)D insufficiency [defined as serum 25(OH)D <20 μg/L (1)] was 55%. Serum 25(OH)D characteristics among African Americans in the MESA replication cohort were similar (mean: 19.0 μg/L; prevalence of participants at risk of vitamin D insufficiency: 60%). Health ABC Study participant characteristics for African Americans are further described in Table 1.
TABLE 1.
Population characteristics of African-American (n = 989) participants of the Health, Aging, and Body Composition Study1
| Variable | Values |
| Serum 25(OH)D, μg/L | 20.7 ± 9.0 |
| Age, y | 74.5 ± 2.9 |
| Female, % | 57.3 |
| Study site, % Pittsburgh | 55.2 |
| Season of blood draw, % | |
| Winter | 23.6 |
| Spring | 31.9 |
| Summer | 17.4 |
| Fall | 27.2 |
| Current smokers, % | 14.6 |
| BMI, kg/m2 | 28.6 ± 5.5 |
| BMI category, % | |
| <25 | 26.1 |
| 25–30 | 38.7 |
| >30 | 35.2 |
| Dietary vitamin D intake, IU/d | 197 ± 143 |
| Dietary calcium intake, mg/d | 769 ± 413 |
| Vitamin D supplement, % | 5.8 |
| Multivitamin, % | 23.9 |
| Calcium supplement, % | 10.9 |
| Dairy consumption, % | |
| No dairy | 32.3 |
| 1–3 servings/d | 63.6 |
| >3 servings/d | 4.2 |
| Cereal consumption, % | |
| No cereal | 12.6 |
| 1–4 times/mo | 32.9 |
| >1 time/wk | 54.5 |
| Healthy eating score, % | |
| Poor | 10.7 |
| Needs improvement | 76.2 |
| Good | 13.0 |
| Time walking, min/wk | 107 ± 222 (median = 10) |
| Walking time >180 min/wk, % | 3.3 |
Values are means ± SDs or percentages for categorical variables. Population characteristics for the 980 participants in the genetic analyses are nearly identical and thus are not presented here. 25(OH)D, 25-hydroxyvitamin D.
Environmental predictors of circulating 25(OH)D.
Twenty-five percent of the variation in serum 25(OH)D in Health ABC Study African Americans was explained by a multivariate model (Table 2) including predictor variables significantly associated with 25(OH)D at P < 0.05 in single variable models. Although age had little or no association with serum 25(OH)D, male gender, residence in Memphis, and summer season of blood draw were all positively associated with 25(OH)D status. BMI had a nonlinear association with serum 25(OH)D such that the strongest inverse association of BMI with 25(OH)D status was observed in obese individuals.
TABLE 2.
Multiple linear regression coefficients for predictors of serum 25(OH)D of African-American participants (n = 989) of the Health, Aging, and Body Composition Study
| Variable | β1 | 95% CI1 | P-trend2 | R2,3 % |
| Age | 0.03 | −0.1, 0.2 | 0.70 | 0.01 |
| Male | 2.2 | 1.2, 3.3 | <0.0001 | 1.3 |
| Site, Memphis | 1.7 | 0.7, 2.7 | 0.001 | 0.8 |
| Season of blood draw | — | — | 0.02 | 0.7 |
| Summer | Referent | |||
| Winter | −2.0 | −3.6, −0.5 | — | — |
| Spring | −0.7 | −2.2, 0.7 | — | — |
| Fall | −0.1 | −1.6, 1.5 | — | — |
| BMI | 0.8 | 0.1, 1.4 | 0.02 | 0.4 |
| BMI2 | −0.01 | −0.03, 0.0 | 0.007 | 0.6 |
| Multivitamin use | 6.3 | 5.1, 7.5 | <0.0001 | 8.3 |
| Vitamin D supplement use | 5.2 | 2.5, 7.9 | 0.0002 | 1.1 |
| Calcium supplement use | 3.9 | 1.9, 6.0 | 0.0002 | 1.1 |
| Healthy Eating Index | — | — | 0.03 | 0.6 |
| Poor, <51 | Referent | |||
| Needs improvement, 51–80 | 2.0 | 0.3, 3.6 | — | — |
| Good, ≥81 | 2.8 | 0.7, 4.9 | — | — |
| Dairy consumption | — | — | 0.0008 | 1.1 |
| No dairy | Referent | |||
| 1–3 servings/ d | 2.0 | 0.9, 3.1 | — | — |
| >3 servings/d | 3.3 | 0.7, 5.9 | — | — |
| Cereal consumption | — | — | 0.004 | 0.9 |
| No cereal | Referent | |||
| ≤1 time/wk | 1.1 | −0.6, 2.7 | — | — |
| >1 time/wk | 2.5 | 0.9, 4.1 | — | — |
| Walking time >180 min/wk | 3.1 | 0.4, 5.9 | 0.03 | 0.9 |
| Total model R2 | 25.4 |
Estimated change in 25(OH)D in μg/L per unit increase in predictor variable, adjusted for all other covariates in model. 25(OH)D, 25-hydroxyvitamin D.
P-trend, adjusted for all other covariates in model.
R2 individual variables, adjusted for all other covariates in a multivariate model. Because of correlation between some variables, the sum of the individual variable R2 values does not add up to the total R2.
The strongest predictor of serum 25(OH)D status among African Americans in the Health ABC Study population was multivitamin supplement use, which accounted for about 8% of the variability; the use of either vitamin D or calcium supplements also made significant contributions to the model. Both cereal consumption and dairy consumption were significantly associated with serum vitamin D; furthermore, participants with an HEI score categorized as good had about 3-μg/L higher serum 25(OH)D concentrations compared with participants categorized as poor. Over the full range of values, physical activity had little or no association with serum 25(OH)D, but walking briskly >180 min/wk was positively associated with serum status. Total intake of vitamin D (micronutrient variable estimated from FFQ reflecting all foods/supplements containing vitamin D) was associated with serum 25(OH)D in bivariate models, but the association of the micronutrient variable was captured well by a few dietary components (e.g., dairy intake). We did not observe a significant association of smoking status with continuous serum 25(OH)D.
Genetic predictors of serum 25(OH)D.
Two recent GWASs of serum 25(OH)D in Caucasians identified genome-wide significant SNPs in or near cytochrome P450, family 2, subfamily R, polypeptide 1 (CYP2R1), group-specific component (vitamin D binding protein) (GC), and 7-dehydrocholesterol reductase (DHCR7)/NAD synthetase 1 (NADSYN1); we investigated the SNPs most strongly associated with serum 25(OH)D from each study (total of 10 SNPs, only 8 of which were available in Health ABC Study data) in relation to serum 25(OH)D in Health ABC Study African Americans. None of the SNPs identified in past GWASs of Caucasians were associated with serum 25(OH)D in this population at a statistical significance threshold of P < 0.05 (Table 3), but the rs7041 SNP in GC was near the significance threshold (P = 0.08). Given differences in genetic structure between Caucasians and African Americans, we further tested 205 imputed SNPs in the identified GWAS genes; none of the imputed SNPs met significance thresholds adjusted for multiple testing (Supplemental Table 1 shows the 15 SNPs at Pnominal < 0.10).
TABLE 3.
SNP associations in African-American participants of the Health, Aging, and Body Composition Study for SNPs reported in published GWASs of the serum 25(OH)D phenotype in Caucasians
| SNP | Gene | Chr | Position | Coded allele | Freq. | β1 ± SE | P |
| rs70412,3 | GC | 4 | 72837198 | T4 | 0.82 | −0.93 ± 0.53 | 0.08 |
| rs22826792,3 | GC | 4 | 72827247 | G | 0.10 | 0.07 ± 0.71 | 0.92 |
| rs11555632,3 | GC | 4 | 72862352 | C | 0.11 | 0.12 ± 0.66 | 0.85 |
| rs20607933 | CYP2R1 | 11 | 14871886 | A | 0.37 | −0.10 ± 0.42 | 0.81 |
| rs107416572 | CYP2R1 | 11 | 14871454 | A | 0.28 | −0.04 ± 0.46 | 0.92 |
| rs19931162,3 | CYP2R1 | 11 | 14866810 | A | 0.28 | −0.06 ± 0.46 | 0.90 |
| rs127858782 | DHCR7/ NADSYN1 | 11 | 70845097 | G | 0.73 | −0.28 ± 0.46 | 0.55 |
| rs38292513 | DHCR7/ NADSYN1 | 11 | 70872207 | A | 0.23 | −0.23 ± 0.48 | 0.63 |
Estimated change in serum 25(OH)D per copy of coded allele calculated in a multivariate regression model adjusted for age, gender, study site, season of blood draw, and principal components. A, adenine; C, cytosine; Chr, chromosome; CYP2R1, cytochrome P450, family 2, subfamily R, polypeptide 1; DHCR7, 7-dehydrocholesterol reductase; Freq., frequency; G, guanine; GC, group-specific component (vitamin D binding protein); GWAS, genome-wide association study; NADSYN1, NAD synthetase 1; SNP, single nucleotide polymorphism; T, thymine; 25(OH)D, 25-hydroxyvitamin D.
Associated with serum 25(OH)D in Wang et al. (7).
Associated with serum 25(OH)D in Ahn et al. (8).
rs7041 was coded in the Health ABC Study as an A/C SNP, but in this manuscript it is referred to as the equivalent T/G SNP to maintain consistency with the literature.
Because rs7041 is a functional, nonsynonymous coding SNP in GC, and because it was near the significance threshold for the first set of SNPs tested, we explored gene × nutrient interactions between rs7041 and multivitamin supplement use, the strongest nongenetic predictor of serum 25(OH)D status in Health ABC Study African Americans. Given the low prevalence of the rs7041 G minor allele (MAF: 18%), the TT genotype group was compared to the GT/GG genotype. Mean serum 25(OH)D was 3.7 μg/L higher in multivitamin supplement users with the rs7041 GG/GT genotype compared with supplement users with the TT genotype (29 vs. 25.3 μg/L, respectively; t test comparison of means, P = 0.002), but there was little to no difference in mean 25(OH)D by genotype among nonsupplement users (19.5 vs. 18.7 μg/L, respectively; P = 0.24). In multivariate linear regression models including an rs7041 × multivitamin supplement interaction term, adjusted for age, study site, gender, season of blood draw, and principal components, mean serum 25(OHD) was higher in supplement users with the rs7041 GG/GT genotype and the interaction term was statistically significant (P = 0.04). Given that rs7041 was the strongest genetic predictor of 25(OH)D, we also explored variance explained in a joint gene-environment model that included the nongenetic predictors of 25(OH)D, the rs7041 genotype, and the rs7041 × multivitamin supplement use interaction (n = 880 participants with both genetic and nongenetic complete data); this model explained 28.2% of the variance in serum 25(OH)D (data not shown).
Although the primary goal of this study was to test SNPs associated with the 25(OH)D phenotype in prior GWASs in Caucasian populations, we also tested the rs4588 SNP because it is a nonsynonymous SNP in GC. Thus, we conducted an exploratory analysis of this SNP in relation to serum 25(OH)D. The A allele of rs4588 (frequency of 11%) had no association with serum 25(OH)D in models adjusted for age, gender, study site, season of blood draw, and principal components (P = 0.76).
Genome-wide prediction of serum 25(OH)D.
Initial models estimated that about 25% (95% CI: 0%, 74%) of the serum 25(OH)D variance is attributed to additive genetic variation in Health ABC Study African Americans (Table 4); these models do not include adjustments for principal components because of concerns about overadjusting for population of origin effects that could be proxies for skin color and hence UV absorption. In models adjusting for population substructure, the point estimate was reduced to a near-null value of 0.6% (95% CI: 0%, 65%).
TABLE 4.
Estimate of variance in log-transformed 25(OH)D explained by all genome-wide autosomal SNPs1 calculated using a linear mixed model for GCTA (37)1
| n |
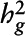 2 2
|
SE | 95% CI3 | P4 | |
| Health ABC Study | 889 | 0.25 | 0.25 | 0, 74 | — |
| MESA | 1198 | 0.21 | 0.19 | 0, 59 | — |
| Meta-analysis, both cohorts | 2087 | 0.23 | 0.15 | 0, 52 | 0.14 |
Total number of genotyped SNPs in the Health ABC Study (before exclusion for MAF <0.01%): 1,024,986. GCTA model covariates included age, gender, study site, and season of blood draw. GCTA, genome-wide complex trait analysis; Health ABC, Health, Aging, and Body Composition; MAF, minor allele frequency; MESA, Multi-Ethnic Study of Atherosclerosis; SNP, single nucleotide polymorphism; 25(OH)D, 25-hydroxyvitamin D.
Estimated proportion of phenotypic variance explained by additive genetic variation.
Lower CI bound set at 0 because the genetic association with 25(OH)D serum concentrations cannot be <0.
Meta-analyzed P value for 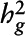 estimate.
estimate.
We replicated the GCTA findings in 1198 participants from the MESA African-American cohort. In the MESA, about 21% of serum 25(OH)D variance is attributed to genetic variation (95% CI: 0%, 59%). Similar to the Health ABC Study findings, in models adjusting for population substructure, the GCTA point estimate was reduced to 0%.
Meta-analysis of the findings from the 2 cohorts led to an estimate of 23% for the variance in serum 25(OH)D explained by additive genetic variation (95% CI: 0%, 52%), after adjusting for age, gender, study site, and season of blood draw (Table 4).
Discussion
We examined both environmental and genetic predictors of 25(OH)D in elderly African Americans. Approximately 25% of the total variability in serum 25(OH)D was explained by environmental determinants. Multivitamin supplement use was an important predictor of serum 25(OH)D, and supplement users had 6.3-μg/L higher serum 25(OH)D concentration compared with nonusers. GWAS-identified SNPs predictive of serum 25(OH)D in Caucasians did not reach P value thresholds for statistical significance in Health ABC Study African Americans, but a suggestive finding for rs7041 in GC was identified, including a statistically significant interaction with multivitamin supplement use. Using a novel analytic approach, we estimated that about 23% of serum 25(OH)D variability is explained by additive genome-wide genetic variation in a pooled analysis of Health ABC Study and MESA African-American participants, although we could not separate the influence of population substructure from direct genetic influences on vitamin D.
Consideration of nongenetic predictors of serum 25(OH)D in the current study builds on a prior study that investigated vitamin D insufficiency in African Americans (20); we found that predictors associated with insufficiency had associations over the full range of serum vitamin D concentrations. We identified dietary predictors of circulating 25(OH)D in this cohort of elderly African Americans, including frequency of cereal and dairy consumption, suggesting that regular consumption of vitamin D–fortified foods may be an important contributor to serum 25(OH)D status in this population. Higher BMI was associated with lower serum 25(OH)D, likely because of vitamin D storage in adipose tissue (39, 40), with evidence for nonlinearity given a steeper inverse association at higher BMI. Although in Caucasians aging skin is associated with a decreased ability to synthesize vitamin D (6), age was not significantly associated with 25(OH)D in elderly African Americans; the lack of association may reflect the limited 9-y age range of Health ABC Study participants. Finally, physical activity had a limited association with 25(OH)D compared to study site and season of blood draw, suggesting that the latter 2 variables may be better proxies for sun exposure in this elderly population.
Although we demonstrated a consistent estimate of the effect of genetic variation on serum 25(OH)D in African Americans from 2 independent cohorts, we did not observe statistically significant associations between Caucasian GWAS-associated SNPs and surrounding gene regions and serum 25(OH)D. SNP frequencies vary by ancestry (Supplemental Table 2), limiting the possibility that specific genetic associations will be replicable across races. Furthermore, genes associated with 25(OH)D in African Americans may differ from those identified in Caucasians, reflecting divergent genetic adaptations to ancestral environments. For instance, genes related to skin pigmentation may be most strongly linked to serum 25(OH)D concentrations in African Americans, as suggested by research demonstrating a significant correlation between skin tone and serum 25(OH)D response to UVB light exposure (41). Considering patterns of genetic variation more broadly, African ancestry populations are typically more genetically diverse than Caucasian ancestry populations, with more rare SNPs, lower levels of linkage disequilibrium, and shorter haplotype blocks (42); larger sample sizes may be needed to demonstrate associations. In the Health ABC Study, the GC gene had lower levels of linkage disequilibrium compared with European Americans (Supplemental Figure 1), which may explain the lack of association of previously identified SNPs in this gene (7, 8).
This study tentatively identified a gene × nutrient interaction in the Health ABC cohort that is worthy of further follow-up. In unadjusted bivariate comparisons, serum 25(OH)D status differed by rs7041 genotype in multivitamin supplement users, and in multivariate linear models the findings were consistent. Because supplement use was not assessed in the MESA, we were not able to replicate these findings. rs7041 is a nonsynonymous SNP in GC associated with a difference in affinity for vitamin D metabolites (43), and the SNP (T allele) was previously associated with lower serum 25(OH)D in both African Americans (17) and Caucasians (44–47). A recent study in Caucasian females demonstrated gene-environment interactions with rs7041; thus, the associations of both dietary vitamin D and season of blood draw with serum 25(OH)D differed by the rs7041 genotype (14). As in the current study, the T allele of rs7041 was associated with lower serum 25(OH)D, supporting the hypothesis that rs7041 genotype modifies the serum 25(OH)D response to environmental exposures in multiple racial groups. If confirmed by further research, our finding that participants with the TT genotype had a lower serum 25(OH)D response to multivitamin supplementation has potentially important public health and clinical implications, given that populations with African ancestry are at higher risk of inadequate vitamin D status and have a higher frequency of the T allele compared with Caucasian populations (48).
The rs4588 SNP has not been identified in prior GWASs, but it is a nonsynonymous SNP in GC with evidence of association with 25(OH)D (14, 17, 44–46, 49); the C allele is associated with higher 25(OH)D. In Health ABC Study African Americans, the C allele of rs4588 was most often found in combination with the T allele of rs7041, and the latter allele was associated with lower 25(OH)D status in our population. We hypothesize that the lack of association between rs4588 and 25(OH)D in Health ABC Study African Americans suggests that rs7041 is a stronger predictor of 25(OH)D in this population. Furthermore, the frequency of the GC2 isoform of the vitamin D binding protein which comprises both the A allele of rs4588 and the T allele of rs7041, is rare in populations of African ancestry (48); indeed, only 1.3% of the Health ABC cohort was homozygous for the GC2 isoform. The GC1f (C allele of rs4588, T allele of rs7041) and GC1s (C allele of rs4588, G allele of rs7041) isoforms of GC predominate in African-American populations (48), and this was true in our population.
In a pooled analysis, combining data from the Health ABC Study and MESA, 23% of serum 25(OH)D variation was estimated to be due to additive genetic factors. In comparison, a recent genome-wide complex trait meta-analysis in Caucasians estimated that 9% (95% CI: 0%, 22%) of 25(OH)D variation was attributable to additive genetic variation, with adjustment for population substructure (11). We considered the GCTA with and without adjustment for ancestry principal components, and adjusting for ancestry led to an essentially null value with a wide CI for the GCTA estimate. Skin pigmentation affects endogenous skin synthesis of 25(OH)D (41), and ancestry informative markers correlate strongly with both skin pigmentation and serum 25(OH)D in African Americans (50, 51). Thus, including ancestry principal components in the model is likely to be an overadjustment for any trait that covaries with population ancestry, such as skin pigmentation. The true estimate of direct, additive genetic influences on serum 25(OH)D may be <23%, but we were unable to arrive at a more accurate lower bound.
Important strengths include use of a large sample size of elderly African Americans for a hypothesis-driven investigation of genetic and nongenetic predictors of 25(OH)D. A limitation is that dosage information for the amount of vitamin D in the multivitamin and vitamin D supplements was not available, precluding consideration of dose-response associations and limiting the interpretation of the suggestive rs7041-multivitamin interaction. Neither were there direct data on sun exposure, sunscreen use, or outdoor physical activity, although study site, season of blood draw, and time spent walking were reasonable proxies for UV exposure. Although we explained a substantial amount of variation in serum 25(OH)D, there was unexplained variability in 25(OH)D that could be attributable to differences in sun exposure or other unmeasured variables, or to gene × environment interactions.
In conclusion, we identified several modifiable factors including diet and supplement use that explain variability in serum 25(OH)D in an elderly African-American population. Additionally, the identification of a common genotype associated with serum 25(OH)D concentrations among multivitamin supplement users is a promising area for future research, with the potential to inform ongoing and future clinical trials of vitamin D supplementation. Finally, we used a novel genetic analysis to estimate that up to 23% of the variability in 25(OH)D can be attributed to additive genetic variation, supporting the need to further study the genetic architecture of 25(OH)D in African Americans in larger cohorts.
Supplementary Material
Acknowledgments
JGH and PAC designed the study and were the primary manuscript authors; JGH, WT, and PAC conducted the analysis of genetic and environmental predictors of 25(OH)D in the Health ABC Study; KCH, PMB, and DKH contributed to interpretation of the statistical analysis; SBK, TBH, MG, KL, and YL conducted the Health ABC GWAS, which provided data for this paper; and JGH, IHdB, BRK, CR-C, DSS, and PAC conducted the GCTA replication analysis in the MESA. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CYP2R1, cytochrome P450, family 2, subfamily R, polypeptide 1; DHCR7, 7-dehydrocholesterol reductase; GC, group-specific component (vitamin D binding protein); GCTA, genome-wide complex trait analysis; GWAS, genome-wide association study; Health ABC, Health, Aging, and Body Composition; HEI, Healthy Eating Index; MAF, minor allele frequency; MESA, Multi-Ethnic Study of Atherosclerosis; NADSYN1, NAD synthetase 1; SNP, single nucleotide polymorphism; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Ross AC, Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 2005;19:2685–95. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 2001;86:888–94. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief, no. 59. Hyattsville (MD): National Center for Health Statistics; 2011. [PubMed]
- 6.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985;76:1536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19:2739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr 1997;66:929–36. [DOI] [PubMed] [Google Scholar]
- 10.McCullough ML, Weinstein SJ, Freedman DM, Helzlsouer K, Flanders WD, Koenig K, Kolonel L, Laden F, Le Marchand L, Purdue M, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraki LT, Major JM, Chen C, Cornelis MC, Hunter DJ, Rimm EB, Simon KC, Weinstein SJ, Purdue MP, Yu K, et al. Exploring the genetic architecture of circulating 25-hydroxyvitamin d. Genet Epidemiol 2013;37:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007;85:860–8. [DOI] [PubMed] [Google Scholar]
- 13.van Dam RM, Snijder MB, Dekker JM, Stehouwer CD, Bouter LM, Heine RJ, Lips P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr 2007;85:755–61. [DOI] [PubMed] [Google Scholar]
- 14.Engelman CD, Meyers KJ, Iyengar SK, Liu Z, Karki CK, Igo RP Jr, Truitt B, Robinson J, Sarto GE, Wallace R, et al. Vitamin D intake and season modify the effects of the GC and CYP2R1 genes on 25-hydroxyvitamin D concentrations. J Nutr 2013;143:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin A, Moriakova A, Akhter N, Rao D, Xie H, Kukreja S, Barengolts E. Determinants of 25-hydroxyvitamin D levels in African-American and Caucasian male veterans. Osteoporos Int 2009;20:1795–803. [DOI] [PubMed] [Google Scholar]
- 16.Chan J, Jaceldo-Siegl K, Fraser GE. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control 2010;21:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab 2008;93:3381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 2000;85:4125–30. [DOI] [PubMed] [Google Scholar]
- 19.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States). Cancer Causes Control 2008;19:527–35. [DOI] [PubMed] [Google Scholar]
- 20.Shea MK, Houston DK, Tooze JA, Davis CC, Johnson MA, Hausman DB, Cauley JA, Bauer DC, Tylavsky F, Harris TB, et al. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the health, aging and body composition study. J Am Geriatr Soc 2011;59:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman DM, Cahoon EK, Rajaraman P, Major JM, Doody MM, Alexander BH, Hoffbeck RW, Kimlin MG, Graubard BI, Linet MS. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide U.S. study. Am J Epidemiol 2013;177:180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wjst M, Altmuller J, Braig C, Bahnweg M, Andre E. A genome-wide linkage scan for 25-OH-D(3) and 1,25-(OH)2–D3 serum levels in asthma families. J Steroid Biochem Mol Biol 2007;103:799–802. [DOI] [PubMed] [Google Scholar]
- 23.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino RB Sr, Ordovas JM, O'Donnell CJ, Dawson-Hughes B, Vasan RS, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr 2009;63:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, Vieth R, Sadovnick AD, Ebers GC. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 2008;88:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter D, De Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 2001;16:371–8. [DOI] [PubMed] [Google Scholar]
- 26.Signorello LB, Shi J, Cai Q, Zheng W, Williams SM, Long J, Cohen SS, Li G, Hollis BW, Smith JR, et al. Common variation in vitamin D pathway genes predicts circulating 25-hydroxyvitamin D levels among African Americans. PLoS ONE 2011;6:e28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batai K, Murphy AB, Shah E, Ruden M, Newsome J, Agate S, Dixon MA, Chen HY, Deane LA, Hollowell CM, et al. Common vitamin D pathway gene variants reveal contrasting effects on serum vitamin D levels in African Americans and European Americans. Hum Genet 2014;133:1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Kritchevsky SB, Tylavsky FA, Harris T, Everhart J, Simonsick EM, Rubin SM, Newman AB. Weight-loss intention in the well-functioning, community-dwelling elderly: associations with diet quality, physical activity, and weight change. Am J Clin Nutr 2004;80:466–74. [DOI] [PubMed] [Google Scholar]
- 30.The Healthy Eating Index. Washington (DC): USDA Center for Nutrition Policy and Promotion; 1995.
- 31.Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013;97:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artigas MS, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, Zhai G, Zhao JH, Smith AV, Huffman JE, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 2011;43:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korn JM, Kuruvilla FG, McCarroll SA, Wysoker A, Nemesh J, Cawley S, Hubbell E, Veitch J, Collins PJ, Darvishi K, et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet 2008;40:1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009;5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen-Torvik LJ, Guo X, Bowden DW, Bertoni AG, Sale MM, Yao J, Bluemke DA, Goodarzi MO, Chen YI, Vaidya D, et al. Fasting glucose GWAS candidate region analysis across ethnic groups in the Multiethnic Study of Atherosclerosis (MESA). Genet Epidemiol 2012;36:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiner AP, Lettre G, Nalls MA, Ganesh SK, Mathias R, Austin MA, Dean E, Arepalli S, Britton A, Chen Z, et al. Genome-wide association study of white blood cell count in 16,388 African Americans: the continental origins and genetic epidemiology network (COGENT). PLoS Genet 2011;7:e1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011;88:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine 2008;33:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol 2007;57:588–93. [DOI] [PubMed] [Google Scholar]
- 42.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet 2008;9:403–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta 2006;372:33–42. [DOI] [PubMed] [Google Scholar]
- 44.Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr 2009;89:634–40. [DOI] [PubMed] [Google Scholar]
- 45.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010;65:215–20. [DOI] [PubMed] [Google Scholar]
- 46.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin D status. Cancer Epidemiol Biomarkers Prev 2008;17:1339–43. [DOI] [PubMed] [Google Scholar]
- 47.Wood AM, Bassford C, Webster D, Newby P, Rajesh P, Stockley RA, Thickett DR. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax 2011;66:205–10. [DOI] [PubMed] [Google Scholar]
- 48.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet 1986;72:281–93. [DOI] [PubMed] [Google Scholar]
- 49.Litonjua AA. Vitamin D and the lung: mechanisms and disease associations. New York: Humana Press; 2012. [Google Scholar]
- 50.Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet 2004;36:S54–60. [DOI] [PubMed] [Google Scholar]
- 51.Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, Hargreaves MK, Hollis BW, Blot WJ. Blood vitamin D levels in relation to genetic estimation of African ancestry. Cancer Epidemiol Biomarkers Prev 2010;19:2325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


