Abstract
Emerging evidence has suggested a possible physiologic role for peripheral glucagon-like peptide 1 (GLP-1) in regulating glucose metabolism and food intake. The likely site of action of GLP-1 is on vagal afferent neurons (VANs). The vagal afferent pathway is the major neural pathway by which information about ingested nutrients reaches the central nervous system and influences feeding behavior. Peripheral GLP-1 acts on VANs to inhibit food intake. The mechanism of the GLP-1 receptor (GLP-1R) is unlike other gut-derived receptors; GLP-1Rs change their cellular localization according to feeding status rather than their protein concentrations. It is possible that several gut peptides are involved in mediating GLP-1R translocation. The mechanism of peripheral GLP-1R translocation still needs to be elucidated. We review data supporting the role of peripheral GLP-1 acting on VANs in influencing glucose homeostasis and feeding behavior. We highlight evidence demonstrating that GLP-1 interacts with ghrelin and leptin to induce satiation. Our aim was to understand the mechanism of peripheral GLP-1 in the development of noninvasive antiobesity treatments.
Keywords: glucagon-like peptide 1, ghrelin, leptin, food intake, vagal afferent neurons
Introduction
The gastrointestinal tract is an important site in which nutrients are digested, absorbed, and assimilated. Enteroendocrine cells, found in the gastrointestinal epithelial layer, are the first level of integration of information from the gut lumen. They secrete hormones in response to nutrient stimuli such as carbohydrates, lipids, and proteins.
Gut hormones influence gastrointestinal function and feeding behavior by either directly acting on target tissues via the circulation or activating intrinsic and extrinsic neurons in a paracrine manner. A major target for gut-derived hormones is the vagal afferent neurons (VANs)5. The vagus nerve is a major link between the gastrointestinal tract and central nervous system (CNS); its nerve endings lie in the mucosa of the gut and terminate in the nucleus of the solitary tract. The vagus nerve expresses receptors for many gut hormones and there is strong evidence that gut-derived hormones can act on VANs to regulate food intake. Studies have demonstrated that ablation of VANs abolishes cholecystokinin (CCK)-induced inhibition of food intake (1, 2), highlighting the importance of VANs in the control of food intake.
Among the gut hormones, glucagon-like peptide 1 (GLP-1) is released in response to a meal from enteroendocrine cells, and GLP-1 receptors (GLP-1Rs) are found in both the periphery and the CNS. GLP-1 has an extremely short half-life, possibly suggesting a peripheral site of action on vagal afferent fiber endings. Considerable attention has focused on GLP-1 as an incretin hormone, and GLP-1 analogs regulate glucose homeostasis in patients with type 2 diabetes. Emerging evidence suggests a possible physiologic role for GLP-1 in regulating food intake. Exogenous administration of GLP-1 or its long-acting analogues dose-dependently inhibits food consumption and administration of a GLP-1R antagonist has been shown, under certain conditions such as after a meal preload, to increase food intake (3). In addition, GLP-1 concentrations in the plasma are increased after bariatric surgery, and this is associated with elevated satiating signals leading to weight loss and ameliorations in glycemia.
This review is focused on the current understanding of GLP-1 signaling on VANs and outlines the phenotypic changes induced by gut-derived hormones on VANs according to feeding status. It reviews the interaction of GLP-1 with ghrelin and leptin and the interaction of these peptides in mediating energy homeostasis. Understanding the mechanisms by which peripheral GLP-1 regulates glucose metabolism and food intake can help in developing noninvasive antiobesity treatments.
GLP-1 Secretion in the Gastrointestinal Tract
GLP-1 is derived from the expression of the transcriptional product of the preproglucagon gene in intestinal L cells and pancreatic A cells. Preproglucagon is cleaved into several fragments; the main translational products are glucagon-containing glicentin, GLP-1, and glucagon-like peptide 2 in the gastrointestinal tract, and glucagon and glicentin-related polypeptide in the pancreas (4, 5).
GLP-1 is released by L cells in response to nutrient ingestion (6). L cells are an open type of endocrine cell whose base lies on the basement membrane and cytoplasmic processes project into the gut lumen. These processes have microvilli, and it is hypothesized that the microvilli are part of the nutrient-sensing machinery in these cells, resulting in the sensing of the luminal content discharge of granules on the basolateral side. Once stimulated, L cells secrete peptides into the interstitial space. They are found in close proximity to both neurons and the systemic circulation in the intestine, which allows them to be influenced by both neural and humoral signals (7, 8). L cells are found throughout the gut; the highest expression is found in the ileum and distal colon, with fewer cells in the proximal gut (9). GLP-1 is colocalized in intestinal L cells with peptide YY, glucose-dependent insulinotropic peptide (GIP), and insulin-like peptide 5 (10–12).
Once secreted, GLP-1 is released into the lamina propria and enters the capillary bed or lymphatics. Preprandial plasma concentrations of GLP-1 are very low and increase with nutrient ingestion. Multiple studies have demonstrated that GLP-1 is quickly degraded into its inactive form by dipeptidylpeptidase IV (DDP-IV) (13). DDP-IV is abundant in the brush border and endothelial cells that line the capillaries (14, 15). It is estimated that ∼50% of GLP-1 released into the capillaries in vivo is transformed into its inactive form, N-terminally truncated GLP-1 9–36 amide, before it reaches the hepatic portal vein. Further degradation takes place in the liver, leaving only 10–15% of intact GLP-1 by the time it reaches the systemic circulation. In the circulation, GLP-1 has a 2–3 min half-life due to the presence of DDP-IV (13). Inhibiting DDP-IV prevents GLP-1 degradation in the porcine ileum by 46% at baseline (16, 17). GLP-1 can cross the blood–brain barrier (18) but given that it is degraded so quickly, it is unlikely that a substantial amount of active GLP-1 released from the periphery can reach the brain. In addition, GLP-1 concentrations are higher in intestinal lymphatics than in the hepatic portal vein, likely because lymph flow is lower than portal blood flow and there is less DDP-IV in lymphatics than in blood vessels (19). The concentration of GLP-1 in the intestinal lymphatics reflects interstitial concentrations and is increased after meal ingestion (19). This evidence supports the hypothesis that GLP-1 acts in a paracrine way on VANs (20). Indeed, Punjabi et al. (21) demonstrated that systemic active GLP-1 concentrations do not increase in response to a regular unpurified diet meal in rats.
GLP-1 and its receptor are found at central and peripheral sites. Currently there is only one known GLP-1R, which has high single binding affinity for GLP-1 (22). The GLP-1R was originally cloned from pancreatic islet cells (23). It is a G protein–coupled receptor that is distributed in various tissues, both centrally and peripherally (24, 25). It is most abundant in the lungs, brain, taste cells, and the distal gastrointestinal tract. There are 2 different signaling pathways downstream of the GLP-1R. In the hindbrain and the pancreas, GLP-1 binds to its receptor and activates adenylyl cyclase to induce the cAMP pathway (26, 27). In muscle and liver, the GLP-1R may activate a cAMP independent pathway (28, 29). Thus, although there is evidence for a single receptor for GLP-1, there are differences in signal transduction in different tissues. The biological activities of GLP-1 include maintaining glucose homeostasis, regulating cardiovascular function, and regulating gastric motility and food intake. The insulinotropic effect of GLP-1 is mainly mediated through the pancreas, whereas the satiating effect of GLP-1 is mainly mediated through the vagus nerve.
The Insulinotropic Activity of GLP-1
GLP-1 is a major player in regulating glucose homeostasis. It is partly responsible for inducing the incretin effect, in which an oral glucose load substantially increases plasma insulin concentrations compared with the same amount of glucose administered intravenously (30, 31). The incretin effect is regulated by both GLP-1 and GIP. GIP is released from K cells in the duodenum in response to nutrients and activates insulin secretion in a glucose-dependent manner (32). The release of GLP-1 and GIP from the gut after an oral glucose load accounts for 60% of insulin secretion (33). GLP-1 and GIP are both released in response to nutrient stimuli and degraded by DDP-IV in the circulation. These 2 peptides work synergistically to potentiate glucose-stimulated insulin secretion. This is confirmed through GLP-1R and glucose-dependent insulinotropic peptide receptor (GIPR) knockout mice. GLP-1R knockout mice exhibit rather modest perturbations in glucose homoeostasis; they have mild hyperglycemia, glucose intolerance, and abnormal glycemic excursions in response to glucose (34). Isolated pancreatic β cells from GLP-1R knockout mice preserve insulin storage and glucose-dependent insulin secretion (35). GLP-1R knockout mice exhibit a compensatory mechanism by which glucose homeostasis is maintained. GIP and GLP-1 signaling is substantially upregulated in the pancreatic β cells of knockout mice, possibly explaining why GLP-1R knockout mice only have a mild change in phenotype (31). Likewise, GIPR knockout mice display a mild change in phenotype with reduced glucose tolerance and glucose-induced insulin secretion. In contrast with GLP-1R knockout mice, GIPR knockout mice have normal glycemia when deprived of food and normal glucose excursion (31, 34, 36). Together these studies demonstrate the compensatory mechanisms that exist between GLP-1 and GIP in vivo. To date, GLP-1 and GIP are the only hormones that fulfill the definition of an incretin hormone in rodents and humans.
The GLP-1R is expressed in β-pancreatic islet cells; this has been demonstrated by immunohistochemistry (37, 38). Pancreatic-specific GLP-1R knockout mice have normal glucose tolerance after oral and intraperitoneal glucose tolerance tests. Pancreatic GLP-1R signaling was restored in pancreatic-specific GLP-1R ex vivo islet extracts compared with whole-body GLP-1R knockout islet extracts (39). GLP-1 regulates glucose homeostasis by inhibiting glucagon, stimulating insulin release, increasing insulin biosynthesis, increasing β cell proliferation, and decreasing β cell apoptosis in rodents (40). In β cells, GLP-1 binds to its receptor to stimulate adenylate cyclase and cAMP. Subsequently, cAMP activation leads to protein kinase A and cAMP-regulated guanine nucleotide exchange factor II, which elevates intracellular calcium concentrations, leading to exocytosis of insulin-containing granules (4, 41). As with other G protein–coupled receivers, the GLP-1R undergoes ligand-induced internalization by complex and numerous mechanisms. In vitro studies have demonstrated that the GLP-1R in mouse insulinoma 6 cells (MIN6), a pancreatic cell line, is endocytosed upon activation via both clathrin-coated–dependent and caveolin-1–dependent mechanisms (42). In resting MIN6 cells, the receptor is constitutively cycled between the plasma membrane and the cytoplasm (42).
The idea that the incretin effect of GLP-1 is predominantly mediated by its effect on pancreatic β cells has been debated. The fact that GLP-1 is rapidly metabolized and its postprandial concentrations are considerably lower than GIP concentrations brings into question how much intact peptide actually reaches the pancreas. Studies demonstrate that the activation of extrapancreatic GLP-1Rs may be necessary in maintaining glucose homeostasis. For example, activation or attenuation of the GLP-1R in the CNS exerts profound effects on glucose-dependent insulin secretion. Of interest, GLP-1Rs have been localized in the portal vein, and a blockade of these GLP-1Rs substantially impairs glucose tolerance in rodents (43). Peripheral GLP-1 administration potently stimulates insulin secretion and improves glucose tolerance in rodents and humans (44, 45). In vitro studies indicate that GLP-1 and its agonist can act directly on pancreatic β cells (46, 47). In vivo, GLP-1 and its receptor agonist also modulate glucose metabolism (48, 49). In addition, GLP-1 acts through sensory nerves to regulate glucose homeostasis. Infusions of the active form of GLP-1 into the hepatic vein stimulate vagal afferent and efferent fibers innervating the pancreas; this effect is attenuated by ganglion blockade (50). Furthermore, infusions of a low dose of GLP-1 in mice with intact vagal fibers induces insulin secretion; this effect is attenuated in capsaicin-treated mice (51). Together, these studies provide evidence that the peripheral insulinotropic effect of GLP-1 is at least partly mediated through VANs.
GLP-1 and the Control of Food Intake
Plasma GLP-1 concentrations are low in fasting conditions and rapidly increase postprandially, especially in the presence of carbohydrates and fat (20). There is evidence that exogenous GLP-1 inhibits food intake. Acute peripheral GLP-1R activation by exendin-4 (Ex-4) and native GLP-1 inhibits food intake in a dose-dependent manner in rodents and humans (52, 53). Indeed, a daily dose of liraglutide, a GLP-1 agonist, to obese patients led to substantial and sustained weight loss (54). Results from studies that made use of GLP-1 analogues such as Ex-4 and liraglutide may be enhanced by longer half-life and additional actions on central sites after crossing the blood–brain barrier. Studies in rodents indicate that peripheral administrations of native GLP-1 induce satiation but require higher doses than synthetic long-acting GLP-1R agonists (55, 56). Several lines of evidence also support the notion that native gut-derived GLP-1 plays a physiologic role in satiety. Peripheral administrations of native GLP-1 that mimic its release from the gastrointestinal tract under physiologic conditions decrease food intake in a dose-dependent manner (57–59). Blockade of peripheral GLP-1Rs attenuates satiation after a nutrient preload or peripheral GLP-1 administration (60). However, there are discrepancies in the literature regarding whether endogenous gut-derived GLP-1 plays a functional role, because no effect from various doses of peripheral native GLP-1 on food intake was observed in some studies (61), whereas others show a substantial decrease in food intake at a lower dose in rats (62). In addition, GLP-1R knockout mice exhibit normal body weight and no change in overall food intake. However, a thorough analysis of meal pattern has not been done and it is possible that GLP-1 can have effects on meal size and duration, consistent with other gut peptides, such as CCK. Consistent with other studies, we have recently demonstrated that peripheral, native GLP-1 requires either a postprandial state or an ongoing meal to induce satiation (59, 63–65). Prolonged fasting attenuates the satiating effects of GLP-1. This concept could explain the discrepancies in the literature regarding the satiating effects of peripheral GLP-1. Consequently, rodents in a postprandial phase will respond to GLP-1, whereas rodents deprived of food for 24 h and 48 h do not respond to various doses of native acute GLP-1 (56, 57). GLP-1 inhibits food intake in mice consuming food ad libitum up to 30 min before GLP-1 administration (66) and a short bout of eating before administration of GLP-1 decreases food intake in rats.
The site of action of GLP-1 with respect to its effect on food intake remains to be discussed. Central mechanisms are important in regulating the anorexigenic effects of GLP-1 and activation of central pathways that affect behavior is necessary to mediate the downstream responses irrespective of the site of action of GLP-1. Peripheral native GLP-1 administration activates c-fos expression in the hindbrain and hypothalamus in rodents (66–68), indicating that peripheral GLP-1 actions are activating central circuits. Blockade of either the central or peripheral GLP-1R attenuates GLP-1–induced satiation (60, 69, 70). Likewise, central administration of native GLP-1 and its agonist, Ex-4, substantially reduces food intake in rodents (26, 71). Activation of central GLP-1Rs plays a role in mediating food intake; intracerebroventricular administrations of Ex-4 into the third ventricle induces satiation and activates c-fos expression in hypothalamic regions (72). Lesions to the brainstem–hypothalamic pathway attenuate GLP-1–induced satiation in rats, indicating the importance of central regions mediating the effect of systemic GLP-1 (73). GLP-1Rs are colocalized with pro-opiomelanocortin (POMC) neurons located in the hypothalamus (72). Central administrations of GLP-1 prevent fasting-induced upregulation of hypothalamic neuropeptide Y (NPY) and agouti-related peptide (AgRP) and fasting-induced downregulation of POMC and cocaine and amphetamine–regulated transcript (CART) (74, 75). Altogether, these studies highlight the important role in which central pathways are necessary to mediate the inhibitory effects of GLP-1.
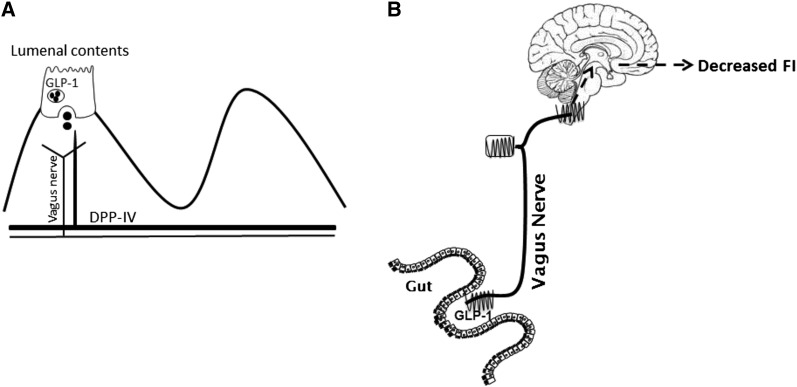
It is likely that endogenous gut-derived GLP-1 suppresses food intake by acting in a paracrine manner on adjacent GLP-1Rs expressed on vagal afferents (Figure 1). Evidence to support this hypothesis includes the following: 1) active GLP-1 is rapidly degraded, resulting in an extremely short half-life (76), and 2) subdiaphragmatic vagal deafferentation prevents GLP-1 from inhibiting food intake (65, 73). Ruttimann et al. (65) demonstrated that intraperitoneal rather than intravenous administration, which more accurately mimics the endogenous route of action of GLP-1, requires intact vagal afferent fibers to induce satiation. In addition, administration of GLP-1 will increase the electrophysiologic activity of VANs in vitro and in vivo (77, 78). GLP-1Rs are present in VANs; indirect evidence through mRNA levels, as well as histologic and most recently direct immunohistochemical evidence, demonstrates that VANs express 42% of the GLP-1R (64, 78). GLP-1Rs are functional in VANs; however, their mechanism is unlike other G-coupled protein receptors. Gut-derived hormones induce neurochemical changes in VANs by regulating a phenotypic “switch.” VANs exist in states that either promote orexigenic or anorexigenic phenotypes (79, 80). In a food-deprived condition, anorexigenic receptor expression decreases as orexigenic receptor expression increases. Conversely, these changes are reversed by refeeding through a CCK-dependent mechanism. However, we have demonstrated that GLP-1Rs are constitutively expressed and that GLP-1Rs alter cellular localization according to feeding status. Under fasting conditions, the majority of GLP-1Rs are located in the cytoplasm, whereas, in a postprandial state, there is a 42% increase in GLP-1Rs at the plasma membrane (64). However, the exact mechanism of the translocation of GLP-1 remains unknown. We hypothesize that either the satiating effect of GLP-1 and its receptor translocation to the plasma membrane is either inhibited in a fasted state or potentiated in a refed state.
FIGURE 1.
A schematic representation of peripheral endogenous GLP-1 acting in a paracrine way on VANs. (A) In response to a meal, GLP-1 is released from L cells into the lamina propria and enters the capillaries, where it is quickly degraded into its inactive form. GLP-1 can act in a paracrine manner on nearby vagal afferent fibers expressing GLP-1Rs. (B) Endogenous gut-derived GLP-1 suppresses food intake by acting in a paracrine manner on adjacent GLP-1Rs expressed on vagal afferents. DPP-IV, dipeptidylpeptidase IV; FI, food intake; GLP-1, glucagon-like peptide 1; GLP-1R, glucagon-like peptide 1 receptor; VAN, vagal afferent neuron.
Gut-derived hormones interact with each other at the level of VANs in order to regulate energy homeostasis. Specifically, several studies indicate that GLP-1 interacts with several gut peptides to regulate energy homeostasis and glucose homeostasis.
Evidence that Ghrelin Modulates GLP-1–Induced Actions
Ghrelin is a circulating orexigenic hormone. Ghrelin is a 28–amino acid polypeptide produced mainly by endocrine A-like cells in the gastric epithelium (81). Although the stomach is the main site of secretion, ghrelin is also secreted by the pituitary, hypothalamus, lungs, heart, and pancreas. Native ghrelin undergoes a unique post-translational acylation of the third serine residue, converting it into its active form. The enzyme responsible for the acylation of ghrelin is the ghrelin O-acyltransferase (GOAT) enzyme. Acetylated ghrelin is an endogenous ligand for growth hormone secretagogue receptor (GHS-R), which is constitutively expressed. GHS-R is principally found in the pituitary and hypothalamus. The highest density of GOAT mRNA expression is found in gastric gastrin cells, indicating a high association between ghrelin and GOAT (82). The biological functions of ghrelin are widespread; it plays a role in lipid metabolism, glucose homeostasis, and growth hormone release. Additionally, ghrelin stimulates appetite, body weight gain, and adiposity. The acylated form of ghrelin has been recognized as the major active orexigenic molecule. Circulating concentrations of acylated ghrelin do not increase with prolonged fasting, whereas deacylated ghrelin accounts for up to 90% of the majority of circulating ghrelin (83). Endogenous acylated ghrelin serves as a gastric sensor and increases appetite and food intake, which indicates that ghrelin acts as a physiologic hunger signal (84).
Plasma concentrations of ghrelin are high during fasting and robustly decrease in a postprandial state, suggesting that ghrelin is a main player in meal initiation. Exogenous ghrelin is known to stimulate food intake; central and peripheral administrations of ghrelin will increase energy consumption and body weight in rodents (85). Intravenous injections of ghrelin will stimulate appetite and food consumption in humans (86). In rats, ghrelin enhances weight gain by decreasing energy expenditure. The regulation of food intake by ghrelin is dependent on feeding status; exogenous ghrelin will stimulate food intake in rats consuming food ad libitum but not in food-deprived rats (87). Ghrelin acts centrally in the arcuate nucleus (ARC) of the hypothalamus, a region known to regulate feeding behavior. Intracerebroventricular administrations of ghrelin in the third ventricle will increase food intake and activate c-fos expression in the hypothalamus (88). Immunohistochemical evidence has found ghrelin-expressing neurons in multiple regions of the hypothalamus. Evidence supports the notion that mRNA levels of AgRP and NPY are increased in response to an injection of ghrelin into the third ventricle (85). Given that there are central and peripheral distributions of ghrelin, several mechanisms have been proposed in which ghrelin will activate its receptor in the hypothalamus, including crossing the blood–brain barrier, activating VANs, or synthesizing locally. The rate at which peripheral ghrelin crosses the blood–brain barrier is very low, further supporting the concept that ghrelin may act both at peripheral and central sites (89).
GHS-R is expressed in 40% of VANs and is colocalized with the orexigenic melanin-concentrating hormone (MCH) and cannabinoid-1 (CB-1) receptors, which are involved in food initiation (90). Date et al. (91) found that the destruction of vagal afferent fibers by capsaicin or lesions to subdiaphragmatic vagal fibers abolished ghrelin-induced feeding and substantially decreased c-fos expression in the ARC, where vagal afferents terminate, in response to ghrelin in capsaicin-treated rats. Furthermore, ghrelin increased vagal electrophysiologic activity in isolated vagal segments (91). It is well established that ghrelin influences changes in the phenotypic switch of VANs. The administration of ghrelin to rats deprived of food before refeeding prevents the downregulation of MCH and CB-1 receptors in VANs, suggesting that ghrelin mediates the expression of orexigenic receptors to induce food intake (90). Moreover, exogenous ghrelin inhibits the CCK-stimulated upregulation of CART by inhibiting phosphorylation of cAMP response element–binding protein in the nucleus of VANs (92). Taken together, these studies support the idea that that ghrelin can influence VAN activity induced by CCK to modulate food intake. Studies have demonstrated that ghrelin interacts with other gut peptides to control energy balance as well as glucose homeostasis. GHS-Rs are expressed on VANs and coexpress with other gut peptides such as CCK. Ghrelin interacts with numerous gut-derived peptides on VANs. For example, CB-1 and MCH expression levels decrease in a refed state; ghrelin will attenuate the decrease of expression in refed rats (90, 93). Electrophysiologic studies reveal that as CCK increases vagal activity, ghrelin attenuates it (91). Systemic infusions of ghrelin dose-dependently attenuate the anorexigenic effects of GLP-1 in rats (94). Conversely, native GLP-1 infusions in humans inhibited postprandial increase in ghrelin plasma concentrations (95). In rats deprived of food for 72 h, GLP-1R activation potently reduced ghrelin plasma concentrations (56). Together, these studies indicate that there is a clear interaction between ghrelin and GLP-1 to regulate food intake; however, the exact mechanism of action is unknown.
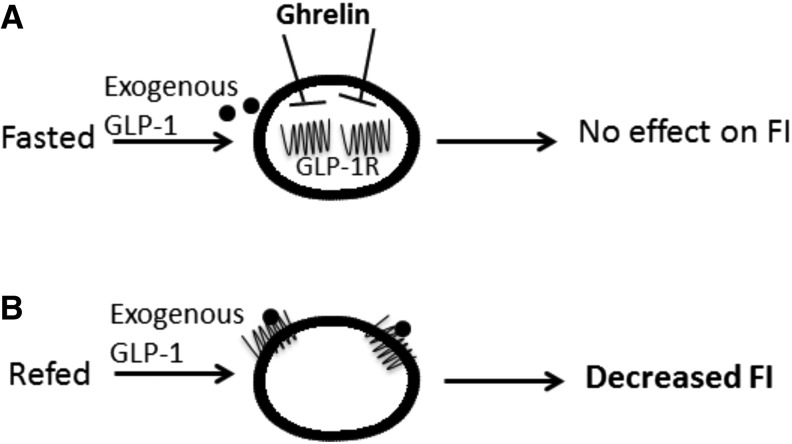
Insulin secretion from pancreatic cells is modulated by gut peptides such as ghrelin and GLP-1. GLP-1 induces insulin secretion and ghrelin attenuates the release and increases blood glucose concentrations. There is evidence that there is an interaction between ghrelin and GLP-1 to regulate the insulinotropic effects. In the pancreas, GLP-1 has been shown to counteract the endogenous and exogenous actions of ghrelin. GLP-1 stimulated glucose-induced insulin release, and cAMP production in β cells is attenuated by ghrelin. Furthermore, the presence of Dly3GHRP6, a ghrelin receptor antagonist, markedly enhances the insulinotropic effects of GLP-1 (96). We have preliminary data to support the fact that ghrelin inhibits GLP-1R translocation according to nutrient availability in vitro. Under fed conditions, ghrelin brings the GLP-1R into the cytoplasm in VAN cell cultures; prior blockade of ghrelin blocks this effect. It is well established that ghrelin has the ability to block VANs from responding to anorexigenic signals. For example, CCK increases electrophysiologic activity of the vagus, whereas ghrelin attenuates the neuronal excitation. We have demonstrated that peripheral administration of a GSH-R antagonist, DLy3GHRP6, before GLP-1 administration will substantially decrease food intake in rats deprived of food. Given that GLP-1 requires a refed state in order to induce satiation, it is likely that ghrelin plays a role in mediating the satiating effects of GLP-1; GLP-1 will induce satiation in animals deprived of food when ghrelin is blocked before administration of GLP-1 (CC Ronveaux, G DeLartigue, and HE Raybould, unpublished results, 2014) and, we have preliminary evidence to suggest that ghrelin restricts GLP-1Rs on VANs to the cytoplasm and that CCK will move GLP-1Rs to the plasma membrane (Figure 2).
FIGURE 2.
Ghrelin mediates GLP-1R localization on VANs. (A) In a fasted state, ghrelin restricts GLP-1Rs on VANs to the cytoplasm; therefore, exogenous GLP-1 cannot access its receptors and has no effect on food intake. (B) In a refed state, when ghrelin concentrations are low, GLP-1Rs are present in the plasma membrane; therefore, exogenous GLP-1 can bind to its receptor and induce satiation. FI, food intake; GLP-1, glucagon-like peptide 1; GLP-1R, glucagon-like peptide 1 receptor; VAN, vagal afferent neuron.
Evidence that Leptin Interacts with GLP-1 Actions
Leptin is a 127–amino acid peptide mainly secreted by adipocytes and to a lesser degree from the stomach (97). Leptin is known to suppress appetite, body weight gain, and adiposity in humans, rodents, and monkeys (83, 98, 99). Circulating concentrations of leptin correlates with body adiposity. In rodents and humans, leptin signaling in the brain results in decreased energy intake and increased energy expenditure to maintain the body fat store (83, 100). Leptin acts on leptin receptors (LepRs) which are abundantly found in the hypothalamus. Leptin easily crosses the blood–brain barrier through a saturable transport and acts on hypothalamic neurons; it inhibits expression of orexigenic AgRP, NPY, and MCH and stimulates anorexigenic POMC and CART (101).
Studies demonstrate that peripheral acute administrations of leptin substantially inhibit food intake (99, 102). Plasma concentrations of leptin increase hours after a meal and, in humans, several days after overfeeding, suggesting that leptin acts both at short term and long term on food intake. Leptin concentrations exhibit a circadian rhythm pattern in which the highest concentrations of circulating leptin are at night (83). Leptin deficiency in ob/ob mice leads to an obesogenic phenotype. The ARC is required for leptin-induced anorexia because ARC-lesioned ob/ob mice are irresponsive to central infusions of leptin (103). Deficiency in leptin signaling leads to altered expression hypothalamic neuropeptides. For example, ob/ob mice have increased degrees of orexigenic AgRP expression and decreased anorexigenic POMC expression (104, 105). Mice lacking LepRs in POMC neurons are mildly obese, have hyperleptinemia, and surprisingly have decreased orexigenic AgRP and NPY mRNA levels (106). Leptin replacement restores energy homeostasis in ob/ob mice but not db/db mice that have a mutation of the LepR (107). Similarly, in humans, obese individuals have increased fat mass and elevated leptin concentrations. However, individuals continue to overeat and increase body weight regardless of their elevated leptin concentrations (83). Likewise, high fat diet–induced obesity in mice leads to hyperleptinemia and hyperphagia (108). Leptin resistance is the inability of obese individuals or diet-induced obese models to respond to exogenous and endogenous leptin. In most models of obesity, leptin concentrations are elevated, indicating the importance of leptin resistance in the pathogenesis of obesity.
In addition, LepR is expressed in other tissues such as the stomach and the vagus nerve. In the vagus nerve, leptin receptor is expressed in the nodose ganglion. Leptin signaling in VANs has been demonstrated to play an important role in regulating energy homeostasis. Leptin increases the electrophysiologic activity of VANs and increases calcium release in culture (109). We have demonstrated that leptin resistance in VANs leads to an obese phenotype. First, we found that there was a substantial increase in body weight and food intake in parallel with a decrease in phosphorylation of signal transducer and activator of transcription 3, a marker of leptin signaling, in VANs in response to leptin (111). Leptin resistance in the ARC did not develop until after the onset of the obesogenic phenotypes. Whether leptin resistance in VANs drives hyperphagia and eventually leads to an obese phenotype was addressed by specifically knocking down LepR from sensory neurons in mice. The LepR-sensory neuron knockout mice exhibited an increase in body weight, food intake, and adiposity compared with their control littermates (102). Furthermore, LepR-sensory neuron knockout mice have a constitutive upregulation of orexigenic receptors (MCH and CB-1 receptors) and downregulation of anorexigenic receptors (Y2 receptor and CART) on VANs. These studies indicate that disruption of leptin signaling on VANs leads to hyperphagia and obesity. Together, these studies highlight the importance of leptin signaling on VANs in regulating energy homeostasis.
Leptin has been found to enhance the inhibitory effects of various anorexigenic gut hormones. For example, in VANs, CCK stimulates the expression of CART peptide, which induces its inhibitory effects. CCK in the presence of leptin will stimulate CART peptide concentrations at significantly lower concentrations than when CCK acts alone (92). It seems that the interaction of leptin with other gut hormones is necessary in order to induce short-term satiation. Specifically, leptin has been demonstrated to interact with GLP-1 and its receptor antagonist to induce satiation. Leptin receptors are found in endocrine L cells and neurons secreting GLP-1 (112), and leptin was found to stimulate GLP-1 release in L cells. In brain centers, LepRs were found in GLP-1R–expressing neurons in the nucleus of the solitary tract and leptin was found to stimulate these neurons (113, 114). Food deprivation decreases leptin plasma concentrations concurrently with GLP-1 expression in the hypothalamus, and it is possible that GLP-1 released from leptin-stimulated neurons modulates hypothalamic brain centers involved in appetite. Peripheral sites of mechanism of action of leptin interaction with GLP-1 seem to play an important role in modulating appetite. Peripheral blockade of the GLP-1R will attenuate leptin-induced satiation and body weight gain in rats (115). GLP-1 inhibitory effects are abolished in leptin-deficient rats. In normal rats, leptin alone has no effect on food intake; however, leptin together with Ex-4 and GLP-1 substantially potentiates its anorexigenic effect. Furthermore, the inhibitory actions of native GLP-1 and Ex-4 are attenuated in rats deprived of food; however, pretreatment with leptin restored the satiating effects of GLP-1 and Ex-4 (59).
Conclusion
This review focused on the mechanism of the action of GLP-1 on VANs and its relation with glucose metabolism and the control of food intake. Numerous studies have shown that GLP-1Rs are present in VANs and that GLP-1 stimulates electrophysiological activity on VANs. Interestingly, GLP-1Rs change their cellular localization according to feeding status rather than their protein expression levels, and the translocation of GLP-1Rs on VANs in response to feeding is likely to be mediated by other gut-derived hormones. We have evidence suggesting that ghrelin inhibits the satiating effect of GLP-1 in a fasted state by acting on the translocation of GLP-1Rs on VANs. However, it is plausible that ghrelin is not the only peptide modulating GLP-1R translocation, given that studies have highlighted the interaction of GLP-1 and leptin. The mechanism of GLP-1R translocation is still unclear and needs to be elucidated further. To date, there are limited studies that use native GLP-1. Synthetic GLP-1 analogues escape degradation and are effective in decreasing food intake through an additional mechanism, which native GLP-1 does not induce. It is necessary to understand which GLP-1Rs are activated under physiologic conditions in order to effectively design noninvasive antiobesity treatments.
Acknowledgments
CCR, DT, and HER wrote the paper. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AgRP, agouti-related peptide; ARC, arcuate nucleus; CART, cocaine and amphetamine–regulated transcript; CB-1, cannabinoid-1; CCK, cholecystokinin; CNS, central nervous system; DDP-IV, dipeptidylpeptidase IV; Ex-4, exendin-4; GCPR, G-coupled protein receptor; GHS-R, growth hormone secretagogue receptor; GIP, glucose-dependent insulinotropic peptide; GIPR, glucose-dependent insulinotropic peptide receptor; GLP-1, glucagon-like peptide 1; GLP-1R, glucagon-like peptide 1 receptor; GOAT, ghrelin O-acyltransferase; LepR, leptin receptor; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; VAN, vagal afferent neuron.
References
- 1.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol 1997;272:R1245–51. [DOI] [PubMed] [Google Scholar]
- 2.Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol 1985;248:R501–4. [DOI] [PubMed] [Google Scholar]
- 3.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009;150:2654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–39. [DOI] [PubMed] [Google Scholar]
- 5.Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept 2005;128:97–107. [DOI] [PubMed] [Google Scholar]
- 6.Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell. Diabetes 2006;63:S70–7. [Google Scholar]
- 7.Näslund E, Hellstrom PM. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol Behav 2007;92:256–62. [DOI] [PubMed] [Google Scholar]
- 8.Hofer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. News in physiological sciences: an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society.1999; 14:18–23. [DOI] [PubMed]
- 9.Hansen CF, Vrang N, Sangild PT, Jelsing J. Novel insight into the distribution of L-cells in the rat intestinal tract. American journal of translational research.2013; 5(3):347–358. [PMC free article] [PubMed]
- 10.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 2003;114:189–96. [DOI] [PubMed] [Google Scholar]
- 11.Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013;56:1413–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GrosseJ, Heffron H, Burling K, Akhter Hossain M, Habib AM, Rogers GJ, Richards P, Larder R, Rimmington D, Adriaenssens AA, et al. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc Natl Acad Sci USA 2014;111:11133–8. [DOI] [PMC free article] [PubMed]
- 13.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995;136:3585–96. [DOI] [PubMed] [Google Scholar]
- 14.Bai JP. Distribution of brush-border membrane peptidases along the rabbit intestine: implication for oral delivery of peptide drugs. Life Sci 1993;52:941–7. [DOI] [PubMed] [Google Scholar]
- 15.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 2005;48:612–5. [DOI] [PubMed] [Google Scholar]
- 16.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999;140:5356–63. [DOI] [PubMed] [Google Scholar]
- 17.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 1995;80:952–7. [DOI] [PubMed] [Google Scholar]
- 18.Orskov C, Poulsen SS, Moller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes 1996;45:832–5. [DOI] [PubMed] [Google Scholar]
- 19.D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 2007;293:R2163–9. [DOI] [PubMed] [Google Scholar]
- 20.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 1993;138:159–66. [DOI] [PubMed] [Google Scholar]
- 21.Punjabi M, Arnold M, Ruttimann E, Graber M, Geary N, Pacheco-Lopez G, Langhans W. Circulating glucagon-like peptide-1 (GLP-1) inhibits eating in male rats by acting in the hindbrain and without inducing avoidance. Endocrinology 2014;155:1690–9. [DOI] [PubMed] [Google Scholar]
- 22.Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes 1993;42:1678–82. [DOI] [PubMed] [Google Scholar]
- 23.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 1992;89:8641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunphy JL, Taylor RG, Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 1998;141:179–86. [DOI] [PubMed] [Google Scholar]
- 25.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999;403:261–80. [DOI] [PubMed] [Google Scholar]
- 26.Hayes MR, et al. . Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 2011;13:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ME Doyle. Mechanisms of Action of GLP-1 in the Pancreas. Pharmacotherapy 2007;113:546–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgado E, Luque MA, Alcantara A, Trapote MA, Clemente F, Galera C, Valverde I, Villanueva-Penacarrillo ML. Glucagon-like peptide-1 binding to rat skeletal muscle. Peptides 1995;16:225–9. [DOI] [PubMed] [Google Scholar]
- 29.Villanueva-Peñacarrillo ML, Delgado E, Trapote MA, Alcantara A, Clemente F, Luque MA, Perea A, Valverde I. Glucagon-like peptide-1 binding to rat hepatic membranes. J Endocrinol 1995;146:183–9. [DOI] [PubMed] [Google Scholar]
- 30.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–4. [DOI] [PubMed] [Google Scholar]
- 31.Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 2005;128:125–34. [DOI] [PubMed] [Google Scholar]
- 32.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009;52:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986;63:492–8. [DOI] [PubMed] [Google Scholar]
- 34.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 1996;2:1254–8. [DOI] [PubMed] [Google Scholar]
- 35.Flamez D, Van Breusegem A, Scrocchi LA, Quartier E, Pipeleers D, Drucker DJ, Schuit F. Mouse pancreatic beta-cells exhibit preserved glucose competence after disruption of the glucagon-like peptide-1 receptor gene. Diabetes 1998;47:646–52. [DOI] [PubMed] [Google Scholar]
- 36.Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 1999;96:14843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hörsch D, Goke R, Eissele R, Michel B, Goke B. Reciprocal cellular distribution of glucagon-like peptide-1 (GLP-1) immunoreactivity and GLP-1 receptor mRNA in pancreatic islets of rat. Pancreas 1997;14:290–4. [DOI] [PubMed] [Google Scholar]
- 38.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996;137:2968–78. [DOI] [PubMed] [Google Scholar]
- 39.Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest 2012;122:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–57. [DOI] [PubMed] [Google Scholar]
- 41.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 2004;53:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Syme CA, Zhang L, Bisello A. Caveolin-1 regulates cellular trafficking and function of the glucagon-like Peptide 1 receptor. Mol Endocrinol 2006;20:3400–11. [DOI] [PubMed] [Google Scholar]
- 43.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 2007;148:4965–73. [DOI] [PubMed] [Google Scholar]
- 44.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–6. [DOI] [PubMed] [Google Scholar]
- 45.Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, Li B, Mahbod P, Sandoval D, Perez-Tilve D, Tamarina N, et al. The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab 2014;19:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 1987;79:616–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 2005;146:2069–76. [DOI] [PubMed] [Google Scholar]
- 48.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–30. [DOI] [PubMed] [Google Scholar]
- 49.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003;88:3082–9. [DOI] [PubMed] [Google Scholar]
- 50.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol 2000;279:R1449–54. [DOI] [PubMed] [Google Scholar]
- 51.Ahrén B, Holst JJ, Mari A. Characterization of GLP-1 effects on beta-cell function after meal ingestion in humans. Diabetes Care 2003;26:2860–4. [DOI] [PubMed] [Google Scholar]
- 52.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2012;62:1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 1999;44:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–51. [DOI] [PubMed] [Google Scholar]
- 55.Rodriquez de Fonseca F, et al. . Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 2000;49:709–17. [DOI] [PubMed] [Google Scholar]
- 56.Pérez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, Leiras R, Tovar S, Dieguez C, Mallo F. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes 2007;56:143–51. [DOI] [PubMed] [Google Scholar]
- 57.Poleni PE, Akieda-Asai S, Koda S, Sakurai M, Bae CR, Senba K, Cha YS, Furuya M, Date Y. Possible involvement of melanocortin-4-receptor and AMP-activated protein kinase in the interaction of glucagon-like peptide-1 and leptin on feeding in rats. Biochem Biophys Res Commun 2012;420:36–41. [DOI] [PubMed] [Google Scholar]
- 58.Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, et al. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 2005;146:5120–7. [DOI] [PubMed] [Google Scholar]
- 59.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 2006;55:3387–93. [DOI] [PubMed] [Google Scholar]
- 60.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 2009;150:1680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72. [DOI] [PubMed] [Google Scholar]
- 62.Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res 1998;779:75–83. [DOI] [PubMed] [Google Scholar]
- 63.Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D'Alessio DD, Seeley RJ. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS ONE 2012;7:e51870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ronveaux CC, de Lartigue G, Raybould HE. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol Behav 2014;135:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 2009;150:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asarian L. Loss of cholecystokinin and glucagon-like peptide-1-induced satiation in mice lacking serotonin 2C receptors. Am J Physiol Regul Integr Comp Physiol 2009;296:R51–6. [DOI] [PubMed] [Google Scholar]
- 67.Baumgartner I, Pacheco-Lopez G, Ruttimann EB, Arnold M, Asarian L, Langhans W, Geary N, Hillebrand JJ. Hepatic-portal vein infusions of glucagon-like peptide-1 reduce meal size and increase c-Fos expression in the nucleus tractus solitarii, area postrema and central nucleus of the amygdala in rats. J Neuroendocrinol 2010;22:557–63. [DOI] [PubMed] [Google Scholar]
- 68.Parker JA, McCullough KA, Field BC, Minnion JS, Martin NM, Ghatei MA, Bloom SR. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes (Lond) 2013;37:1391–8. [DOI] [PubMed] [Google Scholar]
- 69.Peters CT, Choi YH, Brubaker PL, Anderson GH. A glucagon-like peptide-1 receptor agonist and an antagonist modify macronutrient selection by rats. J Nutr 2001;131:2164–70. [DOI] [PubMed] [Google Scholar]
- 70.Meeran K, O’Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 1999;140:244–50. [DOI] [PubMed] [Google Scholar]
- 71.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol 1996;271:R848–56. [DOI] [PubMed] [Google Scholar]
- 72.Dalvi PS, Nazarians-Armavil A, Purser MJ, Belsham DD. Glucagon-like peptide-1 receptor agonist, exendin-4, regulates feeding-associated neuropeptides in hypothalamic neurons in vivo and in vitro. Endocrinology 2012;153:2208–22. [DOI] [PubMed] [Google Scholar]
- 73.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005;1044:127–31. [DOI] [PubMed] [Google Scholar]
- 74.Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J 2008;55:867–74. [DOI] [PubMed] [Google Scholar]
- 75.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 2008;57:2046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol 1996;271:E458–64. [DOI] [PubMed] [Google Scholar]
- 77.Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci 2002;102:39–44. [DOI] [PubMed] [Google Scholar]
- 78.Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellstrom PM. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil 2009;21:978–e78. [DOI] [PubMed] [Google Scholar]
- 79.Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–21. [DOI] [PubMed] [Google Scholar]
- 80.Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci 2008;28:11583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. [DOI] [PubMed] [Google Scholar]
- 82.Delporte C. Structure and physiological actions of ghrelin. Scientifica. 2013;2013:518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2007;8(1):21–34. [DOI] [PubMed]
- 84.Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut 2006;55:135. [PMC free article] [PubMed] [Google Scholar]
- 85.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001;50:2438–43. [DOI] [PubMed] [Google Scholar]
- 86.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992. [DOI] [PubMed] [Google Scholar]
- 87.Alen F, Crespo I, Ramirez-Lopez MT, Jagerovic N, Goya P, de Fonseca FR, de Heras RG, Orio L. Ghrelin-induced orexigenic effect in rats depends on the metabolic status and is counteracted by peripheral CB1 receptor antagonism. PLoS ONE 2013;8:e60918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003;144:1506–12. [DOI] [PubMed] [Google Scholar]
- 89.Banks WA. The blood-brain barrier: connecting the gut and the brain. Regul Pept 2008;149:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 2006;290:G1289–97. [DOI] [PubMed] [Google Scholar]
- 91.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002;123:1120–8. [DOI] [PubMed] [Google Scholar]
- 92.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 2010;151:3589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci 2007;27:2876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chelikani PK, Haver AC, Reidelberger RD. Ghrelin attenuates the inhibitory effects of glucagon-like peptide-1 and peptide YY(3–36) on food intake and gastric emptying in rats. Diabetes 2006;55:3038–46. [DOI] [PubMed] [Google Scholar]
- 95.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept 2007;143:64–8. [DOI] [PubMed] [Google Scholar]
- 96.Damdindorj B, Dezaki K, Kurashina T, Sone H, Rita R, Kakei M, Yada T. Exogenous and endogenous ghrelin counteracts GLP-1 action to stimulate cAMP signaling and insulin secretion in islet beta-cells. FEBS Lett 2012;586:2555–62. [DOI] [PubMed] [Google Scholar]
- 97.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, et al. . The stomach is a source of leptin. Nature 1998;394:790–3. [DOI] [PubMed] [Google Scholar]
- 98.Tang-Christensen M, Havel PJ, Jacobs RR, Larsen PJ, Cameron JL. Central administration of leptin inhibits food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab 1999;84:711–7. [DOI] [PubMed] [Google Scholar]
- 99.Barrachina MD, Martinez V, Wei JY, Tache Y. Leptin-induced decrease in food intake is not associated with changes in gastric emptying in lean mice. Am J Physiol 1997;272:R1007–11. [DOI] [PubMed] [Google Scholar]
- 100.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996;98:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Molecular metabolism.2014; 3(6):595–607. [DOI] [PMC free article] [PubMed]
- 103.Choi S, Sparks R, Clay M, Dallman MF. Rats with hypothalamic obesity are insensitive to central leptin injections. Endocrinology 1999;140:4426–33. [DOI] [PubMed] [Google Scholar]
- 104.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev 1997;11:593–602. [DOI] [PubMed] [Google Scholar]
- 105.Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 1997;138:5063–6. [DOI] [PubMed] [Google Scholar]
- 106.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004;42:983–91. [DOI] [PubMed] [Google Scholar]
- 107.Coleman DL. A historical perspective on leptin. Nat Med 2010;16:1097–9. [DOI] [PubMed] [Google Scholar]
- 108.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004;53: Suppl 3:S215–9. [DOI] [PubMed] [Google Scholar]
- 109.Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol 2006;290:R1544–9. [DOI] [PubMed] [Google Scholar]
- 110.Bates SH, Myers MG Jr. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends in endocrinology and metabolism. TEM 2003;14:447–52. [DOI] [PubMed] [Google Scholar]
- 111.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab 2011;301:E187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 2003;52:252–9. [DOI] [PubMed] [Google Scholar]
- 113.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 2011;121:2413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 2012;36:1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bojanowska E, Nowak A. Interactions between leptin and exendin-4, a glucagon-like peptide-1 agonist, in the regulation of food intake in the rat. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society.2007; 58(2):349–360. [PubMed]