Abstract
Background: Individuals with prediabetes mellitus (PreDM) and low circulating 25-hydroxyvitamin D [25(OH)D] are at increased risk of type 2 diabetes mellitus (T2DM).
Objective: We aimed to determine whether low 25(OH)D concentrations are associated with defects in insulin action and insulin secretion in persons with PreDM.
Methods: In this cross-sectional study, we stratified 488 nondiabetic subjects as having PreDM or normal fasting glucose (NFG) and a 25(OH)D concentration ≤20 ng/mL (deficient) or >20 ng/mL (sufficient). We determined insulin resistance by steady state plasma glucose (SSPG) concentration and homeostasis model assessment of insulin resistance (HOMA-IR) and insulin secretion by homeostasis model assessment of β-cell function (HOMA-β). We compared insulin resistance and secretion measures in PreDM and NFG groups; 25(OH)D-deficient and 25(OH)D-sufficient groups; and PreDM-deficient, PreDM-sufficient, NFG-deficient, and NFG-sufficient subgroups, adjusting for age, sex, race, body mass index, multivitamin use, and season.
Results: In the PreDM group, mean SSPG concentration and HOMA-IR were higher and mean HOMA-β was lower than in the NFG group (P < 0.001 for all comparisons). In the 25(OH)D-deficient group, mean SSPG concentration was higher (P < 0.001), but neither mean HOMA-IR nor HOMA-β was significantly different from that in the 25(OH)D-sufficient group. In the PreDM-deficient subgroup, mean (95% CI) SSPG concentration was higher (P < 0.01) than in the PreDM-sufficient, NFG-deficient, and NFG-sufficient subgroups [192 (177–207) mg/dL vs. 166 (155–177) mg/dL, 148 (138–159) mg/dL, and 136 (127–144) mg/dL, respectively]. Despite greater insulin resistance, mean HOMA-β was not significantly higher in the PreDM-deficient subgroup than in the PreDM-sufficient, NFG-deficient, and NFG-sufficient subgroups [98 (85–112) vs. 91 (82–101), 123 (112–136), and 115 (106–124), respectively].
Conclusion: Subjects with PreDM and low circulating 25(OH)D concentrations are the subgroup of nondiabetic individuals who are the most insulin resistant and have impaired β-cell function, attributes that put them at enhanced risk of T2DM.
Keywords: 25-hydroxyvitamin D, HOMA-β, HOMA-IR, insulin resistance, insulin secretion, prediabetes
Introduction
Understanding the relation between low 25-hydroxyvitamin D [25(OH)D]7 concentrations and development of type 2 diabetes mellitus (T2DM) has been advanced by recent findings (1–4) showing that the adverse impact of low 25(OH)D concentrations on glucose homeostasis is most apparent in individuals with prediabetes mellitus (PreDM). Subjects with PreDM tend to be heavier and to have abnormalities in insulin action and/or insulin secretion (5–9). Low concentrations of 25(OH)D have also been associated with the same abnormalities: obesity, insulin resistance, and defective insulin secretion (10–13). However, there is no consensus as to which of these abnormalities are characteristic of subjects with low concentrations of 25(OH)D. Furthermore, the relative importance of the role that each of them might play in making the subset of PreDM individuals most likely to develop T2DM in the setting of low 25(OH)D concentration has not been defined. This matter has been further confounded by results of studies in which administration of vitamin D to individuals with low 25(OH)D concentrations and/or high risk of T2DM yielded little or no clinical benefit or changes in insulin action or secretion (12, 14–19).
Thus, although subjects with PreDM are the population of nondiabetic individuals at greatest risk of developing T2DM in the presence of low concentrations of 25(OH)D, the pathophysiologic changes that link PreDM, low concentrations of 25(OH)D, and T2DM remain obscure. Possibly contributing to this lack of clarity is that many studies enrolling a large number of participants have not differentiated between subjects with normal fasting glucose (NFG) and PreDM and have not performed physiologic measurements of both insulin action and secretion. Because T2DM occurs when insulin-resistant individuals cannot sustain the degree of hyperinsulinemia necessary to overcome this defect (20, 21), the goal of this study was to test the hypothesis that low plasma concentrations of 25(OH)D in individuals with PreDM are associated with defects in insulin action and insulin secretion, putting them at increased risk of developing T2DM.
Methods
Study subjects.
The study sample consisted of 488 persons without diabetes who had participated in our studies of insulin resistance between November 2000 and April 2008. The study participants were recruited from the San Francisco Bay Area (lat 37°N) through newspaper advertisements. The volunteers were all apparently healthy and had normal findings on medical history, physical examination, and laboratory tests. Individuals were included in the study if they had a BMI (in kg/m2) between 18.5 and 39.9, had a fasting plasma glucose (FPG) concentration <126 mg/dL, had undergone an insulin suppression test, and had a frozen plasma sample available for measurement of 25(OH)D concentration. On the basis of the criteria of the American Diabetes Association (22), individuals with FPG concentrations of 100–125 mg/dL were classified as having PreDM and those with FPG concentrations <100 mg/dL were classified as having NFG. An oral glucose tolerance test was not performed. On the basis of the Institute of Medicine guidelines for evaluation of vitamin D status, individuals with 25(OH)D concentration ≤20 ng/mL were defined as 25(OH)D deficient and those with 25(OH)D concentrations >20 ng/mL were defined as 25(OH)D sufficient (23). The Stanford University’s Human Subjects Committee approved the study protocols, and all subjects gave written informed consent.
Experimental measurements.
Height and weight were determined while subjects were wearing light clothing and no shoes, and BMI was calculated by dividing weight (in kilograms) by height (meter squared).
FPG concentrations were measured by using a Beckman glucose analyzer. Fasting plasma insulin (FPI) concentrations were determined by using the LC-MS/MS method (24). Concentrations of 25-hydroxyergocalciferol [25(OH)D2] and 25-hydroxycholecalciferol [25(OH)D3] were measured by using an LC-MS/MS method that used a ThermoElectron Cohesive TLX-4 LC system coupled to ThermoElectron TSQ Ultra mass spectrometer (Thermo Fisher Scientific). Analysis was completed by using 2 multiple-reaction monitoring transitions per analyte, calculating the area under each individual peak, ratioing those numbers to the added stable-isotope–labeled vitamin D analogs, and comparing the resultant ratios to a calibration curve. Individual results for both 25(OH)D2 and 25(OH)D3 were calculated and summed to provide a total 25(OH)D result (25). For 25(OH)D2 and 25(OH)D3, the intra-assay CVs were 6–10% and 4–9%, respectively; the interassay CVs were 9–12% and 10%, respectively; and the analytic sensitivity was 4 ng/mL. The assay was 100% specific for 25(OH)D2 and 25(OH)D3. There was no cross-reaction with vitamin D2 or vitamin D3; 1α,25-dihydroxyergocalciferol; 1α,25-dihydroxycholecalciferol, calcitriol; 25,26-dihydroxycholecalciferol; 1α-hydroxyergocalciferol, doxercalciferol; or 1α-hydroxycholecalciferol, alfacalcidol. The laboratory measuring 25(OH)D concentrations was certified by the Vitamin D External Quality Assessment Scheme. FPI and 25(OH)D concentrations were measured on heparin plasma samples that had been frozen at −80°C and had not been thawed before the measurements. Blood samples for the measurement of 25(OH)D, insulin, and glucose concentrations were obtained in a fasting state on the same morning as the insulin suppression test described later.
The ability of insulin to dispose of a continuous intravenous glucose infusion was quantified by a modified version (26) of the insulin suppression test as introduced and validated by our research group (27, 28). After an overnight fast, an intravenous catheter was placed in 1 arm for a 180-min infusion of octreotide acetate (0.27 μg ⋅ m−2 ⋅ min−1), insulin (32 mU ⋅ m−2 ⋅ min−1), and glucose (267 mg ⋅ m−2 ⋅ min−1) and another catheter was placed in the contralateral arm to obtain blood for measurement of plasma glucose and insulin concentrations before and 150, 160, 170, and 180 min after starting the infusion. The mean of the 4 values obtained during the last 30 min of the infusion provided the steady state plasma glucose (SSPG) and steady state plasma insulin concentrations for each individual. Because octreotide suppresses endogenous insulin secretion, steady state plasma insulin concentrations were similar, both qualitatively and quantitatively, in all individuals. Consequently, the height of the SSPG concentration provided a direct measure of how effective insulin was in mediating disposal of the infused glucose, a value that is highly correlated with the results of the euglycemic, hyperinsulinemic clamp (28, 29).
Insulin resistance was also estimated by homeostasis model assessment of insulin resistance (HOMA-IR) as described by Mathews et al. (30): [fasting insulin concentration (μU/mL) × FPG (mmol/L)]/22.5. Pancreatic β-cell function was assessed by the homeostasis model assessment of β-cell function (HOMA-β) by using the following formula: [20 × fasting insulin concentration (μU/mL)]/[FPG (mmol/L) − 3.5] (30).
Statistical methods.
Summary statistics are presented as number (percent) of subjects, arithmetic mean ± SEM, or geometric mean (95% CI) unless otherwise indicated. FPI, HOMA-IR, and HOMA-β values were log transformed to approximate normal distribution. Characteristics of PreDM and NFG groups and 25(OH)D-deficient and 25(OH)D-sufficient groups were compared by independent sample t test (continuous variables) and χ2 test (proportions). For outcome measures (SSPG concentration, HOMA-IR, and HOMA-β), 2-factor ANCOVA models were used to evaluate the main effects of PreDM vs. NFG diagnosis, 25(OH)D status (deficient vs. sufficient), and their interaction. In addition, 1-factor ANCOVA models were used to compare mean SSPG concentration, HOMA-IR, and HOMA-β in the 4 subgroups created on the basis of PreDM vs. NFG diagnosis and 25(OH)D status: 1) PreDM, 25(OH)D deficient (n = 60); 2) PreDM, 25(OH)D sufficient (n = 106); 3) NFG, 25(OH)D deficient (n = 121); and 4) NFG, 25(OH)D sufficient (n = 201). All ANCOVA models included the following covariates: age, sex, race (white, non-Hispanic vs. nonwhite), BMI, multivitamin supplement use, and season of sample collection [summer–fall (June 1–November 30) vs. winter–spring (December 1–May 31)]. Pairwise comparisons were performed by using least-significant-difference pairwise comparison tests. Statistical analyses were performed with IBM SPSS Statistics software, version 22.0.
Results
Demographic and metabolic characteristics of participants divided on the basis of PreDM vs. NFG diagnosis or 25(OH)D status are compared in Table 1. The PreDM group was significantly older on average, had a significantly lower proportion of women, and had a significantly higher mean BMI than the NFG group. Mean FPI, HOMA-IR, and SSPG concentrations were significantly higher and mean HOMA-β was significantly lower in subjects with PreDM than in those with NFG. It should be emphasized that the 25(OH)D concentrations were essentially identical in the PreDM and NFG groups as was the proportion of individuals taking multivitamins. The 25(OH)D-deficient group was significantly younger on average, had a significantly lower proportion of non-Hispanic white subjects, and had a significantly higher mean BMI than the 25(OH)D-sufficient group. Although mean FPG concentrations did not differ as a function of 25(OH)D status, mean FPI and SSPG concentrations and mean HOMA-IR and HOMA-β values were significantly higher in the 25(OH)D-deficient group than in the 25(OH)D-sufficient group. In addition, a significantly lower proportion of 25(OH)D-deficient subjects were taking multivitamins and had the 25(OH)D samples collected during summer and fall.
TABLE 1.
Demographic and metabolic characteristics of study participants by PreDM vs. NFG diagnosis or 25(OH)D status (n = 488)1
| Glycemic status |
25(OH)D status |
|||||
| Variable | PreDM (n = 166) | NFG (n = 322) | P | Deficient (n = 181) | Sufficient (n = 307) | P |
| FPG, mg/dL | ≥100 | <100 | — | 96 ± 1 | 96 ± 1 | 0.98 |
| Plasma 25(OH)D, ng/mL | 23.6 ± 0.7 | 22.9 ± 0.5 | 0.44 | ≤20 | >20 | — |
| Multivitamin use, n (%) | 62 (37.3) | 125 (38.8) | 0.75 | 47 (26.0) | 140 (45.6) | <0.0001 |
| Age, y | 54 ± 1 | 50 ± 1 | <0.001 | 50 ± 1 | 52 ± 1 | 0.03 |
| Women, n (%) | 87 (52.4) | 200 (62.1) | 0.04 | 114 (63.0) | 173 (56.4) | 0.15 |
| White, non-Hispanic, n (%) | 120 (72.3) | 213 (66.1) | 0.17 | 94 (51.9) | 239 (77.9) | <0.001 |
| BMI, kg/m2 | 30.7 ± 0.3 | 28.9 ± 0.2 | <0.001 | 30.1 ± 0.3 | 29.2 ± 0.2 | 0.02 |
| FPI, μU/mL | 11.9 (10.9, 13.0) | 8.7 (8.2, 9.7) | <0.001 | 10.7 (9.8, 11.7) | 9.1 (8.5, 9.8) | 0.005 |
| SSPG, mg/dL | 183 ± 5 | 136 ± 4 | <0.001 | 169 ± 5 | 143 ± 4 | <0.001 |
| HOMA-IR | 3.12 (2.85, 3.42) | 1.95 (1.83, 2.09) | <0.001 | 2.54 (2.31, 2.79) | 2.17 (2.02, 2.33) | 0.009 |
| HOMA-β | 99 (90, 108) | 115 (107, 123) | 0.009 | 122 (111, 133) | 102 (95, 109) | 0.002 |
| Samples collected during summer/fall, n (%) | 77 (46.4) | 147 (45.7) | 0.88 | 56 (30.9) | 168 (54.7) | <0.001 |
Data are arithmetic mean ± SEM or geometric mean (95% CI) unless otherwise indicated. Means were compared by independent sample t test and proportions by χ2 test. FPG, fasting plasma glucose; FPI, fasting plasma insulin; HOMA-β, homeostasis model assessment of β-cell function; NFG, normal fasting glucose; PreDM, prediabetes mellitus; SSPG, steady state plasma glucose; 25(OH)D, 25-hydroxyvitamin D.
In the entire group (n = 488), the mean plasma 25(OH)D concentration was significantly higher in samples collected during summer and fall than in those collected during winter and spring (25.6 ± 0.6 ng/mL vs. 21.1 ± 0.5 ng/mL; P < 0.001). The mean 25(OH)D concentrations were also significantly higher in non-Hispanic white subjects than in nonwhite subjects (25.1 ± 0.4 ng/mL vs. 19.1 ± 0.7 ng/mL; P < 0.001) and in individuals who were taking multivitamin supplements than in those who were not (25.4 ± 0.6 ng/mL vs. 21.8 ± 0.5 ng/mL; P < 0.001).
The independent effects of PreDM vs. NFG diagnosis and 25(OH)D status on insulin resistance and insulin secretory function are shown in Table 2. Focusing initially on insulin resistance, the results of 2-factor ANCOVA demonstrate that individuals with PreDM had a higher mean SSPG concentration and a higher mean HOMA-IR value (more insulin resistant) than those who had NFG, after adjusting for 25(OH)D status and the covariates (significant main effects of PreDM vs. NFG diagnosis). Table 2 also shows that 25(OH)D-deficient subjects were more insulin resistant by SSPG concentration but not by HOMA-IR than those who were 25(OH)D sufficient, after accounting for PreDM vs. NFG diagnosis and the covariates [significant main effect of 25(OH)D status in case of SSPG concentration]. Furthermore, the differences in means of SSPG concentration and HOMA-IR between the PreDM and NFG groups did not depend on the 25(OH)D status (nonsignificant interaction effects; P = 0.24 for SSPG concentration and P = 0.62 for HOMA-IR).
TABLE 2.
Impact of PreDM vs. NFG diagnosis and 25(OH)D status on insulin resistance (SSPG concentration and HOMA-IR) and insulin secretion (HOMA-β; n = 488)1
| Glycemic status2 |
25(OH)D status3 |
|||||
| Variable | PreDM4 (n = 166) | NFG4 (n = 322) | P | Deficient5 (n = 181) | Sufficient5 (n = 307) | P |
| SSPG,6 mg/dL | 179 ± 5 | 142 ± 3 | <0.001 | 170 ± 5 | 151 ± 4 | 0.002 |
| HOMA-IR6 | 2.94 (2.71, 3.18) | 2.05 (1.93, 2.17) | <0.001 | 2.55 (2.35, 2.77) | 2.35 (2.21, 2.50) | 0.13 |
| HOMA-β7 | 94 (87, 103) | 119 (112, 126) | <0.001 | 110 (101, 119) | 102 (96, 109) | 0.21 |
Data are arithmetic mean ± SEM or geometric mean (95% CI). Means were compared by using 2-factor ANCOVA adjusting for age, sex, race, BMI, multivitamin use, and season (covariates). There was no significant glycemic status × 25(OH)D status interaction. HOMA-β, homeostasis model assessment of β-cell function; NFG, normal fasting glucose; PreDM, prediabetes mellitus; SSPG, steady state plasma glucose; 25(OH)D, 25-hydroxyvitamin D.
FPG concentration ≥100 mg/dL, PreDM, and <100 mg/dL, NFG.
25(OH)D concentration ≤20 ng/mL, 25(OH)D deficient, and > 20 ng/mL, 25(OH)D sufficient.
Means adjusted for 25(OH)D status and covariates.
Means adjusted for glycemic status and covariates.
Higher values indicate increased insulin resistance.
Lower values indicate decreased β-cell function.
Results of a similar analytic approach, applied to our estimate of insulin secretory function, demonstrated that insulin secretory function (mean HOMA-β value) was also lower in subjects with PreDM than in those with NFG, after adjusting for 25(OH)D status and covariates (significant main effect of PreDM vs. NFG diagnosis; Table 2). In contrast, mean HOMA-β values of the 25(OH)D-deficient and -sufficient groups were similar, after accounting for PreDM vs. NFG diagnosis and the covariates [nonsignificant main effect of 25(OH)D status]. Moreover, the difference in HOMA-β means between the PreDM and the NFG groups did not depend on the 25(OH)D status (nonsignificant interaction effect; P = 0.98).
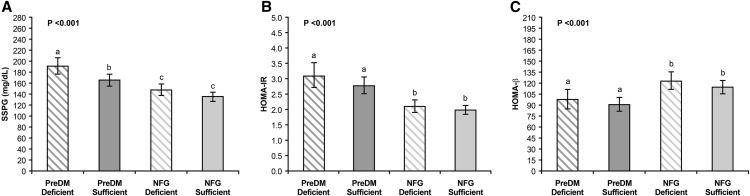
The comparison of measures of insulin action and insulin secretion in subgroups created on the basis of PreDM vs. NFG diagnosis and 25(OH)D status is depicted in Figure 1. Subjects with both PreDM and deficient 25(OH)D were significantly more insulin resistant by SSPG concentration but not by HOMA-IR than those with both PreDM and sufficient 25(OH)D. On the other hand, mean SSPG concentration and HOMA-IR did not differ significantly by 25(OH)D status in the NFG group. Furthermore, individuals with both PreDM and deficient 25(OH)D were significantly more insulin resistant by SSPG concentration and HOMA-IR than those with NFG and deficient or sufficient 25(OH)D. Figure 1 also compares insulin secretory function in the various experimental subgroups and demonstrates that mean HOMA-β did not differ significantly by 25(OH)D status in the PreDM group or the NFG group. Finally, subjects with both PreDM and deficient 25(OH)D had a significantly lower mean HOMA-β value than those with NFG and deficient or sufficient 25(OH)D.
FIGURE 1.
Insulin resistance (SSPG concentration and HOMA-IR) and insulin secretion (HOMA-β) in individuals with PreDM or NFG divided on the basis of 25(OH)D status. Values are arithmetic means (SSPG concentration) or geometric means (HOMA-IR and HOMA-β) and their 95% CIs (error bars). Higher values of SSPG concentration (A) and HOMA-IR (B) indicate increased insulin resistance and lower values of HOMA-β (C) indicate decreased β-cell function. In each panel, the P value denotes the overall difference in means among the 4 subgroups by 1-factor ANCOVA, adjusted for age, sex, race, BMI, multivitamin use, and season. Pairs of means without a common letter differ, P < 0.05 by least-significant-difference pairwise comparison test. FPG concentration ≥100 mg/dL, PreDM, and <100 mg/dL, NFG. 25(OH)D concentration ≤20 ng/mL, 25(OH)D deficient, and >20 ng/mL, 25(OH)D sufficient. Deficient, 25(OH)D deficient; HOMA-β, homeostasis model assessment of β-cell function; NFG, normal fasting glucose; PreDM, prediabetes mellitus; SSPG, steady state plasma glucose; sufficient, 25(OH)D sufficient; 25(OH)D, 25-hydroxyvitamin D.
Discussion
The results of this cross-sectional study provide new information concerning the relation between plasma 25(OH)D concentration and insulin action and secretion in nondiabetic individuals and enable us to propose a pathophysiologic explanation for why T2DM is more likely to develop in association with low 25(OH)D concentrations in subjects with PreDM compared with persons with NFG.
First, the results in Table 1 show that 25(OH)D concentrations in subjects with PreDM were not different from concentrations in those with NFG. Thus, the enhanced diabetes risk of persons with PreDM when compared with those with NFG cannot be a simple function of a priori differences in 25(OH)D status. However, subjects with PreDM had higher SSPG concentrations and lower values for HOMA-β than those with NFG (Table 2); they were more insulin resistant with lower insulin secretory function. These differences were independent of 25(OH)D status and other covariates. Because insulin resistance and impaired insulin secretion are the predictors of T2DM (20, 21), the basic metabolic characteristics of subjects with PreDM increase their risk of T2DM compared with individuals with NFG.
Although subjects with PreDM were insulin resistant as a group compared with NFG, when each group was separated into 2 subgroups on the basis of having a deficient or sufficient 25(OH)D concentration (Figure 1), individuals with both PreDM and deficient 25(OH)D concentration had the highest mean SSPG concentration—they were the most insulin resistant. Comparisons of HOMA-β measurements in the 4 subgroups (Figure 1) are particularly important and show that despite a greater degree of insulin resistance, subjects with both PreDM and 25(OH)D deficiency did not have a significantly higher insulin secretory function than the other subgroups. Thus, subjects with PreDM and deficient 25(OH)D concentrations appear to be at increased risk of T2DM because of a higher degree of insulin resistance and an inadequate insulin secretory function to compensate for insulin resistance.
Multiple potential mechanisms whereby 25(OH)D deficiency could contribute to the inability of the subjects with PreDM to adequately increase insulin secretory function and to cause worse insulin resistance have been documented (31, 32). Vitamin D acts on multiple pathways that regulate insulin and glucose homeostasis including 1) insulin synthesis, 2) insulin signaling, 3) systemic and adipose inflammation, and 4) adipose tissue homeostasis. Vitamin D receptors (VDRs) have been identified in pancreatic β-cells and a vitamin D response element is present in the promoter of the insulin gene (33). Mice with absent VDR (VDR null mice) exhibit impaired insulin secretory capacity (34). Pancreatic β-cells express CYP27B1 [the gene encoding the enzyme 25(OH)D3-1α-hydroxylase], giving these cells the ability to synthesize active 1,25-dihydroxyvitamin D [1,25(OH)2D] from circulating 25(OH)D, which can then act locally in a paracrine fashion within the islets to regulate target genes (35). 1,25(OH)2D has also been shown to regulate insulin receptors in target cells (36). Thus, activated vitamin D exhibits the ability to stimulate both insulin synthesis and insulin signaling. 1,25(OH)2D has been shown to exhibit many systemic anti-inflammatory actions (37, 38) and it is well documented that systemic and adipose tissue inflammation increase insulin resistance (39–41). Finally, vitamin D deficiency alters adipose cells in multiple ways including modulating differentiation, inflammation, adipogenesis, and adipocyte secretion and is associated with increased fat mass and infiltration into muscle, all actions that would increase insulin resistance (31, 42). These findings collectively provide a mechanistic rationale for subjects with 25(OH)D deficiency exhibiting increased risk of transitioning from PreDM to T2DM.
Our study had several limitations that should be addressed. It was a cross-sectional in design and was based on specimens collected from earlier studies. Therefore, a causal relation between low circulating 25(OH)D concentrations and defects in insulin action and insulin secretion cannot be inferred from our findings. Second, we used the Institute of Medicine suggested 25(OH)D concentration cutoff point of 20 ng/mL to classify individuals as 25(OH)D deficient or sufficient. Although this is a logical choice, it is also significant because it is a value approximating both the median 25(OH)D concentration of our study sample (23 ng/mL) and the value of 26 ng/mL that Sorkin et al. (43) identified as being associated with loss of insulin sensitivity. Finally, although we quantified insulin resistance (SSPG concentration) directly, we determined insulin secretion by a widely used surrogate estimate (HOMA-β).
On the other hand, we measured 25(OH)D concentrations, insulin action, and insulin secretion in a large sample of nondiabetic individuals, subdivided into groups with either PreDM or NFG, in an attempt to explain why the PreDM group is put at greatest risk of developing T2DM in association with lower 25(OH)D concentrations. We are unaware of any published study that has tried to do that.
Because the prevalence of insulin resistance is increased in subjects with PreDM (9), it is not surprising that persons with PreDM represent the subset of nondiabetic individuals most likely to develop T2DM in the face of low plasma 25(OH)D concentrations. Based on this pathophysiologic insight, it seems reasonable to hypothesize that if vitamin D repletion was effective in preventing the onset of T2DM in nondiabetic individuals with low plasma 25(OH)D concentrations, it would be most likely to succeed in those at highest risk, i.e., persons with PreDM. On the other hand, not all persons with PreDM are insulin resistant (9), and this may help explain why the vitamin D repletion study by Davidson et al. (19) did not reduce the development of T2DM in subjects with PreDM. Specifically, the vitamin D–treated subjects in their study were chosen solely on the basis of having PreDM, not whether or not they were insulin resistant, and in this otherwise excellent study, surrogate estimates, not direct measures of insulin sensitivity, were used.
Thus, to test the hypothesis that it is a combination of insulin resistance and low 25(OH)D concentrations that increases the risk of T2DM in individuals with PreDM, nutritional scientists can conduct a pilot study by enrolling insulin-resistant volunteers with PreDM and low plasma 25(OH)D concentrations, using specific methodology to make sure that all volunteers are insulin resistant. Volunteers meeting these criteria can then be randomly assigned to treatment with vitamin D or placebo, and the degree to which vitamin D repletion improves insulin action and/or secretion can be compared. In other words, to successfully evaluate the proposed hypothesis, the experimental population should consist of subjects with PreDM who are both insulin resistant and have low 25(OH)D concentrations and have also undergone detailed assessments of nutritional status and other variables that influence vitamin D status.
Acknowledgments
We thank Jeff Radcliff of Quest Diagnostics Nichols Institute for providing editorial assistance and Butch Colyear of Butch Colyear Designs and Illustration for preparing the figure. FA researched the data, had full access to the data, and takes responsibility for the integrity of the data and the accuracy of data analysis. FA and GMR designed the study. MPC and FMH supervised the 25(OH)D measurements. FA, CB, and FMH analyzed the data. FA, CB, DF, MPC, FMH, and GMR interpreted the data, contributed to the discussion, and reviewed/edited the manuscript. FA, DF, and GMR wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CYP27B1, the gene encoding the enzyme 25(OH)D3-1α-hydroxylase; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HOMA-β, homeostasis model assessment of β-cell function; NFG, normal fasting glucose; PreDM, prediabetes mellitus; SSPG, steady state plasma glucose; T2DM, type 2 diabetes mellitus; VDR, vitamin D receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol.
References
- 1.Gupta AK, Brashear MM, Johnson WD. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care 2011;34:658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care 2011;34:1114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittas AG, Nelson J, Mitri J, Hillmann W, Garganta C, Nathan DM, Hu FB, Dawson-Hughes B; Diabetes Prevention Program Research Group. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care 2012;35:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Ostenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia 2012;55:1668–78. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–5. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B; American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–9. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Reaven GM. Isolated impaired fasting glucose and peripheral insulin sensitivity: not a simple relationship. Diabetes Care 2008;31:347–52. [DOI] [PubMed] [Google Scholar]
- 10.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–5. [DOI] [PubMed] [Google Scholar]
- 11.Kayaniyil S, Retnakaran R, Harris SB, Vieth R, Knight JA, Gerstein HC, Perkins BA, Zinman B, Hanley AJ. Prospective associations of vitamin D with beta-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes 2011;60:2947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 2011;94:486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, Perkins BA, Harris SB, Zinman B, Hanley AJ. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33:1379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljunghall S, Lind L, Lithell H, Skarfors E, Selinus I, Sorensen OH, Wide L. Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance–a prospective randomized double-blind study. Acta Med Scand 1987;222:361–7. [DOI] [PubMed] [Google Scholar]
- 15.Lind L, Pollare T, Hvarfner A, Lithell H, Sorensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res 1989;11:141–7. [PubMed] [Google Scholar]
- 16.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–6. [DOI] [PubMed] [Google Scholar]
- 17.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 2008;31:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai K, Need AG, Horowitz M, Chapman IM. Glucose tolerance and vitamin D: effects of treating vitamin D deficiency. Nutrition 2008;24:950–6. [DOI] [PubMed] [Google Scholar]
- 19.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013;36:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607. [DOI] [PubMed] [Google Scholar]
- 21.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–92. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Asociation. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36(Suppl 1):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington (DC): The National Academies Press; 2011. [PubMed] [Google Scholar]
- 24.Chen Z, Caulfield MP, McPhaul MJ, Reitz RE, Taylor SW, Clarke NJ. Quantitative insulin analysis using liquid chromatography-tandem mass spectrometry in a high-throughput clinical laboratory. Clin Chem 2013;59:1349–56. [DOI] [PubMed] [Google Scholar]
- 25.Taylor RL, Grebe SK, Singh RJ. Throughput analysis of 25-hydroxyvitamins D2 and D3 by LC-MS/MS using an automated on-line extraction. Clin Chem 2005;51:A231–2. [Google Scholar]
- 26.Pei D, Jones CN, Bhargava R, Chen YD, Reaven GM. Evaluation of octreotide to assess insulin-mediated glucose disposal by the insulin suppression test. Diabetologia 1994;37:843–5. [DOI] [PubMed] [Google Scholar]
- 27.Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest 1970;49:2151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes 1981;30:387–92. [DOI] [PubMed] [Google Scholar]
- 29.Knowles JW, Assimes TL, Tsao PS, Natali A, Mari A, Quertermous T, Reaven GM, Abbasi F. Measurement of insulin-mediated glucose uptake: direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism 2013;62:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, Schoonjans K, Verstuyf A. Vitamin D and energy homeostasis—of mice and men. Nat Rev Endocrinol 2014;10:79–87. [DOI] [PubMed] [Google Scholar]
- 32.Muscogiuri G, Mitri J, Mathieu C, Badenhoop K, Tamer G, Orio F, Mezza T, Vieth R, Colao A, Pittas A. Mechanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur J Endocrinol 2014;171:R101–10. [DOI] [PubMed] [Google Scholar]
- 33.Maestro B, Davila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 2003;84:223–30. [DOI] [PubMed] [Google Scholar]
- 34.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 2003;17:509–11. [DOI] [PubMed] [Google Scholar]
- 35.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 2004;89–90:121–5. [DOI] [PubMed] [Google Scholar]
- 36.Calle C, Maestro B, Garcia-Arencibia M. Genomic actions of 1,25-dihydroxyvitamin D3 on insulin receptor gene expression, insulin receptor number and insulin activity in the kidney, liver and adipose tissue of streptozotocin-induced diabetic rats. BMC Mol Biol 2008;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 2011;51:311–36. [DOI] [PubMed] [Google Scholar]
- 38.Ding C, Wilding JP, Bing C. 1,25-Dihydroxyvitamin D3 protects against macrophage-induced activation of NFkappaB and MAPK signalling and chemokine release in human adipocytes. PLoS ONE 2013;8:e61707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141–50. [DOI] [PubMed] [Google Scholar]
- 41.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014;41:36–48. [DOI] [PubMed] [Google Scholar]
- 42.Marcotorchino J, Tourniaire F, Astier J, Karkeni E, Canault M, Amiot MJ, Bendahan D, Bernard M, Martin JC, Giannesini B, et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem 2014;25:1077–83. [DOI] [PubMed] [Google Scholar]
- 43.Sorkin JD, Vasaitis TS, Streeten E, Ryan AS, Goldberg AP. Evidence for threshold effects of 25-hydroxyvitamin D on glucose tolerance and insulin resistance in black and white obese postmenopausal women. J Nutr 2014;144:734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]