Abstract
Background: An estimated one-third of the world’s children who are wasted live in India. In Bihar state, of children <5 y old, 27.1% are wasted and 8.3% have severe acute malnutrition (SAM). In 2009, Médecins Sans Frontières (MSF) initiated a community-based management of acute malnutrition (CMAM) program for children aged 6–59 mo with SAM.
Objective: In this report, we describe the characteristics and outcomes of 8274 children treated between February 2009 and September 2011.
Design: Between February 2009 and June 2010, the program admitted children with a weight-for-height z score (WHZ) <−3 SD and/or midupper arm circumference (MUAC) <110 mm and discharged those who reached a WHZ >−2 SDs and MUAC >110 mm. These variables changed in July 2010 to admission on the basis of an MUAC <115 mm and discharge at an MUAC ≥120 mm. Uncomplicated SAM cases were treated as outpatients in the community by using a WHO-standard, ready-to-use, therapeutic lipid-based paste produced in India; complicated cases were treated as inpatients by using F75/F100 WHO-standard milk until they could complete treatment in the community.
Results: A total of 8274 children were admitted including 5149 girls (62.2%), 6613 children aged 6–23 mo (79.9%), and 87.3% children who belonged to Scheduled Caste, Scheduled Tribe, or Other Backward Caste families or households. Of 3873 children admitted under the old criteria, 41 children (1.1%) died, 2069 children (53.4%) were discharged as cured, and 1485 children (38.3%) defaulted. Of 4401 children admitted under the new criteria, 36 children (0.8%) died, 2526 children (57.4%) were discharged as cured, and 1591 children (36.2%) defaulted. For children discharged as cured, the mean (±SD) weight gain and length of stay were 4.7 ± 3.1 and 5.1 ± 3.7 g · kg−1 · d−1 and 8.7 ± 6.1 and 7.3 ± 5.6 wk under the old and new criteria, respectively (P < 0.01). After adjustment, significant risk factors for default were as follows: no community referral for admission, more severe wasting on admission, younger age, and a long commute for treatment.
Conclusions: To our knowledge, this is the first conventional CMAM program in India and has achieved low mortality and high cure rates in nondefaulting children. The new admission criteria lower the threshold for severity with the result that more children are included who are at lower risk of death and have a smaller WHZ deficit to correct than do children identified by the old criteria. This study was registered as a retrospective observational analysis of routine program data at http://www.isrctn.com as ISRCTN13980582.
Keywords: community-based management of acute malnutrition, India, severe acute malnutrition, severe wasting, mean upper arm circumference
INTRODUCTION
According to the third Indian National Family Health Survey-3 conducted in 2005–2006, 19.8% of children <5 y of age were wasted and 6.4% suffered from severe acute malnutrition (SAM)4 in India. The situation appeared worse in the state of Bihar, where in children <5 y of age, 27.1% were wasted and 8.3% suffered from SAM (1). These findings suggest that, at any one point in time, an average of 8 million children in India <5 y of age suffer from severe wasting, which is the most common form of SAM. This number constitutes one-third of the global burden (2). The Indian government and a number of nongovernmental organizations (NGOs) are currently implementing various initiatives that address SAM across the country. Although the principal strategy being deployed remains inpatient care through Nutritional Rehabilitation Centers and Malnutrition Treatment Centers (3–6), there is a growing consensus within India that the adoption of community-based management of acute malnutrition (CMAM) is crucial to achieving widespread, effective coverage and treatment of all children with SAM (7, 8). Furthermore, standardized inpatient management of SAM has been associated with worse outcomes than has management in the community, which is attributable partly to the risk of children with SAM acquiring serious infections from other hospitalized patients and partly because of families’ lack of acceptance of hospital-based care (9, 10).
After widespread flooding in Bihar, Médecins Sans Frontières (MSF) conducted a household-based survey in Darbhanga, which is a district of 3.9 million people and one of the poorest in Bihar. The survey showed that the prevalence of wasting and SAM in children age <5 y was 19.4% and 4.8%, respectively (11). Consequently, under a memorandum of understanding with the district authorities and, thereafter, consent from the Bihar State Health Society, in February 2009, MSF initiated a CMAM program in Biraul block, Darbhanga district. The CMAM model has been extensively used outside India, with >50 countries adopting this approach as their first-line strategy for treating SAM (12). CMAM is based on principles that acknowledge both the need for prolonged treatment and the small proportion of children with SAM who require inpatient care for medical reasons and evidence that ambulatory management in the community can be a cost-effective solution from both societal and health care provider perspectives (13–15). A standard CMAM program consists of treatment sites close to the community where the children with uncomplicated SAM, who constitute the majority of patients, can be seen weekly and an inpatient facility [Stabilization Center (SC)] that admits only children with SAM plus associated medical complications that require specialist medical attention and keeping the children only until they recover enough to continue treatment as outpatients in the community.
However, a major obstacle to the widespread adoption of this strategy in India is that the vast majority of evidence on the effectiveness of CMAM programs comes from African settings. Therefore, we conducted an observational, retrospective cohort study to assess key programmatic variables and clinical outcomes of India’s first (and currently only to our knowledge) conventional setting CMAM program, which treated 8274 children between February 2009 and September 2011. We also analyzed the impact of adopting simpler admission and discharge criteria on the profile of admitted children and treatment outcomes.
METHODS
Overview of the program
The CMAM program opened in Biraul block, which has a population of ∼286,000 people, in February 2009. The inpatient SC was established within the Biraul Primary Health Center (PHC), and over the next 3 y, 5 ambulatory treatment centers [called Ambulatory Therapeutic Feeding Centers (ATFCs)] were established at different community settings within the block. Within the existing health infrastructure, government general nurse midwives (GNMs), auxiliary nurse midwives (ANMs), and accredited social health activists (ASHAs) were trained to use midupper arm circumference (MUAC) tapes (provided by MSF) to screen children aged 6–59 mo for SAM and refer identified cases to the CMAM program. Within the CMAM program, MSF used GNMs and ANMs who were also trained in the use of Salter scales (precision to 100 g) and locally produced height boards (precision to 0.1 cm) to record weight and height/length, respectively, and as a way of monitoring progress throughout treatment. Salter scales were regularly calibrated and replaced as per manufacturer guidelines. Children admitted to the program were a mixture of those screened in the community and referred by ASHAs, those who self-presented for SAM screening, and those visiting the sites to seek health care for other reasons. On admission, information on caste, which is a form of social stratification used in India, was also recorded by using the following categories and definitions: Scheduled Caste and Scheduled Tribe (terms used for 2 groups of historically disadvantaged people recognized in India’s Constitution), Other Backward Class (a collective term used by the Indian government for castes that are educationally and socially disadvantaged but not specifically mentioned in the Constitution), and General Category (not considered disadvantaged). The first 3 groups combined account for ∼60% of India’s population.
Treatment centers were open on an average of 1 d/wk at each location, generally on the same day each week to provide consistency for caregivers. Once a child was newly diagnosed with SAM, his or her details were entered into a register, and health education was provided to the caregiver while an ANM or GNM repeated anthropometric measurements, took the child’s vital signs, and performed a basic triage including a standardized appetite test to determine the child’s ability and willingness to eat (Table 1). The purpose of this test is to provide the child with a small amount of food to see if a child with severe wasting has the desire and strength to eat; if this is not the case, the child is considered to have anorexia, and, therefore, be at higher risk of complications, and thus is admitted directly for inpatient care in the SC. All children were examined by a physician for complications that might require either admission into the SC or additional medication beyond that provided during the systematic initial treatment. The initial treatment comprised albendazole, amoxicillin, vitamin A (given once edema resolved in affected children), folic acid, measles vaccination, and screening for malaria.
TABLE 1.
Criteria for diagnosis of complicated severe acute malnutrition1
| Category | Description of symptom/circumstance |
| Appetite | Poor appetite or refusal or inability to eat test dose of ready-to-use lipid-based paste |
| Medical complications | Intractable vomiting |
| Severe dehydration (on the basis of history and clinical signs) | |
| Fever >39°C or hypothermia <36°C | |
| Lower respiratory tract infection (as per IMCI guidelines) | |
| Severe anemia (very pale; difficulty breathing) | |
| Profound weakness, apathy, unconsciousness, or convulsions | |
| Edema ++/+++2 | |
| Caregiver choice | Offered the choice, caregiver refuses ambulatory care |
| Transfer in from ATFC | Medical complication (see above) |
| Static weight or weight loss for 2 consecutive weeks | |
| No recovery after 2 mo in ambulatory program |
ATFC, ambulatory therapeutic treatment center; IMCI, Integrated Management of Childhood Illness.
Edema was graded on a scale from + to +++, reflecting mild to most severe, respectively. The mildest form (+) was treated in the community.
Children were considered complicated cases if they required admission to the SC, either immediately on diagnosis of SAM or at any point during the community treatment phase, on the basis of the exhibition of one or more symptoms listed in Table 1.
Treatment at community-level ATFCs
Caregivers of children considered uncomplicated were counseled regarding the program and given a 1-wk supply of WHO-standard (16), prepackaged F100-equivalent (per kcal), lipid-based, ready-to-use therapeutic paste produced in India (Eezeepaste; Compact). The quantity provided depended on the child’s weight as detailed in Table 2. Caregivers were informed that the paste was medicine, not food, and, therefore, should not be shared with other family members. To guard against refeeding syndrome (a group of clinical symptoms caused by fluid and electrolyte imbalances resulting from too rapid a rate of nutritional supplementation in severely malnourished patients), caregivers were also educated about dividing the daily ration into frequent small feeds with approximate gaps of 3 h to augment the therapy with local foods if children remained hungry and ensure that children had plenty of drinking water during and after eating. Continued breastfeeding of children ≤24 mo old was encouraged for those who were not yet fully weaned.
TABLE 2.
Treatment schedule for ready-to-use lipid-based paste in the community ambulatory setting
| Ready-to-use lipid-based paste sachet (92-g sachet containing 500 kcal) |
||
| Weight | Sachets/d | Sachets/wk |
| 3.0–3.4 kg | 1.25 | 9 |
| 3.5–4.9 kg | 2 | 14 |
| 5.0–6.9 kg | 3 | 21 |
| 7.0–9.9 kg | 4 | 28 |
| ≥10.0 kg | 6 | 42 |
Caregivers were asked to return to the ATFC at weekly intervals, at which time anthropometric measurements were repeated and children examined for complications. Caregivers were also advised to seek medical advice or attend the SC if a child became unwell between scheduled visits.
Treatment of children at the inpatient SC
Admission into the 24-h SC unit could occur at the initial presentation or any point during the community-treatment phase. All children who presented with one or more conditions listed in Table 1 were admitted directly to the SC and provided 24-h medical care and nutritional treatment until their clinical condition stabilized enough for transfer (or return) to an ATFC. Within the SC, children were treated according to the standard MSF inpatient-treatment protocol for SAM, which is very similar to the later published Indian Ministry of Health 2011 operational guidelines for facility-based management (17). Both protocols divide the treatment of complicated cases into the following phases as shown in Table 3: phase 1 is used to restore metabolic function and stabilize clinical status by using frequent feeds of WHO-standard F75 milk (18); a transition phase of increased energy intake within the same volume of feed is used to restore lost tissue and reduce risk of refeeding syndrome and fluid overload; and phase 2 is used to promote weight gain by increasing nutritional intake and re-integration into the social environment before transfer to the ATFC by using F100-equivalent lipid-based paste augmented with local foods.
TABLE 3.
Summary of inpatient treatment protocol used in Stabilization Centers
| Treatment phase | Treatment | Duration, d |
| Phase 1 | 8 meals/d (every 3 h), F75 milk | 1–7 |
| 100 kcal · kg−1 · d−1 | ||
| (135 mL F75 milk · kg−1 · d−1) | ||
| Transition phase | 8 meals/d (every 3 h), F100 milk | 1–3 |
| 135 kcal · kg−1 · d−1 | ||
| (135 mL F100 milk · kg−1 · d−1) | ||
| Phase 2 | Ready-to-use lipid-based paste plus one local meal | 1–2 |
| >200 kcal · kg−1 · d−1 |
Classification of exits
To be discharged as cured, children had to meet the discharge criteria on 2 consecutive visits. Defaulters were defined as those who failed to attend the AFTC for 2 consecutive weeks or who left the SC and did not return for 2 consecutive days. Caregivers who refused consent for their children to be admitted into the SC or removed them from the SC prematurely but agreed to continue treatment in the ATFC were not considered defaulters. Death was defined as children who died while registered in the program.
Children were classified as nonresponders if their nutritional status did not improve over an extended period of time despite their having good appetite and no identified underlying medical conditions or reason for the lack of weight gain. Children treated in the community who either did not gain or lost weight over a 2-wk period were offered elective admission to the SC so that feeding practices could be observed and a more detailed medical examination (including diagnostic tests) performed. If no causes for failure to thrive were shown, the child was classified as a nonrespondent. However, this elective admission to the SC was not widely accepted by caregivers, and therefore, a large proportion of children classified as nonresponders were based only on prolonged periods of unsupervised community treatment.
Community information, education, and communication strategies
A critical component of the CMAM program involved regular, active engagement of dedicated teams with communities across Biraul block by using information, education, and communication (IEC) tools to explain CMAM. This engagement included mobilizing community leaders to disseminate information regarding severe malnutrition and providing details on the availability and schedule of ATFCs. A variety of messaging tools were developed for the local context including plays, songs recorded in the local language by local artists, and a show by local artists featuring paintings related to local concepts of malnutrition (and subsequently used for delivering IEC messages to the wider community). Over the course of the program, the majority of ASHAs in the block were sensitized about the CMAM program and relayed messages about SAM to the community.
Admission and discharge criteria
The program initially aimed to treat all children between 6 and 59 mo of age who presented with a weight-for-height z score (WHZ) <−3 and/or MUAC <110 mm and/or bilateral edema. Children were discharged after maintaining a WHZ >−2 and MUAC >110 mm with no edema for 1 wk and in good clinical condition with a good appetite (referred to here as the old criteria).With the use of updated recommendations from WHO and UNICEF (19) and to allow for wider screening coverage and simplicity, in July 2010 the admission criteria were changed to an MUAC <115 mm and/or bilateral edema; discharge criteria were changed to maintaining an MUAC ≥120 mm with no edema for 1 wk with good clinical condition and good appetite (new criteria). The MUAC has been implemented throughout the world as a simple, sensitive, and easy to use tool to screen and identify children most at risk of death from SAM (20).
Data handling and statistical analysis
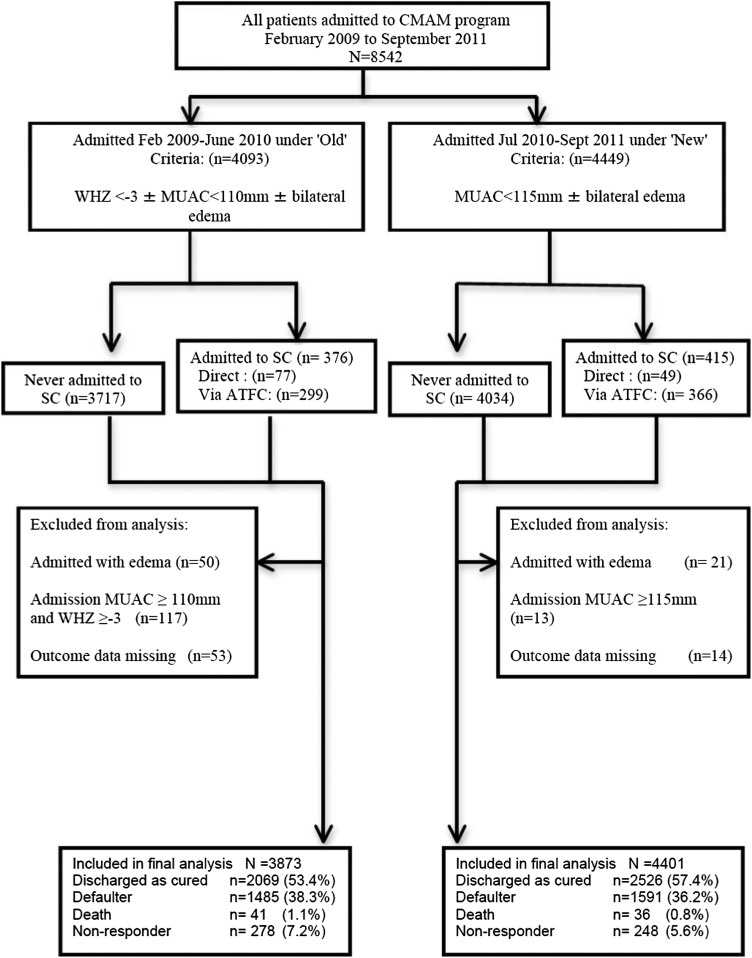
Dedicated and frequently trained data-entry operators entered all data into a standard Microsoft Excel 2010 database; however, double data entry was not done. An epidemiologist ensured that the database was well maintained by performing regular audits on the quality of data transfer and integrity of the database. Regular database cleaning comprised checks for inconsistencies relative to source documents when necessary, and all patient files were maintained securely throughout the program for this purpose. WHO Anthro software (v.3.2.2) was used to calculate the WHZ. A retrospective analysis of all routinely collected program data were conducted with SPSS version 19 statistical software (IBM). A multivariate logistic regression model was also developed to determine risk factors significantly (P < 0.05) associated with being a defaulter on the bivariate analysis. Variables that were justified a priori or associated with default in other studies were also included and added stepwise in the multivariate analysis. Children admitted despite not meeting admission criteria were excluded from the final analysis (Figure 1) as were children admitted into the program with edema because of to their very limited numbers (n = 71, 0.8%).
FIGURE 1.
Flowchart of analysis. ATFC, Ambulatory Therapeutic Feeding Center; CMAM, community-based management of acute malnutrition; MUAC, midupper arm circumference; SC, Stabilization Center; WHZ, weight-for-height z score.
Ethics statement
This analysis met the MSF Institutional Ethics Review Committee criteria for a study involving routinely collected program data. The program used a widely recognized treatment model (CMAM) for SAM and was conducted under a memorandum of understanding with the district authorities and, thereafter, with consent of the Bihar State Health Society, which is the usual procedure for NGOs operating in this context. All electronic data were analyzed anonymously.
RESULTS
A total of 8542 children were admitted into the program between February 2009 and September 2011 (Figure 1) with admissions following a similar seasonal pattern each year.
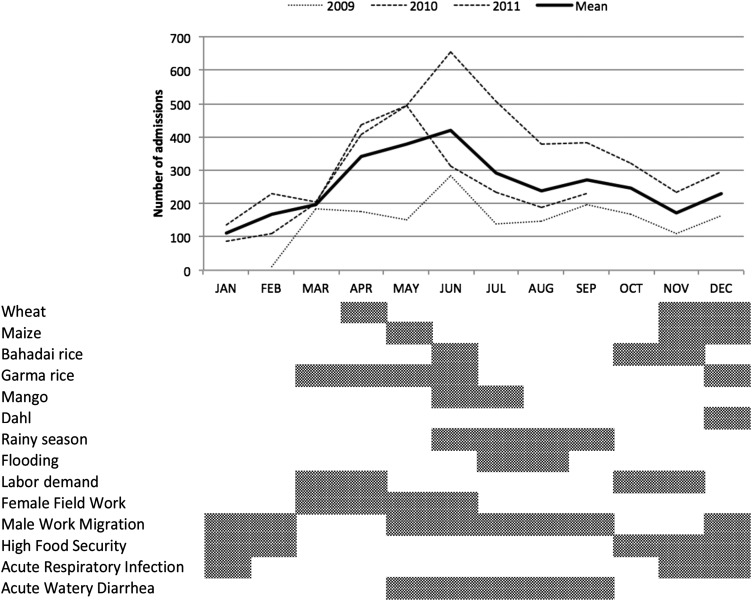
Figure 2 illustrates local seasonal activities relative to the numbers of children admitted in the program. There are 2 main agricultural seasons, one before the monsoon rains of March that lasts through to May and represents the worst food-security period and another at the end of the monsoon rains in October through November that marks the beginning of the best food-security period.
FIGURE 2.
Seasonal variations in admissions (top) and associated local socio-environmental factors (bottom) that may influence admission trends (n = 8542).
Patient characteristics
Of children who met the inclusion criteria for the analysis, 5149 children (62.2%) admitted into the program were girls, which was a proportion substantially higher than in the background population (47.12% girls) (21). The mean (±SD) age was 16.4 ± 9.4 mo (range: 6–60 mo); 79.9% of all admitted children were 6–23 mo old, of whom 36.5% were <12 mo old; 6793 children (87.3%) belonged to Scheduled Caste, Scheduled Tribe, or Other Backward Caste families/households. A total of 791 (9.3%) children required admission to the SC, 126 (1.5%) of whom were admitted with complicated SAM at initial presentation and the remainder admitted at some point during community treatment. Just under one-half the children (n = 3989; 48.2%) came from within Biraul block, whereas the remainder commuted from outside.
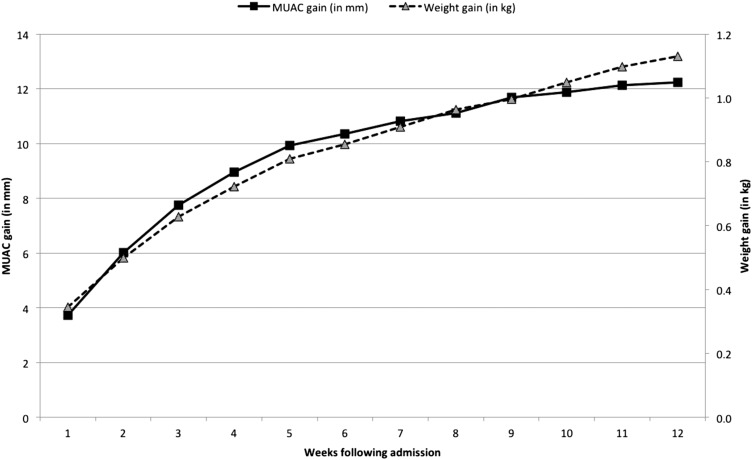
Of the 5198 children who did not default from the program, 88.4% of children were discharged as cured with a mean (±SD) weight gain of 4.9 ± 3.4 g · kg−1 · d−1 and length of stay (LOS) of 7.9 ± 5.9 wk; the median weight gain was 4.1 g · kg−1 · d−1 (IQR: 2.6–6.3 g · kg−1 · d−1) and LOS was 6 wk (IQR: 4–10 wk). Weight and MUAC followed the same trend throughout recovery and was most rapid in the early stages of admission (Figure 3).
FIGURE 3.
Mean increase in weight and MUAC over time for children discharged as cured (n = 4595). MUAC, midupper arm circumference.
Profile and outcomes of children admitted and discharged under the old criteria
Table 4 shows profiles and outcomes of the 3873 children admitted between February 2009 and June 2010 with a WHZ <−3 and/or MUAC <110 mm and/or bilateral edema. Children were discharged as cured when they had attained a WHZ >−2 and MUAC >110 mm with no edema for 1 wk and were in good clinical condition with a good appetite.
TABLE 4.
Admission characteristics and outcomes of children admitted under old criteria stratified by admission MUAC1
| MUAC |
||||||
| <90 mm | 90 to <100 mm | 100 to <110 mm | 110 to <115 mm | ≥115 mm | Total | |
| Children admitted, n (%) | 153 (4.0) | 420 (10.8) | 1980 (51.1) | 694 (17.9) | 626 (16.2) | 3873 |
| Sex, n (%) | ||||||
| F | 112 (73.2) | 292 (69.5) | 1329 (67.1) | 297 (42.8) | 178 (28.4) | 2208 (57) |
| M | 41 (26.8) | 128 (30.5) | 651 (32.9) | 397 (57.2) | 448 (71.6) | 1665 (43) |
| Age (mo), n (%) | ||||||
| 6–11 | 65 (43.6) | 140 (33.3) | 504 (25.5) | 94 (13.5) | 45 (7.2) | 848 (21.9) |
| 12–23 | 61 (40.9) | 198 (47.1) | 1092 (55.2) | 400 (57.6) | 289 (46.2) | 2040 (52.7) |
| 24–35 | 20 (13.4) | 57 (13.6) | 260 (13.1) | 139 (20 | 140 (22.4) | 616 (15.9) |
| 36–59 | 3 (2.0) | 25 (6.0) | 124 (6.3) | 61 (8.8) | 151 (24.2) | 364 (9.4) |
| Age, mo | 12 (7.5–17)2 | 12 (9–18) | 14 (11–18) | 18 (12–24) | 18 (15–31.5) | 17 (12–24) |
| Nutritional status at admission | ||||||
| MUAC, mm | 81.9 ± 10.63 | 95.2 ± 2.8 | 105.6 ± 2.7 | 112.0 ± 1.7 | 119.4 ± 3.4 | 106.9 ± 9.0 |
| WHO WHZ | −4.5 ± 1.1 | −4.1 ± 0.9 | −3.2 ± 0.9 | −3.5 ± 0.4 | −3.5 ± 0.4 | −3.5 ± 0.8 |
| Admissions with <−3 WHZ, % | 89.4 | 88.8 | 62.8 | 100 | 100 | 79.3 |
| WHO HAZ | −5.6 ± 1.7 | −4.7 ± 1.8 | −4.0 ± 1.6 | −3.4 ± 1.6 | −2.9 ± 1.7 | −3.8 ± 1.7 |
| Admitted to SC during ATFC treatment, n (%) | 20 (13.1) | 39 (9.3) | 117 (5.9) | 49 (7.1) | 47 (7.5 | 272 (7.0) |
| Direct referral to SC at admission, n (%) | 19 (12.4) | 7 (1.7) | 16 (0.8) | 3 (0.4) | 2 (0.3) | 47 (1.2) |
| Outcomes, n (%) | ||||||
| Defaulter | 77 (50.3) | 146 (34.8) | 739 (37.3) | 278 (40.1) | 245 (39.1) | 1485 (38.3) |
| Nonresponder | 10 (6.5) | 33 (7.9) | 144 (7.3) | 43 (6.2) | 48 (7.7) | 278 (7.2) |
| Death | 9 (5.9) | 12 (2.9) | 14 (0.7) | 2 (0.3) | 4 (0.6) | 41 (1.1) |
| Cured | 57 (37.3) | 229 (54.5) | 1083 (54.7) | 371 (53.5) | 329 (52.6) | 2069 (53.4) |
| Nutritional status at discharge of children discharged as cured | ||||||
| Attained ≥15% of body weight, n (%) | 55 (96.5) | 223 (97.4) | 716 (66.1) | 292 (78.7) | 183 (55.6) | 1469 (71) |
| MUAC, mm | 117.5 ± 5.4 | 118.4 ± 6.0 | 119.9 ± 5.6 | 124.5 ± 5.2 | 128.7 ± 5.6 | 121.9 ± 6.6 |
| WHO WHZ | −1.3 ± 1.3 | −1.4 ± 1.0 | −1.5 ± 0.7 | −1.7 ± 0.6 | −1.9 ± 0.6 | −1.6 ± 0.7 |
| WHZ <−3 at discharge, n (%) | 5 (8.9) | 7 (3.1) | 18 (1.7) | 8 (2.2) | 14 (4.3) | 52 (2.5) |
| WHO HAZ | −5.1 ± 1.6 | −4.7 ± 2.1 | −4.2 ± 1.5 | −3.6 ± 1.5 | −3.3 ± 1.3 | −4.0 ± 1.6 |
| Treatment-response indicators (cured) | ||||||
| Mean weight gain, g · kg−1 · d−1 | 7.4 ± 3.7 | 5.9 ± 3.8 | 4.8 ± 3.0 | 4.0 ± 2.6 | 3.7 ± 2.8 | 4.7 ± 3.2 |
| Mean length of stay, wk | 13.6 ± 8.0 | 11.1 ± 6.8 | 7.7 ± 5.6 | 9.3 ± 5.7 | 9.3 ± 6.5 | 8.7 ± 6.1 |
| Length of stay, wk | 12.0 (7.1–17.9) | 9.0 (6.0–14.1) | 6.0 (4.0–9.7) | 8.0 (5.0–12.0) | 8.0 (4.9–11.9) | 7.0 (4.1–11.1) |
| MUAC gain, mm/d | 0.48 ± 0.31 | 0.40 ± 0.25 | 0.38 ± 0.26 | 0.28 ± 0.21 | 0.23 ± 0.21 | 0.34 ± 0.25 |
| Height gain, cm/wk | 0.32 ± 0.31 | 0.25 ± 0.24 | 0.15 ± 0.26 | 0.12 ± 0.17 | 0.11 ± 0.12 | 0.15 ± 0.33 |
ATFC, Ambulatory Therapeutic Feeding Center; HAZ, height-for-age z score; MUAC, midupper arm circumference; SC, Stabilization Center; WHZ, weight-for-height z score.
Median; IQR in parentheses (all such values).
Mean ± SD (all such values).
Profile and outcomes of children admitted and discharged under the new criteria
Table 5 shows profiles and outcomes of 4401 children admitted between July 2010 and September 2011 with an MUAC <115 mm and/or bilateral edema. Children were discharged as cured when they reached an MUAC ≥120 mm with no edema for 1 wk and were in good clinical condition with a good appetite. WHZ targets were not incorporated in the new criteria.
TABLE 5.
Admission and outcome characteristics of children admitted under new criteria stratified by admission MUAC1
| MUAC |
|||||
| <90 mm | 90 to <100 mm | 100 to <110 mm | 110 to <115 mm | Total | |
| Children admitted, n (%) | 115 | 361 | 1330 | 2595 | 4401 |
| Sex, n (%) | |||||
| F | 76 (66.1) | 264 (73.1) | 899 (67.6) | 1702 (65.6) | 2941 (66.8) |
| M | 39 (33.9) | 97 (26.9) | 431 (32.4) | 893 (34.4) | 1460 (33.2) |
| Age (mo), n (%) | |||||
| 6–11 | 74 (64.9) | 166 (46.0) | 559 (42.0) | 768 (29.6) | 1567 (35.6) |
| 12–23 | 30 (26.3) | 151 (41.8) | 600 (45.1) | 1377 (53.1) | 2158 (49.0) |
| 24–35 | 7 (6.1) | 29 (8.0) | 121 (9.1) | 326 (12.6) | 483 (11.0) |
| 36–59 | 3 (2.6) | 15 (4.2) | 50 (3.8) | 124 (4.8) | 192 (4.4) |
| Age, mo | 9 (7–12)2 | 12 (8–18) | 12 (9–18) | 12 (10–18) | 12 (10–18) |
| Nutritional status at admission | |||||
| MUAC, mm | 82.4 ± 9.13 | 95.2 ± 2.8 | 105.2 ± 2.7) | 112.6 ± 1.6 | 108.2 ± 7.2 |
| WHO WHZ | −4.5 ± 1.3 | −4.0 ± 1.1 | −3.3 ± 0.9 | −2.9 ± 0.7 | −3.1 ± 0.9 |
| WHZ <−3 at admission, % | 90.3 | 84.4 | 67.9 | 43.2 | 55.2 |
| WHO HAZ | −5.5 ± 1.7 | −4.6 ± 1.6 | −3.9 ± 1.5 | −3.3 ± 1.4 | −3.7 ± 1.5 |
| Admitted to SC during ATFC treatment, n (%) | 21 (18.3) | 53 (14.7) | 123 (9.2) | 159 (6.1) | 356 (8.1) |
| Direct referral to SC at admission, n (%) | 11 (9.6) | 7 (1.9) | 6 (0.5) | 9 (0.3) | 33 (0.7) |
| Outcome, n (%) | |||||
| Defaulter | 59 (51.3) | 175 (48.5) | 584 (43.9) | 773 (29.8) | 1591 (36.2) |
| Nonresponder | 5 (4.3) | 34 (9.4) | 102 (7.7) | 107 (4.1) | 248 (5.6) |
| Death | 8 (7.0) | 6 (1.7) | 11 (0.8) | 11 (0.4) | 36 (0.8) |
| Cured | 43 (37.4) | 146 (40.4) | 633 (47.6) | 1704 (65.7) | 2526 (57.4) |
| Nutritional status at discharge of children discharged as cured | |||||
| Attained ≥15% of body weight, n (%) | 43 (100) | +145 (99.3) | 561 (88.6) | 671 (39.4) | 1420 (56.2) |
| MUAC, mm | 122.0 ± 1.3 | 122.9 ± 3.1 | 123.0 ± 2.5 | 123.2 ± 2.3 | 123.1 ± 2.4 |
| WHO WHZ | −0.7 ± 0.9 | −1.1 ± 1.1 | −1.4 ± 0.8 | −1.6 ± 0.7 | −1.5 ± 0.8 |
| WHZ <−3 at discharge, n (%) | 1 (2.3) | 3 (2.1) | 7 (1.1) | 46 (2.7) | 57 (2.3) |
| WHZ <−2 at discharge, n (%) | 3 (7.0) | 28 (19.2) | 128 (20.3) | 463 (27.2) | 622 (24.6) |
| WHO HAZ | −5.0 ± 1.4 | −4.6 ± 1.4 | −4.1 ± 1.4 | −3.5 ± 1.4 | −3.7 ± 1.4 |
| Treatment-response indicators (cured) | |||||
| Weight gain, g · kg−1 · d−1 | 7.5 ± 3.0 | 6.6 ± 4.4 | 5.1 ± 2.9 | 4.9 ± 3.8 | 5.1 ± 3.7 |
| Length of stay, wk | 15.3 ± 8.0 | 12.4 ± 7.6 | 9.0 ± 5.9 | 6.0 ± 4.5 | 7.3 ± 5.6 |
| Length of stay, wk | 13.3 (9.1–20.6) | 11.0 (7.0–15.8) | 7.9 (5.0–11.1) | 4.7 (3.0–7.3) | 6.0 (3.3–9.3) |
| MUAC gain, mm/d | 0.44 ± 0.20 | 0.43 ± 0.26 | 0.39 ± 0.26 | 0.40 ± 0.32 | 0.40 ± 0.30 |
| Height/length gain, cm/wk | 0.30 ± 0.18 | 0.22 ± 0.39 | 0.17 ± 0.19 | 0.13 ± 0.41 | 0.15 ± 0.36 |
ATFC, Ambulatory Therapeutic Feeding Center; HAZ, height-for-age z score; MUAC, midupper arm circumference; SC, Stabilization Center; WHZ, weight-for-height z score.
Median; IQR in parentheses (all such values).
Mean ± SD (all such values).
Profile and outcomes of children admitted into the SC
Of 8542 children admitted during the study period, 791 children (9.3%) required admission to the SC at some point during treatment; of those admitted, 698 children met the inclusion criteria for analysis. Profiles and outcomes of these children are summarized in Table 6.
TABLE 6.
Admission and outcome characteristics of children admitted into the SC (n = 698)1
| MUAC |
||||||
| <90 mm | 90 to <100 mm | 100 to <110 mm | 110 to <115 mm | ≥115 mm | Total | |
| Children admitted, n (%) | 61 (8.7) | 72 (10.3) | 187 (26.8) | 113 (54.9) | 77 (44.8) | 384 (55.0) |
| Sex, n (%) | ||||||
| F | 38 (62.3) | 41 (56.9) | 115 (61.5) | 113 (54.9) | 77 (44.8) | 384 (55.0) |
| M | 23 (37.7) | 31 (43.1) | 72 (38.5) | 93 (45.1) | 95 (55.2) | 314 (45.0) |
| Age (mo), n (%) | ||||||
| 6–11 | 38 (62.3) | 35 (48.6) | 81 (43.3) | 70 (34.0) | 43 (25.0) | 267 (38.3) |
| 12–23 | 20 (32.8) | 24 (33.3) | 75 (40.1) | 107 (51.9) | 84 (48.8) | 310 (44.4) |
| 24–35 | 2 (3.3) | 10 (13.9) | 18 (9.6) | 21 (10.2) | 16 (9.3) | 67 (9.6) |
| 36–59 | 1 (1.6) | 3 (4.2) | 13 (7.0) | 8 (3.9) | 29 (16.9) | 54 (7.7) |
| Age, mo | 9.1 (7.0–15.9)2 | 12.0 (8.6–18.4) | 12.5 (9.5–18.1) | 14.0 (10.7–19.0) | 16.9 (11.9–25.1) | 13.5 (9.9–19.4) |
| Direct Admission to SC, n (%) | 30 (49.2) | 14 (19.4) | 20 (10.7) | 9 (4.4) | 4 (2.3) | 77 (11.0) |
| Admission during ATFC stay, n (%) | 31 (50.8) | 58 (80.6) | 167 (89.3) | 197 (95.6) | 168 (97.7) | 621 (89.0) |
| Nutritional status at time of admission to SC | ||||||
| MUAC, mm | 79.7 ± 6.4 | 94.4 ± 3.1 | 104.8 ± 2.6 | 112.2 ± 1.6 | 119.4 ± 3.6 | 107.3 ± 11.8 |
| WHO WHZ | −5.1 ± 2.2 | −4.3 ± 1.4 | −3.4 ± 1.0 | −3.0 ± 1.0 | −2.7 ± 1.1 | −3.4 ± 1.4 |
| WHZ <−3, n (%) | 59 (97.7) | 70 (97.2) | 185 (98.9) | 193 (93.7) | 138 (80.2) | 645 (92.4) |
| WHO HAZ | −5.4 ± 2.9 | −4.6 ± 2.9 | −4.2 ± 1.5 | −3.8 ± 1.6 | −3.5 ± 1.4 | −4.0 ± 1.9 |
| Outcome, n (%) | ||||||
| Transfer to ATFC | 42 (68.9) | 44 (61.1) | 180 (96.3) | 200 (97) | 167 (97.1) | 651 (93.3) |
| Defaulted from SC | 10 (16.4) | 19 (1.4) | 1 (0.5) | 3 (1.5) | 0 (0) | 15 (2.1) |
| Death in SC | 9 (14.8) | 9 (12.7) | 6 (3.2) | 3 (1.5) | 5 (2.9) | 32 (4.6) |
| Treatment-response indicators | ||||||
| Weight gain, g · kg−1 · d−1 | 5.0 ± 51.7 | 6.1 ± 13.8 | 7.1 ± 12.3 | 6.5 ± 10.7 | 7.5 ± 13.8 | 6.7 ± 19.3 |
| Length of stay, d | 8.9 ± 8.9 | 5.9 ± 4.3 | 5.5 ± 3.5 | 5.3 ± 3.5 | 5.0 ± 3.5 | 5.7 ± 4.4 |
| MUAC gain, mm/d | 0.78 ± 1.6 | 0.44 ± 20.8 | 0.67 ± 1.2 | 0.55 ± 0.9 | 0.30 ± 0.9 | 0.53 ± 1.1 |
ATFC, Ambulatory Therapeutic Feeding Center; HAZ, height-for-age z score; MUAC, midupper arm circumference; SC, Stabilization Center; WHZ, weight-for-height z score.
Median; IQR in parentheses (all such values).
Mean ± SD (all such values).
For children who required care in the SC at any stage during their treatment, the mean MUAC at time of admission into the program (104. 0 mm ± 12.6) was lower than that in children treated solely in the ATFC [107.9 ± 7.5 mm; mean difference: 3.9 mm (95% CI: 2.9, 4.8); P < 0.01]. The mean weight gain during SC stay was 6.7 ± 19.3 g · kg−1 · d−1, and the mean LOS was 5.7 ± 4.4 d. The mean MUAC increase during SC stay appeared to be higher in children with more-severe wasting. Default rates from the SC were low (n = 15; 2.1%) as was the overall mortality rate (n = 32; 4.6%). The mortality rate of children admitted directly to the SC at time of entry into the program was 15% compared with 3.3% in children admitted to the SC during ATFC treatment.
Analysis of defaulters
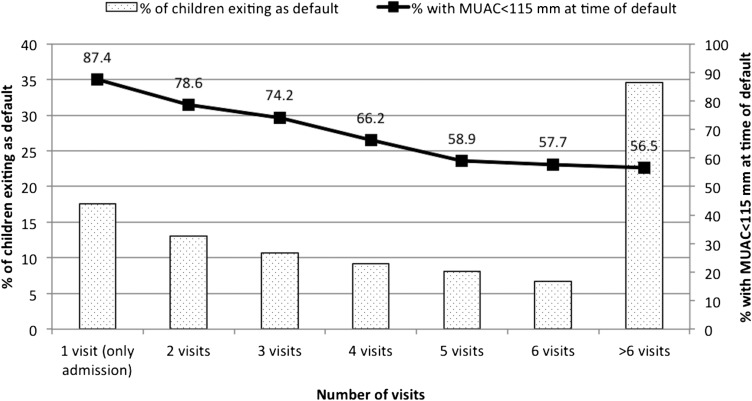
Of 8274 children who met the inclusion criteria during the study period, 3076 children (37.2%) defaulted. Of 3076 defaulters, 542 children (17.6%) defaulted immediately after the initial admission visit. For the remaining 2534 defaulters, the mean (±SD) time to default was 6.2 ± 5.2 wk, and the median time was 5 wk (IQR: 2.3–8.4 wk). Just over one-third of children (34.6%) defaulted ≥6 wk after admission. At the time of default, 67.8% of children had an MUAC <115 mm (Figure 4), 25.1% of children had an MUAC >115–120 mm, and 7% of children had an MUAC >120 mm. The nutritional status of children who defaulted improved as the LOS increased before default. Of 1063 children who were in the program ≥6 wk before defaulting, 43.5% of subjects had an MUAC ≥115 mm on the last visit before default.
FIGURE 4.
Length of stay before defaulting and associated MUAC at time of default (n = 3076). MUAC, midupper arm circumference.
The adjusted odds (95% CI) of children aged <12 mo for defaulting were 1.5 times (1.3, 1.7 times) those of children aged ≥24 mo of age (P < 0.01), whereas the adjusted odds (95% CI) of defaulting in children who presented with an MUAC <100 mm were 1.6 times (1.4, 1.9) times higher than in children admitted with an MUAC between 110 and <115 mm (P < 0.01). On bivariate analysis, a younger age, admission WHZ <−3, lower admission MUAC, residence outside Biraul block, nonreferral to the program by an ASHA, and admission under the old criteria were significantly associated with default. Female sex (OR: 1.02; 95% CI: 0.9, 1.1; P = 0.712) and lower caste did not appear to be associated with higher risk of default. All variables significant in the bivariate analysis remained significant after the multiple logistic regression analysis, although with the old admission criteria became slightly protective (OR: 0.8; 95% CI: 0.7, 0.9; P < 0.01) against default (Table 7).
TABLE 7.
Risk factors for default from CMAM program1
|
n (%) |
||||
| Risk factor | Defaulters | Cured | Adjusted OR (95% CI) | P |
| Age group (n = 7671) (mo) | ||||
| 6–11 | 966 (31.4) | 1228 (26.7) | 1.5 (1.3, 1.7) | <0.01 |
| 12–23 | 1551 (50.4) | 2364 (52.4) | 1.2 (1.1, 1.4) | <0.01 |
| >24 | 559 (18.2) | 1003 (21.8) | 1 (—) | — |
| Caste category (n = 7199) | ||||
| Scheduled Caste | 1294 (44.8) | 1778 (41.3) | 1.1 (0.97, 1.3)2 | 0.106 |
| Other Backward Class | 1244 (43.0) | 1971 (45.8) | 0.99 (0.9, 1.2) | 0.907 |
| General | 356 (12.2) | 559 (13.0) | 1 (—) | — |
| WHO WHZ on admission (n = 7654) | ||||
| <−3 | 2184 (71.3) | 2887 (62.9) | 1.5 (1.3, 1.6) | <0.01 |
| ≥−3 to <−2 | 728 (23.8 | 1414 (30.8) | 1 (—) | — |
| ≥−2 | 151 (4.9) | 290 (6.3) | 0.99 (0.8, 1.2) | 0.910 |
| MUAC on admission (n = 7657) (mm) | ||||
| <100 | 446 (14.6) | 474 (10.3) | 1.6 (1.4, 1.9) | <0.01 |
| 100 to <110 | 1323 (43.2) | 1716 (37.4) | 1.5 (1.3, 1.6) | <0.01 |
| 110 to <115 | 1049 (34.2) | 2075 (45.2) | 1 (—) | — |
| ≥115 | 245 (8.0) | 329 (7.2) | 1.4 (1.2, 1.8) | <0.01 |
| Child’s residence (n = 7671) | ||||
| Adjacent block | 1754 (57.0) | 2264 (49.3) | 1.1 (1.01, 1.2) | 0.025 |
| Biraul block | 1322 (43.0) | 2331 (50.7) | 1 (—) | — |
| Admission criteria (n = 7671) | ||||
| Old | 1485 (48.3) | 2069 (45.0) | 0.8 (0.7, 0.9) | <0.01 |
| New | 1591 (51.7) | 2526 (55.0) | 1 (—) | — |
| Referred into the program by ASHA (n = 7656) | ||||
| No | 3029 (98.9) | 4074 (88.7) | 10.2 (6.9, 14.9) | <0.01 |
| Yes | 33 (1.1) | 520 (11.3) | 1 (—) | — |
Nonresponders were not included in this analysis. CMAM, community-based management of acute malnutrition; MUAC, midupper arm circumference; WHZ, weight-for-height z score.
Included in regression model as borderline significance on univariate analysis (P = 0.08).
Impact of change of admission and discharge criteria
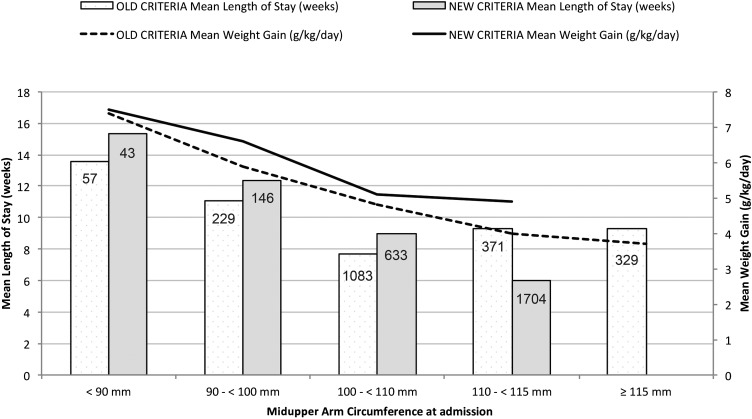
There was a higher proportion of girls admitted [66.8% compared with 57.0%; RR: 1.17 (95% CI: 1.1, 1.2); P < 0.01] and a shift toward admission of younger age groups [mean age: 15 compared with 18 mo; mean difference: 3.0 mo (95% CI: 2.6, 3.4 mo); P < 0.01] under the new criteria. The change had a substantial impact on decreasing the LOS and increasing the mean weight gain in children discharged as cured (Figure 5). The mean WHZ at discharge decreased from −1.6 to −1.5, whereas the mean percentage increase in body weight fell from 22% to 19.4% under the new criteria (Table 8).
FIGURE 5.
Mean weight gain and length of stay for children discharged as cured stratified by admission midupper arm circumference cutoff (n = 4595).
TABLE 8.
Comparison of outcomes of old compared with new criteria for admission and discharge1
| Old criteria (n = 3873) | New criteria (n = 4401) | Values | P | Combined (n = 8274) | |
| Outcome, n (%) | |||||
| Cured | 2069 (53.4) | 2526 (57.4) | — | — | 4595 (55.5) |
| Dead | 41 (1.1) | 36 (0.8) | 0.7 (0.5, 1.1)2 | 0.150 | 77 (0.9) |
| Defaulter | 1485 (38.3) | 1591 (36.2) | 0.92 (0.88, 0.98) | <0.01 | 3076 (37.2) |
| Nonresponder | 278 (7.2) | 248 (5.6) | 0.8 (0.6, 0.9) | <0.01 | 526 (6.4) |
| Treatment response if cured | |||||
| WG, g · kg−1 · d−1 | 4.7 ± 3.23 | 5.1 ± 3.7 | 0.34 [0.15, 0.54]4 | <0.01 | 4.9 ± 3.4 |
| WG, g · kg−1 · d−1 | 3.9 (2.5–6.2)5 | 4.2 (2.7–6.4) | — | 4.1 (2.6–6.3) | |
| LOS, wk | 8.7 ± 6.1 | 7.3 ± 5.6 | −1.5 [−1.8, −1.2] | <0.01 | 7.9 ± 5.9 |
| LOS, wk | 7 (4–11) | 6 (3.3–9.3) | — | — | 6 (4–10) |
| MUAC gain, mm/d | 0.34 ± 0.25 | 0.40 ± 0.30 | 0.06 [0.05, 0.08] | <0.01 | 0.38 ± 0.28 |
| Children who gained ≥15% BW, % | 71 | 56.2 | 1.5 (1.4, 1.6) | <0.01 | 62.9 |
| Percentage increase in BW | 22.0 ± 12.9 | 19.4 ± 12.9 | −2.6 [−3.4, 1.9] | <0.01 | 20.6 ± 13.0 |
| Nutrition status at discharge | |||||
| MUAC, mm | 121.9 ± 6.6 | 123.1 ± 2.4 | 1.2 [1.0, 1.5] | <0.01 | 122.6 ± 4.8 |
| WHO WHZ | −1.6 ± 0.7 | −1.5 ± 0.8 | 0.05 [0.003, 0.09] | 0.035 | −1.5 ± 0.8 |
| WHO HAZ | −4.0 ± 1.6 | −3.7 ± 1.5 | 0.30 [0.21, 0.39] | <0.01 | −3.9 ± 1.5 |
BW, body weight; HAZ, height-for-age z score; LOS, length of stay; MUAC, midupper arm circumference; WG, weight gain; WHZ, weight-for-height z score.
RR; 95% CI in parentheses (all such values).
Mean ± SD (all such values).
Mean difference; 95% CI in brackets (all such values).
Median; IQR in parentheses (all such values).
DISCUSSION
As home to nearly one-third of the world’s children with SAM, India’s approach to treating SAM has relied almost exclusively on inpatient care, whereas the community-based approaches shown to be effective and feasible in African settings have not been well tested, studied, or implemented in India. This article describes the clinical outcomes of 8274 children with SAM treated in an MSF-implemented and -supported CMAM program in a rural area of one of the poorest districts in Bihar (22). This large observational study represents, to the authors’ best knowledge, the only one from India to describe the outcomes of a conventional setting CMAM program by using WHO standard ready-to-use therapeutic lipid-based paste produced in India. Our findings suggested that CMAM was an effective strategy, which led to cures for 88.4% of children who completed the treatment although hindered by substantial default rates. Because these results represent operational field outcomes achieved despite challenges that might also be present if other large-scale CMAM initiatives are undertaken in India, they suggest that CMAM has significant, under-exploited potential as a strategy for scaling up.
Overall outcomes
Demographic data showed a disproportionate share of girls (62.2%) in children admitted to the program and reinforced the importance of social identity (caste) as a predisposing factor for SAM. The data also confirmed observations in other published Indian studies that suggested a much higher burden of SAM in children <2 y old (3, 4), which was consistent with data from African settings (23). The severity of stunting correlated directly with the severity of wasting on admission, with a mean (±SD) height-for-age z score of −3.9 ± 1.5 for the overall cohort. That 79.9% of children admitted into the program were <2 y of age has major public health implications for the prevention and reversal of stunting and also suggests that active case-detection strategies may benefit from focusing on this age group.
The introduction of an MUAC <115 mm and/or edema as sole admission criteria in July 2010 resulted in a change in the demographic of patients admitted into the program. Notably, a higher proportion of girls and younger age groups were admitted after the new criteria were implemented. Both phenomena were noted in other contexts (23) and also within India (3). The new admission criteria lowered the threshold for severity with the result that more children were included who are at lower risk of death and have a smaller WHZ deficit to correct (WHZ: 3.1 compared with 3.5) than do children identified by the old criteria. Consequently, the mortality and time to recovery can be expected to be lower in children identified by the new criteria.
Although 9.3% of children required admission to the SC at some point during treatment, the proportion admitted immediately to the SC on entering the program (0.9%; n = 77) was far lower than the 10–20% reported in African settings (24). This result suggested that the morbidity associated with SAM may be less severe in the Indian context. During their SC stay, average weight gain was lower and the LOS was higher in children with more-severe wasting than in children with a higher MUAC on admission to the SC, reflecting longer periods spent in phase 1 treatment (because of more-severe illness) when weight gain was not expected.
The overall in-program mortality was 0.9%, which was well within the bounds of standards set out in both national (<5% mortality) and international (<10% mortality) standards of care (17, 25). Although excluded from the analysis, the mortality rate was far higher (15.5%) in the 71 children admitted with edema in keeping with other studies from the Indian context (4). In addition, the case fatality rate of children admitted directly into the SC at the time of entry into the program (n = 11 of 77; 15%) was substantially higher than in children admitted to the SC after a period of treatment in the ATFC (n = 21 of 621; 3.3%), which could have reflected a need for better standard of care for children who presented in a more-critical condition. As shown in Figure 3, weight and MUAC followed the same trend throughout recovery and was most rapid in the early stages of admission as noted elsewhere (26). This result supports conclusions of other studies that the MUAC may be useful as a program-monitoring tool for recovery from SAM (23). Height gain correlated well with average weight gain and the mean MUAC gain across different severities of wasting, suggesting that it may be a useful indicator of proportionate growth during recovery.
However, a substantial number of children (37.2%) defaulted from care, a figure that failed to meet national and international standards of care (<15%) but was consistent with outcomes from other inpatient SAM treatment programs in India (3, 4); for example, the Nutritional Rehabilitation Centers in Uttar Pradesh reported a defaulter rate as high as 47.2% (5). Adjusted odds of defaulting were slightly higher for children commuting to Biraul block for treatment than for those living within Biraul block, suggesting that the increased distance from ATFCs posed a barrier to completing treatment. This finding was consistent with the results of successive Semi-Quantitative Assessment of Access and Coverage surveys conducted by MSF in Biraul block, which identified caregiver time constraints along with poor perception of self-recovery and seasonal agricultural labor demands as important contributors to nonadherance with treatment (27).
Risk factors for defaulting were similar to those shown in other studies that described the default from other inpatient nutritional programs in India (4), although in this study, identity as Other Backward Class did not appear to be a risk factor for default. Notably, seasonal trends showed an increase in defaulters during months when women’s field work is in high demand (March through June) and during the monsoon rains and yearly floods. This period also includes the wedding season (May through June), which could have had an impact on the default rate because of the temporary migration of mothers (28). Children who were identified and referred to the program by ASHAs had 10-fold lower odds of defaulting, which supported a central role for these community workers in future CMAM initiatives in India. On the basis of this finding, the program put substantial effort into ASHA training and involvement in 2012 that, along with improved community messaging and earlier identification of children at high risk of default, likely contributed to the sharp decline in default rates to <20% seen in 2013 (MSF; unpublished data).
Implications for SAM treatment strategies in India
Because of the high burden of SAM seen in India, the current strategy of inpatient treatment programs alone is unlikely to provide care for all of these SAM children at higher risk of death. A 2012 study of 93 children treated in the state of Madhya Pradesh concluded that, although the compulsory 14-d inpatient stay succeeded in improving the condition of admitted children, the improvement was not sustained after discharge. Perhaps more crucial was the supposition that even if all 175 dedicated inpatient nutritional facilities were running at full capacity, it would take 15.5 y to provide this treatment method to the 1.3 million children in the state suffering from SAM (6).
There are a limited number of other observational studies in the literature that described the outcomes of other models of inpatient care elsewhere in India. A 2012 observational study that described the inpatient management of 3595 children with SAM in Malnutrition Treatment Centers across the state of Jharkhand showed a mortality rate of 0.6%, default rate of 18.4%, mean in-program weight gain of 9.6 g · kg−1 · d−1, and mean LOS of 16 d. No priority was given to the admission of children with complicated SAM, and the main discharge criteria were the good clinical condition of the child, toleration of feeds of 120–130 kcal · kg−1 · d−1, and gain of 5 g · kg−1 body weight · d−1 (3, 4).
An additional 2013 study in Madhya Pradesh described the outcomes of 2740 children randomly sampled from the 44,017 children treated for SAM in 2010. This program model admitted all children for 14 d of inpatient care in Nutrition Rehabilitation Centers and discharged them to a community program in which Ministry of Health frontline workers monitored their progress and ensured that they benefited from the Integrated Child Development Services Supplementary Nutrition Program. No priority was given to admitting children with complicated SAM, and discharge was automatic after 60 d of community follow-up. Overall, the program reported a 0.4% overall mortality rate with a 32% default, 23.7% discharged-as-nonrecovered, and 43.9% discharged-as-recovered rates. The mean weight gain was 2.7 g · kg−1 · d−1, whereas the mean LOS was 75.8 d. The mean weight gain during the community-treatment phase was 1.6 g · kg−1 body weight · d−1 (6).
Limitations and future direction
A major strength of this study was the data that were maintained throughout the program with relatively few missing data points for the final analysis. However, because the study was a routine NGO-supported program rather than a clinical trial, there were a number of limitations in interpreting the data. Although most children’s admission anthropometric data were measured by 3 individuals at the time of admission, only one measurement was recorded, which meant that no interobserver and intra-observer reliability could be analyzed.
Many children defaulted from the program and, therefore, were lost to follow-up. Although data were used from defaulting children to identify risk factors for default, not knowing the eventual outcome of this group of children could have created a potential bias in presenting overall outcomes of the program (e.g., by underestimating mortality if children were defaulting because of death in the community). Although not systematically recorded, very few children classed as defaulters were reported to have died when traced by the IEC team to encourage them to return to the program. A longer-term follow-up of these patients after exit from the program would be crucial to ascribe a more accurate survival status to this important cohort.
However, the major challenge in interpretation was that these findings came from an externally supported program and may not have been achieved if the program was transferred to government facilities. In one comprehensive review of 33 studies of community-based nutritional rehabilitation programs over a 25-y period, none of the programs operating within routine health systems and without external assistance were shown to be adequate (29). However, this result suggests that potential sustainable solutions may be shown through private-public partnerships between the government and local NGOs, which is a model that has already been adopted for the Nutritional Rehabilitation Centers in Bihar. In addition, this model assumes that governance (leadership, resources, and accountability) for nutritional interventions will remain low in the future, whereas already some state governments in India have been able to implement more-complex programs such as Madhya Pradesh Newborn Care Units. To this effect, the CMAM model has the capacity to be adopted into an existing government capacity; for example, ANMs at the PHC and additional PHC level can be trained and empowered to provide this service with appropriate training if adequate institutional support is provided. Local ownership and community understanding and acceptance of both messaging and treatment will likely also be critical in developing sustainable solutions (30). A cost-effectiveness analysis that compares routine CMAM projects with the current hospital-based model of care would be a useful next step in providing policy makers more evidence for future planning strategies.
In conclusion, the absence of Indian national guidelines regarding CMAM makes the scale up of SAM treatment difficult. The implementation of an effective public health approach for addressing SAM in India will require a significant investment by policymakers to develop state-specific sustainable, effective models of care that provide sufficient coverage and capacity to treat the large burden of children with SAM that exist in the country. More broadly, it will also require a holistic approach that incorporates preventive measures such as poverty reduction, hygiene promotion, clean water, prevention of prenatal macronutrient and micronutrient deficiencies, and the promotion of age-appropriate foods and feeding practices for infants and young children.
Acknowledgments
We thank Sanjay Kumar, Executive Director, and Dr. NK Mishra, State Program Officer at the State Health Society of Bihar at the time of analysis, for their support. We thank Dr. Om Prakash and specialist pediatricians at the Darbhangha Medical College Hospital and Dr. Amrendra Kumar Singh, Medical Officer in Charge at Biraul Block PHC, who have all been pivotal in assisting the work of MSF. We also thank Patricia Kahn for editing assistance.
The authors' responsibilities were as follows—SB and RM: analyzed data and wrote the manuscript; SB, TS, EM, CS, MT, KK, PM, AJ, NS, and KNM: were directly involved in the program design and implementation and reviewed the manuscript; and all authors: read and approved the final manuscript. No donor had any part in the study design or implementation. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ANM, auxiliary nurse midwife; ASHA, accredited social health activist; ATFC, Ambulatory Therapeutic Feeding Center; CMAM, community-based management of acute malnutrition; GNM, general nurse midwife; IEC, information, education, and communication; LOS, length of stay; MSF, Médecins Sans Frontières; MUAC, midupper arm circumference; NGO, nongovernmental organization; PHC, Primary Health Center; SAM, severe acute malnutrition; SC, Stabilization Center; WHZ, weight-for-height z score.
REFERENCES
- 1.International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005-2006. Mumbai (India): IIPS; 2007. [Google Scholar]
- 2.UNICEF. Tracking progress on child and maternal nutrition: a survival and development priority. New York: UNICEF; 2009. [Google Scholar]
- 3.Aguayo VM, Agarwal V, Agnani M, Das Agrawal D, Bhambhal S, Rawat AK, Gaur A, Garg A, Badgaiyan N, Singh K. Integrated program achieves good survival but moderate recovery rates among children with severe acute malnutrition in India. Am J Clin Nutr 2013;98:1335–42. [DOI] [PubMed] [Google Scholar]
- 4.Aguayo VM, Jacob S, Badgaiyan N, Chandra P, Kumar A, Singh K. Providing care for children with severe acute malnutrition in India: new evidence from Jharkhand. Public Health Nutr 2014;17:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh K, Badgaiyan N, Ranjan A, Dixit HO, Kaushik A, Aguayo VM, Kushwaha KP. Management of children with severe acute malnutrition in India: experience of Nutrition Rehabilitation Centres in Uttar Pradesh, India. Indian Pediatr 2014;51:21–5. [DOI] [PubMed]
- 6.Taneja G, Dixit S, Khatri A, Yesikar V, Raghunath D, Chourasiya S. A study to evaluate the effect of nutritional intervention measures on admitted children in selected nutrition rehabilitation centers of indore and ujjain divisions of the state of Madhya Pradesh (India). Indian J Community Med 2012;37:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indian Academy of Pediatrics, Dalwai S, Choudhury P, Bavdekar SB, Dalal R, Kapil U, Dubey AP, Ugra D, Agnani M, Sachdev HP, et al. Consensus Statement of the Indian Academy of Pediatrics on integrated management of severe acute malnutrition. Indian Pediatr 2013;50:399–404. [DOI] [PubMed]
- 8.Kapil U, Sachdev HP. Management of children with severe acute malnutrition a national priority. Indian Pediatr 2010;47:651–3. [DOI] [PubMed] [Google Scholar]
- 9.Chapko MK, Prual A, Gamatie Y, Maazou AA. Randomized clinical trial comparing hospital to ambulatory rehabilitation of malnourished children in Niger. J Trop Pediatr 1994;40:225–30. [DOI] [PubMed] [Google Scholar]
- 10.Collins S, Dent N, Binns P, Bahwere P, Sadler K, Hallam A. Management of severe acute malnutrition in children. Lancet 2006;368:1992–2000. [DOI] [PubMed] [Google Scholar]
- 11.Espié E, Pujol CR, Masferrer M, Saint-Sauveur JF, Urrutia PP, Grais RF. Acute malnutrition and under-5 mortality, northeastern part of India. J Trop Pediatr 2011;57:389–91. [DOI] [PubMed] [Google Scholar]
- 12.Emergency Nutrition Network. Special focus on government experiences of CMAM scale up. Oxford (United Kingdom): Field Exchange; 2012. p. 43.
- 13.Collins S, Sadler K, Dent N, Khara T, Guerrero S, Myatt M, Saboya M, Walsh A. Key issues in the success of community-based management of severe malnutrition. Food Nutr Bull 2006;27:S49–82. [DOI] [PubMed] [Google Scholar]
- 14.Wilford R, Golden K, Walker DG. Cost-effectiveness of community-based management of acute malnutrition in Malawi. Health Policy Plan 2012;27:127–37. [DOI] [PubMed] [Google Scholar]
- 15.Tekeste A, Wondafrash M, Azene G, Deribe K. Cost effectiveness of community-based and in-patient therapeutic feeding programs to treat severe acute malnutrition in Ethiopia. Cost Eff Resour Alloc 2012 Mar 19 (Epub ahead of print; DOI: 10.1186/1478-7547-10-4). [DOI] [PMC free article] [PubMed]
- 16.Golden MH, Briend A. Treatment of malnutrition in refugee camps. Lancet 1993;342:360. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health and Family Welfare. Operational guidelines on facility-based management of children with severe acute malnutrition. New Delhi (India): Government of India; 2011. [Google Scholar]
- 18.World Health Organization. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva (Switzerland): WHO; 1999.
- 19.World Health Organization, United Nations Children's Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children; a joint statement by the World Health Organization and the United Nations Children's Fund. Geneva (Switzerland): WHO; 2009. [PubMed]
- 20.Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull 2006;27:S7–23. [DOI] [PubMed] [Google Scholar]
- 21.International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), India, 2005-06: Bihar. Mumbai (India): IIPS; 2008. [Google Scholar]
- 22.Ministry Of Health and Family Welfare. Government of India. Bihar State Report (cited 27 Nov 2013). Available from: http://Mohfw.Nic.In/Nrhm/State%20files/Bihar.Htm.
- 23.Goossens S, Bekele Y, Yun O, Harczi G, Ouannes M, Shepherd S. Mid-upper arm circumference based nutrition programming: evidence for a new approach in regions with high burden of acute malnutrition. PLoS ONE 2012;7:e49320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tectonidis M. Crisis in Niger–outpatient care for severe acute malnutrition. N Engl J Med 2006;354:224–7. [DOI] [PubMed] [Google Scholar]
- 25.The Sphere Project. Humanitarian charter and minimum standards in humanitarian response: 2011 edition. Bangalore (India): The Sphere Project in India; 2011. [Google Scholar]
- 26.Prudhon C, Prinzo ZW, Briend A, Daelmans BM, Mason JB. WHO, UNICEF, and SCN Informal Consultation on Community-Based Management of Severe Malnutrition in Children. Food Nutr Bull 2006;27(3 Suppl):S99–104. [DOI] [PubMed] [Google Scholar]
- 27.Marino E, Salse N, Jha A, Burza S. Using SQUEAC methodology to assess CMAM for SAM programme coverage in India (cited 20 Sep 2014). Available from: http://issuu.com/msfuk/docs/squeac.
- 28.Burtscher D, Burza S. Health-seeking behaviour and community perceptions of childhood undernutrition and a Community Management of Acute Malnutrition (CMAM) programme in rural Bihar, India: a qualitative study. Public Health Nutr. In press. [DOI] [PMC free article] [PubMed]
- 29.Ashworth A. Efficacy and effectiveness of community-based treatment of severe malnutrition. Food Nutr Bull 2006;27:S24–48. [DOI] [PubMed] [Google Scholar]
- 30.Manary MJ. Local production and provision of ready-to-use therapeutic food (RUTF) spread for the treatment of severe childhood malnutrition. Food Nutr Bull 2006;27:S83–9. [DOI] [PubMed] [Google Scholar]