Abstract
Background: Elevated concentrations of serum uric acid are associated with increased risk of gout and renal and cardiovascular diseases. Genetic studies in adults have consistently identified associations of solute carrier family 2, member 9 (SLC2A9), polymorphisms with variation in serum uric acid. However, it is not known whether the association of serum uric acid with SLC2A9 polymorphisms manifests in children.
Objective: The aim was to investigate whether variation in serum uric acid is under genetic influence and whether the association with SLC2A9 polymorphisms generalizes to Hispanic children of the Viva La Familia Study.
Design: We conducted a genomewide association study with 1.1 million genetic markers in 815 children.
Results: We found serum uric acid to be significantly heritable [h2 ± SD = 0.45 ± 0.08, P = 5.8 × 10−11] and associated with SLC2A9 variants (P values between 10−16 and 10−7). Several of the significantly associated polymorphisms were previously identified in studies in adults. We also found positive genetic correlations between serum uric acid and BMI z score (ρG = 0.45, P = 0.002), percentage of body fat (ρG = 0.28, P = 0.04), fat mass (ρG = 0.34, P = 0.02), waist circumference (ρG = 0.42, P = 0.003), and waist-to-height ratio (ρG = 0.46, P = 0.001).
Conclusions: Our results show that variation in serum uric acid in Hispanic children is under considerable genetic influence and is associated with obesity-related phenotypes. As in adults, genetic variation in SLC2A9 is associated with serum uric acid concentrations, an important biomarker of renal and cardiovascular disease risk, in Hispanic children.
Keywords: hyperuricemia, obesity, metabolic syndrome, SNP association, urate transporter
INTRODUCTION
Uric acid is the end product of dietary and endogenous purine metabolism (1). Purines are nitrogen-containing bases that form the backbone of nucleic acids, DNA, and RNA. Hyperuricemia or elevated concentrations of serum uric acid (SUA)5 in adults have been linked to greater risk of metabolic disorders such as hypertension, inflammation, type 2 diabetes, metabolic syndrome, and renal and cardiovascular disease (CVD) (2–5). Studies in adults have also shown SUA to be an independent risk factor for CVD (6, 7). In the past few decades, SUA concentrations, which are highly affected by lifestyle factors such as diet, physical activity, and medications, have increased in the United States (8).
SUA concentrations have been strongly associated with the metabolic syndrome and its individual components in children and adolescents from the United States (9, 10) and other countries (11, 12). SUA in children is dependent on age and puberty stages. Concentrations of SUA increase from early childhood and stabilize by ∼15–17 y of age (13). However, it was also observed that SUA concentrations are higher in overweight and obese children and increase at rates on par with normo-uremic and normal-weight children (14). In adults, hyperuricemia predicts hypertension (15, 16). Likewise, childhood hyperuricemia is strongly predictive of adult blood pressure (17). Jones et al. (18) showed that SUA concentrations in childhood are associated with adult blood pressure, particularly systolic, and thus may be an important tool to assess CVD risk in adulthood.
Variation in SUA concentrations is affected by genetic as well as environmental factors. Population studies have shown that SUA concentrations are significantly heritable, with heritability estimates ranging between 15% and 73% (19–22). Genomewide or candidate gene association studies in these populations have shown SUA concentrations to be associated with single nucleotide variants [single nucleotide polymorphisms (SNPs)] in various uric acid transporter encoding genes. All of these genetic studies were conducted in adult populations. Genetic studies in children conducted thus far mainly focused on familial hyperuricemic nephropathy or juvenile gout. Thus, to identify genes and SNPs associated with variation in SUA concentrations, we conducted a genomewide association analysis of SUA concentrations in Hispanic children of the Viva La Familia Study (VFS) (23, 24).
METHODS
Study design and participants
The VFS was designed to investigate genetic and environmental factors affecting obesity and its comorbidities in Hispanic children. Its enrollment was not limited by country of origin; however, the majority of families were of Mexican-American descent. To qualify, families had to have at least 1 obese proband aged 4–19 y. Thus, the VFS represents a family-based cohort highly enriched for obesity. A total of 319 Hispanic families enrolled in the VFS in 2000–2004. The majority of the parents were either overweight (34%) or obese (57%), and 51% of the children were classified as obese (>95th BMI percentile) with BMI z scores ranging from 2.3 to 4.5. Anthropometric and body composition measurements were conducted in parents and children. Fasting blood samples were drawn for biochemical profiling of the children and for genotyping of children and parents. Subjects and study procedures are described in detail in previous publications (23, 24). The demographic, genetic, and phenotypic details of the adult populations used for comparison in this article were previously published (21, 22, 25, 26).
Ethics
All of the children and their parents gave written informed consent or assent. The protocol was approved by the Institutional Review Boards for Human Subject Research of Baylor College of Medicine and Affiliated Hospitals and the Texas Biomedical Research Institute. The VFS began in 2001 and is not registered.
Measurement of SUA and other phenotypes
Uric acid was oxidized to allantoin by uricase with the production of hydrogen peroxide. The peroxide reacts with 4-aminoantipyrine and 2,4,6-tribromo-3-hydroxy benzoic acid (Fisher Diagnostics) in the presence of peroxidase to yield a quinoneimine dye. The resulting change in absorbance is proportional to uric acid concentration in the sample. Methods used to measure fasting concentrations of biomarkers in blood are described elsewhere (27, 28). In addition, blood pressure measurements were taken by using an automated monitor. Anthropometric measurements were performed by using standardized techniques according to Lohman et al. (29). Body composition was determined by dual-energy X-ray absorptiometry (30).
Genotyping in Hispanic children
Genotyping of the 815 children for 1.1 million SNPs was conducted by using marker assays included on the Illumina HumanOmni1-Quad v1.0 BeadChips (30). Genotype calls were obtained after scanning on the Illumina BeadStation 500GX and analyzed by using the GenomeStudio software. Our genotyping error rate (based on duplicates) was 2 per 100,000 genotypes. The average call rate per individual sample was 97%. Specific markers were removed from analysis if they had call rates <95% (∼4000 SNPs) or deviated from Hardy-Weinberg equilibrium at a 5% false-discovery rate (12 SNPs). SNP genotypes were checked for Mendelian consistency by using the program SimWalk2 (31). The estimates of the allele frequencies and their SEs were obtained by using the software program Sequential Oligogenic Linkage Analysis Routines (SOLAR version 7.6.2) (32).
Statistical analyses
Quantitative genetic analysis (univariate and bivariate)
A variance components decomposition method was used to estimate heritability of SUA and CVD-related phenotypes. This method is implemented in the software program SOLAR. To estimate the genetic contribution to the variation in SUA, its heritability was estimated. Total phenotypic variance can be partitioned into its genetic and environmental components. The fraction of total phenotypic variance (σ2P) resulting from additive genetic effects (σ2G) is called the heritability and is denoted by h2 = σ2G/σ2P (33).
A variance components approach was also used to estimate phenotypic and genetic correlations between SUA and CVD-related phenotypes. The approach is described in detail elsewhere (33). In short, the phenotypic correlation between SUA and other phenotypes can be explained in terms of its genetic and environmental correlation components. A model in which all of the variables are estimated is compared with a model in which the genetic correlation is constrained to zero. If the result of this statistical test is significant, then we infer that the phenotypes share effects of a common set of genes. For this comparison, the likelihood ratio test is distributed asymptotically again as a 1/2:1/2 mixture of a chi-square variable with 1 df and a point mass at zero (34).
Genomewide association analysis using measured genotype analysis
Association analyses were performed by using the SOLAR program (version 7.6.2). Each marker genotype was converted to a covariate measure equal to 0, 1, or 2 copies of the minor allele (or, for missing genotypes, the weighted covariate based on imputation). These covariates were included in the variance components mixed models for measured genotype analyses (35) vs. null models that incorporated the random effect of kinship and fixed effects such as age, sex, their interaction and higher order terms. For the initial genomewide association screen, we tested each SNP covariate independently as a 1-df likelihood ratio test. Empirical thresholds for genomewide significant and suggestive evidence of association were based on the distribution of P values from 10,000 simulated null genomewide association studies (GWASs; i.e., simulations of a heritable trait with no modeled SNP covariate effects using the VFS pedigree and genotypes). The threshold for significance (P < 1 × 10−7) was defined as the cutoff for the lower 5% tails of the empirical distribution, and the threshold for suggestive evidence (P < 1 × 10−6) was the minimum P value obtained not more than once per genome scan. The linkage disequilibrium (LD) was computed in SOLAR by using information for all genotyped SNPs on all individuals. The effective number of SNPs given LD was calculated by the method of Moskvina and Schmidt (36), as implemented in SOLAR. The average ratio of the SNP effective number to the actual number obtained from analysis of 1989 nonoverlapping bins of SNPs was used to calculate the genomewide effective number of tests and thus the significance threshold for genomewide association. We performed a quantitative transmission disequilibrium test (implemented in SOLAR) to test for population stratification (37). An initial genomewide association screen was conducted on a residual trait after accounting for the covariates age, sex, their interaction and higher order terms. Also, to determine the covariates that might have a significant effect on modulating SUA concentrations, we tested several CVD risk factors. Of these, BMI z score and systolic blood pressure were found to be significant covariates for SUA. Thus, we used age, sex, and their interaction and higher order terms; BMI z scores; and systolic blood pressure to regress SUA concentrations and used the residual trait in our GWAS.
RESULTS
SUA concentrations in VFS
The study included data from 815 children participating in the VFS. The mean (±SD) SUA concentration in participating children was 5.2 ± 1.7 mg/dL. Significant heritability was detected for SUA concentrations [h2 = 0.45 (0.08), P = 5.8 × 10−11]. The prevalence of hyperuricemia as classified by SUA concentrations >2 SDs from the mean was 25%.
SUA concentrations and CVD risk factors
Overall, SUA concentrations were significantly correlated phenotypically with several CVD risk factors. We partitioned the phenotypic correlation into genetic and environmental correlations. Significant positive genetic and environmental correlations were seen between SUA and obesity-related measurements such as BMI z score, percentage of body fat, and waist circumference. The genetic correlations indicate the presence of common genetic effects acting on SUA and each of these traits. However, for other phenotypes (liver function, lipids, systolic blood pressure, and diabetes-related phenotypes), only environmental correlations with SUA were significant (Table 1).
TABLE 1.
Genetic and phenotypic correlations of serum uric acid with cardiovascular disease risk factors1
| Phenotype | Mean ± SD | ρG (SE) | P | ρE (SE) | P | ρP (SE) | P |
| Serum uric acid, mg/dL | 5.22 ± 1.7 | — | — | — | — | — | — |
| Age, y | 10.61 ± 3.9 | — | — | — | — | — | — |
| Anthropometric | |||||||
| Birth weight, kg | 3.49 ± 0.6 | −0.01 (0.12) | 9.5 × 10−1 | 0.21 (1.3) | 8.0 × 10−1 | 0.009 (0.03) | 8.2 × 10−1 |
| BMI z score | 1.51 ± 1.0 | 0.45 (0.12) | 2.0 × 10−3 | 0.45 (0.07) | 3.6 × 10−6 | 0.45 (0.03) | 9.6 × 10−38 |
| Fat, % | 0.33 ± 0.09 | 0.28 (0.12) | 3.9 × 10−2 | 0.41 (0.08) | 3.0 × 10−5 | 0.35 (0.03) | 1.0 × 10−23 |
| Fat mass, g | 19.28 ± 12.2 | 0.34 (0.12) | 1.7 × 10−2 | 0.50 (0.07) | 3.7 × 10−7 | 0.43 (0.03) | 9.4 × 10−36 |
| Waist circumference, cm | 76.01 ± 18.0 | 0.42 (0.11) | 3.0 × 10−3 | 0.51 (0.07) | 7.2 × 10−7 | 0.47 (0.03) | 2.0 × 10−43 |
| Waist-to-height ratio | 0.53 ± 0.09 | 0.46 (0.11) | 1.0 × 10−3 | 0.45 (0.08) | 1.8 × 10−5 | 0.45 (0.03) | 1.1 × 10−39 |
| Serum lipids, mg/dL | |||||||
| Triglycerides | 105.32 ± 56.8 | 0.17 (0.12) | 2.0 × 10−1 | 0.55 (0.08) | 2.4 × 10−7 | 0.37 (0.03) | 5.8 × 10−26 |
| HDL cholesterol | 46.83 ± 11.1 | −0.05 (0.11) | 6.5 × 10−1 | −0.49 (0.10) | 1.5 × 10−5 | −0.25 (0.03) | 1.3 × 10−12 |
| LDL cholesterol | 103.88 ± 29.1 | −0.04 (0.12) | 7.1 × 10−1 | 0.21 (0.12) | 8.6 × 10−2 | 0.06 (0.03) | 7.1 × 10−2 |
| Total cholesterol | 171.76 ± 34.4 | 0.005 (0.11) | 9.7 × 10−1 | 0.26 (0.14) | 8.0 × 10−2 | 0.09 (0.03) | 9.0 × 10−3 |
| Adipokines/inflammatory markers | |||||||
| Leptin, ng/mL | 17.96 ± 15.0 | 0.24 (0.13) | 9.8 × 10−2 | 0.42 (0.08) | 9.2 × 10−6 | 0.34 (0.03) | 2.0 × 10−23 |
| TNF-α, pg/mL | 8.32 ± 2.4 | 0.01 (0.09) | 9.2 × 10−2 | 0.02 (0.22) | 9.1 × 10−1 | 0.01 (0.03) | 7.7 × 10−1 |
| ICAM-1, pg/mL | 279.92 ± 113 | −0.06 (0.11) | 5.9 × 10−1 | 0.35 (0.12) | 4.0 × 10−3 | 0.11 (0.03) | 3.0 × 10−3 |
| IL-6, pg/mL | 2.10 ± 2.1 | 0.23 (0.14) | 1.1 × 10−1 | 0.12 (0.09) | 1.9 × 10−1 | 0.17 (0.03) | 7.2 × 10−7 |
| MCP-1, pg/mL | 312.80 ± 90.2 | 0.22 (0.12) | 5.0 × 10−2 | −0.14 (0.12) | 2.2 × 10−1 | 0.06 (0.03) | 8.1 × 10−2 |
| CRP, ng/mL | 1188.5 ± 1427 | 0.24 (0.15) | 1.2 × 10−1 | 0.28 (0.08) | 1.0 × 10−3 | 0.26 (0.03) | 5.3 × 10−15 |
| Liver function markers, U/L | |||||||
| ALT | 24.34 ± 24.3 | 0.08 (0.15) | 5.9 × 10−1 | 0.41 (0.08) | 9.6 × 10−6 | 0.28 (0.03) | 1.2 × 10−15 |
| AST | 25.11 ± 15.1 | −0.07 (0.13) | 5.8 × 10−1 | 0.36 (0.09) | 3.0 × 10−4 | 0.15 (0.03) | 1.1 × 10−5 |
| Diabetes-related phenotypes | |||||||
| Glucose, mg/dL | 92.31 ± 12.1 | 0.03 (0.12) | 8.2 × 10−1 | 0.17 (0.11) | 1.1 × 10−1 | 0.10 (0.03) | 5.0 × 10−3 |
| Insulin, μU/mL | 22.43 ± 18.5 | 0.26 (0.14) | 9.2 × 10−2 | 0.45 (0.07) | 1.8 × 10−6 | 0.37 (0.03) | 7.7 × 10−27 |
| C-peptide, ng/mL | 2.69 ± 1.8 | 0.002 (0.12) | 9.9 × 10−1 | 0.80 (0.14) | 2.1 × 10−8 | 0.31 (0.03) | 3.6 × 10−19 |
| HOMA | 5.26 ± 4.9 | 0.23 (0.14) | 1.3 × 10−1 | 0.46 (0.07) | 1.3 × 10−6 | 0.36 (0.03) | 7.2 × 10−26 |
| Blood pressure, mm Hg | |||||||
| Systolic | 108.13 ± 10.9 | 0.19 (0.14) | 1.9 × 10−1 | 0.23 (0.08) | 1.0 × 10−2 | 0.21 (0.03) | 3.6 × 10−10 |
| Diastolic | 51.01 ± 6.9 | −0.03 (0.15) | 8.5 × 10−1 | 0.04 (0.09) | 6.8 × 10−1 | 0.01 (0.03) | 7.6 × 10−1 |
Estimates of the allele frequencies and their SEs were obtained by using the software program Sequential Oligogenic Linkage Analysis Routines (SOLAR), version 7.6.2 (32). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; ρE, estimate of environmental correlations; ρG, estimate of genetic correlations; ρP, estimate of phenotypic correlations.
Genomewide association analysis
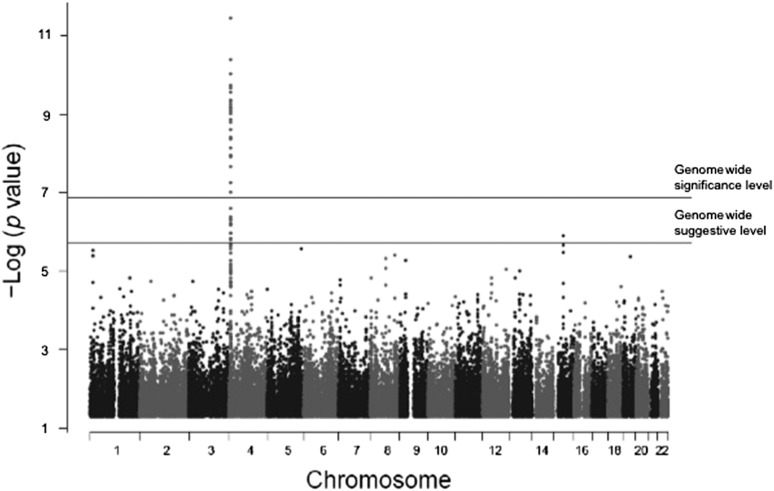
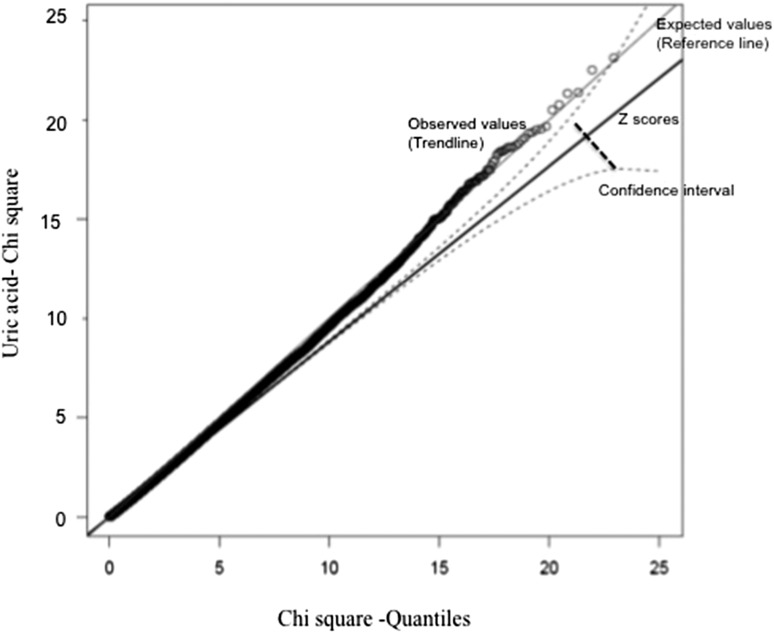
Genomewide association analysis of SUA concentrations showed strong associations with SNPs in solute carrier protein 2 family, member 9 (SLC2A9), on chromosome 4 (Figure 1). A total of 400 SNPs showed an association with SUA concentrations at a genomewide significance level of P < 1 × 10−7. All of the 400 SNPs were in the chromosome 4 region that spanned ∼46 kb and covered both SLC2A9 and the adjacent gene WD repeat-containing protein 1 (WDR1). Genotype distributions of all significantly associated SNPs conformed to Hardy-Weinberg equilibrium. Population stratification was not significant as per the quantitative transmission disequilibrium test and therefore did not confound our associations. The distribution of P values from GWASs of SUA showed no evidence of inflation due to population stratification (Figure 2). The relation pairs (n = 1242) that were used in the analysis are shown in Table 2. The top 20 significant associations are shown in Table 3. Frequencies of the minor alleles ranged between 0.24 and 0.49, whereas effect sizes (proportion of the residual phenotypic variance accounted by minor allele of the SNP) ranged between 8.5% and 10% (Table 3). Genotype-specific means of SUA concentrations showed that minor alleles of most of the SNPs are associated with lower concentrations of SUA, which is consistent with adult studies. Details of risk and minor alleles are given in Table 3. To uncover other signals and to offset the overwhelming association of SLC2A9 and flanking SNPs, we conducted another GWAS with adjustment for the top 20 significant SNPs (from the chromosome 4 region) from the previous genomewide association analysis. Evidence of a suggestive association with SUA concentrations was found for 2 SNPs: rs12965305 from docking protein 6 (DOK6) and rs3796879 from glutamyl aminopeptidase (ENPEP). None of the SNPs were associated with SUA at a significance level of P < 1 × 10−7.
FIGURE 1.
Genomewide association analysis of serum uric acid in Hispanic children.
FIGURE 2.
A Q-Q plot showing the absence of inflation due to population stratification and batch or clustering effects.
TABLE 2.
Relation pairs used in this analysis
| Relationships | Pairs, n |
| Identical sibling pair | 2 |
| Siblings | 888 |
| Half siblings and first cousins | 2 |
| Double first cousins | 1 |
| Half avuncular | 5 |
| First cousins | 338 |
| Half first cousins | 6 |
| Total | 1242 |
TABLE 3.
Significant associations of SLC2A9 SNPs with serum uric acid concentrations1
| Genotype-specific means of serum uric acid concentrations5 |
||||||||
| SNP | Base-pair position2 | MGA P value | Minor allele/frequency | Risk allele3 | Effect size,4 % | 11 | 12 | 22 |
| rs11723388 | 9804900 | 3.3 × 10−16 | A/0.26 | G | 10 | 4.21 (2.3) | 5.02 (2.1) | 5.54 (2.5) |
| rs11721501 | 9798233 | 5.7 × 10−16 | A/0.26 | G | 9.9 | 4.17 (2.3) | 5.02 (2.1) | 5.55 (2.5) |
| rs6843466 | 9537003 | 1.2 × 10−15 | A/0.49 | G | 9.6 | 4.67 (1.7) | 5.32 (1.6) | 5.75 (1.9) |
| rs17251963 | 9751659 | 1.3 × 10−15 | G/0.25 | A | 9.7 | 4.12 (1.5) | 5.04 (1.6) | 5.53 (1.7) |
| rs13129697 | 9536065 | 1.4 × 10−15 | A/0.49 | A | 9.6 | 5.74 (1.9) | 5.29 (1.6) | 4.66 (1.7) |
| rs7696983 | 9604927 | 1.5 × 10−15 | A/0.26 | G | 9.3 | 4.03 (2.3) | 5.06 (1.6) | 5.51 (2.5) |
| rs13113918 | 9607591 | 1.5 × 10−15 | A/0.26 | G | 9.3 | 4.03 (2.3) | 5.06 (1.6) | 5.51 (2.5) |
| rs7683856 | 9610045 | 1.5 × 10−15 | A/0.26 | G | 9.3 | 4.03 (2.3) | 5.06 (1.6) | 5.51 (2.5) |
| rs9991278 | 9611763 | 1.5 × 10−15 | A/0.26 | G | 9.3 | 4.03 (2.3) | 5.06 (2.1) | 5.51 (2.5) |
| rs13111638 | 9605988 | 2.1 × 10−15 | A/0.25 | G | 9.1 | 3.97 (1.4) | 5.07 (1.6) | 5.49 (1.7) |
| rs4481233 | 9565177 | 2.4 × 10−15 | A/0.24 | G | 9.0 | 3.96 (2.3) | 5.04 (1.6) | 5.51 (1.8) |
| rs1978274 | 9854185 | 2.5 × 10−15 | C/0.29 | A | 9.5 | 4.28 (2.3) | 5.06 (2.1) | 5.56 (2.5) |
| rs7669607 | 9606899 | 2.4 × 10−15 | A/0.26 | G | 8.5 | 4.03 (2.3) | 5.06 (1.6) | 5.51 (2.5) |
| rs9991278 | 9611817 | 3.0 × 10−15 | A/0.26 | G | 9.1 | 4.03 (2.3) | 5.07 (2.1) | 5.51 (2.5) |
| rs11723439 | 9560917 | 3.5 × 10−15 | A/0.25 | G | 8.6 | 3.98 (1.3) | 5.08 (1.7) | 5.49 (1.7) |
| rs4697745 | 9914179 | 5.1 × 10−15 | A/0.27 | G | 9.1 | 4.24 (1.6) | 5.04 (1.6) | 5.55 (1.8) |
| rs7675964 | 994134 | 5.7 × 10−15 | G/0.49 | G | 9.2 | 5.73 (1.9) | 5.28 (1.6) | 4.69 (1.7) |
| rs938552 | 9925524 | 7.0 × 10−15 | A/0.41 | G | 8.9 | 4.53 (1.6) | 5.23 (1.7) | 5.60 (1.7) |
| rs6449213 | 9994215 | 1.3 × 10−14 | G/0.25 | A | 8.6 | 4.02 (1.4) | 5.05 (1.6) | 5.50 (1.7) |
| rs12510549 | 9885815 | 2.0 × 10−14 | G/0.26 | A | 8.5 | 4.32 (1.6) | 5.04 (1.6) | 5.53 (1.8) |
The top 20 associations are shown. Estimates of the allele frequencies and their SEs were obtained by using the software program Sequential Oligogenic Linkage Analysis Routines (SOLAR), version 7.6.2 (32). The final model included age, age-squared, sex, age × sex, age-squared × sex, BMI z score, and systolic blood pressure as covariates. MGA, measured genotype analysis; SLC2A9, solute carrier family 2, member 9; SNP, single nucleotide polymorphism.
Position based on NCBI Genome Build 36.3.
Risk allele = allele that is associated with increased serum uric acid concentrations (mg/dL).
Effect size = proportion of residual phenotypic variance explained by the SNP.
1 = minor allele; 2 = major allele.
Extension of the SLC2A9 association with SUA to children
For several of the significantly associated SNPs (rs6449213, rs10805346, rs1014290, and rs737267), an association with SUA was previously reported in Mexican Americans of the San Antonio Family Heart Study, American Indians of the Strong Heart Family Study, Western Alaska Natives of the Genetics of Coronary Artery Disease in Alaska Natives, Zuni Indians of the Genetics of Kidney Disease in Zuni Indians, and in European adult populations (Table 4). Interestingly, the effect sizes in Hispanic children were much higher than those in adults (3–5%) (38, 39).
TABLE 4.
SLC2A9 SNP associations in VFS children that replicate results observed in adults1
| VFS | SAFHS | SHFS | GOCADAN | GKDZI | Studies in Europeans, P | Reference for European studies | |
| Age, y | 11.0 ± 4.02 | 47.9 ± 14.8 | 39.5 ± 17.0 | 48.9 ± 14.8 | 36.8 ± 13.7 | — | — |
| Serum uric acid, mg/dL | 5.2 ± 1.7 | 5.4 ± 1.4 | 5.14 ± 1.5 | 5.25 ± 1.3 | 5.9 ± 1.7 | — | — |
| Hyperuricemia,3 % | 25 | 22 | 17 | 14 | 31 | — | — |
| Associated SLC2A9 SNPs,4 P value | |||||||
| rs13129697 | 9.9 × 10−16 | — | — | — | 1.8 × 10−5 | 2.3 × 10−19 | (52) |
| rs13124563 | 2.0 × 10−12 | 1.1 × 10−5 | — | — | — | — | — |
| rs7660895 | 1.2 × 10−14 | 1.5 × 10−4 | — | — | 1.6 × 10−6 | — | — |
| rs11723439 | 8.8 × 10−15 | — | — | — | 3.1 × 10−7 | — | — |
| rs13111638 | 3.5 × 10−15 | — | — | — | 1.5 × 10−7 | 1.1 × 10−9 | (39) |
| rs4697701 | 2.8 × 10−15 | — | — | — | 1.4 × 10−6 | — | — |
| rs6832439 | 3.8 × 10−14 | 6.0 × 10−9 | 7.7 × 10−31 | 2.2 × 10−5 | 8.5 × 10−8 | — | — |
| rs737267 | 7.7 × 10−14 | 4.2 × 10−8 | 2.9 × 10−29 | 7.9 × 10−6 | 4.5 × 10−7 | 2.5 × 10−9 | (38) |
| rs6449213 | 2.1 × 10−14 | 1.6 × 10−6 | 1.5 × 10−29 | 1.3 × 10−6 | 4.3 × 10−8 | 6.1 × 10−10 | (39) |
| rs10805346 | 8.4 × 10−13 | 1.8 × 10−5 | 5.4 × 10−28 | 9.5 × 10−4 | 1.2 × 10−4 | — | — |
| rs1014290 | 1.0 × 10−13 | 1.9 × 10−4 | — | — | 1.6 × 10−5 | 5.6 × 10−8 | (38) |
| rs13125029 | 1.9 × 10−10 | 4.3 × 10−5 | — | — | 5.6 × 10−7 | — | — |
| rs4447863 | 7.4 × 10−13 | 2.7 × 10−2 | — | — | 1.4 × 10−6 | — | — |
| rs4697695 | 7.1 × 10−14 | 4.0 × 10−2 | — | — | 1.3 × 10−4 | — | — |
GKDZI, Genetics of Kidney Disease in Zuni Indians; GOCADAN, Genetics of Coronary Artery Disease in Alaska Natives; SAFHS, San Antonio Family Heart Study; SHFS, Strong Heart Family Study; SLC2A9, solute carrier family 2, member 9; VFS, Viva La Familia Study.
Mean ± SD (all such values).
Based on 2 SDs above the serum uric acid means in children and serum uric acid >7 and >6 mg/dL in men and women, respectively.
SNPs for the VFS, SAFHS, and GKDZI were genotyped as part of genomewide association studies; SNPs for the SHFS and GOCADAN were generated for candidate gene studies.
DISCUSSION
To our knowledge, this is the first GWAS of SUA concentrations in children and the first to extend the strong association of variants in the SLC2A9 genetic locus with SUA concentrations to children in a family-based study. GWASs have been conducted in several adult populations to identify loci associated with SUA concentrations. Most of these studies showed associations of SLC2A9 SNPs with SUA concentrations. Vitart et al. (40) showed the association of SLC2A9 SNPs with SUA concentrations in a Croatian sample and then replicated the result in a sample from the island of Orkney. Similar associations were reported in individuals from Germany (41), Sardinia (42), and the United Kingdom (43). Our group has replicated the association of SUA with SLC2A9 SNPs in Mexican American (38), American Indian (39), Western Alaska Native, and Zuni Indian adult populations (44, 45). The main aim of all these family-based studies was to identify genetic determinants of complex diseases, mainly CVD and chronic kidney disease (21, 22, 25, 44, 45). All of these cohorts, except for Zuni Indians, were recruited without regard to disease status. Nevertheless, the obesity rates were still high in these cohorts.
The concentrations of SUA in children are dependent on age, sex, and puberty stage. Hyperuricemia cutoffs in children are different from adults and are defined as SUA concentrations >2 SDs above the mean. In this study, ∼25% children were hyperuricemic with the use of this criterion. The serum uric acid profile also varied according to age, with older children (≥13 y) having serum uric acid concentrations similar to adults. Hyperuricemia is caused by either increased production of uric acid or decreased renal excretion or a combination of both. In recent times, children with hyperuricemia increasingly present with obesity, as is the case in adults. Elevated SUA concentrations in children and adults are often associated with obesity and the metabolic syndrome (8, 11, 12, 14, 19, 46, 47). Several investigators have advocated for uric acid to be a component of the metabolic syndrome. In a cohort of obese children and adolescents, Denzer et al. (14) found positive correlations between SUA concentrations and BMI, serum triglycerides, and systolic blood pressure. They also pointed out that childhood hyperuricemia may be an indicator for pre–metabolic syndrome in obese youth and an unfavorable cardiovascular profile in obese adults. In Japanese junior high school students, hyperuricemia was strongly associated with cardiometabolic risk factors but only in boys (48). Consistent with other publications, we found positive correlations of SUA with all components of the metabolic syndrome, except for HDL-cholesterol concentrations. Likewise, SUA concentrations in childhood have been strongly associated with blood pressure (1, 15, 49–53) and are predictive of adult hypertension (51). We also found positive correlations of SUA with systolic and diastolic blood pressure. Soletsky and Feig (52) showed that uric acid–lowering therapy was effective in reducing blood pressure and systemic vascular resistance in obese adolescents. Our GWAS results, when additionally adjusted for BMI z score and systolic blood pressure, showed associations between SUA and SLC2A9 SNPs with stronger effect sizes. This may be a reflection of the previous reports of a biological link between blood pressure, adiposity, and SUA, especially in children (8, 15, 17, 47, 51, 52). Our results of genetic correlations of SUA with obesity measurements, particularly waist circumference, are consistent with our results in Mexican-American adults (21).
The association of SUA concentrations with adiposity and inflammation is well recognized. Although the mechanism is not yet clear, obese adults and children have higher SUA concentrations as observed in this study and in other studies (14, 53, 54). Gillum (53) also found independent associations of SUA concentrations with systolic blood pressure and waist-to-hip ratio. Lyngdoh et al. (54) suggested that adiposity might be the link between hyperuricemia and hypertension. Wasilewska et al. (55) showed that children with hyperuricemia tend to have higher concentrations of circulating monocyte chemoattractant protein-1 and C-reactive protein, which is consistent with our results. In our study we found strong genetic correlations between SUA and adiposity measures, suggesting a common set of genes affecting both of these measures. We did not detect any significant genetic correlations of SUA with blood pressure, inflammation markers, or other cardiometabolic risk factors, although we did find strong phenotypic correlations between them.
This is the first GWAS of SUA in children. The heritability estimate for SUA concentrations in this cohort of Hispanic children was 45%, which is in the same range as reported in adults (10, 19–22). The variation in several uric acid transporter genes have been associated with SUA concentrations in adults (European and non-European), with variants in SLC2A9 being the most commonly associated across populations (39–43, 56). Interestingly, we replicated the SLC2A9 findings; however, the associations and effect sizes were stronger in children. One of the SNPs, rs938552, is in strong LD with a missense polymorphism, rs16890979. Because strong genetic correlations were observed between obesity phenotypes and SUA concentrations, we conducted association analysis between them. None of the SLC2A9 SNPs were associated with obesity phenotypes, which was in contrast to our results in Mexican-American adults in whom SLC2A9 SNPs were significantly associated with BMI and waist circumference (38). In another study, Brandstätter et al. (57) showed that the association between SLC2A9 and SUA concentrations was modified by BMI.
We conducted an additional GWAS for SUA, conditioned on significant SLC2A9 SNPs, to uncover novel signals otherwise masked by SLC2A9 SNPs. Although we did not find significant associations between SUA and other genes, we found suggestive evidence of an association of SUA concentrations with DOK6 and ENPEP. None of these have been previously associated with SUA concentrations either in adults or in children. However, they have been shown to be associated with renal function (58) and blood pressure regulation (59), respectively. In a GWAS in individuals of African ancestry, urinary albumin-creatinine ratio was associated with DOK6 (58). DOK6 encodes a member of a family of intracellular adaptor proteins, expressed highly in human kidneys, that plays a role in rearranged during transfection signaling cascade (60). ENPEP encodes the glutamyl aminopeptidase that plays an important role in blood pressure regulation by facilitating conversion of angiotensin II to angiotensin III (59).
The majority of candidate biomarkers that are used in drug development are usually studied in adults. Given the association between SUA concentrations and increased risk of gout, hypertension, renal disease, and CVD in adults, and the ability to predict adult hypertension on the basis of pediatric SUA concentrations, the identification of such biomarkers will aid in the development of new treatment strategies that can be applied early in life. Although we were able to replicate results from studies in adults, lack of similar studies in children makes it difficult to replicate or validate our results. In conclusion, our genomewide association results of SUA concentrations in children replicate findings in adults and support SLC2A9 genetic variation as an important pediatric biomarker of renal disease and CVD risk.
Acknowledgments
We thank Grace-Ellen Meixner and Maria del Pilar Villegas. for technical assistance.
The authors’ responsibilities were as follows—VSV, SAC, NFB, and AGC: designed the research and had primary responsibility for final content; VSV, SL, and KH: conducted the research; KH, NRM, SAC, and NFB: provided essential reagents; VSV and SL: analyzed data or performed statistical analysis; VSV, NFB, and AGC: wrote the manuscript; and SL, KH, NRM, and SAC: read and provided edits to the manuscript. The authors declared no conflicts of interest.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DOK6, docking protein 6; ENPEP, glutamyl aminopeptidase; GWAS, genomewide association study; LD, linkage disequilibrium; SLC2A9, solute carrier family 2, member 9; SNP, single nucleotide polymorphism; SOLAR, Sequential Oligogenic Linkage Analysis Routine; SUA, serum uric acid; VFS, Viva La Familia Study.
REFERENCES
- 1.Menè P, Punzo G. Uric acid: bystander or culprit in hypertension and progressive renal disease? J Hypertens 2008;26:2085–92. [DOI] [PubMed] [Google Scholar]
- 2.Ogbera AO, Azenabor AO. Hyperuricaemia and the metabolic syndrome in type 2DM. Diabetol Metab Syndr 2010;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutional considerations. Semin Nephrol 2011;31:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS ONE 2013;8:e56864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanbay M, Segal M, Afsar B, Kang DH, Ridriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013;99:759–66. [DOI] [PubMed] [Google Scholar]
- 6.Kivity S, Kopel E, Maor E, Abu-Bachar F, Segev S, Sidi Y, Olchovsky D. Association of serum uric acid and cardiovascular disease in healthy adults. Am J Cardiol 2013;111:1146–51. [DOI] [PubMed] [Google Scholar]
- 7.Baldree LA, Stapleton FB. Uric acid metabolism in children. Pediatr Clin North Am 1990;37:391–418. [DOI] [PubMed] [Google Scholar]
- 8.Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Semin Nephrol 2011;31:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007;115:2526–32. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Köttgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet 2010;3:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MS, Wahlqvist ML, Yu HL, Pan WH. Hyperuricemia and metabolic syndrome in Taiwanese children. Asia Pac J Clin Nutr 2007;16:594–600. [PubMed] [Google Scholar]
- 12.Civantos Modino S, Guijarro de Armas MG, Monereo Mejias S, Montano Martinez JM, Iglesias Bolanos P, Merina Viveros M, Ladero Quesada JM. Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinol Nutr 2012;59:533–8. [DOI] [PubMed] [Google Scholar]
- 13.Harlan WR, Cormoni-Huntley J, Leaverton PE. Physiologic determinants of serum urate levels in adolescence. Pediatrics 1979;63:569–75. [PubMed] [Google Scholar]
- 14.Denzer C, Muche R, Mayer H, Heinze E, Debatin KM, Wabitsch M. Serum uric acid levels in obese children and adolescents: linkage to testosterone levels and pre-metabolic syndrome. J Pediatr Endocrinol Metab 2003;16:1225–32. [DOI] [PubMed] [Google Scholar]
- 15.Jossa F, Farinaro E, Panico S, Krogh V, Celentano E, Galasso R, Mancini M, Trevisan M. Serum uric acid and hypertension: the Olivetti Heart Study. J Hum Hypertens 1994;8:677–81. [PubMed] [Google Scholar]
- 16.Selby JV, Friedman GD, Quesenberry CP Jr. Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol 1990;131:1017–27. [DOI] [PubMed] [Google Scholar]
- 17.Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005;45:34–8. [DOI] [PubMed] [Google Scholar]
- 18.Jones DP, Richey PA, Alpert BS, Li R. Serum uric acid and ambulatory blood pressure in children with primary hypertension. Pediatr Res 2008;64:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W, Miller MB, Rich SS, North KE, Pankow JS, Borecki IB, Myers RH, Hopkins PN, Leppert M, Arnett DK; National Heart, Lung and Blood Institute Family Heart Study. Linkage analysis of a composite factor for the multiple metabolic syndrome: the National Heart, Lung and Blood Institute Family Heart Study. Diabetes 2003;52:2840–7. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Guo CY, Cupples A, Levy D, Wilson PW, Fox CS. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism 2005;54:1435–41. [DOI] [PubMed] [Google Scholar]
- 21.Voruganti VS, Nath SD, Cole SA, Thameem F, Jowett JB, Bauer R, MacCluer JW, Blangero J, Comuzzie AG, Abboud HE, et al. Genetics of variation in serum uric acid and cardiovascular risk factors in Mexican-Americans. J Clin Endocrinol Metab 2009;94:632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voruganti VS, Goring HHH, Mottl A, Franceschini N, Haack K, Laston S, Almasy L, Fabsitz RR, Lee ET, Best LG, et al. Genetic influence on variation in serum uric acid in American Indians: the Strong Heart Family Study. Hum Genet 2009;126:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr 2006;84:646–54. [DOI] [PubMed] [Google Scholar]
- 24.Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, Butte NF. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS ONE 2012;7:e51954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voruganti VS, Cole SA, Ebbesson SOE, Goring HH, Haack K, Laston S, Wenger CR, Tejero ME, Devereux RB, Fabsitz RR, et al. Genetic variation in APOJ, LPL, and TNFRSF10B affects plasma fatty acid distribution in Alaskan Eskimos. Am J Clin Nutr 2010;91:1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacCluer JW, Scavini M, Shah VO, Cole SA, Laston SL, Voruganti VS, Paine SS, Eaton AJ, Comuzzie AG, Tentori F, et al. Heritability of measures of kidney disease among Zuni Indians: the Zuni Kidney Project. Am J Kidney Dis 2010;56:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, Ellis KJ, Comuzzie AG. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva La Familia Study. Am J Clin Nutr 2007;85:1478–85. [DOI] [PubMed] [Google Scholar]
- 28.Cai G, Cole SA, Butte NF, Smith CW, Mehta NR, Voruganti VS, Proffitt JM, Comuzzie AG. A genetic contribution to circulating cytokines and obesity in children. Cytokine 2008;44:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual: Champaign. Champaign (IL): Human Kinetics Books; 1988. [Google Scholar]
- 30.Butte NF, Christiansen E, Sorensen TI. Energy imbalance underlying the development of childhood obesity. Obesity (Silver Spring) 2007;15:3056–66. [DOI] [PubMed] [Google Scholar]
- 31.Sobel E, Lange K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet 1996;58:1323–37. [PMC free article] [PubMed] [Google Scholar]
- 32.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998;62:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet 1982;46:373–83. [DOI] [PubMed] [Google Scholar]
- 34.Self SG, Liang KY. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J Am Stat Assoc 1987;82:605–10. [Google Scholar]
- 35.Boerwinkle E, Chakraborty R, Sing CF. The use of measured genotype information in the analysis of quantitative phenotypes in man. I. Models and analytical methods. Ann Hum Genet 1986;50:181–94. [DOI] [PubMed] [Google Scholar]
- 36.Moskvina V, Schmidt KM. On multiple-testing correction in genome-wide association studies. Genet Epidemiol 2008;32:567–73. [DOI] [PubMed] [Google Scholar]
- 37.Havill LM, Dyer TD, Richardson DK, Mahaney MC, Blangero J. The quantitative trait linkage disequilibrium test: a more powerful alternative to the quantitative transmission disequilibrium test for use in the absence of population stratification. BMC Genet 2005;6(Suppl 1):S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voruganti VS, Kent JW Jr, Debnath S, Cole SA, Haack K, Göring HHH, Carless MA, Curran JE, Johnson MP, Almasy L, et al. Genome-wide association analysis confirms and extends the association of SLC2A9 with serum uric acid levels to Mexican Americans. Front Genet 2013;4:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voruganti VS, Franceschini N, Haack K, Laston S, MacCluer JW, Umans JG, Comuzzie AG, North KE, Cole SA. Replication of the effect of SLC2A9 genetic variation on serum uric acid levels in American Indians. Eur J Hum Genet 2014;22:938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437–42. [DOI] [PubMed] [Google Scholar]
- 41.Döring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 2008;40:430–6. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orru M, Albai G, et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 2007;3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, Ahmadi K, Dobson RJ, Marcano AC, Hajat C, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 2008;82:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laston S, Voruganti VS, Haack K, Shah VO, Bobelu A, Bobelu J, Ghahate D, Harford AM, Paine SS, Tentori F, et al. Genetics of kidney disease and related phenotypes in Zuni Indians: a genome-wide association study—the Zuni Kidney Project. Front Genet. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voruganti VS, Laston SL, Haack K, Cole SA, Kent JW, Ebbesson SOE, MacCluer JW, Blangero J, Zager P, Umans JG, et al. Association of serum uric acid genetic risk score with chronic kidney disease in Mexican American, American Indian and Alaska Native populations. J Am Soc Nephrol 2013;24:158A. [Google Scholar]
- 46.Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innnocent bystander? Metabolism 2006;55:1293–301. [DOI] [PubMed] [Google Scholar]
- 47.Tang L, Kubota M, Nagai A, Mamoto K, Tokuda M. Hyperuricemia in obese children and adolescents: the relationship with metabolic syndrome. Pediatr Rep 2010;2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hongo M, Hidaka H, Sakaguchi S, Nakanishi K, Ichikawa M, Hirota N, Tanaka N, Yazaki Y, Kinoshita O, Ikeda U, et al. Association between serum uric acid levels and cardiometabolic risk factors among Japanese junior high school. Circ J 2010;74:1570–7. [DOI] [PubMed] [Google Scholar]
- 49.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 2003;42:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong AP, Choi KC, Ho CS, Chan MH, Ozaki R, Chan CW, Chan JC. Associations of uric acid and gamma-glutamyltransferase (GGT) with obesity and components of metabolic syndrome in children and adolescents. Pediatr Obes 2013;8:351–7. [DOI] [PubMed] [Google Scholar]
- 51.Feig DI, Mazzali M, Kang DH, Nakagawa T, Price K, Kannelis J, Johnson RJ. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol 2006;17:S69–73. [DOI] [PubMed] [Google Scholar]
- 52.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolscents. Hypertension 2012;60:1148–56. [DOI] [PubMed] [Google Scholar]
- 53.Gillum RF. The association of the ratio of waist to hip girth with blood pressure, serum cholesterol and serum uric acid in children and youths aged 6-17 years. J Chronic Dis 1987;40:413–20. [DOI] [PubMed] [Google Scholar]
- 54.Lyngdoh T, Vuistiner P, Mrques-Vidal P, Rousson V, Waeber G, Vollenweider P, Bochud M. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS ONE 2012;7:e39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wasilewska A, Tenderenda E, Taranta-Janusz K, Tobolczyk J, Stypulkowska J. Markers of systemic inflammation in children. Acta Paediatr 2012;101:497–500. [DOI] [PubMed] [Google Scholar]
- 56.Karns R, Zhang G, Sun G, Indugula SR, Cheng H, Havas-Augustin D, Novokmet N, Rudan D, Durakovic Z, Missoni S, et al. Genome-wide association of serum uric acid concentration: replication of sequence variants in an island population of the Adriatic coast of Croatia. Ann Hum Genet 2012;76:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandstätter A, Kiechl S, Kollerits B, Hunt SC, Heid IM, Coassin S, Willeit J, Adams TD, Illig T, Hopkins PN, et al. Sex-specific association of the putative fructose transporter SLC2A9 variants with uric acid levels is modified by BMI. Diabetes Care 2008;31:1662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu CT, Garnaas MK, Tin A, Kottgen A, Franceschini N, Peralta CA, de Boer IH, Lu X, Atkinson E, Ding J, et al. Genetic association for renal traits among participants of African Ancestry reveals new loci for renal function. PLoS Genet 2011;7:e1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crowder RJ, Enomoto H, Yang M, Johnson EM Jr., Milbrandt J. Dok-6, a novel p62 Dok family member, Ret-medicated neurite outgrowth. J Biol Chem 2004;279:42072–81. [DOI] [PubMed]
- 60.Mizutani S, Ishii M, Hattori A, Nomura S, Numaguchi Y, Tsujimoto M, Kobayshi H, Murohara T, Wright JW. New insights into the importance of aminopeptidase A in hypertension. Heart Fail Rev 2008;13:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]