Abstract
Background: Folic acid prevents neural tube closure defects (NTDs), but the causal metabolic pathways have not been established. Serine hydroxymethyltransferase 1 (SHMT1) is an essential scaffold protein in folate-dependent de novo thymidylate synthesis in the nucleus. SHMT1-deficient mice provide a model to investigate folic acid–responsive NTDs wherein disruption of de novo thymidylate synthesis impairs neural tube closure.
Objective: We examined the effects of maternal supplementation with the pyrimidine nucleosides uridine, thymidine, or deoxyuridine with and without folate deficiency on NTD incidence in the Shmt1 mouse model.
Design: Shmt1+/+ and Shmt1−/− female mice fed folate-replete or folate-deficient diets and supplemented with uridine, thymidine, or deoxyuridine were bred, and litters (n = 10–23 per group) were examined for the presence of NTDs. Biomarkers of impaired folate status and metabolism were measured, including plasma nucleosides, hepatic uracil content, maternal plasma folate concentrations, and incorporation of nucleoside precursors into DNA.
Results: Shmt1+/− and Shmt1−/− embryos from dams fed the folate-deficient diet were susceptible to NTDs. No NTDs were observed in litters from dams fed the folate-deficient diet supplemented with deoxyuridine. Surprisingly, uridine supplementation increased NTD incidence, independent of embryo genotype and dietary folic acid. These dietary nucleosides did not affect maternal hepatic uracil accumulation in DNA but did affect plasma folate concentrations.
Conclusions: Maternal deoxyuridine supplementation prevented NTDs in dams fed the folate-deficient diet, whereas maternal uridine supplementation increased NTD incidence, independent of folate and embryo genotype. These findings provide new insights into the metabolic impairments and mechanisms of folate-responsive NTDs resulting from decreased Shmt1 expression.
Keywords: SHMT1, deoxyuridine, folic acid, neural tube defects, uridine
INTRODUCTION
Low maternal folate status is among the strongest environmental determinants of neural tube closure defects (NTDs)6 in human populations (1) and interacts with specific gene variants to confer risk for an NTD-affected pregnancy (2–5). Although it known that genetic variants interact with folate status to influence NTD risk, most of the genetic risk has yet to be identified (6). Clinical trials have established that periconceptional maternal folic acid supplementation prevents the occurrence and recurrence of NTDs by up to 70% (7, 8). Folic acid fortification of enriched grains has been introduced in more than 60 countries (9) and has significantly reduced the rates of neural tube defects (7, 8). However, most but not all NTDs are responsive to dietary folic acid (10). Other risk factors for NTD-affected pregnancies include environmental and food-based toxins (11, 12), obesity (13), and maternal diabetes (14). Deficiencies in other nutrients that biochemically interact with folate may also contribute to NTD risk (15, 16), although their role has not been established.
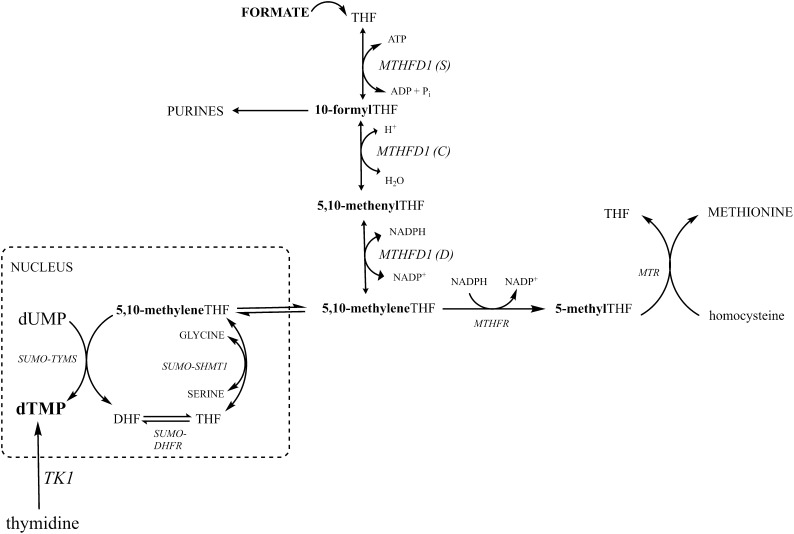
Folates function as enzyme cofactors that carry and chemically activate one-carbon units for a network of pathways collectively known as one-carbon metabolism. One-carbon metabolism is essential for de novo purine and thymidylate (dTMP) biosynthesis and for the remethylation of homocysteine to methionine (Figure 1). Responsiveness to maternal folic acid supplementation has been determined in only a few of the many mouse models (17) that exhibit NTDs (17–19). Two NTD models, Pax3 and Shmt1 loss of function, exhibit folic acid–responsive NTDs and impairments in de novo dTMP biosynthesis. The splotch mutant (Pax3Sp) demonstrates impaired de novo dTMP (19, 20) and purine nucleotide biosynthesis (20). NTDs in the splotch mutant can be rescued with dietary folic acid, indicating that folic acid prevents NTDs by rescuing de novo dTMP and/or purine synthesis in this mouse model (18, 19). Embryonic serine hydroxymethyltransferase 1 (Shmt1) gene disruptions also cause folate-responsive NTDs in mice (20, 21). This is the only mouse model with a genetic disruption in a gene encoding a folate-dependent enzyme that causes folic acid–responsive NTDs (20). This gene-diet interaction resembles the interactions found in human studies of NTD pathogenesis, including incomplete penetrance, subtle alterations in one-carbon metabolism, and folic acid responsiveness (4, 5). Shmt1 generates one-carbon units from the enzymatic cleavage of serine to glycine that are preferentially shunted to dTMP biosynthesis (22) and is an essential scaffold protein required for the formation of a metabolic complex containing the de novo dTMP synthesis pathway at the replication fork (23, 24). Shmt1−/− and Shmt1+/− embryos are sensitized to folic acid–responsive NTDs and demonstrate impaired de novo dTMP biosynthesis (20).
FIGURE 1.
One-carbon metabolism in the cytoplasm and nucleus. One-carbon metabolism is required for the synthesis of purines, dTMP, and methionine. Mitochondrial-derived formate can enter the cytoplasm and function as a one-carbon unit for folate metabolism through the activity of MTHFD1. At S-phase, the enzymes of the de novo dTMP synthesis pathway undergo SUMO-dependent translocation to the nucleus. DHF, dihydrofolate; DHFR, dihydrofolate reductase; dTMP, thymidylate; MTHFD1, methylenetetrahydrofolate dehydrogenase 1; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; S, C, D, synthetase, cyclohydrolate, and dehydrogenase activities of MTHFD1, respectively; SHMT1, serine hydroxymethyltransferase 1; SUMO, small ubiquitin-like modifier; THF, tetrahydrofolate; TYMS, thymidylate synthase.
The mechanism whereby disruption of de novo dTMP biosynthesis causes NTDs in the Shmt1 mouse model is not known. Previously, one study indicated that thymidine supplementation prevented NTDs in splotch homozygotes (19), but another did not observe a reduced risk of NTDs with thymidine supplementation in the splotch mouse model (18). In this study, we examined the effects of the dietary nucleosides uridine, thymidine, and deoxyuridine on NTD risk in Shmt1+/+ and Shmt1−/− dams fed a folate-replete or folate-deficient AIN-93G diet.
METHODS
Mouse models
The Shmt1 mouse model and its susceptibility to folic acid–responsive NTDs has been described previously (20, 25). Shmt1+/+ and Shmt1−/− mice were generated from Shmt1+/− breeding pairs maintained as a heterozygote breeding colony on a pure 129SvEv background.
Experimental animals and diets
All animal experiments were approved by the Cornell Institutional Animal Care and Use Committee (Cornell University, Ithaca, NY) according to the guidelines of the Animal Welfare Act and all applicable federal and state laws. Mice were maintained on a 12-h light/dark cycle in a temperature-controlled room. For studies investigating Shmt1 disruption and NTDs, Shmt1+/+ and Shmt1−/− female dams were randomly assigned to one of 8 diets, described in Table 1: AIN-93G as control (C); control diet supplemented with uridine (C+U), thymidine (C+T), or 2′-deoxyuridine (C+dU); AIN-93G lacking folic acid (FD); or folate-deficient diet supplemented with uridine (FD+U), thymidine (FD+T), or 2′-deoxyuridine (FD+dU) (Dyets) (Supplemental Table 1). The levels of dietary nucleoside supplementation were informed by previous studies of immune responsiveness in rodents (26–28).
TABLE 1.
NTDs, resorptions, and crown-rump length as a function of diet and maternal genotype1
| Diet and maternal genotype | Litters, n | Implants, n | Embryos, n | NTDs,2 n (%) | Resorptions,3 n (%) | Crown-rump,4 mm |
| C | 21 | 148 | 120 | 0 (0) (FD, U*) | 28 (18.9) (FD, dU*) | 8.13 ± 0.09 (FD*) |
| Shmt1+/+ | 11 | 74 | 67 | 0 (0) | 7 (9.5) | 8.13 ± 0.11 |
| Shmt1−/− | 10 | 74 | 53 | 0 (0) | 21 (28.4) | 8.13 ± 0.15 |
| C+T | 22 | 143 | 120 | 0 (0) | 23 (16.1) | 7.20 ± 0.06 (C*) |
| Shmt1+/+ | 11 | 74 | 60 | 0 (0) | 14 (18.9) | 7.20 ± 0.09 |
| Shmt1−/− | 11 | 69 | 60 | 0 (0) | 9 (13.0) | 7.20 ± 0.08 |
| C+U | 23 | 154 | 119 | 8 (6.7) (C*) | 35 (22.7) | 7.44 ± 0.06 (C*) |
| Shmt1+/+ | 13 | 87 | 69 | 5 (7.3) | 18 (20.7) | 7.47 ± 0.09 |
| Shmt1−/− | 10 | 67 | 50 | 3 (6.00) | 17 (25.4) | 7.39 ± 0.08 |
| C+dU | 21 | 153 | 93 | 0 (0) | 60 (39.2) (C*) | 7.04 ± 0.07 (C*) |
| Shmt1+/+ | 10 | 77 | 39 | 0 (0) | 38 (49.4) | 6.87 ± 0.14 |
| Shmt1−/− | 11 | 76 | 54 | 0 (0) | 22 (29.0) | 7.15 ± 0.08 |
| FD | 21 | 151 | 92 | 8 (8.7) (C, dUFD*) | 61 (40.4) (C*) | 6.78 ± 0.08 (C, dUFD*) |
| Shmt1+/+ | 11 | 80 | 42 | 1 (2.4) | 38 (47.5) | 6.98 ± 0.11 |
| Shmt1−/− | 10 | 71 | 50 | 7 (14.0) | 23 (32.4) | 6.61 ± 0.12 |
| FD+T | 21 | 126 | 81 | 5 (4.0) | 46 (36.5) | 7.19 ± 0.12 |
| Shmt1+/+ | 11 | 67 | 49 | 2 (4.0) | 18 (26.9) | 7.12 ± 0.10 |
| Shmt1−/− | 10 | 59 | 32 | 3 (9.4) | 28 (47.5) | 7.35 ± 0.32 |
| FD+U | 21 | 143 | 101 | 13 (12.9) | 44 (30.8) | 7.43 ± 0.10 |
| Shmt1+/+ | 10 | 73 | 56 | 4 (7.1) | 17 (23.3) | 7.52 ± 0.14 |
| Shmt1−/− | 11 | 70 | 45 | 9 (20.0) | 27 (38.6) | 7.29 ± 0.15 |
| FD+dU | 23 | 149 | 85 | 0 (0) (FD*) | 64 (43.0) | 7.36 ± 0.12 (FD*) |
| Shmt1+/+ | 10 | 66 | 38 | 0 (0) | 28 (42.4) | 7.60 ± 0.14 |
| Shmt1−/− | 13 | 83 | 47 | 0 (0) | 36 (43.4) | 7.18 ± 0.18 |
Frequency of NTDs and resorptions, and crown rump length observed in litters isolated from crosses of Shmt1-deficient mice on a 129/SvEv background on gestational day 11.5. The effects of maternal diet, maternal Shmt1 genotype, and the interaction between diet and maternal genotype on NTDs and resorptions were assessed by using generalized estimating equation models with a binomial distribution and logit-link function, controlling for litter. Models of NTD occurrence also included embryo genotype as an independent variable. A set of a priori comparisons between diets was assessed, and a Bonferroni correction was applied (C+T, C+U, and C+dU vs. C; FD vs. C; FD+T, FD+U, and FD+dU vs. FD). Exact P values were used where needed. A mixed model was used to assess the effects of maternal diet, maternal Shmt1 genotype, and the interaction between diet and maternal genotype on crown-rump length, controlling for a random litter effect. A set of a priori comparisons between diets with a Bonferroni correction was applied. *Significant comparisons are preceded by the diet of comparison, P < 0.05. C, control diet; C+dU, control diet supplemented with 2′-deoxyuridine; C+T, control diet supplemented with thymidine; C+U, control diet supplemented with uridine; FD, AIN-93G lacking folic acid; FD+dU, folate-deficient diet supplemented with 2′-deoxyuridine; FD+T, folate-deficient diet supplemented with thymidine; FD+U, folate-deficient diet supplemented with uridine; NTD, neural tube defect.
NTDs: n = 34/811. There was a significant effect of diet on NTD occurrence (P < 0.0001). The effect of maternal genotype on NTD occurrence (P > 0.05) and the interaction between diet and maternal genotype (P > 0.05) were not significant. *The frequency of NTDs was significantly higher with the FD diet than with the C diet (FD vs. C: 8.70% vs. 0%; unadjusted P = 0.0011, adjusted P = 0.0077). The frequency of NTDs was significantly higher with the C+U diet than with the C diet (C+U vs. C: 6.72% vs. 0%; unadjusted P = 0.0033, adjusted P = 0.0231). The frequency of NTDs was significantly lower with the FD+dU diet than with the FD diet (FD+dU vs. FD: 0% vs. 8.70%; unadjusted P = 0.0069, adjusted P = 0.0483).
Resorptions: n = 361/1167. There was a significant effect of diet on resorption occurrence (P = 0.0004). The effect of maternal genotype on resorption occurrence was not significant (P = 0.33), and the interaction between diet and maternal genotype was significant (P = 0.045). *The frequency of resorptions was significantly higher with the FD diet (FD vs. C: 40.40% vs. 18.92%; unadjusted P = 0.0004, adjusted P = 0.0028) and the dU diet (dU vs. C: 39.22% vs. 18.92%; unadjusted P = 0.0058, adjusted P = 0.0406) than with the C diet.
Values are means ± SDs. Crown-rump length: n = 610. There was a significant effect of diet on crown-rump length (P < 0.0001). The effect of maternal genotype on crown-rump length was not significant (maternal genotype: P = 0.41). The interaction between diet and maternal genotype was not significant (P > 0.05). *The crown-rump length with the FD diet was significantly lower than with the C diet (FD vs. C: −1.38 ± 0.26 mm; unadjusted P < 0.0001, adjusted P < 0.0007). The crown-rump lengths with the C+T, C+U, and C+dU diets were also significantly lower than that with the C diet [C+T vs. C: −0.91 ± 0.25 mm (unadjusted P = 0.0004, adjusted P = 0.0028); C+U vs. C: −0.73 ± 0.25 mm (unadjusted P = 0.0037, adjusted P = 0.0259); C+dU vs. C: −1.11 ± 0.25 mm (unadjusted P < 0.0001, adjusted P < 0.0007)].
Female mice were maintained on one of the 8 diets from weaning throughout the breeding period and gestation until killed. Virgin female mice aged 70–120 d were housed overnight with males. The following morning, females were examined for the presence of a vaginal plug. Gestational day 0.5 (E0.5) was designated at 0900 on the day of the plug appearance. Pregnant females were sacrificed by cervical dislocation at E11.5, and blood was collected by cardiac puncture. Gravid uteri were removed, and all implants and resorption sites were recorded. Embryos were examined for NTDs and morphologic abnormalities at E11.5 and measured for crown-rump length. All yolk sacs were collected for subsequent genotyping. Once analyzed and recorded, embryos were randomly divided into one of 2 groups. One group was fixed in 10% neutral buffered formalin for preservation, and the other group was frozen in liquid nitrogen (and stored at or below −80°C) for biochemical analyses.
Genotype analysis
Genotyping for embryo sex was performed by using established protocols (29–31). Genotyping for Shmt1+/+, Shmt1+/−, and Shmt1−/− alleles was performed by using a previously described protocol (25).
Analysis of plasma folate concentrations
Folate concentrations in plasma samples were quantified using a Lactobacillus casei microbiological assay as previously described (32).
Uracil content in hepatic nuclear DNA and HeLa cells
HeLa cells were maintained and routinely passaged in minimal essential media, α modification (Hyclone) supplemented with 10% fetal bovine serum (FBS; Hyclone). To measure uracil in nuclear DNA, we plated cells in triplicate in 150-mmol/L plates and allowed them to grow for 4 doublings in defined minimal essential medium (DMEM, formulated without glycine, serine, methionine, pyridoxine, folic acid, and all nucleosides/nucleotides; Hyclone) supplemented with 10% dialyzed FBS and 200 μmol/L methionine and 1 mg/L pyridoxine. DMEM was also supplemented with 50 μmol/L uridine or 50 μmol/L deoxyuridine, both with and without 1 mg/L folic acid. DNA was extracted from either HeLa cell pellets or 25–50 mg flash-frozen maternal liver tissue by using a DNeasy Kit (Qiagen), including an incubation with RNase A (Sigma) and RNase T1 (Ambion) for 30 min at 37°C, and then treated with 1 U uracil DNA glycosylase (Epicentre) for 1 h at 37°C. Immediately following incubation, 10 pg [15N2]-uracil (Cambridge Isotopes) was added to each sample as an internal standard, and the sample was dried completely in a desiccator. Then, 50 μL acetonitrile, 10 μL triethylamine, and 1 μL 3,5-bis(trifluormethyl)benzyl bromide were added to each sample and incubated for 25 min at 30°C with shaking at 500 rpm. Next, 50 μL water, followed by 100 μL isooctane, was added to each sample. Following extraction of the organic phase, uracil content in nuclear DNA was analyzed by gas chromatography–mass spectrometry, as previously described (25).
Determination of plasma uridine, deoxyuridine, and thymidine
Plasma from mice was collected, flash frozen, and stored at or below −80°C until analyses were conducted by HPLC with ultraviolet detection. Then, 50 μL plasma was diluted with an equal volume of 50 mmol/L ammonium acetate (pH 5.6) and spiked with 10 μmol/L 5-fluoruridine as an internal standard. The diluted plasma was clarified by using an Amicon Ultra centrifugal filter with a molecular weight cutoff of 3000 kDa and centrifuged at 14,000 × g for 30 min at 4°C. The flowthrough was collected, and 20 μL was injected into a Supelco Supelcosil LC 18-T 25 cm × 4.6–mm 5-μm column by using a binary buffer system at 1 mL/min. Buffer A consisted of 100 mmol/L ammonium acetate (pH 5.6), and buffer B was 100 mmol/L ammonium acetate and 20% methanol. The nucleosides were eluted with a linear gradient from 1 to 30 min starting with 0% buffer B to 75% at 30 min, followed by a linear gradient from 30 to 35 min, decreasing buffer B from 75% to 0%. Uridine, deoxyuridine, and thymidine concentrations were quantified by using a Shimadzu diode array detector, with a starting wavelength of 240 nm and ending at 300 nm, and analyzed by using a 5-point standard curve for each analyte. All values were corrected for sample loss by using the internal standard.
Pyrimidine incorporation assay
HeLa cells were maintained and routinely passaged in minimal essential media, α modification (Hyclone) supplemented with 10% FBS (Hyclone). Mouse embryonic fibroblast cells were generated from embryos isolated 10–14 d after coitus from Shmt1+/− intercrosses and were maintained and routinely passaged in minimal essential media, α modification (Hyclone) supplemented with 10% FBS (Hyclone). During the pyrimidine incorporation assay, cells were plated in triplicate in 100-mm plates and allowed to grow for 4 doublings in DMEM (formulated without glycine, serine, methionine, pyridoxine, folic acid, and all nucleosides/nucleotides; Hyclone) supplemented with 10% dialyzed FBS and 200 μmol/L methionine, 1 mg/L pyridoxine, 2.35 nM [2,8-3H]-2′-deoxyadenosine (Moravek), and either 33 nM [5-3H]-uridine (American Radiochemicals) or 33 nM [5-3H]-2′-deoxyuridine (American Radiochemicals). Nuclear DNA was isolated by using a DNeasy kit (Qiagen) with RNase A treatment per the manufacturer’s instructions. DNA was digested to nucleosides, as previously described (33), and 25 μL was injected into a Phenomenex Synergi 4-μm Fusion-RP 80A 150 × 4.6–mm column by using a binary buffer system with a flow rate of 1 mL/min. Buffer A consisted of 20 mmol/L ammonium acetate (pH 4.5), and buffer B was acetonitrile. Buffer B concentration increased from 5% to 11% over the 0- to 4-min runtime, to 11–13% between 4 and 8.5 min, and finally to 35% between 8.5 and 10 min. Nucleotides were detected by using a Shimadzu SPD-M20A diode array detector, monitoring wavelengths from 220 to 300 nm. Then, 100-μL fractions were collected by using a Shimadzu FRC-10A fraction collector; elution times were verified by using tritiated nucleoside standards. Fractions were mixed with 4 mL Ecoscint (National Diagnostics) scintillation fluid and radioactivity quantified on a Beckman LS-6500 liquid scintillation counter. Data are shown of the ratio of the 3H counts per minute in the dT fraction to the 3H counts per minute in the dA fraction, and the means and SDs of 3 biological replicates are presented.
Statistical analyses
The effects of maternal diet, maternal Shmt1 genotype, and the interaction between diet and maternal genotype on the incidence of NTDs and resorptions were assessed by using generalized estimating equation models with a binomial distribution and logit-link function, controlling for litter. Models of NTD occurrence included embryo genotype as an independent variable. The effect of embryonic Shmt1 genotype was evaluated by using an exact test. Mixed models were used to evaluate the effects of maternal diet, maternal Shmt1 genotype, and the interaction between diet and maternal genotype on embryonic crown-rump length. General linear models were used to model the effects of maternal diet, maternal Shmt1 genotype, and the interaction between diet and maternal genotype on plasma folate, uridine, thymidine, and deoxyuridine and hepatic uracil concentrations. A set of a priori comparisons between diets was assessed (C+T, C+U, and C+dU vs. C; FD vs. C; FD+T, FD+U, and FD+dU vs. FD), and a Bonferroni correction for multiple comparisons was applied (Supplemental Table 1). The χ2 test was used to evaluate potential deviation from Mendelian ratios for embryo genotype. For HeLa cell analyses, statistical significance was determined by using a Student’s t test with a Bonferroni correction for multiple comparisons. Statistical analyses were performed with SAS software, version 9.4 (SAS Institute).
RESULTS
Dietary nucleosides modify risk of folic acid–responsive NTDs
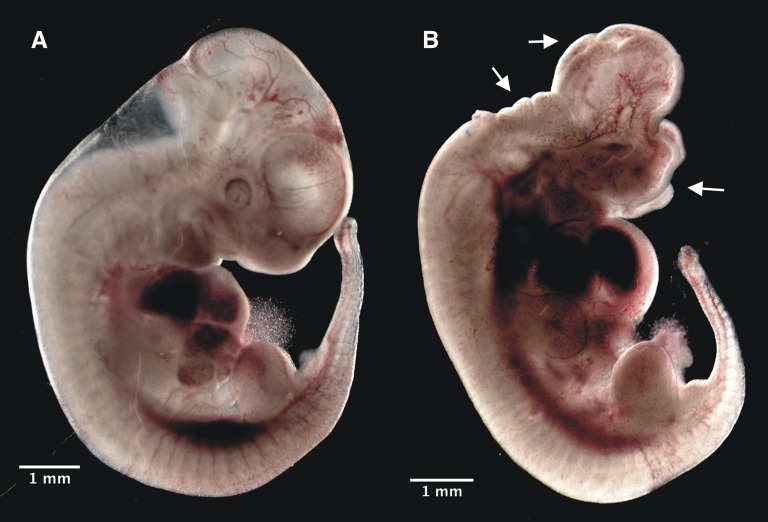
On the basis of knowledge of the de novo and salvage thymidylate synthesis pathways, we proposed and tested a priori hypotheses regarding the impact of maternal dietary nucleotide supplementation on NTD incidence (Supplemental Table 1). Maternal diet significantly influenced NTD incidence (P < 0.0001) (Table 1). As we have previously reported, the frequency of NTDs was significantly higher in dams fed the FD diet compared with dams fed the C diet (FD vs. C: 8.7% vs. 0%; unadjusted P = 0.001, adjusted P = 0.0077). All observed NTDs were exencephaly (Figure 2). In control groups, there were no significant differences in NTD incidence between dams fed the C+dU diet (C+dU vs. C: 0% vs. 0%) or T diet (C+T vs. C: 0% vs. 0%) compared with the C diet. However, the incidence of NTDs was significantly higher in dams fed the C+U diet (C+U vs. C: 6.7% vs. 0%; unadjusted P = 0.0033, adjusted P = 0.0231). Unexpectedly, NTDs were observed in Shmt1+/+ embryos from dams fed the C+U diet (Table 2).
FIGURE 2.
Neural tube defects in Shmt1-deficient embryos at gestational day 11.5 (E11.5). (A) Representative unaffected embryo. (B) Shmt1+/− embryo from a dam fed a folate-deficient diet supplemented with thymidine diet exhibited failure of rostral neural tube closure beyond the hindbrain boundary. Scale bars indicate 1 mm. Arrows indicate extent of lesions.
TABLE 2.
Frequency of NTDs as a function of embryonic Shmt1 genotype1
| Embryo Shmt1+/+ |
Embryo Shmt1+/− |
Embryo Shmt1−/− |
||||
| Diet and maternal genotype | NTDs, n (%) | Embryos, n | NTDs, n (%) | Embryos, n | NTDs, n (%) | Embryos, n |
| C | 0 (0) | 31 | 0 (0) | 62 | 0 (0) | 27 |
| Shmt1+/+ | 0 (0) | 31 | 0 (0) | 36 | — | — |
| Shmt1−/− | — | — | 0 (0) | 26 | 0 (0) | 27 |
| C+T | 0 (0) | 25 | 0 (0) | 63 | 0 (0) | 32 |
| Shmt1+/+ | 0 (0) | 25 | 0 (0) | 35 | — | — |
| Shmt1−/− | — | — | 0 (0) | 28 | 0 (0) | 32 |
| C+U | 1 (2.9) | 35 | 6 (10.3) | 58 | 1 (3.9) | 26 |
| Shmt1+/+ | 1 (2.9) | 35 | 4 (11.8) | 34 | — | — |
| Shmt1−/− | — | — | 2 (8.3) | 24 | 1 (3.9) | 26 |
| C+dU | 0 (0) | 16 | 0 (0) | 50 | 0 (0) | 27 |
| Shmt1+/+ | 0 (0) | 16 | 0 (0) | 23 | — | — |
| Shmt1−/− | — | — | 0 (0) | 27 | 0 (0) | 27 |
| FD | 0 (0) | 22 | 6 (12.2) | 49 | 2 (9.5) | 21 |
| Shmt1+/+ | 0 (0) | 22 | 1 (5.0) | 20 | — | — |
| Shmt1−/− | — | — | 5 (17.2) | 29 | 2 (9.5) | 21 |
| FD+T | 1 (4.6) | 22 | 2 (4.4) | 45 | 2 (14.3) | 14 |
| Shmt1+/+ | 1 (4.6) | 22 | 1 (3.7) | 27 | — | — |
| Shmt1−/− | — | — | 1 (5.6) | 18 | 2 (14.3) | 14 |
| FD+U | 2 (8.0) | 25 | 5 (8.9) | 56 | 6 (30.0) | 20 |
| Shmt1+/+ | 2 (8.0) | 25 | 2 (6.5) | 31 | — | — |
| Shmt1−/− | — | — | 3 (12.0) | 25 | 6 (30.0) | 20 |
| FD+dU | 0 (0) | 17 | 0 (0) | 50 | 0 (0) | 18 |
| Shmt1+/+ | 0 (0) | 17 | 0 (0) | 21 | — | — |
| Shmt1−/− | — | — | 0 (0) | 29 | 0 (0) | 18 |
Frequency of NTDs observed in litters isolated from crosses of Shmt1-deficient mice on a 129/SvEv background on gestational day 11.5. The effect of embryo genotype on NTD occurrence was not significant (P > 0.05). A set of a priori hypotheses between diets (C+T, C+U, and C+dU vs. C; FD vs. C; FD+T, FD+U, and FD+dU vs. FD) within each embryo genotype was tested by using exact tests. A Bonferroni correction was applied as a correction for multiple comparisons. C, control diet; C+dU, control diet supplemented with 2′-deoxyuridine; C+T, control diet supplemented with thymidine; C+U, control diet supplemented with uridine; FD, AIN-93G lacking folic acid; FD+dU, folate-deficient diet supplemented with 2′-deoxyuridine; FD+T, folate-deficient diet supplemented with thymidine; FD+U, folate-deficient diet supplemented with uridine; NTD, neural tube defect; —, embryonic genotype not observed from these dams.
In folate-deficient groups, there were no NTDs observed in the FD+dU diet (FD+dU vs. FD: 0.0% vs. 8.7%; unadjusted P = 0.0069, adjusted P = 0.0483). There were no significant differences in NTD incidence observed in dams fed either the FD+U diet (FD+U vs. FD: 12.9% vs. 8.7%; P > 0.05) or the FD+T diet (FD+T vs. FD: 4.0% vs. 8.7%; P > 0.05) compared with the FD diet. Similarly, the interaction between diet and maternal genotype (P > 0.05) was not significant. The number of observed Shmt1+/+, Shmt1+/−, and Shmt1−/− embryos at E11.5 did not deviate from expected Mendelian inheritance for all crosses examined.
In summary, folate deficiency and uridine supplementation caused NTDs. Maternal thymidine supplementation did not affect NTD incidence. No NTDs were observed in the dU-supplemented groups (i.e., C+dU or FD+dU), and FD+dU was the only folate-deficient group without NTDs.
Crown-rump length and resorptions
There was a significant effect of diet on crown-rump length (P < 0.0001) (Tables 1 and 2). The effect of maternal genotype on crown-rump length was not significant (P = 0.41), and the interaction between diet and maternal genotype was not significant (P > 0.05). Embryos isolated from dams fed the FD diet were significantly smaller than embryos from dams fed the C diet (FD vs. C: −1.38 ± 0.26 mm; unadjusted P < 0.0001, adjusted P < 0.0007). Embryos from dams fed the C+T, C+U, and C+dU diets also had significantly smaller crown-rump length compared with the C diet [C+T vs. C: −0.91 ± 0.25 mm (unadjusted P = 0.0004, adjusted P = 0.0028); C+U vs. C: −0.73 ± 0.25 mm (unadjusted P = 0.0037, adjusted P = 0.0259); C+dU vs. C: −1.11 ± 0.25 mm (unadjusted P < 0.0001, adjusted P < 0.0007)]. Neither weight of dams nor the number of implantation sites was significantly affected by diet or genotype. However, there was a significant effect of diet on resorption occurrence (P = 0.0004). The frequency of resorptions was significantly higher in the FD diet (FD vs. C: 40.40% vs. 18.92%; unadjusted P = 0.0004, adjusted P = 0.0028) and the C+dU diet (dU vs. C: 39.22% vs. 18.92%; unadjusted P = 0.0058, adjusted P = 0.0406) compared with the C diet.
Effects of diet on plasma nucleoside and folate concentrations
The effects of dietary folic acid and nucleosides on plasma folate, uridine, deoxyuridine, and thymidine concentrations are presented in Table 3.
TABLE 3.
Effects of diet on folate, uracil, uridine, thymidine, and deoxyuridine concentrations by maternal genotype1
| Diet and maternal genotype | Folate,2 fmol/μL | Thymidine,3 μmol/L | Uridine,4 μmol/L | Deoxyuridine,5 μmol/L | Uracil,6 pg/μg DNA |
| C | 165.12 ± 12.30 (FD*) | 1.28 ± 0.23 | 8.17 ± 1.24 | 1.55 ± 0.16 | 0.23 ± 0.03 |
| Shmt1+/+ | 170.79 ± 9.32 | 1.43 ± 0.31 | 7.87 ± 1.60 | 1.60 ± 0.22 | 0.23 ± 0.04 |
| Shmt1−/− | 159.45 ± 24.03 | 1.03 ± 0.33 | 8.58 ± 2.11 | 1.45 ± 0.12 | 0.24 ± 0.03 |
| C+T | 260.07 ± 26.54 (C*) | 1.02 ± 0.10 | 9.88 ± 1.25 | 2.10 ± 0.09 | 0.21 ± 0.02 |
| Shmt1+/+ | 263.51 ± 50.54 | 1.25 ± 0.14 | 11.35 ± 1.39 | 1.97 ± 0.13 | 0.21 ± 0.02 |
| Shmt1−/− | 258.01 ± 34.69 | 0.82 ± 0.07 | 8.13 ± 2.09 | 2.22 ± 0.12 | 0.21 ± 0.03 |
| C+U | 163.25 ± 19.86 | 1.01 ± 0.09 | 5.70 ± 0.54 | 1.51 ± 0.12 | 0.18 ± 0.01 |
| Shmt1+/+ | 180.48 ± 27.74 | 0.78 ± 0.02 | 5.56 ± 0.87 | 1.54 ± 0.12 | 0.17 ± 0.01 |
| Shmt1−/− | 146.02 ± 29.27 | 1.24 ± 0.08 | 5.81 ± 0.74 | 1.48 ± 0.22 | 0.20 ± 0.03 |
| C+dU | 261.21 ± 19.93 (C*) | 1.12 ± 0.11 | 5.27 ± 1.02 | 2.55 ± 0.32 | 0.19 ± 0.01 |
| Shmt1+/+ | 272.29 ± 27.65 | 1.13 ± 0.15 | 5.91 ± 1.49 | 2.10 ± 0.22 | 0.18 ± 0.01 |
| Shmt1−/− | 253.30 ± 29.31 | 1.09 ± 0.08 | 3.98 ± 0.65 | 3.45 ± 0.68 (C*) | 0.19 ± 0.02 |
| FD | <10 (C*) | 0.89 ± 0.12 | 4.86 ± 0.32 | 1.66 ± 0.09 | 0.20 ± 0.03 |
| Shmt1+/+ | <10 | 0.73 ± 0.06 | 4.87 ± 0.38 | 1.54 ± 0.12 | 0.18 ± 0.02 |
| Shmt1−/− | <10 | 1.04 ± 0.24 | 4.84 ± 0.58 | 1.77 ± 0.12 | 0.24 ± 0.07 |
| FD+T | <10 | 1.36 ± 0.44 | 10.42 ± 1.79 (*FD) | 1.75 ± 0.25 | 0.20 ± 0.02 |
| Shmt1+/+ | <10 | 0.79 ± 0.16 | 11.20 ± 3.03 | 1.67 ± 0.58 | 0.16 ± 0.01 |
| Shmt1−/− | <10 | 1.82 ± 0.75 | 9.80 ± 2.42 | 1.82 ± 0.17 | 0.26 ± 0.02 |
| FD+U | <10 | 0.85 ± 0.05 | 8.82 ± 1.59 | 1.52 ± 0.16 | 0.22 ± 0.02 |
| Shmt1+/+ | <10 | 0.85 ± 0.06 | 13.22 ± 2.36 | 1.89 ± 0.27 | 0.20 ± 0.03 |
| Shmt1−/− | <10 | 0.85 ± 0.08 | 5.52 ± 1.26 | 1.24 ± 0.15 | 0.23 ± 0.02 |
| FD+dU | <10 | 1.10 ± 0.16 | 8.61 ± 0.92 | 1.95 ± 0.19 | 0.19 ± 0.01 |
| Shmt1+/+ | <10 | 1.15 ± 0.13 | 8.69 ± 1.39 | 1.62 ± 0.23 | 0.17 ± 0.01 |
| Shmt1−/− | <10 | 1.07 ± 0.28 | 8.53 ± 1.33 | 2.24 ± 0.27 | 0.22 ± 0.01 |
Values are means ± SEs from univariate descriptive statistics. General linear models were used to examine the effect of maternal diet, maternal Shmt1 genotype, and the interaction between diet and maternal genotype on folate, uracil, uridine, thymidine, and deoxyuridine concentrations. A set of a priori comparisons between diets was assessed (C+T, C+U, and C+dU vs. C; FD vs. C; FD+T, FD+U, and FD+dU vs. FD) for folate, uracil, uridine, and thymidine concentrations, and a Bonferroni correction was applied. *Significant comparisons are preceded by the diet of comparison, P < 0.05. C, control diet; C+dU, control diet supplemented with 2′-deoxyuridine; C+T, control diet supplemented with thymidine; C+U, control diet supplemented with uridine; FD, AIN-93G lacking folic acid; FD+dU, folate-deficient diet supplemented with 2′-deoxyuridine; FD+T, folate-deficient diet supplemented with thymidine; FD+U, folate-deficient diet supplemented with uridine.
There was a significant effect of diet on folate concentrations (P < 0.0001). The effect of maternal genotype (P = 0.3622) on folate concentrations and the interaction between diet and maternal genotype (P > 0.05) were not significant. All folate-deficient diets had folate concentrations <10 fmol/μL. *The FD diet had significantly lower folate concentrations than the C diet (unadjusted P < 0.0001, adjusted P < 0.0007). The C+T diet and the C+dU diet had significantly higher folate concentrations than the C diet (C+T vs. C: unadjusted P < 0.0001, adjusted P < 0.0007; C+dU vs. C: unadjusted P < 0.0001, adjusted P < 0.0007).
There were no significant effects of diet (P > 0.05) or maternal genotype (P > 0.05) on plasma thymidine concentrations. The interaction between diet and maternal genotype was not significant (P > 0.05).
There was a significant effect of diet (P = 0.0022) and maternal genotype (P = 0.0397) on plasma uridine concentration. The interaction between diet and maternal genotype was not significant (P > 0.05). *The FD+T diet had significantly higher uridine concentrations than the FD diet (unadjusted P = 0.0014, adjusted P = 0.0098). The FD+U and FD+dU diets also had higher uridine concentrations than the FD diet, although this was not statistically significant after adjusting for multiple comparisons (FD+U vs. FD: unadjusted P = 0.0085, adjusted P = 0.0595; FD+dU vs. FD: unadjusted P = 0.0124, adjusted P > 0.05).
There was a significant effect of diet (P < 0.0001) on deoxyuridine concentrations. There was no significant effect of maternal genotype on deoxyuridine concentrations (P = 0.0836). However, the interaction between diet and maternal genotype was significant [F(7, 72) = 2.88; P = 0.0096]. *The C+dU diet had significantly higher deoxyuridine concentrations than the C diet in the Shmt1−/− maternal genotype (unadjusted P < 0.0001, adjusted P < 0.0007), although there were no significant differences in the Shmt1+/+ maternal genotype (unadjusted P = 0.0954, adjusted P > 0.05).
There was no significant effect of diet on uracil concentrations (P = 0.6). There was a significant effect of maternal genotype on uracil concentrations (P = 0.0135). The interaction between diet and maternal genotype was not significant (P > 0.05).
Folate
The effect of maternal diet on folate concentrations was significant (P < 0.0001). All folate-deficient diets had plasma folate concentrations less than 10 fmol/μL. The FD group had significantly lower folate concentrations compared with the C diet (unadjusted P < 0.0001, adjusted P < 0.0007). Dams fed the C+T diet and C+dU diet had significantly higher plasma folate concentrations compared with dams fed the C diet (C+T vs. C: unadjusted P < 0.0001, adjusted P < 0.0007; C+dU vs. C: unadjusted P < 0.0001, adjusted P < 0.0007). Neither the effect of maternal genotype on plasma folate concentrations (P > 0.05) nor the interaction between diet and maternal genotype (P > 0.05) was significant.
Thymidine
There were no significant effects of diet (P > 0.05) or maternal genotype (P > 0.05) on plasma thymidine concentrations, and the interaction between diet and maternal genotype was not significant (P > 0.05).
Uridine
Maternal diet had a significant effect on plasma uridine concentrations (P = 0.0022). The FD+T group had significantly higher uridine concentrations compared with dams fed the FD diet (unadjusted P = 0.0014; adjusted P = 0.0098). Dams fed the FD+U and FD+dU diets also had higher uridine concentrations vs. the FD diet, although this was not statistically significant after adjusting for multiple comparisons (FD+U vs. FD: unadjusted P = 0.0085, adjusted P = 0.0595; FD+dU vs. FD: unadjusted P = 0.0124, adjusted P > 0.05). There was a significant effect of maternal genotype (P = 0.0397) on uridine concentrations: Shmt1+/+ dams had higher uridine concentrations than Shmt1−/− dams. The interaction between diet and maternal genotype was not significant (P > 0.05).
Deoxyuridine
There was a significant effect of maternal diet on plasma deoxyuridine concentrations (P < 0.0001). There was no significant effect of maternal genotype on deoxyuridine concentrations (P = 0.0836). Plasma deoxyuridine concentrations were invariant for both Shmt1−/− and Shmt1+/+ dams fed the C and FD diets. However, there was a significant interaction between diet and maternal genotype on deoxyuridine concentrations (P = 0.0096). The C+dU group had significantly higher deoxyuridine concentrations compared with the C diet in Shmt1−/− dams (C+dU vs. C: unadjusted P < 0.0001, adjusted P < 0.0007), although there were no significant differences in Shmt1+/+ dams (C+dU vs. C: unadjusted P = 0.0954, adjusted P > 0.05).
Maternal diet did not increase hepatic uracil concentrations
The effects of maternal dietary folic acid and nucleosides in maternal liver uracil in nuclear DNA are presented in Table 3. There was no significant effect of diet on uracil concentrations (P = 0.6) (i.e., neither dietary folic acid nor nucleosides affected maternal uracil concentrations in liver nuclear DNA after consuming the diets for at least 8 wk). The effect of maternal genotype on uracil concentrations was significant: Shmt1−/− dams had higher hepatic uracil concentrations compared with Shmt1+/+ dams (P = 0.0135). The interaction between diet and maternal genotype was not significant (P > 0.05).
Deoxyuridine is incorporated into DNA as thymidine
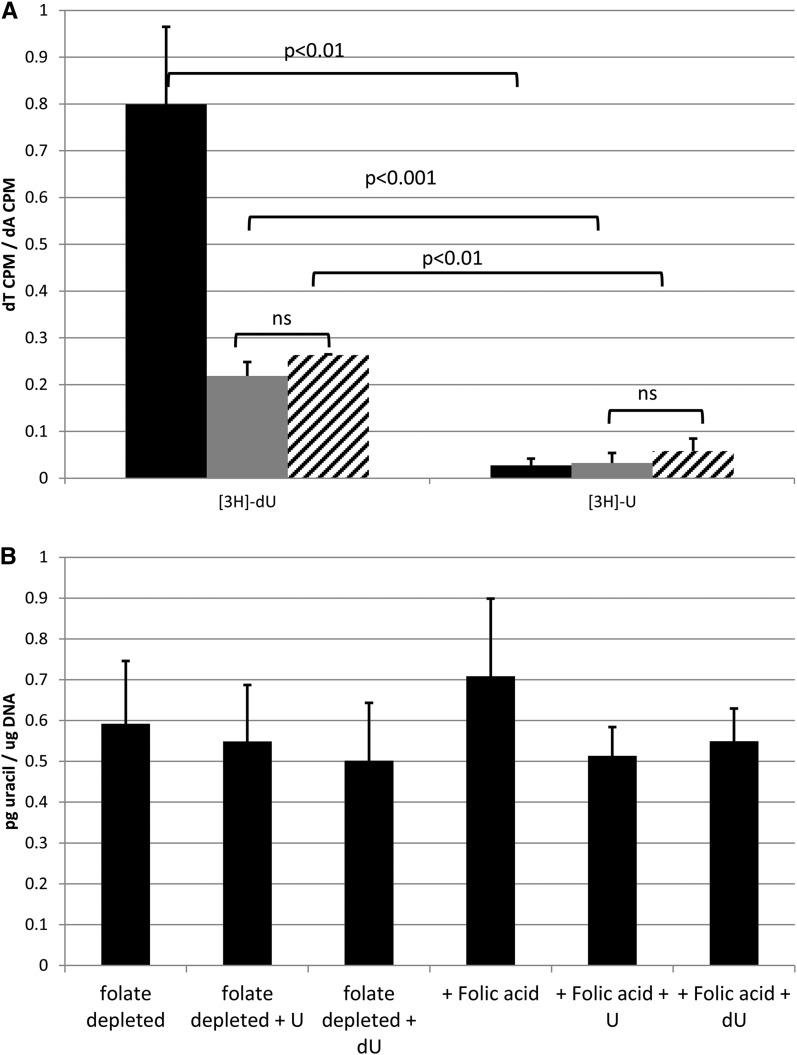
In an in vitro assay to assess differences between incorporation of exogenous U and dU into DNA as the dT base, nearly 30-fold more dU than U was incorporated into DNA as dT (Figure 3A). This suggests that in some cell types (including HeLa cells and mouse embryonic fibroblasts), exogenous dU (compared with exogenous U) is preferentially incorporated into DNA as dT and may not contribute to excess uracil in DNA. Neither exogenous dU nor U resulted in increased uracil in HeLa cell nuclear DNA (Figure 3B), independent of folic acid supplementation.
FIGURE 3.
Deoxyuridine is incorporated into DNA as dT in HeLa and mouse embryonic fibroblast nuclear DNA and does not increase uracil in DNA. (A) HeLa (black bars, n = 3) or mouse embryonic fibroblast (gray bars, Shmt1+/+, n = 3; hatched bars, Shmt1+/−, n = 3) cells were grown for 4 doublings in DMEM supplemented with 2.35 nM [2,8-3H]-2′-dA and either 33 nM [5-3H]-U or 33 nM [5-3H]-2′-dU. DNA was digested to nucleosides, which were then separated via HPLC. Radioactivity from dT and dA fractions were quantified by using a Beckman LS6500 scintillation counter and shown as the ratio of [3H]-dT to [3H]-dA for each treatment. Data are shown as means ± SDs of n = 3 biological replicates, with significance determined by using a Student’s t test with a Bonferroni correction. (B) HeLa cells (n = 3 per condition) were grown for 4 doublings in folate-deficient DMEM supplemented with either 50 μM U or 50 μM dU both with and without 1 mg/L folic acid. DNA was isolated with a DNeasy kit (Qiagen), uracil was excised by using uracil glycosylase, and uracil was measured with gas chromatography–mass spectrometry. Data are shown as means ± SDs of n = 3 biological replicates, with significance determined by using a Student’s t test with a Bonferroni correction. There were no significant differences between the diets. CPM, counts per minute; dA, deoxyadenosine; DMEM, defined minimal essential medium; dT, thymidine; dU, deoxyuridine; U, uridine.
DISCUSSION
Decreased Shmt1 expression impairs de novo dTMP biosynthesis and causes NTDs in response to maternal folate deficiency (20, 21), indicating that impaired de novo dTMP biosynthesis impairs neural tube closure in this mouse model. Therefore, it was hypothesized that administering diets enriched with particular nucleotides in the dTMP synthesis pathway may rescue or exacerbate NTDs and provide new insights into the pathways underlying folate-responsive NTDs. In this study, we investigated the effects of dietary supplemental thymidine, deoxyuridine, and uridine with and without folate deficiency on NTD incidence in Shmt1−/− and Shmt1+/+ dams. The strengths of this study are 1) the use of the Shmt1−/− mouse, which models NTDs in humans in that the NTDs in this model are sporadic and folic acid responsive, and 2) the ability to perform rationally designed dietary manipulations in the dams to elucidate mechanisms and pathways of NTD pathogenesis.
As demonstrated previously, exencephaly was observed in litters from dams maintained on the FD diet but not on the C diet, confirming our previous studies that both maternal folate deficiency and embryonic Shmt1 disruption were required for NTD pathogenesis (20, 34). The results of this study demonstrate that maternal uridine supplementation of the AIN-93G diet caused NTDs independent of fetal Shmt1 genotype and maternal folate status. We also observed that maternal deoxyuridine supplementation prevented NTD occurrence in Shmt1+/− and Shmt1−/− embryos in dams fed the dU+FD diet. In addition, thymidine supplementation had no statistically significant effect on NTD incidence.
The current literature regarding the role of exogenous thymidine in NTD prevention is mixed. One study reported that NTDs in the Splotch mutant could be rescued with thymidine, indicating that folic acid prevents NTDs by rescuing de novo dTMP synthesis in that mouse model, but this finding was not confirmed in another study (18, 19). Another study indicated that de novo purine biosynthesis was impaired to a greater degree than de novo dTMP biosynthesis in the Splotch mutant (20). In this study, maternal thymidine supplementation did not prevent NTDs in embryos from folate-deficient Shmt1+/+ or Shmt1−/− dams. Interestingly, we observed one Shmt1+/+ embryo with exencephaly from a Shmt1+/+ dam fed the FD+T diet. In our previous studies of Shmt1+/− and Shmt1−/− dams fed the FD diet, no NTD-affected Shmt1+/+ embryos were observed (6, 20). We conclude that thymidine is not a maternally derived factor that prevents NTDs in SHMT1-deficient mice and likely increases risk for NTDs.
Surprisingly, supplemental deoxyuridine prevented NTDs in Shmt1−/− and Shmt1+/− embryos from folate-deficient dams. We had initially predicted that dU supplementation would increase, not prevent, NTDs in Shmt1−/− and Shmt1+/− embryos from folate-deficient dams because cellular dUMP accumulation has been proposed to enhance uracil misincorporation into DNA and lead to genome instability (35). However, exogenous [3H]-dU, but not [3H]-U, is robustly incorporated into HeLa cell nuclear DNA as the base dT. Furthermore, dU exposure did not increase uracil in HeLa cell nuclear DNA. This observation does not support the current dogma that uracil misincorporation results simply from an increased dUTP/dTTP ratio and suggests a possible mechanism whereby dU may act to stabilize DNA integrity and prevent NTDs in this mouse model, which is susceptible to increased uracil in DNA. These data suggest that dietary dU rescues NTDs by increasing rates of de novo dTMP synthesis in SHMT1-deficient embryos by mass action through the provision of increased concentrations of dUMP substrate without increasing rates of dU misincorporation into DNA. The mechanisms behind the increased resorption rate in embryos from dU-supplemented dams remain unknown. This increase may not be related to dietary folate or folate-sensitive pathologies, as observed in dams fed both the C+dU and FD diets, compared with the C diet.
Unexpectedly, maternal uridine supplementation caused NTDs independent of maternal folate status and Shmt1 genotype. Exencephaly was observed in litters from both Shmt1−/− and Shmt1+/+ dams fed the U diet. Uridine supplementation caused significantly higher NTD incidence compared with the C diet. The mechanism underlying the role of maternal dietary uridine and NTD risk remains to be established.
It is not clear why plasma nucleoside concentrations do not reflect dietary intake, but it may be because of low nucleoside bioavailability or rapid uptake by tissues. Nonetheless, the results provide additional evidence that the de novo dTMP synthesis pathway underlies NTD pathogenesis in this mouse model. Interestingly, the results also indicate that the dietary nucleosides T and dU have a marked effect on serum folate concentrations in dams fed the folate-replete diets, indicating that these nucleosides may influence whole-body folate homeostasis. Clearly, the inability to understand the metabolic outcomes in individual NTD-affected embryos is a limitation of this study. Developing more sensitive assays to assess uracil in DNA and DNA damage in embryos is the focus of ongoing work.
The most pronounced metabolic phenotype in SHMT1-deficient mice is impaired de novo dTMP biosynthesis and elevated uracil in DNA (20, 36). This study confirms our previous reports that SHMT1 deficiency elevates uracil concentrations in DNA. Although nucleoside supplementation did affect NTD incidence, it did not influence uracil concentrations in maternal liver nuclear DNA. This suggests that uridine supplementation does not cause NTDs by elevating uracil concentrations in DNA, assuming that the fetal neural epithelium and maternal liver respond similarly. Although additional studies are required to establish the mechanism that accounts for uridine teratogenicity, women of reproductive age should avoid over-the-counter uridine dietary supplements until more information is known about their effects on the developing embryo.
Supplementary Material
Acknowledgments
The authors thank Sylvia Allen for technical assistance and especially thank Ashley M Palmer and Anna E Beaudin for thoughtful insight and Françoise Vermeylen for assistance with statistical analysis.
The authors’ responsibilities were as follows—LM: data collection and analysis, manuscript preparation, editing, and revision; MSF: study conception, data collection and analysis, and manuscript editing; JLF: statistical analysis; CAP: data collection; PJS: study conception and design and manuscript editing and revision. The authors declared no conflicts of interest related to this study.
Footnotes
Abbreviations used: C, AIN-93G control diet; DMEM, defined minimal essential medium; dTMP, thymidylate; dU, deoxyuridine; FBS, fetal bovine serum; FD, AIN-93G diet lacking folic acid; NTD, neural tube closure defect; Shmt1, serine hydroxymethyltransferase gene; T, thymidine; U, uridine.
REFERENCES
- 1.Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 1993;86:703–8. [PubMed] [Google Scholar]
- 2.Etheredge AJ, Finnell RH, Carmichael SL, Lammer EJ, Zhu H, Mitchell LE, Shaw GM. Maternal and infant gene-folate interactions and the risk of neural tube defects. Am J Med Genet A 2012;158A:2439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pangilinan F, Molloy AM, Mills JL, Troendle JF, Parle-McDermott A, Signore C, O'Leary VB, Chines P, Seay JM, Geiler-Samerotte K, et al. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC Med Genet 2012;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Relton CL, Wilding CS, Laffling AJ, Jonas PA, Burgess T, Binks K, Tawn EJ, Burn J. Low erythrocyte folate status and polymorphic variation in folate-related genes are associated with risk of neural tube defect pregnancy. Mol Genet Metab 2004;81:273–81. [DOI] [PubMed] [Google Scholar]
- 5.Christensen B, Arbour L, Tran P, Leclerc D, Sabbaghian N, Platt R, Gilfix BM, Rosenblatt DS, Gravel RA, Forbes P, et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 1999;84:151–7. [DOI] [PubMed] [Google Scholar]
- 6.Beaudin AE, Stover PJ. Insights into metabolic mechanisms underlying folate-responsive neural tube defects: a minireview. Birth Defects Res A Clin Mol Teratol 2009;85:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991;338:131–7. [PubMed] [Google Scholar]
- 8.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 10.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 1999;341:1485–90. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt RV. Environmental influence on reproductive health. Int J Gynaecol Obstet 2000;70:69–75. [DOI] [PubMed] [Google Scholar]
- 12.Hutz RJ, Carvan MJ, Baldridge MG, Conley LK, Heiden TK. Environmental toxicants and effects on female reproductive function. Trends Reprod Biol 2006;2:1–11. [DOI] [PMC free article] [PubMed]
- 13.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008;1:170–8. [PMC free article] [PubMed]
- 14.Hendricks KA, Nuno OM, Suarez L, Larsen R. Effects of hyperinsulinemia and obesity on risk of neural tube defects among Mexican Americans. Epidemiology 2001;12:630–5. [DOI] [PubMed] [Google Scholar]
- 15.Stover PJ, Garza C. Bringing individuality to public health recommendations. J Nutr 2002;132(Suppl):2476S–80S. [DOI] [PubMed] [Google Scholar]
- 16.Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev 2004;62(Pt 2):S3–12; discussion S3. [DOI] [PubMed]
- 17.Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol 2007;79:187–210. [DOI] [PubMed] [Google Scholar]
- 18.Wlodarczyk BJ, Tang LS, Triplett A, Aleman F, Finnell RH. Spontaneous neural tube defects in splotch mice supplemented with selected micronutrients. Toxicol Appl Pharmacol 2006;213:55–63. [DOI] [PubMed] [Google Scholar]
- 19.Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science 1998;280:2107–9. [DOI] [PubMed] [Google Scholar]
- 20.Beaudin AE, Abarinov EV, Noden DM, Perry CA, Chu S, Stabler SP, Allen RH, Stover PJ. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr 2011;93:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaudin AE, Abarinov EV, Malysheva O, Perry CA, Caudill M, Stover PJ. Dietary folate, but not choline, modifies neural tube defect risk in Shmt1 knockout mice. Am J Clin Nutr 2012;95:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbig K, Chiang EP, Lee LR, Hills J, Shane B, Stover PJ. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem 2002;277:38381–9. [DOI] [PubMed] [Google Scholar]
- 23.Woeller CF, Anderson DD, Szebenyi DM, Stover PJ. Evidence for small ubiquitin-like modifier-dependent nuclear import of the thymidylate biosynthesis pathway. J Biol Chem 2007;282:17623–31. [DOI] [PubMed] [Google Scholar]
- 24.MacFarlane AJ, Anderson DD, Flodby P, Perry CA, Allan RH, Stabler SP, Stover PJ. Nuclear localization of the de novo thymidylate biosynthesis pathway is required to prevent uracil accumulation in DNA. J Biol Chem 2011;286:44015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacFarlane AJ, Liu X, Perry CA, Flodby P, Allen RH, Stabler SP, Stover PJ. Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J Biol Chem 2008;283:25846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudolph FB, Fanslow WC, Kulkarni AD, Kulkarni SS, Van Buren CT. Effect of dietary nucleotides on lymphocyte maturation. Adv Exp Med Biol 1986;195(Pt A):497–501. [DOI] [PubMed]
- 27.Iwasa Y, Iwasa M, Omori Y, Toki T, Yamamoto A, Maeda H, Kume M, Ogoshi S. The well-balanced nucleoside-nucleotide mixture “OG-VI” for special medical purposes. Nutrition 1997;13:361–4. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni AD, Fanslow WC, Rudolph FB, Van Buren CT. Effect of dietary nucleotides on response to bacterial infections. JPEN J Parenter Enteral Nutr 1986;10:169–71. [DOI] [PubMed] [Google Scholar]
- 29.Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques 2005;38:702, 4, 6. [DOI] [PubMed]
- 30.Machado AF, Zimmerman EF, Hovland DN Jr, Weiss R, Collins MD. Diabetic embryopathy in C57BL/6J mice: altered fetal sex ratio and impact of the splotch allele. Diabetes 2001;50:1193–9. [DOI] [PubMed] [Google Scholar]
- 31.McClive PJ, Sinclair AH. Rapid DNA extraction and PCR-sexing of mouse embryos. Mol Reprod Dev 2001;60:225–6. [DOI] [PubMed] [Google Scholar]
- 32.Suh JR, Oppenheim EW, Girgis S, Stover PJ. Purification and properties of a folate-catabolizing enzyme. J Biol Chem 2000;275:35646–55. [DOI] [PubMed] [Google Scholar]
- 33.Crain PF. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol 1990;193:782–90. [DOI] [PubMed] [Google Scholar]
- 34.Walzem RL, Clifford AJ. Folate deficiency in rats fed diets containing free amino acids or intact proteins. J Nutr 1988;118:1089–96. [DOI] [PubMed] [Google Scholar]
- 35.Stover PJ. One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr 2009;139:2402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macfarlane AJ, Perry CA, McEntee MF, Lin DM, Stover PJ. Shmt1 heterozygosity impairs folate-dependent thymidylate synthesis capacity and modifies risk of Apc(min)-mediated intestinal cancer risk. Cancer Res 2011;71:2098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.