Abstract
Background: Retinopathy of prematurity (ROP) is a vision-threatening disease in premature infants. Serum adiponectin (APN) concentrations positively correlate with postnatal growth and gestational age, important risk factors for ROP development. Dietary ω-3 (n–3) long-chain polyunsaturated fatty acids (ω-3 LCPUFAs) suppress ROP and oxygen-induced retinopathy (OIR) in a mouse model of human ROP, but the mechanism is not fully understood.
Objective: We examined the role of APN in ROP development and whether circulating APN concentrations are increased by dietary ω-3 LCPUFAs to mediate the protective effect in ROP.
Design: Serum APN concentrations were correlated with ROP development and serum ω-3 LCPUFA concentrations in preterm infants. Mouse OIR was then used to determine whether ω-3 LCPUFA supplementation increases serum APN concentrations, which then suppress retinopathy.
Results: We found that in preterm infants, low serum APN concentrations positively correlate with ROP, and serum APN concentrations positively correlate with serum ω-3 LCPUFA concentrations. In mouse OIR, serum total APN and bioactive high-molecular-weight APN concentrations are increased by ω-3 LCPUFA feed. White adipose tissue, where APN is produced and assembled in the endoplasmic reticulum, is the major source of serum APN. In mouse OIR, adipose endoplasmic reticulum stress is increased, and APN production is suppressed. ω-3 LCPUFA feed in mice increases APN production by reducing adipose endoplasmic reticulum stress markers. Dietary ω-3 LCPUFA suppression of neovascularization is reduced from 70% to 10% with APN deficiency. APN receptors localize in the retina, particularly to pathologic neovessels.
Conclusion: Our findings suggest that increasing APN by ω-3 LCPUFA supplementation in total parental nutrition for preterm infants may suppress ROP.
Keywords: ω-3 long-chain polyunsaturated fatty acids, adiponectin, endoplasmic reticulum stress, retinopathy of prematurity, white adipose tissue, neovascularization
INTRODUCTION
Retinopathy of prematurity (ROP)5 is precipitated by persistent inadequate retinal vascularization after premature birth (phase I), resulting in retinal ischemia and tissue hypoxia, stimulating vision-threatening proliferative retinal neovascularization (phase II, classified as stages 1–5) (1, 2). Phase II begins around postmenstrual age (PMA) ∼30–32 wk in very premature infants (3).
Dietary ω-3 long-chain PUFAs (ω-3 LCPUFAs) induce direct inhibition of neovessels through peroxisome proliferator–activated receptor γ (PPARγ) (4), a lipid nuclear receptor that is upstream of adiponectin (APN) (5). After preterm birth, a lack of factors normally available in the third trimester of pregnancy, such as diet-derived ω-3 LCPUFAs (6), is associated with the development of retinopathy (1, 7) and poor neurodevelopment (8). The ω-3 LCPUFA, DHA, is particularly abundant in retina (7). Blood DHA concentrations are decreased by 1 wk after preterm birth and remain low for at least 4 wk (8). In mouse oxygen-induced retinopathy (OIR) modeling human ROP (9), ω-3 LCPUFAs directly suppress retinal neovascularization (4). Clinically, premature infants receiving ω-3 LCPUFA supplementation have reduced retinopathy (10). A mixture of dietary DHA plus arachidonic acid (AA) increases circulating APN concentrations in preterm infants (11). In mouse OIR, dietary ω-3 LCPUFAs’ protective effect mechanism is only partially understood (4, 12–14).
The adipocyte-derived APN concentrations in serum positively correlate with postnatal growth and gestational age, 2 important risk factors for ROP development (15). APN administration suppresses neovascularization in mice (16). APN is produced and assembled into the more bioactive high-molecular-weight (HMW) form in the endoplasmic reticulum (ER) (5, 17). Impaired protein folding and accumulation of unfolded/misfolded proteins under “ER stress” induce a reduction in global protein translation (18, 19). In this study, we hypothesized that ω-3 LCPUFAs may protect against retinopathy through increasing APN concentrations. We found that serum APN concentrations were lower in infants with ROP, and serum APN concentrations positively correlated with serum DHA and EPA concentrations in premature infants. In mice with OIR, attenuating adipose-ER stress with dietary ω-3 LCPUFAs was associated with increased APN concentrations. This study is the first to show the mechanism of dietary ω-3 LCPUFAs leading to increased circulating APN concentrations.
Nutritional strategies to improve growth have not been optimized for very preterm infants, who are usually growth restricted after birth. Parental lipids and milk formulas enriched with DHA were advocated for preterm infants (who are especially sensitive to the effects of dietary fatty acid imbalances) >20 y ago (20). However, current parental nutrition formulations given during the first weeks of life still lack sufficient DHA and result in DHA deficiency (7). Our findings suggest that increased APN concentrations, promoted through ω-3 LCPUFA supplementation, may help protect against ROP development. This study provides further evidence to support the inclusion of ω-3 LCPUFAs in the formulation of total parental nutrition for premature infants.
METHODS
Study design
This translational study aimed to explore the mechanism of ω-3 LCPUFA suppression of proliferative ROP development, with APN as a potential mediator. In the first part of the study, we measured serum APN concentrations in infants with no ROP and ROP (stages 1–3). In the second part, we assessed the effects of ω-3 LCPUFAs on APN concentrations and the effects of APN on retinal vessels in the mouse model of OIR in Apn-knockout (Apn−/−) mice and wild-type (WT) mice with either ω-3 or ω-6 LCPUFA supplementation.
Ethics statement
The clinical study was approved by the Regional Ethical Review Board, Lund, Sweden. Written informed consent was obtained from parents for all study infants. All animal studies adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital.
Patients
Forty-six infants with gestational age (GA) <29 wk at birth and born at a level III neonatal intensive care unit at Skåne University Hospital, Lund, Sweden, were recruited to the study.
Collection of specimens: sampling of neonatal blood
Venous blood samples (0.5 mL) were obtained on postnatal days 3 and 7 and then once weekly until discharge (until a postmenstrual age of at least ∼35 wk) and the serum stored at –80°C until assayed. Samples were obtained from an umbilical or peripheral arterial line during the first postnatal weeks and subsequently obtained weekly from the peripheral vein.
Clinical data
Patients were recruited to the study between January 2005 and May 2007. All pregnancies were dated by ultrasound at 17–18 gestational weeks. Relevant neonatal data were obtained prospectively from the infants’ records in which sex, GA at birth, birth weight, and ROP were registered (Table 1). The infants in the study group were defined as small for gestational age if the deviation in birth weight was >2 SD below that of the population mean adjusted for GA and sex (21).
TABLE 1.
Clinical characteristics of the study group1
| Characteristic | Value |
| Male infants, n (%) | 46 (54) |
| GA at birth, wk | 25.9 ± 1.62 |
| Birth weight, g | 842 ± 237 |
| Birth weight SDS | −1.0 ± 1.3 |
| SGA, n (%) | 11 (24) |
| ROP, % | 19 ± 41 |
GA, gestational age; ROP, retinopathy of prematurity; SDS, SD score; SGA, small for gestational age.
Mean ± SD (all such values).
ROP examination and treatment
ROP screening eye examinations were initiated from a postmenstrual age of 32 wk and continued until the retina was fully vascularized or until ROP regression (22). The eye examinations were performed according to routine protocol consisting of dilated ocular fundus examinations, and outcomes of screening examinations were entered into a study screening protocol. The International Classification of Retinopathy of Prematurity revisited (2) was used for ROP classification, and the recommendations of the Early Treatment of Retinopathy of Prematurity Cooperative Group were followed for treatment (23).
Quantification of serum total APN concentrations by immunoassay
Serum APN concentrations were assayed by using a human APN ELISA kit (E091M; Mediagnost). The intra-assay CVs were 3.8% at 3.9 μg/mL and 4.7% at 13.1 μg/mL, and the interassay CV was 16.3% at 9.9 μg/mL. For values >80 μg/mL, the samples were further diluted by using assay diluent, and the assay was repeated so that the results fell within the recommended range.
Fatty acids analysis
The lipids were extracted from serum with chloroform-methanol 2:1 (vol:vol) containing 0.01% butyrated hydroxytoluene (24). The phospholipids from serum were fractionated on a single SEP-PAK aminopropyl cartridge from Waters Corp. The fatty acid methyl esters were separated by capillary gas–liquid chromatography in a Hewlett-Packard 6890 gas chromatograph as previously described (25). The separation was recorded with Chem Station software (HPGC). C21:0 was used as internal standard, and the fatty acid methyl esters were identified according to the retention times of pure reference substances (Sigma Aldrich Sweden AB and Larodan Fine Chemicals). The amount of each fatty acid was given as mole percentage of the total fatty acid content.
Dietary intervention and mouse model of OIR
Apn−/− and C57BL/6J WT controls were purchased from the Jackson Laboratory. The mice were housed in specific pathogen-free environments, and the temperature in the animal room was 21.7°C ± −16.1°C with a 12-h/12-h light/dark cycle. Micro-Vent Opti (Animal care systems) and PIV racking systems (Allentown) were used. The mice were provided with Carefresh bedding (Scott Distribution) for foraging and nesting and had free access to food (Isopro RMH 3000 irradiated; Scott Distribution) and water (reverse osmosis water with ultraviolet sterilization before distribution). Mouse mothers with pups were fed from postnatal day (P)1 with defined rodent feeds with 10% (w/w) safflower oil containing either 2% ω-3 LCPUFAs (1% DHA and 1% EPA) or 2% ω-6 LCPUFAs (AA) (12). The DHA, EPA, and AA oils were from DSM. DHASCO (R, DHA) and ARASCO (R, AA) provided fatty acids as triglycerides. Fatty acids in MEG-3 (DHA and EPA) were in the form of ethyl. The feed was produced at Research Diets, Inc. and the composition of the feed has been described previously (12). Mice were exposed to 75% oxygen from P7 to P12 and returned to room air (9, 26). Hyperoxia led to vessel loss and relative hypoxia-induced neovascularization (9). Mice were killed by using a lethal intraperitoneal injection of ketamine/xylazine, and eyes were enucleated at P17. Both male and female mouse pups were used, with body weight ranging from 5.5 to 7 g (27). The mice were randomly assigned to either ω-3 or ω-6 LCPUFA-enriched diets with a minimum of 3–4 litters per experimental group. Mice under room air without oxygen exposure were used as controls to compare the differences induced in OIR.
White adipose tissue and serum APN ELISA
At P17, mouse serum was collected and stored at −80°C immediately before use. White adipose tissue (WAT) was homogenized with extraction buffer [1% NP40, 1:1000 protease inhibitor cocktail (P8340; Sigma) in phosphate-buffered saline (PBS)] and analyzed for protein concentration by a bicinchoninic acid protein assay (Pierce). Subsequently, an APN ELISA assay (E091M; Mediagnost) was performed for 1 μL serum or 0.4 μg WAT total protein lysate.
Western blot
P17 WAT lysates (30 μg) were used to detect concentrations of phospho–eukaryotic initiation factor 2α (p-EIF2α, 1:50, 3398P; Cell Signaling Technology), EIF2α (1:500, 5324P; Cell Signaling Technology), C/EBP homologous protein (CHOP) (1:200, 2895P; Cell Signaling Technology), endoplasmic reticulum resident protein (ERP) 44 (1:1000, 3798P; Cell Signaling Technology), ERO1-Lα (1:1000, NB100-2525; Novus), and DSBA-L (1:1000, ab92819; Abcam). For APN, WAT lysates (10 μg), serum (1 μL), or 3T3-L1 cell medium (40 μg) were prepared by incubating with Laemmli’s SDS sample buffer (BP-110R; Boston BioProducts Inc.) for 1 h at room temperature. Primary antibody APN (1:1000, AF1119; R&D) was used. Signals were detected by using 1:5000 corresponding horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescence (Pierce). Four to 6 independent samples per group were used.
3T3-L1 cell culture
3T3-L1 cells (ATCC) were grown on 0.1% gelatin-coated 6-well plates in DMEM containing 10% bovine calf serum and 1% penicillin/streptomycin. Two days after the cells reached confluence, cell differentiation was induced by DMEM containing 10% fetal bovine serum (FBS), 250 μM 3-isobutyl-1-methylxanthine (Sigma), 0.5 μM dexamethasone (Sigma), and 1 μg/mL insulin (Sigma) [day 0]. At day 2, cells were maintained in DMEM containing 10% FBS and 1 μg/mL insulin for 2 d (day 4). Every other day thereafter, medium was changed with DMEM containing 10% FBS only. Approximately by day 8, full differentiation was achieved. DHA (90310; Cayman Chemical) or AA (90010; Cayman Chemical) was delivered to the cells as fatty acid/bovine serum albumin (BSA) complexes. Then, 20 μL 50 mg/mL DHA or AA was added to 1 mL fatty acid-free BSA (0.1 g/mL, A8806; Sigma). This mixture was mixed on a vortex for 2 min and incubated at 37°C for 1 h until the solution was clear (28). For in vitro experiments, fully differentiated 3T3-L1 adipocytes from day 8 were pretreated with vehicle (dilution solution for DHA or AA), AA-BSA, or DHA-BSA for 24 h. ER stress was then induced with 0.1 μg/mL tunicamycin (T7765; Sigma) for another 24 h, and 3T3-L1 cell culture medium was collected.
Quantification of vaso-obliteration and neovascularization
At P17, retinas were dissected and stained overnight at room temperature with 10 μg/mL fluorescent Griffonia Bandeiraea Simplicifolia Isolectin B4 (Alexa Fluor 594, I21413; Molecular Probes) in 1 mmol/L CaCl2 in PBS. For quantification of retinal vaso-obliteration and neovascularization, images of whole mounted retina were taken at 5× magnification on a Zeiss AxioObserver.Z1 microscope and merged to form one image with AxioVision 4.6.3.0 software (Zeiss). Vaso-obliteration and neovascular tuft formation were quantified by comparing the number of pixels in the affected areas with the total number of pixels in the retina by using standard published protocols (27, 29). Percentages of vaso-obliteration and neovascularization from mouse retinas were compared with values for retinas from age-matched control mice with identical oxygen conditions, with n representing the number of eyes quantified.
Laser capture microdissection
P17 eyes from normoxia and OIR conditions were enucleated at P17 and embedded in optimal cutting temperature compound. The eyes were sectioned at 12 μm in a cryostat, mounted on RNase-free polyethylene naphthalate glass slides (11505189; Leica), and immediately stored at −80°C. Slides containing frozen sections were fixed in 50% ethanol for 15 s, followed by 30 s in 75% ethanol, before being washed with diethylpyrocarbonate-treated water for 15 s. Sections were stained with fluoresceinated Isolectin B4 (Alexa Fluor 594, I21413; Molecular Probes; 1:50 dilution in 1 mM CaCl2 in PBS) and treated with RNase inhibitor (03 335 399 001; Roche) at 25°C for 3 min. Laser dissection of retinal blood vessels and different retinal layers was performed immediately thereafter with the Leica LMD 6000 system (Leica Microsystems), and samples were collected directly into lysis buffer from the RNeasy Micro kit (Qiagen) as previously described (30).
Real-time PCR
Total RNA was extracted from isolated subcutaneous WAT by using the RNeasy Lipid Tissue kit (Qiagen) or laser-captured retinal layers and vessels and reverse transcribed into complementary DNA by using reverse transcriptase (Invitrogen). The sequences of primers are Apn (F: 5′-GAA GCC GCT TAT GTG TAT CGC-3′, R: 5′-GAA TGG GTA CAT TGG GAA CAG T-3′), AdipoR1 (F: 5′-TCT TCG GGA TGT TCT TCC TGG-3′, R: 5′-TTT GGA AAA AGT CCG AGA GAC C-3′), AdipoR2 (F: 5′-GGA GTG TTC GTG GGC TTA GG-3′, R: 5′-GCA GCT CCG GTG ATA TAG AGG-3′), T-cadherin (F: 5′-CAT CGA AGC TCA AGA TAT GG-3′, R: 5′-GAT TTC CAT TGA TGA TGG TG-3′), and unchanging control gene Cyclophilin A (F: 5′-CAG ACG CCA CTG TCG CTT T-3′, R: 5′-TGT CTT TGG AAC TTT GTC TGC AA-3′). Quantitative analysis of gene expression was generated by using an Applied Biosystems 7300 Sequence Detection System with the SYBR Green Master mix kit, and gene expression was calculated relative to Cyclophilin A by using the ΔcT method.
Statistical analysis
Clinical data are presented as means ± SEMs. Groups were compared for quantitative variables by the unpaired t test. Correlation analysis was used to examine relations among the variables of interest. Statistical analyses were performed by using the SPSS statistical package (version 20.0; SPSS, Inc.). Animal data are presented as means ± SEMs. All data were used, excluding low-quality images that were not sufficient for analysis. The number per group is shown in the corresponding figure legends. The experimenters were blinded to the treatment conditions. An unpaired t test, ANOVA with Bonferroni’s multiple-comparison test, was used for comparison of results as specified (Prism v5.0; GraphPad Software Inc.). Statistically significant difference was set at P ≤ 0.05.
RESULTS
Lower serum APN concentrations in premature infants are associated with phase II ROP
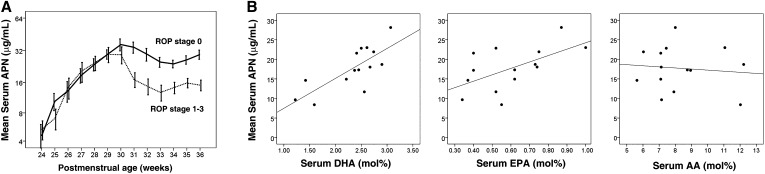
Weekly serum total APN concentrations were measured from birth until discharge in 46 very preterm infants (GA <29 wk at birth). ROP stages were assessed and grouped as no ROP (stage 0) (n = 27) or ROP (stages 1–3) (n = 19). Total APN concentrations in both groups were similar from PMA 24–30 wk (phase I ROP); at ∼PMA 30–32 wk, the onset of phase II (stages 1–3 of ROP) (2, 3), the no-ROP and ROP (stages 1–3) groups diverged. Starting at ∼PMA 30 wk until the last value at PMA 36 wk, infants who developed phase II ROP (stages 1–3) had persistently lower total APN concentrations than did infants without ROP (P < 0.05, Figure 1A and Supplemental Table 1). In addition, the mean HMW APN (most bioactive form) to total APN ratio was higher in premature infants with no ROP than with ROP (n = 9, P = 0.025; Supplemental Figure 1). Infants with ROP had a 25% lower HMW to total APN ratio, whereas infants with no ROP had a slight increase from PMA 31–36 wk.
FIGURE 1.
Mean (±SEM) serum DHA concentrations relate to serum APN concentrations, and lower serum APN concentrations in ROP developed in very preterm infants starting at PMA 30 wk. (A) Longitudinal serum APN concentrations in 46 very preterm infants with GA <29 wk from birth to PMA 36 wk were measured, and ROP development was monitored. n represents different independent samples. Solid line, no ROP (n = 27); dotted line, ROP (stages 1–3) (n = 19, log scale on the y axis). A t test for equality of means was used for comparison of means between 31 and 36 wk. (B) Relation of serum DHA (left, r = −0.759, P = 0.02), EPA (middle, r = −0.583, P = 0.03), and AA (right, r = −0.104, P = NS) concentrations to serum APN concentrations in very preterm infants (n = 14) with ROP development at PMA 30 wk. The Pearson correlation coefficient was used. The result was verified with Spearman’s correlation coefficient. AA, arachidonic acid; APN, adiponectin; GA, gestational age; PMA, postmenstrual age; ROP, retinopathy of prematurity.
Serum DHA, EPA, and APN concentrations positively correlate in premature infants
We measured serum phospholipid fatty acid concentrations in 14 very preterm infants at PMA 30 wk and compared them with the corresponding serum APN concentrations. Serum DHA and EPA concentrations correlated positively with total serum APN concentrations, whereas no correlation was found between serum AA and APN concentrations (Figure 1B).
Serum APN concentrations are increased by dietary intake of ω-3 LCPUFAs in OIR
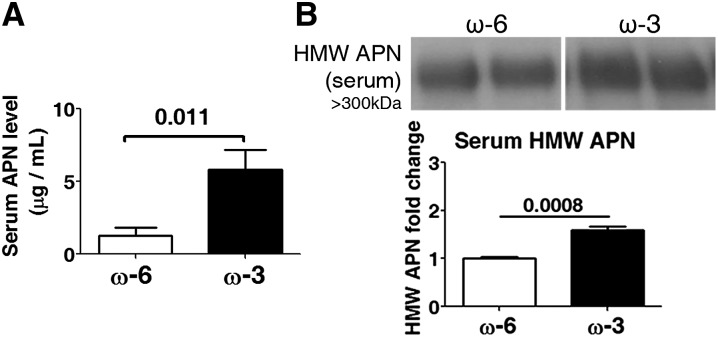
Because clinically we observed a positive correlation between DHA, EPA, and APN concentrations in serum as well as a positive correlation between serum APN concentrations and phase II ROP, the experimental mouse study focused on the effects of ω-3 LCPUFAs on APN concentrations in serum to correspond to the clinical observations. In a mouse model of OIR, ω-3 LCPUFAs directly increased serum APN. We treated mice with OIR with ω-3 LCPUFA-replete vs. ω-3 LCPUFA-deficient diets from P1 to P17. In OIR, mice were exposed to hyperoxia from P7 to P12 and returned to room air from P12 to P17, when we observed maximum neovascularization (9, 27). We observed that occasionally, Apn−/− or WT mothers were negatively affected by the OIR model, resulting in poor care of mouse pups. Therefore, surrogate mothers of the S129 mouse strain were given to every litter because they were more resilient to this OIR model and provided better care of pups. The mouse pups on both diets were ∼6–7 g at P17 after OIR. The defined rodent feeds were isocaloric and identical in composition except for containing either 2% ω-6 LCPUFAs (AA) or 2% ω-3 LCPUFAs (1% DHA and 1% EPA) (12). Lipid content of the milk reflects the mother’s diet lipid profile (12). The ω-3 LCPUFA-fed mice (vs. ω-6) had 4.5-fold higher total serum APN concentrations (P = 0.011; Figure 2A) at P17. Serum APN is present primarily in 3 forms: trimer (67 kDa), hexamer (∼120 kDa), and more bioactive HMW multimer (>300 kDa) (5, 31). In OIR, mice on ω-3 LCPUFA vs. ω-6 LCPUFA feed had a 50% increase in serum HMW APN (P = 0.0008) (Figure 2B) and a 20% decrease in serum hexamer APN concentrations (P = 0.0075; Supplemental Figure 2), with no change in serum trimer APN concentrations (Supplemental Figure 2). Reduced concentrations of hexamer APN with ω-3 LCPUFA feed may result from increased assembly to HMW APN. Our observations suggest that ω-3 LCPUFAs increase serum concentrations of both total and HMW APN in mice with OIR.
FIGURE 2.
ω-3 LCPUFAs increase total serum and HMW APN concentrations in mouse OIR. (A) ELISA for serum APN protein concentrations (n = 6). (B) Western blot for serum HMW APN (n = 4). n represents different independent samples on the same blot. Means ± SEMs are shown (unpaired t test). APN, adiponectin; HMW, high molecular weight; LCPUFA, long-chain PUFA; OIR, oxygen-induced retinopathy.
Both total and HMW APN production and secretion are regulated by ω-3 LCPUFAs
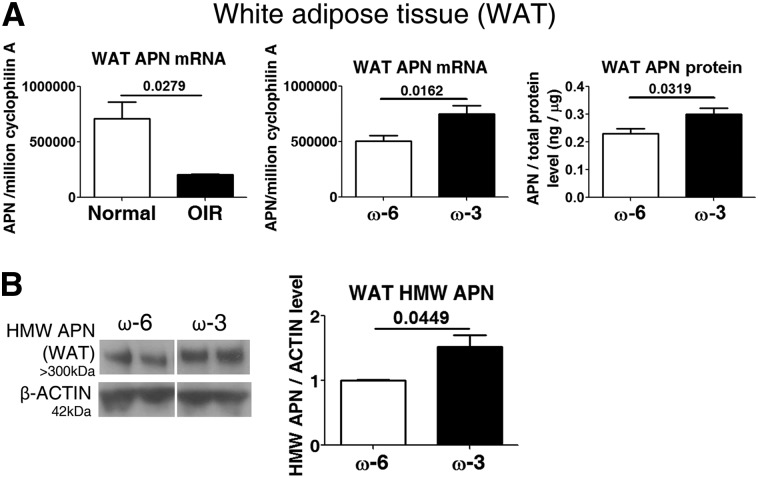
Serum APN is produced predominantly from WAT (32). Therefore, we further investigated how ω-3 LCPUFAs regulated the production of APN in OIR by focusing on WAT. Reduced Apn messenger RNA concentrations were observed in WAT from WT mice with OIR (vs. non–oxygen-exposed controls) (P = 0.0279; Figure 3A). In WAT isolated from OIR mice consuming ω-3 vs. ω-6 LCPUFA feed from P1 to P17, Apn messenger RNA concentrations were increased 1.5-fold (P = 0.0162; Figure 3A) and total APN protein concentrations were increased 1.3-fold at P17 (P = 0.0319; Figure 3A). Importantly, in OIR mice consuming ω-3 LCPUFA vs. ω-6 LCPUFA feed, there was a 1.5-fold increase in HMW APN in WAT (P = 0.0449; Figure 3B).
FIGURE 3.
ω-3 LCPUFAs increase APN concentrations in WAT in OIR. (A) qPCR of Apn in WAT from P17 normal and OIR mice, as well as APN mRNA and protein concentrations in P17 OIR WAT (n = 6–9). (B) Western blot for HMW APN in WAT (n = 3). n represents different independent samples on the same blot. Means ± SEMs are shown (unpaired t test). APN, adiponectin; HMW, high molecular weight; LCPUFA, long-chain PUFA; mRNA, messenger RNA; OIR, oxygen-induced retinopathy; P17, postnatal day 17; qPCR, quantitative polymerase chain reaction; WAT, white adipose tissue.
APN production, assembly, and secretion are regulated by ω-3 LCPUFAs through attenuating adipose-ER stress induction
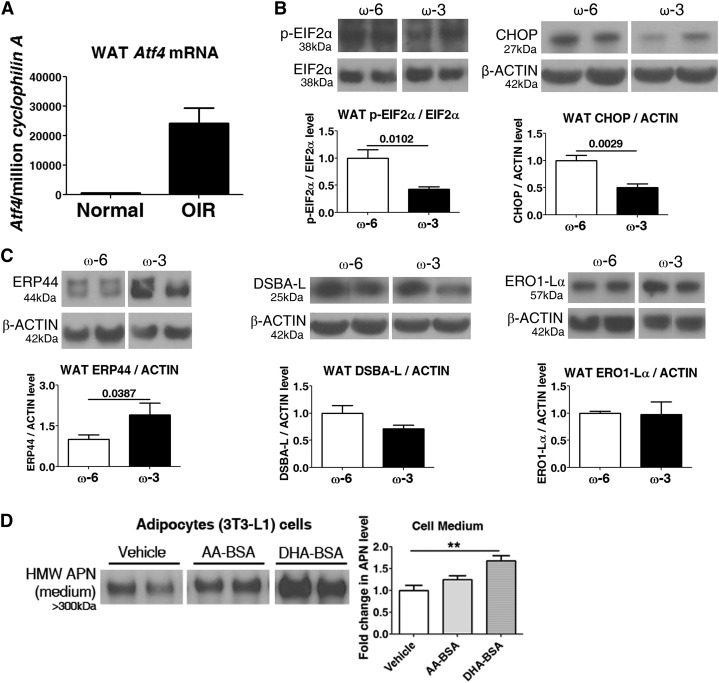
APN production and assembly mainly occurs in the ER (5, 17). In mice with OIR vs. room air controls, ER stress was induced in WAT, indicated by activating transcription factor 4 (Atf4), an ER stress marker (Figure 4A). We investigated in adipocytes whether ω-3 LCPUFAs mediated production of the most bioactive form of APN (HMW APN) through modulating ER stress and increasing production of APN protein as well as increasing assembly into the higher-molecular-weight bioactive form.
FIGURE 4.
ω-3 LCPUFAs modulate endoplasmic-reticulum (ER) stress in WAT. (A) qPCR of ER stress marker Atf4 in WAT from P17 normal and OIR mice (n = 3). In P17 OIR mice: protein concentrations in WAT of ER stress markers (B) p-EIF2α and CHOP (n = 4–6) and ER proteins (C) DSBA-L, ERP44, and ERO1-Lα concentrations (n = 3–7). n represents different independent samples on the same blot. Unpaired t test. (D) ω-3 LCPUFAs (DHA), not ω-6 LCPUFA (AA) modulated HMW APN in ER-stressed adipocytes. Fully differentiated 3T3-L1 cells treated with AA-BSA, DHA-BSA, or vehicle had ER stress induced with tunicamycin. HMW APN concentrations in cell medium were measured by Western blot (n = 4). n represents different independent samples on the same blot. Means ± SEMs, **P < 0.01, ANOVA. AA, arachidonic acid; APN, adiponectin; BSA, bovine serum albumin; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; HMW, high molecular weight; LCPUFA, long-chain PUFA; OIR, oxygen-induced retinopathy; P17, postnatal day 17; p-EIF2α, phospho–eukaryotic initiation factor 2α qPCR, quantitative polymerase chain reaction; WAT, white adipose tissue.
Under ER stress conditions, phosphorylation of EIF2α inhibits recycling of EIF2α and in turn attenuates protein translation (19). The EIF2α signaling pathway plays an essential role in the induction of the proapoptotic transcriptional factor CHOP, a marker for ER stress (19). In our study, phosphorylation of EIF2α and induction of CHOP were decreased 60% and 50%, respectively, in WAT from P17 OIR mice with ω-3 vs. ω-6 LCPUFA feed (p-EIF2α/EIF2α: ω-3/ω-6 = 0.4, P = 0.0102; CHOP: ω-3/ω-6 = 0.5, P = 0.0029; Figure 4B), suggesting that ω-3 LCPUFAs reduced the WAT ER stress in mice with OIR. ER chaperones, including ERP44, DSBA-L, and ERO1-Lα, are involved in APN assembly and secretion (5). Specifically, ERP44 and ERO1-Lα facilitate HMW APN formation and secretion (5). ERP44 concentrations were increased 1.9-fold in WAT from P17 OIR mice on ω-3 vs. ω-6 LCPUFA feed (P = 0.0387; Figure 4C). Thus, ω-3 LCPUFAs may facilitate assembly of HMW APN by reducing ER stress to increase ERP44 in WAT. To corroborate the in vivo studies, we used white adipocyte 3T3-L1 cell culture to analyze the effect of ω-3 LCPUFAs on APN production and secretion. In 3T3-L1 adipose cells pretreated with DHA-BSA (vs. AA-BSA), secreted concentrations of HMW APN were increased 1.7-fold (P < 0.01; Figure 4D) under ER stress conditions induced with tunicamycin (commonly used to induce ER stress in cell culture) (33).
APN helps mediate the protective effects of ω-3 LCPUFAs against neovascularization
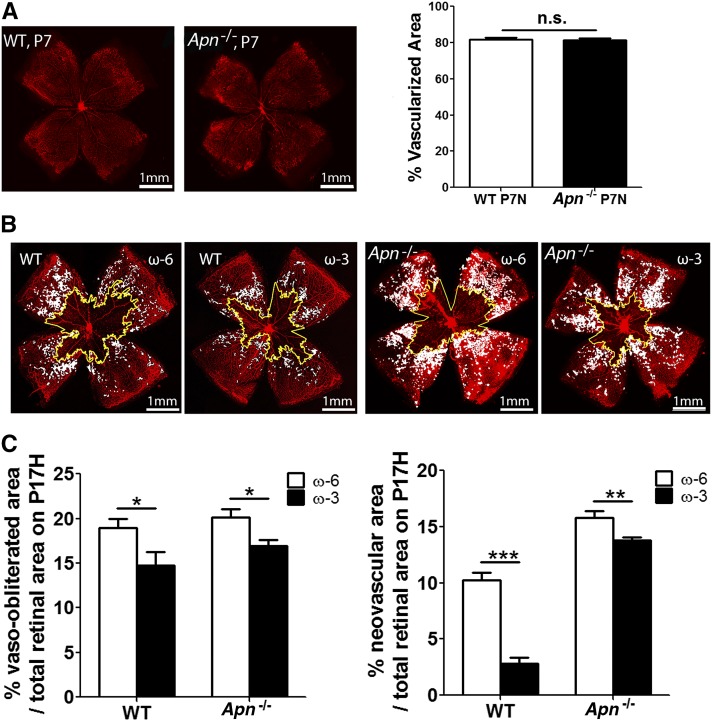
Using Apn−/− and WT mice consuming ω-3 or ω-6 LCPUFA feed, we then determined whether the loss of APN suppresses the protective effects of ω-3 LCPUFA feed against neovascularization. There was no significant difference between 2 genotypes in retinal vascular normal development at P7 (Figure 5A). As expected from our prior studies (4), ω-3 vs. ω-6 LCPUFA feed reduced neovascularization in WT retinas (WT: neovascular area ω-3/ω-6 = 0.3, P < 0.001; Figure 5B, C). However, this 70% protective effect of ω-3 LCPUFAs in reducing neovascularization was reduced to 10% with APN deficiency (Apn−/−: neovascular area ω-3/ω-6 = 0.9, P < 0.01; Figure 5B, C), indicating that ω-3 LCPUFAs protected against neovascularization mediated in large part through APN. Other pathways in addition to APN may also be present because some residual protective effect of ω-3 LCPUFAs against OIR was still observed with APN deficiency.
FIGURE 5.
APN partly mediates the protective effects of ω-3 LCPUFAs against neovascularization. (A) Percentage of superficial vascular coverage over total retinal area at P7 in WT and Apn−/− mouse retinae (n = 10–12). Representative lectin-stained (red) retinal flat-mounts (B) and quantification (C) of vessel loss and neovascularization at P17 from WT and Apn−/− mice with either ω-3 or ω-6 LCPUFA-rich feeds from P1 to P17 (n = 7–18). n represent different independent samples. Means ± SEMs, *P < 0.05, **P < 0.01, ***P < 0.001, NS, ANOVA. Scale bar: 1 mm. APN, adiponectin; LCPUFA, long-chain PUFA; P, postnatal day; WT, wild type.
APN receptors localize in normal and OIR retinas
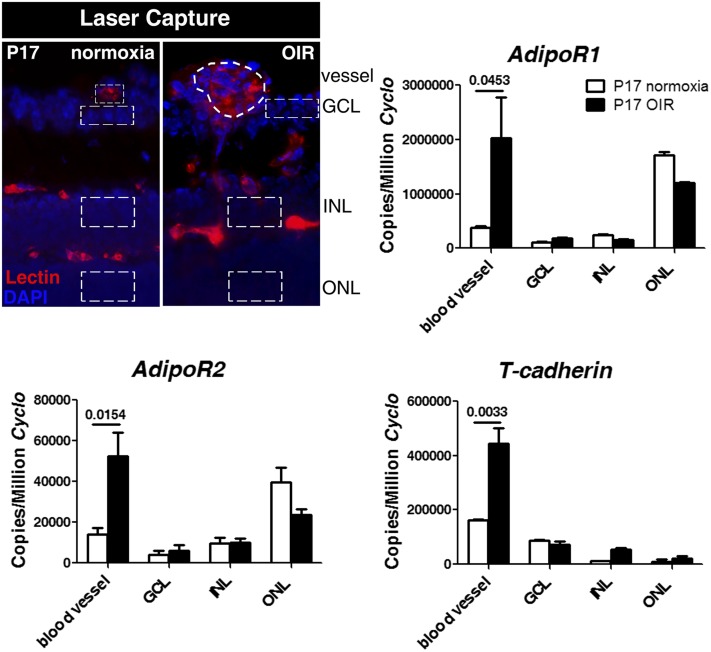
APN binds in tissue to 3 major receptor isoforms: adipoR1, adipoR2, and T-cadherin (34). In retina, expression of these APN receptors was observed in the laser-captured microdissected retinal layers and blood vessels at P17 normoxia and OIR conditions. Significantly increased expression was especially found in the pathologic neovessels (Figure 6).
FIGURE 6.
Localization of APN receptors in the LCM retinal layers and blood vessels at P17. Schematic of the LCM retinal layers (DAPI for nuclei, blue) and blood vessels (lectin, red) isolated is shown (outlined with dotted line). Expression of adipoR1, adipoR2, and T-cadherin was examined by using qPCR. n = 3. Means ± SEMs are shown (t test). APN, adiponectin; GCL, ganglion cell layer; INL, inner nuclear layer; LCM, laser-captured microdissected; OIR, oxygen-induced retinopathy; ONL, outer nuclear layer; P17, postnatal day 17; qPCR, quantitative polymerase chain reaction.
DISCUSSION
Clinically in this study, we found that during the postnatal period associated with the second phase of ROP, infants who developed ROP (stages 1–3) had lower total serum APN and HMW APN concentrations than did infants with no ROP. Serum APN concentrations correlated positively with serum DHA and EPA concentrations. In mouse OIR, ω-3 LCPUFA feed increased total serum APN concentrations, especially the more bioactive HMW APN, by modulating ER stress in WAT. The ω-3 LCPUFA protective effect was largely lost in Apn−/− OIR mice, indicating an important role of APN in controlling the proliferative phase of retinopathy. On the basis of these observations, we suggest that ω-3 LCPUFAs may increase total serum APN concentrations. ω-3 LCPUFAs also increased assembly of HMW APN in WAT through dampening ER stress and increasing the concentrations of ER proteins that promote APN assembly into HMW forms such as ERP44. Increased serum concentrations of higher-order forms of APN acting through retinal APN receptors, in turn, may help reduce neovascularization in OIR (Figure 7).
FIGURE 7.
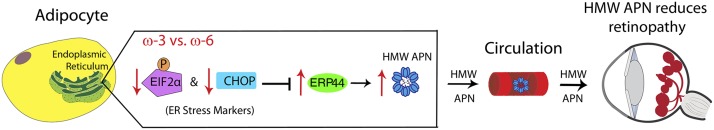
Schematic illustration of proposed pathway for ω-3 LCPUFA regulation of APN production via modulation of ER stress. In ER, activated pERK-EIF2α pathway (an unfolded protein response) in adipocytes upregulates a central transcription factor CHOP, leading to inhibition of ERP44 synthesis, an essential ER protein involved in APN assembly and secretion. Phosphorylation of EIF2α and CHOP upregulation are attenuated with ω-3 LCPUFAs compared with ω-6 LCPUFAs, thereby increasing ERP44 protein concentrations and accelerating APN assembly to a higher-order form and secretion from adipocytes into circulation. Increased serum higher-order forms of APN in turn bind their receptors adipoR1 and adipoR2 in retinal endothelial cells and macrophages, mediating protective effects of ω-3 LCPUFAs on retinal vasculature in retinopathy. APN, adiponectin; CHOP, C/EBP homologous protein; EIF2α, eukaryotic initiation factor 2α ER, endoplasmic reticulum; HMW, high molecular weight; LCPUFA, long-chain PUFA.
Previously, it has been shown in OIR mice and preterm infants that increased dietary ω-3 LCPUFAs can directly suppress neovascularization through PPARγ (4) and that the ω-3 LCPUFA active metabolite 4-HDHA reduces pathologic angiogenesis via PPARγ (4, 13). PPARγ is an upstream mediator of APN (5). Consistent with ω-3 LCPUFAs exerting direct control of proliferative vessels (4) and APN’s antiangiogenic and anti-inflammatory effects in vascular diseases (16, 21), we found higher APN receptor concentrations in neovascular tufts during retinopathy. Our prior studies also demonstrate that ω-3 LCPUFAs protect against neovascularization in mouse OIR with an associated reduction in TNF-α (4). It has been shown that APN ameliorates OIR via TNF-α (16). APN has been found to suppress LPS-mediated stabilization of Tnfα messenger RNA in macrophages in vitro (35). These observations indicate that ω-3 LCPUFAs modulated APN production, which in turn may protect against neovascularization via TNF-α.
APN also influences blood glucose concentrations in a way consistent with a protective effect in ROP. Neonatal hyperglycemia, common in preterm infants, increases the risk of ROP (36–38). Decreased serum APN concentrations are positively correlated with hyperglycemia in extremely premature infants (39). APN increases insulin sensitivity through activating PPARα and AMPK in liver and skeletal muscle (34). Suppression of APN receptor adipoR1 decreases PGC-1α activity and leads to mitochondrial dysfunction through Ca2+ and AMPK/SIRT1 and may contribute to insulin resistance in diabetes (40). Administration of APN receptor agonist reduces plasma glucose concentrations in type 2 diabetic mice (41). Thus, higher APN concentrations in infants without ROP are consistent with a reduced ROP risk with respect to APN effects on insulin sensitivity and blood glucose concentrations. However, further investigations are needed to explore the insulin-sensitizing role of APN in ROP.
In adults, dietary ω-3 LCPUFA or fish oil increases serum APN concentrations (42–44). We found that ω-3 LCPUFAs increased both total and bioactive HMW APN but not trimer or hexamer forms in mouse serum. Although the contribution of APN multimers to specific physiologic processes is not completely understood, considerable evidence suggests that HMW APN is the most bioactive form (31, 45). HMW APN suppresses inflammation in cultured endothelial cells (46), and serum HMW APN concentration is a more potent predictor of endothelial dysfunction than total APN (47). Moreover, HMW APN is the active form in depressing serum glucose concentrations and improving insulin sensitivity in diabetic patients treated with thiazolidinedione, a PPARγ agonist (48). Our current findings suggested that serum HMW APN (vs. other multimers) was specifically increased with dietary ω-3 LCPUFAs in OIR and therefore might help mediate the protective effects of ω-3 LCPUFAs against neovascularization in ROP.
WAT is the major source of circulating APN (32). ER stress is induced in OIR WAT compared with non-OIR mice. Possibly hyperoxia may lead to reduced blood vessel development in adipose tissue, and the relative hypoxia may induce blood vessel proliferation in white fat as in the retina. Oxygen treatment may also influence the redox (reduction/oxidation) state of many molecules in adipocytes. Together, these processes might lead to signals that overwhelm the capacity of ER for protein production related to blood vessel maintenance and growth, or redox regulation, which in turn might further disturb the ER homeostasis. Dietary ω-3 LCPUFAs helped reduce ER stress induction and increased expression of the ER chaperone protein, ERP44. Accumulated unfolded proteins induce phosphorylation of EIF2α, which in turn suppresses general protein translation (18). Previous studies also show that the induced ER stress marker CHOP decreases ER chaperones for APN production, affecting APN concentrations in diabetic mice and 3T3-L1 white adipocytes in vitro (49). ERP44 covalently binds to APN and plays a major role in the assembly of higher-order APN complexes (5, 50). Therefore, we present evidence suggesting that ω-3 LCPUFAs regulated the assembly of HMW APN by dampening ER stress and increasing the ERP44 concentrations that promoted APN assembly into the most bioactive HMW forms (Figure 7).
ROP risk factors include low GA, low birth weight, and poor postnatal growth (15). Interestingly, APN concentrations in neonates also positively correlate with GA and postnatal weight gain (51). Full-term newborns have significantly higher APN concentrations in cord blood than preterm infants, whereas babies who are small for GA have lower APN concentrations than appropriate-for-GA babies (52). There is a 20-fold increase of APN in cord blood between 24 and 40 wk GA (53). In addition, in premature infants, APN concentrations positively correlate with weight gain (51). In premature infants, poor postnatal growth starting at PMA 30 wk is linked with later development of ROP (7). The association of APN and GA and weight gain is consistent with our findings of a strong negative correlation between total serum APN concentrations in infants and ROP development.
Clinically, there is published evidence that fish-oil lipid emulsion supplementation reduces ROP incidence and severity (10, 54, 55). Our clinical results are consistent with a ω-3 LCPUFA protective effect starting at ∼30 wk PMA at the start of phase II of ROP, suggesting suppression of retinal neovascularization. Previously, we found in our mouse model of OIR that dietary ω-3 LCPUFAs directly suppress neovascularization of phase II (4) and also promote revascularization after vessel loss, without a significant effect on oxygen-induced vessel loss (12). In the mouse OIR model, ω-3 LCPUFAs seem not to influence hyperoxia-induced vaso-obliteration of the blood vessels (12). However, the mouse model of OIR may not reflect the hyperglycemia or low concentrations of IGF-1 often seen in preterm neonates, necessitating further evaluation of ω-3 LCPUFA effects on phase I ROP in a model including these variables. Further research in preterm infants is also required.
In summary, our investigations identify a novel role of the adipokine APN on ROP progression, and its serum concentrations can be increased by dietary ω-3 LCPUFAs in OIR. Further exploration, particularly with respect to insulin resistance seen in preterm infants, is needed. We focused on the effects of serum APN in mouse OIR corresponding to our clinical findings. There may also be local production of APN, which might contribute to the antiangiogenic effect. In premature infants, relative hyperoxia after birth may contribute to adipose-ER stress, which may affect APN concentrations. Knowledge of the effects of oxygen on adipose stress in premature infants is limited. Nevertheless, our findings serve as a foundation for a study of adiponectin and ω-3 LCPUFA supplementation in total parental nutrition, which may help prevent ROP development in premature infants. This study also indicates the possible benefits of increasing APN concentrations or ω-3 LCPUFA supplementation for possible treatment of other ocular diseases with proliferative neovascularization.
Supplementary Material
Acknowledgments
We thank Aimee M Juan, Dorothy T Pei, Nathan M Krah, and Colman Hatton for helpful technical contributions. We thank Professor Birgitta Strandvik for her helpful scientific advice and measurement of LCPUFAs in preterm infants.
The authors’ responsibilities were as follows—ZF, CAL, AH, and LEHS: designed research, analyzed the data, and wrote the manuscript; ZF and CAL: conducted the research; ZS, YS, J-SJ, JC, and JPS: provided valuable conceptual input; CGH, RZC, LPE, and KT: executed animal work and retinal analyses; DL and IHP: recruited patients for human serum experiments; ZF, CAL, AH, and LEHS: contributed to the total data analysis for preparation of figures; and all authors: approved the manuscript. The authors declared that they had no conflicts of interest.
Footnotes
Abbreviations used: AA, arachidonic acid; APN, adiponectin; ATF4, activating transcription factor 4; BSA, bovine serum albumin; CHOP, C/EBP homologous protein; EIF2α, eukaryotic initiation factor 2α ER, endoplasmic reticulum; FBS, fetal bovine serum; GA, gestational age; HMW, high molecular weight; LCPUFA, long-chain PUFA; OIR, oxygen-induced retinopathy; P, postnatal day; PBS, phosphate-buffered saline; PMA, postmenstrual age; PPARγ, peroxisome proliferator–activated receptor γ ROP, retinopathy of prematurity; WAT, white adipose tissue; WT, wild type.
REFERENCES
- 1.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis 2007;10:133–40. [DOI] [PubMed] [Google Scholar]
- 2.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–9. [DOI] [PubMed] [Google Scholar]
- 3.Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, Tung B. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991;98:1628–40. [DOI] [PubMed] [Google Scholar]
- 4.Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res 2010;107:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 2010;425:41–52. [DOI] [PubMed] [Google Scholar]
- 6.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n–3 fatty acids in humans. Am J Clin Nutr 2006;83(6 Suppl):1467S–76S. [DOI] [PubMed] [Google Scholar]
- 7.Hård AL, Smith LE, Hellstrom A. Nutrition, insulin-like growth factor-1 and retinopathy of prematurity. Semin Fetal Neonatal Med 2013 Feb 18 (Epub ahead of print; DOI: 10.1016/j.siny.2013.01.006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, Coronel E, Wilschanski M, Stephens AJ, Driscoll DF, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 2011;159:743–9.e1–2. [DOI] [PMC free article] [PubMed]
- 9.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–11. [PubMed] [Google Scholar]
- 10.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized study. JPEN J Parenter Enteral Nutr 2013;38:711–16. [DOI] [PubMed] [Google Scholar]
- 11.Siahanidou T, Margeli A, Lazaropoulou C, Karavitakis E, Papassotiriou I, Mandyla H. Circulating adiponectin in preterm infants fed long-chain polyunsaturated fatty acids (LCPUFA)–supplemented formula—a randomized controlled study. Pediatr Res 2008;63:428–32. [DOI] [PubMed] [Google Scholar]
- 12.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med 2007;13:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med 2011;3:69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao Z, Fu Z, Stahl A, Joyal JS, Hatton C, Juan A, Hurst C, Evans L, Cui Z, Pei D, et al. Cytochrome P450 2C8 omega3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization—brief report. Arterioscler Thromb Vasc Biol 2014;34:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr 2011;23:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circ Res 2009;104:1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 2008;409:623–33. [DOI] [PubMed] [Google Scholar]
- 18.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005;74:739–89. [DOI] [PubMed] [Google Scholar]
- 19.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 2004;11:381–9. [DOI] [PubMed] [Google Scholar]
- 20.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 1992;120:S129–38. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie 2012;94:2137–42. [DOI] [PubMed] [Google Scholar]
- 22.Bråkenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA 2004;101:2476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 2004;94:e27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Gronowitz E, Mellstrom D, Strandvik B. Serum phospholipid fatty acid pattern is associated with bone mineral density in children, but not adults, with cystic fibrosis. Br J Nutr 2006;95:1159–65. [DOI] [PubMed] [Google Scholar]
- 26.Fu ZJ, Li SY, Kociok N, Wong D, Chung SK, Lo AC. Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2012;53:5698–712. [DOI] [PubMed] [Google Scholar]
- 27.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 2009;4:1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kielar ML, Jeyarajah DR, Zhou XJ, Lu CY. Docosahexaenoic acid ameliorates murine ischemic acute renal failure and prevents increases in mRNA abundance for both TNF-alpha and inducible nitric oxide synthase. J Am Soc Nephrol 2003;14:389–96. [DOI] [PubMed] [Google Scholar]
- 29.Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI, Smith LE. Computer-aided quantification of retinal neovascularization. Angiogenesis 2009;12:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahl A, Joyal JS, Chen J, Sapieha P, Juan AM, Hatton CJ, Pei DT, Hurst CG, Seaward MR, Krah NM, et al. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood 2012;120:2925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes 2006;55:1954–60. [DOI] [PubMed] [Google Scholar]
- 32.Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab 2009;296:E22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Spinas GA, Niessen M. ER stress in adipocytes inhibits insulin signaling, represses lipolysis, and alters the secretion of adipokines without inhibiting glucose transport. Horm Metab Res 2010;42:643–51. [DOI] [PubMed]
- 34.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–51. [DOI] [PubMed] [Google Scholar]
- 35.Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor–alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem 2008;283:26850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavez-Valdez R, McGowan J, Cannon E, Lehmann CU. Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants. J Perinatol 2011;31:749–56. [DOI] [PubMed]
- 37.Mohamed S, Murray JC, Dagle JM, Colaizy T. Hyperglycemia as a risk factor for the development of retinopathy of prematurity. BMC Pediatr 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol 2003;23:186–94. [DOI] [PubMed]
- 39.Oberthuer A, Donmez F, Oberhauser F, Hahn M, Hoppenz M, Hoehn T, Roth B, Laudes M. Hypoadiponectinemia in extremely low gestational age newborns with severe hyperglycemia—a matched-paired analysis. PLoS ONE 2012;7:e38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010;464:1313–9. [DOI] [PubMed] [Google Scholar]
- 41.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013;503:493–9. [DOI] [PubMed] [Google Scholar]
- 42.Kondo K, Morino K, Nishio Y, Kondo M, Fuke T, Ugi S, Iwakawa H, Kashiwagi A, Maegawa H. Effects of a fish-based diet on the serum adiponectin concentration in young, non-obese, healthy Japanese subjects. J Atheroscler Thromb 2010;17:628–37. [DOI] [PubMed] [Google Scholar]
- 43.Simão AN, Lozovoy MA, Bahls LD, Morimoto HK, Simao TN, Matsuo T, Dichi I. Blood pressure decrease with ingestion of a soya product (kinako) or fish oil in women with the metabolic syndrome: role of adiponectin and nitric oxide. Br J Nutr 2012;108:1435–42. [DOI] [PubMed] [Google Scholar]
- 44.Silva FM, de Almeida JC, Feoli AM. Effect of diet on adiponectin levels in blood. Nutr Rev 2011;69:599–612. [DOI] [PubMed] [Google Scholar]
- 45.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, et al. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem 2003;278:40352–63. [DOI] [PubMed] [Google Scholar]
- 46.Tomizawa A, Hattori Y, Kasai K, Nakano Y. Adiponectin induces NF-kappaB activation that leads to suppression of cytokine-induced NF-kappaB activation in vascular endothelial cells: globular adiponectin vs. high molecular weight adiponectin. Diab Vasc Dis Res 2008;5:123–7. [DOI] [PubMed] [Google Scholar]
- 47.Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Cheng XW, Numaguchi Y, Murohara T, Okumura K. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clin Endocrinol (Oxf) 2007;67:276–81. [DOI] [PubMed] [Google Scholar]
- 48.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 2004;279:12152–62. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes 2010;59:2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 2007;27:3716–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saito M, Nishimura K, Nozue H, Miyazono Y, Kamoda T. Changes in serum adiponectin levels from birth to term-equivalent age are associated with postnatal weight gain in preterm infants. Neonatology 2011;100:93–8. [DOI] [PubMed] [Google Scholar]
- 52.Siahanidou T, Mandyla H, Papassotiriou GP, Papassotiriou I, Chrousos G. Circulating levels of adiponectin in preterm infants. Arch Dis Child Fetal Neonatal Ed 2007;92:F286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kajantie E, Hytinantti T, Hovi P, Andersson S. Cord plasma adiponectin: a 20-fold rise between 24 weeks gestation and term. J Clin Endocrinol Metab 2004;89:4031–6. [DOI] [PubMed] [Google Scholar]
- 54.Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev 2014;90:27–31. [DOI] [PubMed] [Google Scholar]
- 55.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-Oil Fat Emulsion Supplementation Reduces the Risk of Retinopathy in Very Low Birth Weight Infants: A Prospective, Randomized Study. JPEN J Parenter Enteral Nutr 2013;38:711–6. [DOI] [PubMed] [Google Scholar]
- 56.Pawlik D, Lauterbach R, Hurkala J. The efficacy of fish-oil based fat emulsion administered from the first day of life in very low birth weight newborns. Med Wieku Rozwoj 2011;15:306–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.