Abstract
Background: Little is known about α-tocopherol’s bioavailability as a constituent of food or its dependence on a subject’s age.
Objective: To evaluate the α-tocopherol bioavailability from food, we used collard greens grown in deuterated water (2H collard greens) as a source of deuterium-labeled (2H) α-tocopherol consumed by younger and older adults in a post hoc analysis of a vitamin K study.
Design: Younger (mean ± SD age: 32 ± 7 y; n = 12 women and 9 men) and older (aged 67 ± 8 y; n = 8 women and 12 men) adults consumed a test breakfast that included 120 g 2H collard greens (1.2 ± 0.1 mg 2H-α-tocopherol). Plasma unlabeled α-tocopherol and 2H-α-tocopherol were measured by using liquid chromatography–mass spectrometry from fasting (>12 h) blood samples drawn before breakfast (0 h) and at 24, 48, and 72 h and from postprandial samples collected at 4, 5, 6, 7, 9, 12, and 16 h.
Results: Times (12.6 ± 2.5 h) of maximum plasma 2H-α-tocopherol concentrations (0.82% ± 0.59% total α-tocopherol), fractional disappearance rates (0.63 ± 0.26 pools/d), half-lives (30 ± 11 h), and the minimum estimated 2H-α-tocopherol absorbed (24% ± 16%) did not vary between age groups or sexes (n = 41). Unlabeled α-tocopherol concentrations were higher in older adults (26.4 ± 8.6 μmol/L) than in younger adults (19.3 ± 4.2 μmol/L; P = 0.0019) and correlated with serum lipids (r = 0.4938, P = 0.0012). In addition, 2H-α-tocopherol half-lives were correlated with lipids (r = 0.4361, P = 0.0044).
Conclusions: Paradoxically, α-tocopherol remained in circulation longer in participants with higher serum lipids, but the 2H-α-tocopherol absorbed was not dependent on the plasma lipid status. Neither variable was dependent on age. These data suggest that plasma α-tocopherol concentrations are more dependent on mechanisms that control circulating lipids rather than those related to its absorption and initial incorporation into plasma. This trial was registered at clinicaltrials.gov as NCT0036232.
Keywords: age, bioavailability, cholesterol, pharmacokinetics, triacylglycerides, vitamin E
INTRODUCTION
Vitamin E Dietary Reference Intakes were set in 2000 with the estimated average requirement (EAR)5 equal to 12 mg/d (1). More than 90% of adults in the United States, who do not consume supplements, do not achieve α-tocopherol intakes equal to the EAR (2). Because most of the US population does not apparently suffer from symptoms of α-tocopherol deficiency, there is concern that the EAR is too high. An alternative explanation, on the basis of studies that used 14C-α-tocopherol (3–5), is that the bioavailability of food α-tocopherol is higher than previously estimated by using deuterium-labeled α-tocopheryl acetate-fortified apples (6). As has been noted for vitamin A absorption by humans (7), there are limitations of the various methods for measuring vitamin E absorption. Ideally, a dual-isotope technique that uses an intravenous dose and an oral dose, as has been described for cholesterol (8), would be a useful approach for the quantitation of the fractional absorption of fat-soluble vitamins, but this technique requires that the fat-soluble vitamin be suspended in an intravenous dose that mimics chylomicrons (e.g., a lipid emulsion).
Because of difficulties in estimating the actual α-tocopherol absorption, relative bio-availabilities between various vitamin E forms have been investigated and shown to be useful for identifying α-tocopherol regulatory mechanisms (9–11). In addition, this technique has been used to examine apolipoprotein E-4 effects on vitamin E pharmacokinetics (12). Other than various causes of fat malabsorption that lead to poor α-tocopherol absorption and vitamin E deficiency (13), there is little information with regard to the effect of physiologic factors on vitamin E bioavailability. In addition, little information is available concerning the effects of age on vitamin E bioavailability.
Previous studies showed that collard greens grown in deuterated water contain sufficient 2H-phylloquinone per serving to carry out investigations of absorption and transport of vitamin K (14). Because plants make and store both α-tocopherol and phylloquinone (15), we hypothesized that 2H-α-tocopherol would also be present to allow measurements of α-tocopherol pharmacokinetics and bioavailability from a plant matrix. We further hypothesized that the bioavailability of α-tocopherol endogenously present in food would be greater than what we previously observed by using α-tocopheryl acetate (6), because α-tocopheryl acetate must be hydrolyzed before absorption (16). In addition, it is not known whether absorption of nutrients, especially those that require fat absorption and chylomicron secretion, are affected by aging. Potentially, older participants would not absorb vitamin E as effectively as younger people, as has been shown for lycopene but not α- or β-carotene (17) or vitamin K absorption (18, 19). To evaluate the bioavailability of 2H-α-tocopherol from food, we used collard greens grown in deuterated water as a source of 2H-α-tocopherol as part of a breakfast consumed by both older and younger adults.
METHODS
Materials
HPLC-grade methanol, hexane, and ethanol were obtained from Fisher Scientific. Unlabeled α- and γ-tocopherols, ascorbic acid, potassium hydroxide, and butylhydroxytoluene were from Sigma-Aldrich.
Deuterated collard greens
The cultivation and preparation of the 2H collard greens for consumption was described previously (14). Briefly, collard greens (Brassica oleracea var. acephala, cultivar Georgia) were grown hydroponically by using a nutrient solution enriched with 31 atom% 2H2O. Collard greens were maintained within an acrylic plastic enclosure (situated inside an environmental growth chamber, model PGW36; Conviron) until harvest at 6 wk. At harvest, all leaves were packaged and shipped overnight on ice to the Jean Mayer USDA Human Nutrition Center on Aging at Tufts University. Vegetables were weighed and steamed for 8–12 min until the leaves were completely cooked. Afterward, the vegetables were pureed, portioned, and kept at −80°C until used for the feeding studies. An aliquot was also used for the determination of the 2H-α-tocopherol concentration.
Participants
The Institutional Review Board of New England Medical Center and Tufts University approved the study protocol; all participants gave written informed consent for participation in the study. Only de-identified plasma samples were sent for analysis to the investigators at Oregon State University. This trial was registered at clinicaltrials.gov as NCT0036232.
This study was originally designed to determine dietary and nondietary factors that influence phylloquinone absorption, transport, and utilization (19). Within the parent study, 2H collard greens were used to evaluate phylloquinone bioavailability and lipoprotein transport during periods of phylloquinone restriction and supplementation. The collard greens also contained 2H-α-tocopherol; therefore, α-tocopherol-pharmacokinetics were also investigated by using the same plasma samples that were collected from participants who consumed the collard greens.
As described elsewhere, healthy ambulatory men and women participants in a younger age group (18–40 y) and an older age group (55–80 y) were recruited from the greater Boston area. Women in the older age group were postmenopausal for ≥3 y. All participants fulfilled the following criteria: normal kidney, liver, thyroid, renal, and cardiac function; normal fasting glucose concentrations; and normal clotting times. At the time of the study, participants were not users of the following medications: oral anticoagulants within the previous 12 mo; antibiotics within the previous 3 mo; anticonvulsants, barbiturates, or phenobarbital-containing drugs; herbal preparations; or vitamin E supplements. Participants consumed 600 mg elemental calcium and 10 μg (400 IU) cholecalciferol from 30 d before the study and throughout the study. Subjects consumed a baseline diet for 5 d and a vitamin K restricted diet for 28 d.
Study design
On day 28 of the parent study (d1), participants resided in the Metabolic Research Unit at the Jean Mayer USDA Human Nutrition Center on Aging at Tufts University for 1 d (19). During this residency day (d1), participants were provided with a breakfast, which contained 1 serving fruit yogurt (with 15 g wheat germ), toasted English muffin, butter, honey, skim milk, and decaffeinated coffee and a 120-g serving of 2H collard greens as described previously (19). The breakfast contained 4.7 mg α-tocopherol (unlabeled), 450 kcal, and 14% fat. Over the course of d1, subjects consumed 30.7% kcal from fat and a total of 9.2 mg α-tocopherol from the breakfast and from a standardized lunch and dinner. Participants were free living on days 2, 3, and 4 but were provided with all meals and beverages to minimize interindividual variability.
Fasting (>12 h) blood samples were drawn at 0 h before the ingestion of 2H collard greens. After the consumption of breakfast, blood samples were drawn at 4, 5, 6, 7, 9, 12, and 16 h. Fasting blood samples were subsequently collected at 0800 on days 2, 3, and 4 (corresponding to 24, 48, and 72 h). All blood samples were collected in tubes containing EDTA (0.15% final concentration); plasma was separated by centrifugation and stored frozen until analysis.
Measurement of deuterium-labeled vitamin E
Plasma and collard green α-tocopherols were extracted by using a modified method for saponification and vitamin E extraction (20). Briefly, plasma (50 μL) or collard greens (1 g) were added to ethanol (containing 1% ascorbic acid) and mixed thoroughly and H2O and saturated potassium hydroxide were added. After the addition of a known amount of internal standard (α-tocotrienol), samples were incubated at 60°C for 30 min. After cooling and the addition of 1% ascorbic acid and butylhydroxytoluene, samples were extracted with hexane. An aliquot of the organic phase was dried under nitrogen and resuspended in 200 μL 1:1 ethanol:methanol (vol:vol) for injection into the HPLC system.
The HPLC system (Waters) consisted of a 2695 Separations Module that contained a cooled auto-injector (10°C), a 50-μL sample loop, and a column oven (30°C). The column was a Synergi Hydro-RP (250 mm L × 3.0 mm inside diameter, 4-μm particle size; Phenomenex) with a precolumn (AQ C18, 4 × 3 mm inside-diameter SecurityGuard; Phenomenex). The mobile phase consisted of 100% methanol delivered at 1 mL/min for 10 min. The HPLC was coupled to a ZQ 2000 single-quadrupole mass spectrometer (Micromass) with an atmospheric pressure chemical ionization source operated in negative mode. The corona voltage was set to 25 μA, and the sample cone voltage was set to −35 V. The source temperature was set to 120°C, and the probe temperature was set to 400°C. The desolvation gas (nitrogen) was set to 350 L/h, and the cone gas (nitrogen) was set to 20 L/h. Single-ion recording data were obtained at 429 m/z for unlabeled α-tocopherol and 423 m/z for α-tocotrienol; retention times for α-tocotrienol and α-tocopherol were 5.7 and 8.6 min, respectively.
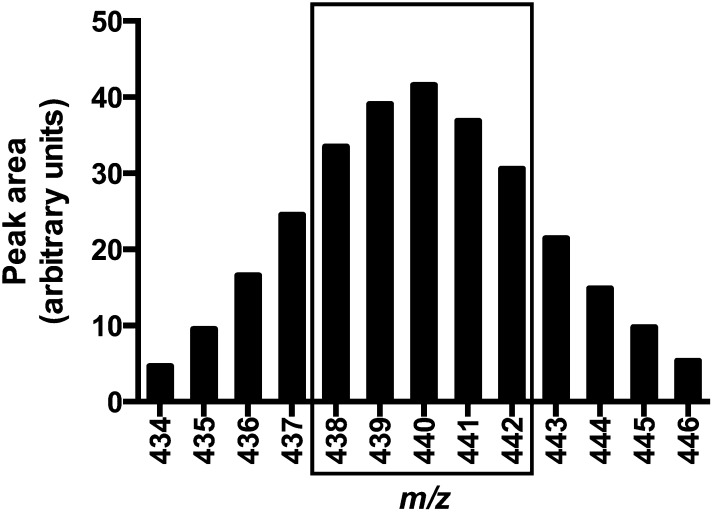
To establish which 2H-α-tocopherols were likely present in the participants’ plasma, isotopic distribution patterns were determined for collard greens similarly to what was described for 2H-phylloquinone (14, 21). The predominant isotopomers in the collard greens’ α-tocopherols were m/z 435–446; no unlabeled α-tocopherol m/z 429 was detected (Figure 1). The ions at m/z 438–442 (equivalent to 9–13 2H atoms, respectively, on the α-tocopherol) were chosen for selected ion recording in plasma extracts because these ions encompassed ∼60% of the total ion abundance for α-tocopherol from the collard greens. The concentration of collard green α-tocopherol was determined by using HPLC with electrochemical detection with the use of authentic standards as described (20). Collard greens were determined to contain 9.8 ± 0.7 mg 2H-α-tocopherol/kg greens, which resulted in 1.2 ± 0.1 mg 2H-α-tocopherol per 120-g serving or ∼2.7 μmol administered to each participant (and no unlabeled collard green α-tocopherol). Subjects also consumed 4.7 mg (10.0 μmol) unlabeled α-tocopherol from the breakfast.
FIGURE 1.
Distribution of 2H-α-tocopherol isotopomers. Single-ion recording mass spectrometer data were used to obtain the isotopic distribution of 2H-α-tocopherol isotopomers extracted from collard greens similarly to what was described for 2H-phylloquinone (14, 21). The predominant isotopomers in the collard green α-tocopherols were m/z 438–442 as indicated by the box; these ions encompassed ∼60% of the total α-tocopherol ion abundance. No unlabeled α-tocopherol (m/z 429) was detected. The ions at m/z 438–442 [equivalent to 9–13 deuterium (2H) atoms, respectively, on the α-tocopherol) were chosen for selected ion recording in plasma extracts.
To estimate plasma α-tocopherols, peak-area data were collected for unlabeled α-tocopherol m/z 429 as well as peak areas for ions at m/z 438–442. These latter peak areas were summed to estimate the majority of 2H-α-tocopherols in the plasma. Areas for α-tocopherol and 2H-α-tocopherol were used to measure α-tocopherols by the ratio of their respective areas to the area internal standard. Deuterated α-tocopherols were corrected to the 100% ion abundance observed in the collard greens. The percentage of labeled to total (labeled and unlabeled) α-tocopherols was calculated for each time point for each participant.
Mathematical and statistical analyses
The AUC of plasma 2H-α-tocopherol concentrations for each person was calculated by using the trapezoidal rule. Maximal concentrations and the time of maximal concentration were identified by visual inspection of the data. α-Tocopherol fractional disappearance rates (FDRs) and half-lives were calculated from the ln of the plasma %2H-α-tocopherol concentrations
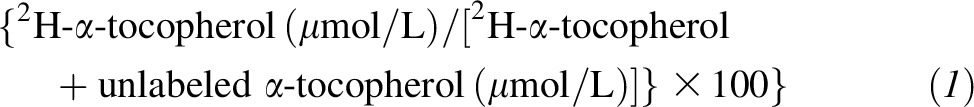 |
as well as the ln of 2H- α-tocopherol concentrations (22). To calculate pools per day, the FDR was multiplied by 24 h. There were no differences in kinetic outcomes whether the actual concentrations or the percentage of 2H-α-tocopherol was used for calculations; therefore, only variables calculated by using %2H-α-tocopherol concentrations are shown. The FDR (the slope of the disappearance curve) was calculated for each individual by using the ln of %2H-α-tocopherol concentrations (or 2H-α-tocopherol concentrations) compared with time. The linest function (Microsoft Excel for Mac 2011, version 14.4.7) was applied by using concentrations from the time of maximum plasma concentration (Tmax) to 72 h; outcomes were acceptable only if the r2 of the fit was >0.9. Two participants had to be excluded because %2H-α-tocopherol concentrations were too low to be fitted reliably; none of their data are included in this study.
The plasma 2H-α-tocopherol concentration was extrapolated back to time zero from the linear regression analysis. This value and the estimated plasma volume were used to calculate the amount of absorbed 2H-α-tocopherol. Plasma volumes (L) were calculated from each participant’s body weight by using separate equations for men
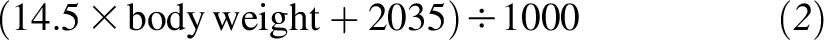 |
and women
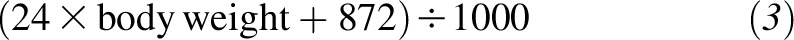 |
as described (23). Fractional absorption was calculated from the estimated amount of 2H-α-tocopherol hypothetically present in the circulation at time zero divided by the amount of 2H-α-tocopherol administered (2.7 μmol).
Data are reported as means ± SDs. Statistical comparisons between groups were performed on logarithmically transformed data with GraphPad Prism software (version 6f; GraphPad Software). The significance of variables was evaluated by using a 2-factor ANOVA followed by Tukey’s post hoc test when significant interactions or main effects (P < 0.05) were observed; most comparisons were not different. Spearman correlations were calculated with GraphPad Prism software.
RESULTS
Baseline characteristics of participants
Participants were recruited with respect to age and sex; the groups included younger women (median age: 34.6 y; range 20.3–40.3 y; n = 12), younger men (median age: 30.6 y; range: 20.6–39.7 y; n = 9), older women (median age: 66.3 y; range: 55.7–66.3 y; n = 8), and older men (median age: 64.0 y; range: 56.8–82.6 y; n = 12; Table 1). With regard to BMI, there were no differences between age groups or between sexes. There were no differences between older and younger adults with respect to fasting serum triacylglycerides, but when these were summed with total cholesterol for each individual, older participants (5.94 ± 0.98 mmol/L) had, on average, ∼15% higher lipid concentrations than those of younger participants (5.16 ± 1.05 mmol/L; P = 0.0088).
TABLE 1.
Participant characteristics at baseline1
| Younger |
Older |
||||||
| Women | Men | Women | Men | P-interaction | P-women compared with men | P-younger compared with older | |
| n | 12 | 9 | 8 | 12 | — | — | — |
| Age, y | 32.9 ± 6.4 | 30.4 ± 7.2 | 67.3 ± 7.9 | 66.3 ± 8.1 | NS | NS | 0.0001 |
| BMI, kg/m2 | 25.5 ± 3.6 | 25.3 ± 3.3 | 25.6 ± 4.8 | 25.2 ± 4.9 | NS | NS | NS |
| Triacylglyceride, mmol/L | 0.70 ± 0.27a | 1.27 ± 0.35b | 1.27 ± 0.80a,b | 1.19 ± 0.66a,b | 0.0249 | 0.0435 | NS |
| Total cholesterol, mmol/L | 4.24 ± 0.94 | 4.18 ± 0.94 | 5.20 ± 0.96 | 4.39 ± 0.78 | NS | NS | 0.0442 |
| LDL cholesterol, mmol/L | 2.35 ± 0.81 | 2.51 ± 0.70 | 3.07 ± 0.66 | 2.58 ± 0.71 | NS | NS | NS |
| Total lipids, mmol/L | 4.94 ± 1.06a | 5.44 ± 1.02a,b | 6.47 ± 1.04b | 5.58 ± 0.78a,b | 0.0322 | NS | 0.0088 |
All values are means ± SDs. The significance of variables was evaluated by using a 2-factor ANOVA followed by Tukey’s post hoc test. For comparisons in a row that do not bear the same superscript letter, P < 0.05.
2H-α-tocopherol kinetics
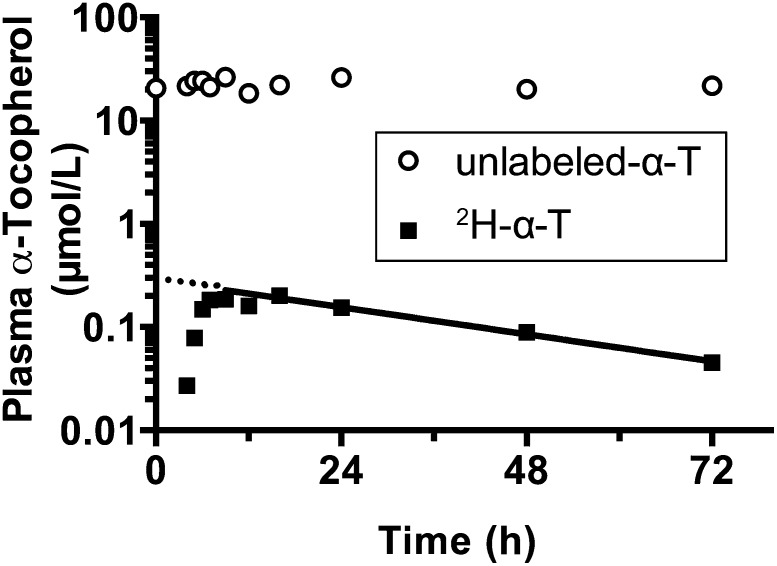
A plot of plasma labeled and unlabeled α-tocopherol concentrations from a representative participant who had kinetic variables similar to the average values of all participants showed that plasma unlabeled α-tocopherol concentrations did not vary appreciably over the course of the 72-h study. By contrast, 2H-α-tocopherol concentrations increased to a maximum and then decreased at an exponential rate as 2H-α-tocopherol left the plasma compartment (Figure 2).
FIGURE 2.
Representative participant plasma labeled and unlabeled α-tocopherol concentrations with an illustration of the curve fitting of 2H-α-tocopherol concentrations postpeak (time of maximum plasma concentration). Plasma unlabeled-α-T (circles) and 2H-α-T (squares) concentrations from a representative participant whose values were similar to average concentrations and kinetic variables are shown. The fractional disappearance rate calculated from the plasma % 2H-α-tocopherol and plasma α-tocopherol concentrations were used to calculate estimated 2H-α-tocopherol concentrations to generate the line shown; the dotted line denotes the extrapolation back to time zero to estimate the hypothetical 2H-α-tocopherol at t = 0 shown in Table 4. 2H-α-T, 2H-α-tocopherol; unlabeled-α-T, unlabeled α-tocopherol.
The AUC of plasma 2H-α-tocopherol concentrations was calculated for each individual; AUCs did not vary between the age or sex groups (Table 2). Indeed, none of the 2H-α-tocopherol kinetic variables, such as Tmax, the maximum plasma concentration (Cmax), FDR, or half-life, varied between age groups or between sexes (Table 2). Overall average values were as follows: AUC, 6.5 ± 4.3 μmol 2H-α-tocopherol/L × h; Tmax, 12.6 ± 2.5 h; Cmax, 0.82 ± 0.59%; FDR, 0.63 ± 0.26 pools/d; and half-life, 30.2 ± 11.1 h.
TABLE 2.
2H-α-tocopherol kinetic variables1
| Younger |
Older |
|||
| Women | Men | Women | Men | |
| n | 12 | 9 | 8 | 12 |
| AUC, μmol 2H-α-tocopherol/L × h | 5.9 ± 3.9 | 5.6 ± 4.5 | 7.7 ± 4.4 | 7.0 ± 4.8 |
| Tmax, h | 12.2 ± 2.1 | 12.2 ± 2.5 | 11.9 ± 2.9 | 13.8 ± 2.5 |
| Cmax, % | 0.94 ± 0.67 | 0.82 ± 0.74 | 0.79 ± 0.48 | 0.71 ± 0.48 |
| y intercept, % | 1.28 ± 0.87 | 1.03 ± 0.78 | 1.07 ± 0.62 | 0.98 ± 0.64 |
| FDR, pools/d | 0.68 ± 0.32 | 0.58 ± 0.18 | 0.66 ± 0.25 | 0.61 ± 0.27 |
| Half-life, h | 28.1 ± 9.8 | 30.6 ± 7.9 | 29.3 ± 12.3 | 32.6 ± 14.0 |
| R2 | 0.9565 ± 0.0322 | 0.9551 ± 0.0364 | 0.9602 ± 0.0343 | 0.9501 ± 0.0387 |
All values are means ± SDs. Cmax is expressed as the percentage of 2H-α-tocopherol per plasma total α-tocopherol concentration; the y intercept and FDR were calculated for each individual from the percentage of 2H-α-tocopherol compared with time curves; the R2 is the average correlation coefficient for the fitting of the curves to the data. There were no significant differences for any of the variables shown (age, sex, or an age × sex interaction (2-factor ANOVA). Cmax, maximum plasma concentration; FDR, fractional disappearance rate; Tmax, time of maximum plasma concentration.
Age groups differed in plasma vitamin E status. Unlabeled α-tocopherol concentrations were greater in older than younger adults when examined at baseline (T = 0, P = 0.0264), averaged over all time points (P = 0.0008), at 24 h (P = 0.0113), averaged over fasting time points (24, 48, and 72 h; P = 0.0016), or at the average of the individual Tmax (for 2H-α-tocopherol concentrations, P = 0.0011; Table 3). When plasma 2H-α-tocopherol concentrations were used to calculate kinetic variables, these values were not different between the 2 age groups (Table 4). The measured 2H-α-tocopherol Cmax from each individual was, on average, 0.17 ± 0.12 μmol/L, the hypothetical (T = 0) Cmax was 0.23 ± 0.15 μmol/L, the estimated absorbed 2H-α-tocopherol was 0.65 ± 0.41 μmol, and the fraction of 2H-α-tocopherol absorbed was 24 ± 15%.
TABLE 3.
Unlabeled α-tocopherol concentrations1
| Younger |
Older |
||||||
| Women | Men | Women | Men | P-interaction | P-women compared with men | P-younger compared with older | |
| n | 12 | 9 | 8 | 12 | — | — | — |
| Unlabeled α-tocopherol | |||||||
| T = 0 h, μmol/L | 18.7 ± 4.8 | 21.6 ± 5.7 | 28.3 ± 11.7 | 24.8 ± 8.6 | NS | NS | 0.0264 |
| Average T = all times, μmol/L | 18.6 ± 3.9 | 20.3 ± 4.7 | 28.8 ± 9.9 | 24.7 ± 7.7 | NS | NS | 0.0008 |
| T = 24 h, μmol/L | 18.6 ± 4.7 | 21.1 ± 6.2 | 27.9 ± 11.0 | 24.3 ± 6.8 | NS | NS | 0.0113 |
| Average at 24, 48, and 72 h, μmol/L | 19.7 ± 4.2 | 20.8 ± 5.7 | 30.3 ± 10.3 | 24.6 ± 7.6 | NS | NS | 0.0016 |
| Tmax,2 μmol/L | 18.6 ± 4.7 | 19.4 ± 5.1 | 28.6 ± 11.9 | 25.5 ± 8.9 | NS | NS | 0.0011 |
All values are means ± SDs. The significance of variables was evaluated by using a 2-factor ANOVA.
Tmax, time of maximum plasma concentration.
TABLE 4.
2H-α-tocopherol concentrations and estimated absorbed 2H-α-tocopherol1
| Younger |
Older |
||||||
| Women | Men | Women | Men | P-interaction | P-women compared with men | P-younger compared with older | |
| n | 12 | 9 | 8 | 12 | — | — | — |
| Cmax2 2H-α-tocopherol, μmol/L | 0.16 ± 0.11 | 0.16 ± 0.16 | 0.20 ± 0.10 | 0.17 ± 0.12 | NS | NS | NS |
| T = 0 h, hypothetical max | 0.22 ± 0.13 | 0.21 ± 0.16 | 0.24 ± 0.06 | 0.23 ± 0.17 | NS | NS | NS |
| Plasma volume, L | 2.51 ± 0.26 | 3.19 ± 0.17 | 2.49 ± 0.36 | 3.27 ± 0.15 | NS | <0.0001 | NS |
| Estimated absorbed 2H-α-tocopherol, μmol | 0.57 ± 0.38 | 0.67 ± 0.48 | 0.59 ± 0.09 | 0.75 ± 0.54 | NS | NS | NS |
| Estimated fraction of 2H-α-tocopherol absorbed, % | 21 ± 14 | 25 ± 18 | 22 ± 3 | 28 ± 20 | NS | NS | NS |
All values are means ± SDs. The significance of variables was evaluated by using a 2-factor ANOVA.
Cmax, maximum plasma concentration.
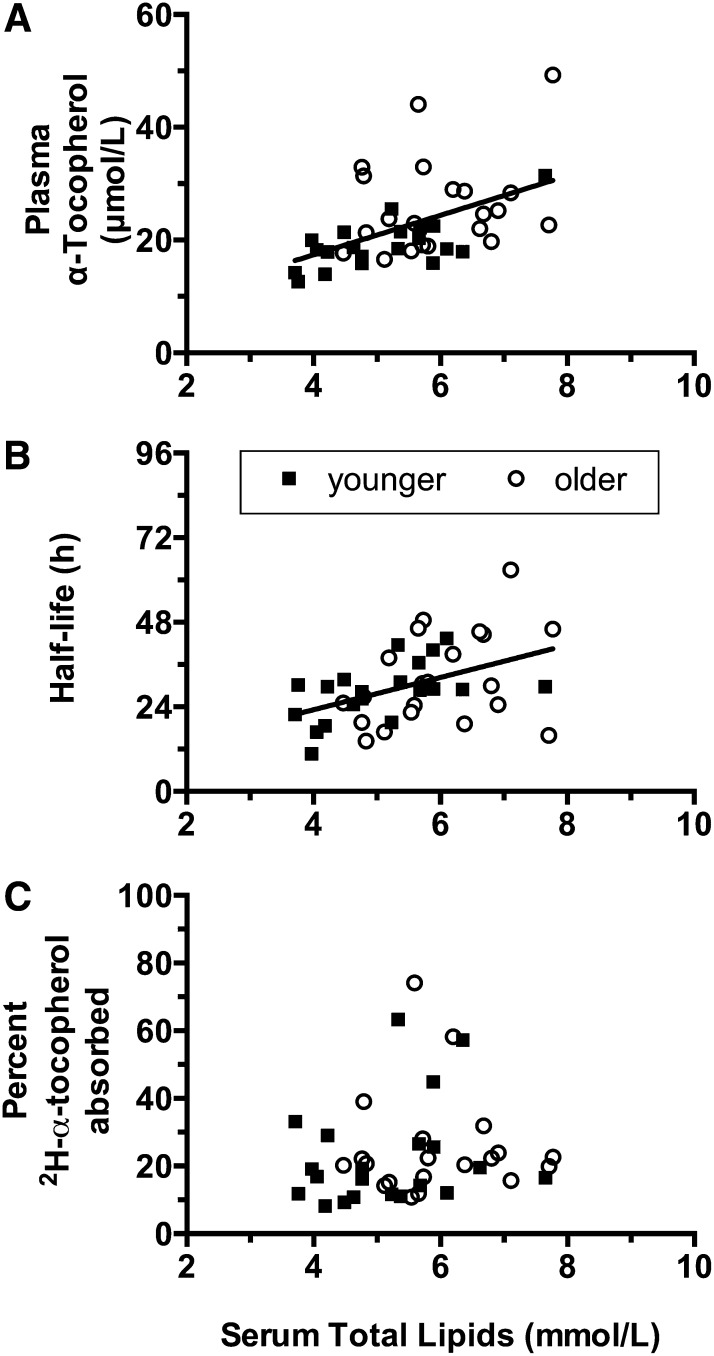
We investigated the reasons underlying the differences in unlabeled α-tocopherol concentrations between age groups and lack of similar differences in 2H-α-tocopherol kinetic variables. Notably, there were significant differences in serum lipids between groups at baseline (Table 1), and serum lipids increased with age (Spearman r = 0.4390; 95% CI: 0.1427, 0.6631; P = 0.0041). Overall average unlabeled α-tocopherol concentrations were correlated with total lipids (Spearman r = 0.5396; 95% CI: 0.2695, 0.7311; P = 0.0003; Figure 3A).
FIGURE 3.
Correlation of overall average plasma α-tocopherol concentrations (A), half-lives (B), or fractional absorption of 2H-α-tocopherol (C) with serum lipids. A: Overall average unlabeled α-tocopherol concentrations were correlated with total lipids (Spearman r = 0.5396; 95% CI: 0.2695, 0.7311; P = 0.0003). B: Calculated half-lives were also correlated with serum lipids (Spearman r = 0.4361; 95% CI: 0.1392, 0.6611; P = 0.0044). Correlations are shown as lines (generated by using a linear regression). C: The lack of relation is shown between serum lipids and the percentage 2H-α-tocopherol absorbed from collard greens.
Unlabeled α-tocopherol concentrations assessed at all the other times shown in Table 3 were also correlated with total lipids (data not shown). Calculated half-lives varied widely (Table 2), but these too were correlated with serum lipids (Spearman r = 0.4361; 95% CI: 0.1392, 0.6611; P = 0.0044; ) as did logarithmically transformed FDR (data not shown). However, baseline serum lipids were not correlated with either the amount of estimated absorbed 2H-α-tocopherol (not shown) or the percentage of the 2H-α-tocopherol dose absorbed (Figure 3C).
DISCUSSION
To our knowledge, this is the first time that α-tocopherol bioavailability from food with naturally incorporated 2H-α-tocopherol was measured, and we showed that the small amounts of collard green 2H-α-tocopherol were absorbed and could be detected after plasma incorporation. Strengths of this study include the large number of participants investigated (n = 41) and ranges of ages (20–82 y) and baseline circulating lipid concentrations (Figure 3). Limitations included the relatively low dose administered, which limited the length of time that the deuterated α-tocopherol could be measured, as well as the lack of measurement of lipids at the time of dose administration. In addition, the study was carried out in subjects consuming a phylloquinone-restricted diet. Nonetheless, it was unlikely that the dietary restriction limited the generalizability because these vitamin K intakes are reported in ∼15–25% of the US adult population (24, 25).
We showed that the α-tocopherol naturally present in plants (e.g., synthesized by the plant) has similar rates of disappearance and half-lives as we observed when we previously tested α-tocopherol bioavailability from food by using exogenously added α-tocopheryl acetate (6, 22). These findings are consistent with Cheeseman et al. (26) who simultaneously compared differently deuterated α-tocopherol and α-tocopheryl acetate. The mean FDR (0.63 ± 0.26) and half-lives (30 ± 11) presented herein (Table 2) were consistent with our previous experiments, but the variability was greater in the current study, which was likely a result of the very low dose of 2H-α-tocopherol administered (1.2 ± 0.1 mg 2H-α-tocopherol/120 g serving collard greens), or perhaps there is greater variability inherent with releasing and absorbing α-tocopherol from a plant matrix. Nonetheless, the kinetic variables showed no major differences in α-tocopherol bio-availabilities between younger and older adults.
To our surprise, despite the lack of significant differences between age groups with respect to the α-tocopherol kinetic variables (Table 2) and 2H-α-tocopherol concentrations (Table 4), plasma unlabeled α-tocopherol concentrations were uniformly higher in older than younger participants (Table 3). Not unexpectedly, plasma unlabeled (but not labeled) α-tocopherol concentrations were highly correlated with baseline serum total lipids (Figure 3A). These data were consistent with α-tocopherol transport in circulating lipoproteins, and differences in lipids concentrations were reflected in the plasma α-tocopherol concentrations (13). Previously, we showed that dietary fat consumption (0–21% in the test breakfast) altered AUCs but not α-tocopherol disappearance rates or half-lives (6). Therefore, what was unexpected in the current study was the significant correlation between α-tocopherol half-lives and serum total lipids (Figure 3B). Apparently, as serum lipids increase, α-tocopherol remains in circulation for a longer time likely because higher lipid concentrations are associated with slower lipoprotein catabolism and uptake by tissues (27). However, the fraction of the dose absorbed was not correlated with serum lipids (Figure 3C).
To compare the current study with the various previously published studies, we showed that many studies reported the Cmax as well as the dose and the amount of fat in the administered breakfast. The values, when expressed as the percentage of dose/L plasma, allowed comparisons of the various labeled dose sizes, which ranged from submicromolar to millimolar (Table 5). Similar values of the Cmax were reported in the current study (6.4 ± 4.4%) and the 14C-α-tocopherol study (5.4 ± 1.6%) (5) despite the widely disparate estimates of fractional absorption rates between the current study (24 ± 15%) and the 14C-α-tocopherol study (81 ± 1%). In contrast, the Cmax of the apple study (6) at the highest fat intake was 11.2 ± 2.5%, which suggested that fat may improve vitamin E absorption when administered as α-tocopheryl acetate and that additional studies are needed that use more-accurate methods to assess fractional absorption. Such measures are needed to estimate how much food vitamin E must be consumed to provide the amounts calculated from biokinetic studies (1). Another limitation of the current study was that estimates derived by using the method of extrapolating the decay curve back to time equal zero are meant for a one-compartment system and are less reliable than those derived for a multicompartment model, which ideally should be used for α-tocopherol pharmacokinetics over a longer time than 72 h.
TABLE 5.
Comparison of concentrations at Cmax from various published studies1
| Study (reference) | n participants | Dose administered, μmol | Matrix | Cmax, percentage of dose/L plasma2 | Fat, g | Percentage of fat calories | Breakfast calories, kcal |
| Collard greens | 41 | 2.7 | Collard greens | 6.4 ± 4.4 | 1.6 | 14 | 450 |
| Apple study (6) | 5 | 50 | “Vacuum impregnation solution” | 3.5 ± 1.8 | 0 | 0 | 47 |
| 6.9 ± 1.5 | 2.4 | 6 | 380 | ||||
| 11.2 ± 2.5 | 11 | 21 | 471 | ||||
| Smoker study (10)3 | 22 | 116 | Encapsulated | 2.3 ± 1.2 | 18.7 | 30 | 560 |
| RBC (37) | 12 | 344 | Encapsulated | 6.0 ± 1.9 | 40 | — | Not stated |
| ApoE4 participants (12) | 10 | 344 | Encapsulated | 3.2 ± 0.5 | 40 | — | Not stated |
| 14C-α-tocopherol (4)4 | 12 | 0.00181 | 2% milk | 5.4 ± 1.6 | 10.4 | 32.6 | 319 |
ApoE4, apolipoprotein E-4; Cmax, maximum plasma concentration; RBC, red blood cell.
All values are means ± SDs.
Both smokers and nonsmokers, all of whom consumed vitamin C supplements.
Fat = 8 g + 2.4 g milk fat; 28% + fat from milk; 252 kcal + 67 kcal from milk.
Currently, available estimates of vitamin E absorption are based on plasma concentrations of labeled α-tocopherol after oral administration (6), which can be considered a minimum absorption estimate, or estimates are based on labeled α-tocopherol present in fecal collections, which can be considered a maximum absorption estimate (4, 5, 28) with the true absorption rate likely between both estimates. The use of radioactive α-tocopherol also has some limitations, because the material has the potential to self-irradiate and, thereby, causes the formation of oxidized tocopherol. Indeed, there have been reports of 30–41% absorption by using estimates of fecal radioactive α-tocopherol, whereas simultaneously, no plasma radioactive α-tocopherol was detected (29). Because of observations that newly absorbed α-tocopherol replaces the endogenous α-tocopherol in human circulation (9), it seems unlikely that the newly absorbed radioactive α-tocopherol would not appear in the circulation especially because the role of the hepatic α-tocopherol transfer protein is to maintain plasma α-tocopherol concentrations. The fractional α-tocopherol absorption is likely to be as high as 55–79% in healthy participants but much-more limited during various forms of fat malabsorption (30).
Unlike the assessment of absorption, there is general agreement as to the kinetic variables for the fast turning-over pool of vitamin E. For example, a mean half-life of 53 h was reported in 1970 as estimated by using radioactive α-tocopherol (30). Of course, multiple pool models estimate longer half-lives for the slower turning-over pools such as adipose tissue or nervous tissues (e.g., spinal cord and brain) (5, 9, 31).
These estimates for vitamin E pharmacokinetics are very different from those of vitamin K. Vitamin K (phylloquinone), which was studied previously by using deuterium-labeled collard greens (14), showed a relatively fast plasma disappearance compared with that of vitamin E; 2H-α-tocopherol persisted in the plasma with a half-life of 30 h ± 11 for the fast turning-over pool, whereas 2H-phylloquinone had returned to baseline by 24 h. In this study, the Tmax for 2H-α-tocopherol was 12 ± 2 h, whereas the Tmax was from 6 to 9 h for 2H-phylloquinone (14). These findings emphasize the importance of the well-established role of the hepatic α-tocopherol transfer protein in maintaining plasma α-tocopherol concentrations in the fast turning-over pool (32) as well as a lack of a similar mechanism for maintaining phylloquinone concentrations (14). Differences in the kinetics of the plasma transport of the 2 vitamins are especially striking because of the similarity in their structures, both of which have a phytyl tail and both are absorbed in chylomicrons (33). Studies that used 2H-phylloquinone showed that liver contains 2H-phylloquinone, suggesting that the unmodified form is transported in triacylglyceride-rich lipoproteins during absorption (34, 35). Currently, specific comparisons between the pharmacokinetics of vitamins E and K are not available for participants in this study.
In conclusion, despite the lack of major differences between younger and older adults in the bioavailability of collard green 2H-α-tocopherol, we showed that 2H-α-tocopherol half-lives were correlated with serum total lipids. Thus, α-tocopherol remained in circulation longer at higher serum lipid concentrations, likely because higher lipid concentrations are associated with slower lipoprotein catabolism and uptake by tissues. These findings have important public health consequences because they highlight a limitation in assessing vitamin E status by using only plasma α-tocopherol concentrations. Lipids were only 15% higher in the older group, but plasma α-tocopherol concentrations were 25% higher, likely because the lipids allowed the plasma to carry more vitamin. A better biomarker of vitamin E status is needed such as the vitamin E metabolite α-carboxyethyl hydroxychromanol (36).
Acknowledgments
We thank James W Peterson for providing excellent technical assistance.
The authors’ responsibilities were as follows—MGT, XF, and SLB: designed research (project conception, development of overall research plan, and study oversight); SWL, ES, MAG, and SLB: conducted the research (hands-on conduct of experiments and data collection); MAG: provided essential reagents or essential materials (by providing, e.g., animals, constructs, and databases, necessary for research); MGT and GB: analyzed data or performed the statistical analysis; MGT: had primary responsibility for the final contents of the manuscript; and all authors: wrote the manuscript. Hermes Arzneimittel GmbH and Tomohiro Saito of Eisai Food and Chemical Co. Ltd. had no role in the study design, study implementation, analysis, or interpretation of data. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: Cmax, maximum plasma concentration; d1, day 28 of the parent study; EAR, estimated average requirement; FDR, fractional disappearance rate; Tmax, time of maximum plasma concentration.
REFERENCES
- 1.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 2.Bailey RL, Fulgoni VL 3rd, Keast DR, Dwyer JT. Examination of vitamin intakes among US adults by dietary supplement use. J Acad Nutr Diet 2012;112:657–63.e4. [DOI] [PMC free article] [PubMed]
- 3.Clifford AJ, de Moura FF, Ho CC, Chuang JC, Follett J, Fadel JG, Novotny JA. A feasibility study quantifying in vivo human alpha-tocopherol metabolism. Am J Clin Nutr 2006;84:1430–41. [DOI] [PubMed] [Google Scholar]
- 4.Chuang JC, Matel HD, Nambiar KP, Kim SH, Fadel JG, Holstege DM, Clifford AJ. Quantitation of [5-14CH3]-(2R, 4'R, 8'R)-alpha-tocopherol in humans. J Nutr 2011;141:1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novotny JA, Fadel JG, Holstege DM, Furr HC, Clifford AJ. This kinetic, bioavailability, and metabolism study of RRR-alpha-tocopherol in healthy adults suggests lower intake requirements than previous estimates. J Nutr 2012;142:2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno RS, Leonard SW, Park SI, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled alpha-tocopheryl acetate. Am J Clin Nutr 2006;83:299–304. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Green MH. Parameter identifiability and Extended Multiple Studies Analysis of a compartmental model for human vitamin A kinetics: fixing fractional transfer coefficients for the initial steps in the absorptive process. Br J Nutr 2014;111:1004–10. [DOI] [PubMed] [Google Scholar]
- 8.Bosner MS, Lange LG, Stenson WF, Ostlund RE Jr. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res 1999;40:302–8. [PubMed] [Google Scholar]
- 9.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr 1998;67:669–84. [DOI] [PubMed] [Google Scholar]
- 10.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med 2006;40:689–97. [DOI] [PubMed] [Google Scholar]
- 11.Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res 1990;31:675–85. [PubMed] [Google Scholar]
- 12.Proteggente AR, Turner R, Majewicz J, Rimbach G, Minihane AM, Kramer K, Lodge JK. Noncompetitive plasma biokinetics of deuterium-labeled natural and synthetic alpha-tocopherol in healthy men with an apoE4 genotype. J Nutr 2005;135:1063–9. [DOI] [PubMed] [Google Scholar]
- 13.Traber MG. Vitamin E. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern nutrition in health and disease. 11th ed. Baltimore (MD): Lippincott Williams & Wilkins; 2014. p. 293–304. [Google Scholar]
- 14.Erkkilä AT, Lichtenstein AH, Dolnikowski GG, Grusak MA, Jalbert SM, Aquino KA, Peterson JW, Booth SL. Plasma transport of vitamin K in men using deuterium-labeled collard greens. Metabolism 2004;53:215–21. [DOI] [PubMed] [Google Scholar]
- 15.Eugeni Piller L, Abraham M, Dormann P, Kessler F, Besagni C. Plastid lipid droplets at the crossroads of prenylquinone metabolism. J Exp Bot 2012;63:1609–18. [DOI] [PubMed] [Google Scholar]
- 16.Burton GW, Ingold KU, Foster DO, Cheng SC, Webb A, Hughes L, Lusztyk E. Comparison of free alpha-tocopherol and alpha-tocopheryl acetate as sources of vitamin E in rats and humans. Lipids 1988;23:834–40. [DOI] [PubMed] [Google Scholar]
- 17.Cardinault N, Tyssandier V, Grolier P, Winklhofer-Roob BM, Ribalta J, Bouteloup-Demange C, Rock E, Borel P. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. Eur J Nutr 2003;42:315–23. [DOI] [PubMed] [Google Scholar]
- 18.Booth SL, O'Brien-Morse ME, Dallal GE, Davidson KW, Gundberg CM. Response of vitamin K status to different intakes and sources of phylloquinone-rich foods: comparison of younger and older adults. Am J Clin Nutr 1999;70:368–77. [DOI] [PubMed] [Google Scholar]
- 19.Truong JT, Fu X, Saltzman E, Al Rajabi A, Dallal GE, Gundberg CM, Booth SL. Age group and sex do not influence responses of vitamin K biomarkers to changes in dietary vitamin K. J Nutr 2012;142:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res 1996;37:893–901. [PubMed] [Google Scholar]
- 21.Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG. Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem 2009;81:5421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard SW, Good CK, Gugger ET, Traber MG. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am J Clin Nutr 2004;79:86–92. [DOI] [PubMed] [Google Scholar]
- 23.Retzlaff JA, Tauxe WN, Kiely JM, Stroebel CF. Erythrocyte volume, plasma volume, and lean body mass in adult men and women. Blood 1969;33:649–61. [PubMed] [Google Scholar]
- 24.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr 2012;3:182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr 1998;128:785–8. [DOI] [PubMed] [Google Scholar]
- 26.Cheeseman KH, Holley AE, Kelly FJ, Wasil M, Hughes L, Burton G. Biokinetics in humans of RRR-alpha-tocopherol: the free phenol, acetate ester, and succinate ester forms of vitamin E. Free Radic Biol Med 1995;19:591–8. [DOI] [PubMed] [Google Scholar]
- 27.Hassing HC, Surendran RP, Mooij HL, Stroes ES, Nieuwdorp M, Dallinga-Thie GM. Pathophysiology of hypertriglyceridemia. Biochim Biophys Acta 2012;1821:826–32. [DOI] [PubMed] [Google Scholar]
- 28.Kelleher J, Losowsky MS. The absorption of vitamin E in man. Biochem J 1968;110:20P–1P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelleher J, Losowsky MS. The absorption of alpha-tocopherol in man. Br J Nutr 1970;24:1033–47. [DOI] [PubMed] [Google Scholar]
- 30.MacMahon MT, Neale G. The absorption of alpha-tocopherol in control subjects and in patients with intestinal malabsorption. Clin Sci 1970;38:197–210. [DOI] [PubMed] [Google Scholar]
- 31.Ingold KU, Burton GW, Foster DO, Hughes L, Lindsay DA, Webb A. Biokinetics of and discrimination between dietary RRR- and SRR-alpha-tocopherols in the male rat. Lipids 1987;22:163–72. [DOI] [PubMed] [Google Scholar]
- 32.Traber MG. Mechanisms for the prevention of vitamin E excess. J Lipid Res 2013;54:2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopec RE, Schweiggert RM, Riedl KM, Carle R, Schwartz SJ. Comparison of high-performance liquid chromatography/tandem mass spectrometry and high-performance liquid chromatography/photo-diode array detection for the quantitation of carotenoids, retinyl esters, alpha-tocopherol and phylloquinone in chylomicron-rich fractions of human plasma. Rapid Commun Mass Spectrom 2013;27:1393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr 2012;142:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farley SM, Leonard SW, Stevens JF, Traber MG. Deuterium-labeled phylloquinone fed to alpha-tocopherol-injected rats demonstrates sensitivity of low phylloquinone-containing tissues to menaquinone-4 depletion. Mol Nutr Food Res 2014;58:1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebold KM, Ang A, Traber MG, Arab L. Urinary α-carboxyethyl hydroxychroman can be used as a predictor of α-tocopherol adequacy, as demonstrated in the Energetics Study. Am J Clin Nutr 2012;96:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeanes YM, Hall WL, Lodge JK. Comparative (2)H-labelled alpha-tocopherol biokinetics in plasma, lipoproteins, erythrocytes, platelets and lymphocytes in normolipidaemic males. Br J Nutr 2005;94(1):92–9. [DOI] [PubMed] [Google Scholar]