Abstract
Tracheal injury is a rare but highly morbid complication of endotracheal intubation. Recent reviews have advocated conservative management of these injuries without operative intervention. Extracorporeal membrane oxygenation may be a useful tool in non-operative management of tracheal injury in the setting of severe respiratory failure and need for prolonged intubation. We present a morbidly obese 33 year-old-female with H1N1 influenza pneumonia complicated by acute respiratory distress syndrome and bacterial super-infection who sustained a post-intubation tracheal injury. Concomitant tracheal injury and acute lung injury pose a difficult ventilation dilemma. This patient was successfully managed by venovenous extracorporeal membrane oxygenation, high frequency oscillator ventilation, proning position and tube thoracostomy. The venovenous extracorporeal membrane oxygenation and ventilator management were essential for this patient’s recovery.
Keywords: ECMO, tracheal injury, H1N1 influenza, traumatic intubation
Introduction
Venovenous extracorporeal membrane oxygenation (VV ECMO) has been well described to manage acute respiratory distress syndrome (ARDS) [1]. The onset of ARDS in the setting of H1N1 influenza is often hyper-acute, requiring emergent ventilatory support and, in severe cases, ECMO cannulation.
In the setting of emergent intubation, tracheal injury is a rare but catastrophic complication, occurring in 1 in 20,000 endotracheal intubations [2]. Historically, urgent surgical intervention has been the mainstay of management of post-intubation tracheal injury. However, more recent reviews advocate a conservative approach, especially in patients who will require prolonged ventilation due to underlying pulmonary pathology [3, 4].
Our case demonstrates successful management of concomitant tracheal injury and H1N1 influenza complicated by bacterial super-infection with VV ECMO supplemented by high-frequency oscillator ventilation (HFOV), prone positioning, and tube thoracostomy in an adult patient.
Case Report
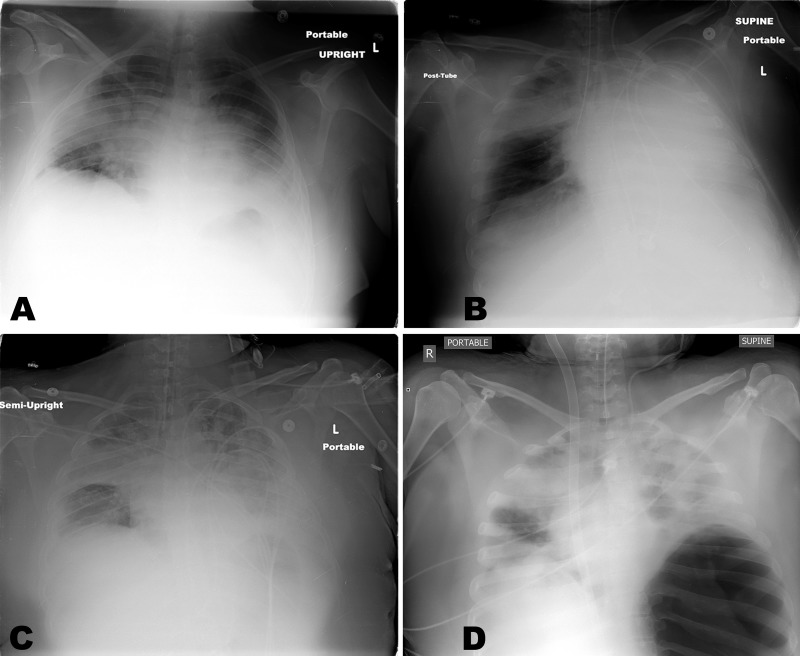
A 33 year-old previously healthy, morbidly obese (body mass index 40, body weight 98 kg, height 157 cm) female presented to an outside emergency department with progressive dyspnea and malaise. In the emergency department, she rapidly developed acute hypoxic respiratory failure with bilateral infiltrates on chest x-ray (Figure 1A). She deteriorated and was emergently intubated. Post-intubation chest x-ray showed the endotracheal tube in the right main stem bronchus (Figure 1B), requiring adjustment (Figure 1C). She remained hypoxic and was transferred to a tertiary center for ARDS management. At arrival, she was hypoxic (arterial blood gas was pH 7.18, PaCO2 58, PaO2 52, saturation 78%), despite fractional inspiratory oxygen (FiO2) of 100%, tidal volume (TV) 600 ml, respiratory rate of 20 positive end expiratory pressure (PEEP) of 20 mm H2O with paralysis and Epoprostenol (50 ng/kg/min) support. Failed medical management necessitated urgent VV ECMO using a bi-caval dual lumen cannula (Maquet, Rastatt, Germany) via the right internal jugular vein, after we obtained consent from her family. Her oxygenation improved (pH 7.35, PaCO2 31, PaO2 176 and saturation >99%) with VV ECMO flow of 4 LPM, sweep of 6 LPM and FiO2 100%. The patient was found to be positive for H1N1 influenza with super imposed methicillin-sensitive Staphylococcus aureus (MSSA) pneumonia; Tamiflu (75 mg q12 hours) and broad-spectrum antibiotic coverage with vancomycin (1.5 gram q12 hours, titrate by the rough level 15-20 mg/L) and piperacillin/tazobactam (3.375 gram q8 hours) were initiated.
Ventilator settings were changed from conventional mode (TV 600 ml, rate 20, FiO2 100%, PEEP 20) to ARDSNet protocol, with 300cc TV (TV=4-6 ml/kg, based on an ideal body weight of 55 kg), respiratory rate 10, and FiO2 of 100% with PEEP 10 for lung protection. Peak airway pressure and mean airway pressure remained elevated (58 cm H2O and 15 cm H2O respectively). Post-ECMO chest x-ray showed a loculated lower left pneumothorax, treated immediately with bedside thoracostomy tube placement (Figure 1D).
Figure 1.
Series of the chest x-rays of the patient. Initial radiograph upon presentation showed bilateral infiltrates (1A). Post intubation x-ray shows endotracheal tube in the right main stem bronchus (1B). After repositioning the endotracheal tube, there is the progression of the bilateral infiltrations (1C). Post ECMO cannulation at arrival to our facility, there is a large loculated pneumothorax in the left thorax (1D).
ECMO = extracorporeal membrane oxygenation.
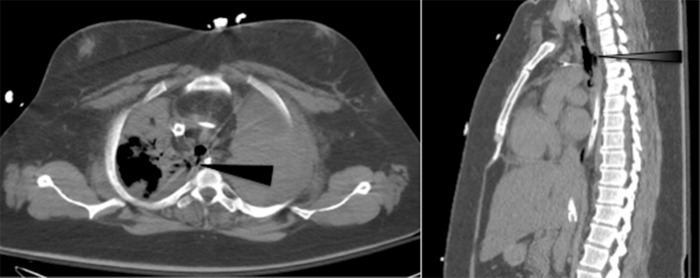
On the following day, bronchoscopy (Figure 2) showed a 3-4 cm injury of the posterior membranous trachea approximately 1 cm above the carina, confirmed by computed tomography (Figure 3).
Figure 2.
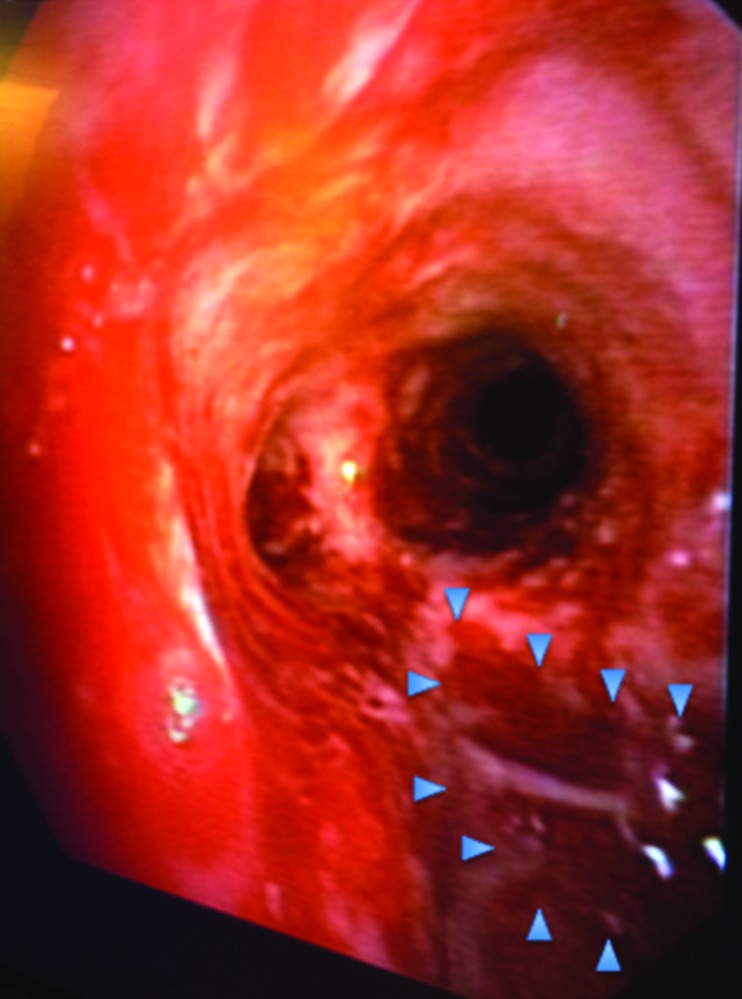
Bronchoscopy confirms membranous defect 1 cm above the carina.
Figure 3.
Axial (A) and sagittal (B) computed tomography images demonstrating 3-4 cm defect in posterior membranous trachea (arrow).
Thoracic surgery was consulted for evaluation of the trachea injury, but the patient was deemed a poor surgical candidate due to severe ARDS requiring ECMO with high positive pressure ventilation.
The patient began to show failure of ARDSnet conventional ventilation (TV = 220-330 cc PEEP 10) with worsening air leak around the endotracheal tube despite of inflation of the cuff, air leak from the chest tube, pneumothorax, pneumomediastinum, and CO2 retention on ARDSNet ventilation (ABG: pH 7.29, PaCO2 59, PaO2 147 and saturation 99%); high frequency oscillatory ventilation (HFOV, setting mean airway pressure 18 cm H2O, amplitude (ΔP) 75 cm H2O. frequency 5 Hz, FiO2 50%.) was initiated. Pneumothorax, pneumomediastinum and chest tube air leak improved, as well as the ABG was improved with the HFOV (pH 7.37, PaCO2 39, PaO2 116, saturation 99%).
The tracheal tear was evaluated daily by bronchoscopy with airway lavage to prevent mucous accumulation. With pneumothorax, pneumomediastinum, and air leak from the chest tube stabilized, on ECMO day 14 and 11 total days of HFOV support, the patient was successfully weaned back to ARDSNet protocol ventilation from HFOV. With ongoing poor lung recruitment and progression of pulmonary infiltrate, the patient was transitioned to prone position ventilation, using the automated RotoProne system (KCI, San Antonio, TX). In prone position, ABG improved to pH 7.46, PaCO2 34, PaO2 105, saturation 98% from pH 7.41, PaCO2 43, PaO2 67, saturation 93% while in supine. Per protocol, the patient was continually rotated to at least 40 degrees for a minimum of 18 hours per day with a maximum prone interval of 3 hours and 15 minutes, interrupted with 45 minutes periods of supine positioning for general nursing care and to minimize facial edema.
The patient’s sedation was maintained with ketamine, fentanyl, and midazolam and she was paralyzed with rocuronium. Enteral nutrition was continued while the patient was paralyzed through a post-pyloric nasal-duodenal tube to minimize risk for aspiration.
The patient’s pulmonary status improved with prone positioning after 6 days and she was successfully weaned and decannulated from VV ECMO on day 20.
At the time of decannulation, bronchoscopy showed improvement of the tracheal injury and esophagogastroscopy confirmed no communication with the esophagus. On day 5 post-decannulation, the patient had a tracheostomy and gastrostomy tube placed for long-term ventilation weaning. On day 20 post decannulation, she was tolerating tracheostomy collar and transferred out of the intensive care unit to the ward. On day 28 post-decannulation, the patient underwent swallow evaluation and was cleared for a diet, tolerating a Passy-Muir valve (Passy-Muir, Irvine, CA).
The patient worked well with physical therapy, ambulating with assistance and was transferred to a rehabilitation facility on post decannulation day 48.
The data presented in this paper was collected under approval from Thomas Jefferson University Internal Review Board.
Discussion
Concurrent acute hypoxic respiratory failure secondary to infection with H1N1 influenza and post-intubation tracheal injury pose a difficult management dilemma due to the requirement for long term positive pressure ventilation. During endotracheal intubation, superficial mucosal tears occur in 18% of patients; however, full thickness post-intubation tracheal injury like this case is rare. Patient risk factors for post-intubation tracheal injury include female gender, short stature ( ≤ 160 cm tall ), difficult airway anatomy, underlying connective tissue disorder and mechanical risk factors include use of rigid stylet, inadequate intubation tube size, cuff over-inflation, emergent intubation, and intubation by non-anesthesiologists. Our patient filled many of these criteria. Emergency intubation and delayed diagnosis have been identified as independent risk factors for mortality after post-intubation tracheal injury [2]. Initial presentation is most often characterized by subcutaneous emphysema, pneumothorax, persistent air leak and hemoptysis-less commonly pneumomediastinum, angina, hypotension and shock [2, 4]. Pneumothorax and pneumomediastinum first aroused suspicion for tracheal injury in our patient.
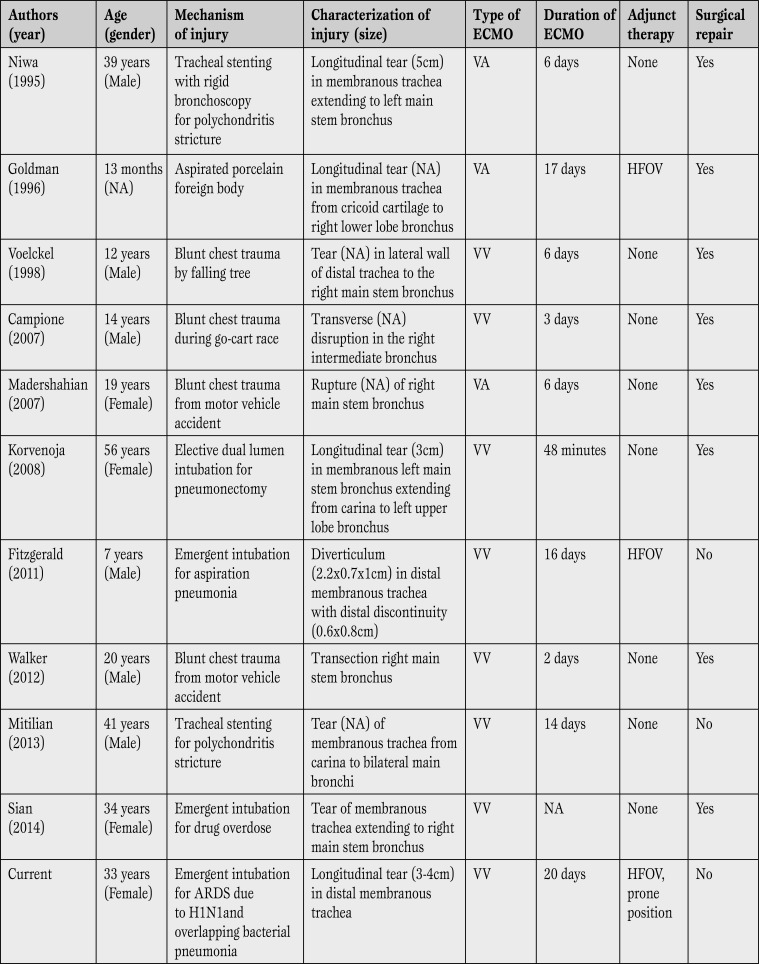
ECMO management for isolated tracheal injury has been previously described, in 10 published reports - 4 pediatric patients and 6 adults (Table 1) [5,6,7,8,9,10,11,12,13,14].
Table 1.
Summary of case reports of tracheal injuries managed by extracorporeal membrane oxygenation.
ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; NA = not available; VA = venoarterial; VV = Venovenous; HFOV = high frequency oscillatory ventilation.
Early reports utilized veno-arterial cannulation, but the majority of patients had isolated pulmonary failure secondary to tracheal tear, requiring only VV ECMO. The majority of iatrogenic injuries resulted in a distinct longitudinal injury to the membranous portion of the trachea, as opposed to blunt chest trauma that resulted in transverse injury with irregular borders [3]. Blunt chest trauma usually required surgical repair, likely due to the nature of tracheal injury especially if anticoagulation and ECMO is required for support [7, 9,10,11,12]. In these papers ECMO support varied from 48min to 20days (Table 1). Although there is likely publication bias, the results from these isolated reports show ECMO to be a promising strategy for tracheal injury with potential for positive outcomes.
Retrospective review has shown a two-fold increase in mortality for surgical management of tracheal injuries detected outside the operating room [2]. Conservative management strategies include low tidal volume ventilation, permissive hypercapnia, endotracheal tube fixation in the distal trachea, double lumen intubation, daily bronchoscopy, HFOV, and even ECMO, as in our case [4]. Early reports utilized these strategies only as a bridge to definitive surgical management. However, current strategies have shifted to reserve surgery only for cases that have failed conservative management - signified by worsening mechanical ventilation requirements, uncontrolled air leak, and active endobronchial bleeding [3, 4]. Surgery for patients requiring ECMO poses added risk due to anticoagulation requirements. Conservative therapy is particularly successful in small injuries, less than 2 cm, with minimal non-progressive symptoms, and no air leakage on spontaneous breathing [2].
VV ECMO provides oxygenation and ventilation allowing for minimal ventilatory support and reduced associated baro- and volu-trauma to the injured bronchial tree. However, VV ECMO requires the lungs to participate in the oxygenation process. Failed oxygenation on VV ECMO may require transition to VA ECMO in order to fully rest the lungs and bronchial tree. In our case, VA ECMO was not ideal due concern to increased infectious risk with femoral cannulation in an morbidly obese patient. In combination with VV ECMO, rescue ventilation therapies may further minimize barotrauma to the injured trachea [6, 11]. Important adjunct therapies to aid in the healing of the tracheal injury in our patient were prone position ventilation and high frequency oscillatory ventilation. A recent randomized control trial showed the benefit of prone positioning on survival in ARDS, but evidence for use with HFOV and concomitant tracheal injury is limited [15].
Tracheal injury, especially in the setting of underlying severe lung injury, is rare and has a high mortality rate. The management has evolved over time, with a movement toward conservative management due to the high morbidity and mortality associated with surgical repair. Here we present a conservative management strategy that incorporates the newest advances in pulmonary critical care, and the first report of successful management of an adult patient with concomitant ARDS, MSSA super-infection and post-intubation tracheal injury with VV ECMO, HFOV and prone position ventilation.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Johnson AP , Cavarocchi NC, Hirose H. Ventilator strategies for VV ECMO management with concomitant tracheal injury and H1N1 influenza. Heart, Lung and Vessels. 2015;7(1):74-80
References
- Peek G L, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany M M. et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- Miñambres E, Burón J, Ballesteros M A, Llorca J, Muñoz P, González-Castro A. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg. 2009;35:1056–1062. doi: 10.1016/j.ejcts.2009.01.053. [DOI] [PubMed] [Google Scholar]
- Conti M, Pougeoise M, Wurtz A, Porte H, Fourrier F, Ramon P. et al. Management of postintubation tracheobronchial ruptures. Chest. 2006;130:412–418. doi: 10.1378/chest.130.2.412. [DOI] [PubMed] [Google Scholar]
- Singh S, Gurney S. Management of post-intubation tracheal membrane ruptures: A practical approach. Indian J Crit Care Med. 2013;17:99–103. doi: 10.4103/0972-5229.114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Masaoka A, Yamakawa Y, Fukai I, Kiriyama M, Shindou J. Esophageal tracheobronchoplasty for membranous laceration caused by insertion of a dumon stent--maintenance of oxygenation by percutaneous cardiopulmonary support. Euro J Cardiothorac Surg. 1995;9:213–215. doi: 10.1016/s1010-7940(05)80148-4. [DOI] [PubMed] [Google Scholar]
- Goldman A P, Macrae D J, Tasker R C, Edberg K E, Mellgren G, Herberhold C. et al. Extracorporeal membrane oxygenation as a bridge to definitive tracheal surgery in children. J Pediatr. 1996;128:386–388. doi: 10.1016/s0022-3476(96)70289-5. [DOI] [PubMed] [Google Scholar]
- Voelckel W, Wenzel V, Rieger M, Antretter H, Padosch S, Schobersberger W. Temporary extracorporeal membrane oxygenation in the treatment of acute traumatic lung injury. Can J Anaesth. 1998;45:1097–1102. doi: 10.1007/BF03012399. [DOI] [PubMed] [Google Scholar]
- Campione A, Agostini M, Portolan M, Alloisio A, Fino C, Vassallo G. Extracorporeal membrane oxygenation in respiratory failure for pulmonary contusion and bronchial disruption after trauma. J Thorac Cardiovasc Surg. 2007;133:1673–1674. doi: 10.1016/j.jtcvs.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Madershahian N, Wittwer T, Strauch J, Franke U F, Wippermann J, Kaluza M. et al. Application of ECMO in multitrauma patients with ARDS as rescue therapy. J Card Surg. 2007;22:180–184. doi: 10.1111/j.1540-8191.2007.00381.x. [DOI] [PubMed] [Google Scholar]
- Korvenoja P, Pitkänen O, Berg E, Berg L. Veno-venous extracorporeal membrane oxygenation in surgery for bronchial repair. Ann Thorac Surg. 2008;86:1348–1349. doi: 10.1016/j.athoracsur.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J C, Topjian A A, McInnes A D, Mattei P, McCloskey J J, Friess S H. et al. Bi-caval dual lumen venovenous extracorporeal membrane oxygenation and high-frequency percussive ventilatory support for postintubation tracheal injury and acute respiratory distress syndrome. J Pediatr Surg. 2011;46:11–15. doi: 10.1016/j.jpedsurg.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Walker J L, Wiersch J, Benson C, Young H A, Dearmond D T, Johnson S B. The successful use of cardiopulmonary support for a transected bronchus. Perfusion. 2012;27:34–38. doi: 10.1177/0267659111420321. [DOI] [PubMed] [Google Scholar]
- Mitilian D, Gonin F, Sage E, Beurtheret S. From relapsing polychondritis to extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2013;146:49–51. doi: 10.1016/j.jtcvs.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Sian K, McAllister B, Brady P. The use of extracorporeal membrane oxygenation therapy in the delayed surgical repair of a tracheal injury. Ann Thorac Surg. 2014;97:338–340. doi: 10.1016/j.athoracsur.2013.04.126. [DOI] [PubMed] [Google Scholar]
- Guérin C, Reignier J, Richard J C, Beuret P, Gacouin A, Boulain T. et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]