Abstract
Prefrontal cortical dysfunction is thought to underlie maladaptive behaviors displayed by chronic drug users, most notably the high propensity for relapse that severely impedes successful treatment of drug addiction. In animal models of drug relapse, exposure to drug-associated stimuli, small amounts of drug, and acute stressors powerfully reinstate drug seeking by critically engaging the prefrontal cortex, with the anterior cingulate, prelimbic, infralimbic, and orbitofrontal subregions making distinct contributions to drug seeking. Hence, from an addiction treatment perspective, it is necessary to fully explicate the involvement of the prefrontal cortex in drug relapse.
Keywords: prefrontal cortex, anterior cingulate, prelimbic, infralimbic, orbitofrontal, extinction, reinstatement, drug seeking
1 Introduction
Clinical studies suggest that structural, physiological, and functional abnormalities in the prefrontal cortex facilitate drug craving and drug seeking, which can be triggered by drug-associated environmental stimuli, small amounts of drug, or stress (Ehrman et al. 1992; Foltin and Haney 2000; Rohsenow et al. 2007). The transition from recreational drug use to drug addiction may be related to neural predisposition to drug addiction or neural plasticity resulting from prolonged drug exposure (Franklin et al. 2002; Volkow et al. 2002). Chronic drug users typically present with decreased gray matter density and reduced baseline blood glucose metabolism in the frontal cortex (London et al. 1999; Volkow and Fowler, 2000; Franklin et al. 2002; Matochik et al. 2003). At the same time, frontal cortical regions of drug users exhibit heightened metabolic activity upon exposure to relapse triggers, which is positively correlated with the intensity of self-reported craving (Grant et al. 1996; Breiter et al. 1997; Childress et al. 1999; Garavan et al. 2000; Bonson et al. 2002; Sinha and Li 2007). In addition to the neural correlates of drug seeking identified in human studies, preclinical studies in rodents directly support the idea that prefrontal cortical subregions make distinct contributions to relapse behaviors (see Table 1), as will be described in this chapter.
Table 1.
PFC Subregional Contribution to Drug-seeking Behavior
| Sub-region | Drug | CS/Extinction | Context/Extinction | Abstinence | Drug Priming | Stress |
|---|---|---|---|---|---|---|
| ACC/PL | Cocaine | Arc ↑, Fos ↑ | Fos ↑, Arc ↑, RGS4 ↑, DAT ↑ | |||
| TTX ↓ | TTX ↓ | BM – | BM ↓, TTX ↓, DA antagonists ↓–, PL lesion ↓ | BM ↓, TTX ↓, D1 antagonist ↓, D2 antagonist – | ||
| Methamphetamine | lidocaine ↓, nACh agonist ↓ | lidocaine ↓, nACh agonist ↓ | ||||
| Heroin | ania-3 ↑, MKP-1 ↑, zif268 ↑, c-fos ↑, Nr4a3 ↑ | Fos ↑ | ||||
| B/M ↓↑ | B/M ↓ | |||||
| Ethanol | Fos ↑ | |||||
| IL | Cocaine | Fos ↑ | Fos ↑, pERK ↑ | |||
| TTX – | TTX – | B/M ↓ | TTX –, BM – | TTX –, BM – | ||
| Methamphetamine | lidocaine – | lidocaine – | ||||
| Heroin | AMPA GluR2 ↓ | |||||
| BM ↓, CB1 antagonist ↓ | BM ↓ | |||||
| OFC | Cocaine | Arc ↑ | Fos ↑ | |||
| lesion –, lOFC: BM ↓ mOFC: BM – | lOFC: BM ↓, lesion ↑ mOFC: BM – | BM –, TTX –, lOFC: lesion ↑ mOFC: lesion ↓ | TTX ↓, D1 antagonist ↓, D2 antagonist – | |||
| Heroin | ania-3 ↑ |
↑ denotes an increase, ↓ denotes a decease, and – denotes no observed change, in gene or protein expression or in drug-seeking behavior. Abbreviations: anterior cingulate cortex (ACC), baclofen plus muscimol (BM), canabinoid 1 (CB1), dopamine transporter protein (DAT), dopamine 1 (D1), dopamine 2 (D2), infralimbic cortex (IL), lateral orbitofrontal cortex (lOFC), medial orbitofrontal cortex (mOFC), nicotinic cholinergic (nACh), phospho-extracellular-related kinase (p-ERK), prelimbic cortex (PL), regulator of G-protein signaling 4 (RGS4), tetrodotoxin (TTX).
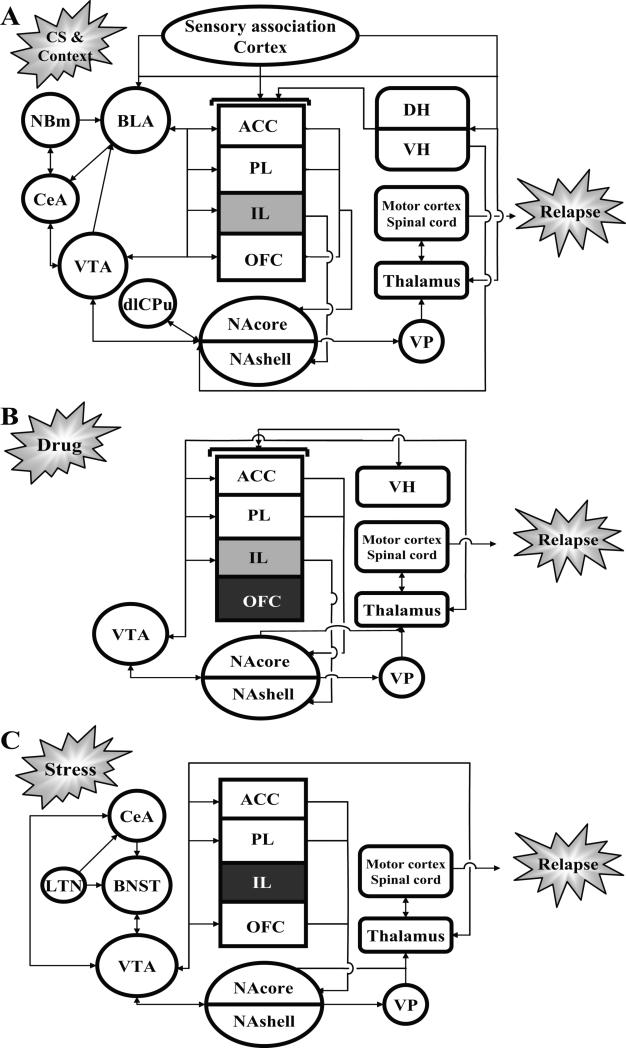
Based on its connectivity, the prefrontal cortex is ideally positioned to receive information about the salience and motivational significance of relapse triggers from limbic and sensory areas and, in turn, to exert executive control over the selection and initiation of drug-seeking behavior via its output to the motor system. The rodent prefrontal cortex is an aggregate of several cortical regions, including the anterior cingulate (ACC), prelimbic (PL), infralimbic (IL), and orbitofrontal cortices (OFC). These prefrontal cortical subregions all receive input from the mediodorsal thalamus (Uylings and van Eden 1990) but make distinct contributions to drug seeking likely based on their differential connectivity with other elements of the cue-induced, drug-induced, and stress-induced relapse circuitries summarized in Figure 1 (for detailed reviews, see Shaham et al. 2003; Schmidt et al. 2005; Feltenstein and See 2008). Importantly, future studies will need to verify the existence of functionally significant interconnections between the circuitry components depicted in the figure.
Fig. 1.
Schematic illustrating the putative neural circuitries of drug-seeking behavior produced by drug-associated environmental stimuli (A), drug itself (B), and stress (C) in rodent models of drug relapse. The anterior cingulate (ACC), prelimbic (PL), infralimbic (IL), and orbitofrontal (OFC) subregions of the prefrontal cortex differentially contribute to these forms of drug seeking. Black shading denotes a lack of demonstrated contribution to cocaine seeking, whereas gray shading denotes limited contribution. Additional abbreviations: basolateral amygdala (BLA), bed nucleus of the stria terminalis (BNST), central amygdaloid nucleus (CeA), dorsal hippocampus (DH), dorsolateral caudate-putamen (dlCPu), lateral tegmental nucleus (LTN), nucleus accumbens core (NAcore), nucleus accumbens shell (NAshell), nucleus basalis of Mynert (NBm), ventral hippocampus (VH), ventral pallidum (VP), ventral tegmental area (VTA).
2 Environmental Stimulus-induced Relapse
Several animal models of cue-induced drug relapse have been developed to assess the neural correlates of incentive motivation for drug elicited by drug-paired environmental stimuli. In these paradigms, rodents are trained to self-administer a drug of abuse by exhibiting an instrumental response. Over the course of self-administration training, drug effects are paired with either the response-contingent presentation of explicit conditioned stimuli (CS) or passive contextual stimulus exposure. Through associative learning processes, these previously neutral stimuli acquire conditioned reinforcing and/or incentive motivational properties, respectively, which permit them to elicit drug seeking in the absence of drug reinforcement (Fuchs et al. 2005; Crombag et al. 2008). Similar to the results from the human neuroimaging studies discussed above, preclinical studies strongly implicate the prefrontal cortex in cue-induced drug seeking. Additionally, these studies suggest that prefrontal cortical subregions exhibit a subregion-specific involvement in mediating drug seeking, depending on the type of cues being utilized and whether drug seeking is assessed following explicit extinction training or after experimenter-enforced abstinence.
2.1 Relapse following Extinction
Numerous studies suggest that drug-associated CSs or environmental contexts critically activate regions of the prefrontal cortex to reinstate extinguished drug seeking. CS-induced cocaine seeking is associated with enhanced Fos protein expression in the ACC and enhanced Arc mRNA expression in the ACC, PL, and OFC (Neisewander et al. 2000; Ciccocioppo et al. 2001; Zavala et al. 2008a). Similarly, CS-induced heroin seeking is paralleled by increased mRNA levels for several immediate-early genes, including c-fos, ania-3, MKP-1, and Nr4a3 in the ACC/PL; zif268 in the PL; and ania-3 in the OFC (Schmidt et al. 2005; Koya et al. 2006). Although a cocaine-associated CS fails to alter Fos expression in the IL, cocaine-context re-exposure enhances IL Fos expression in animals that exhibit context-induced drug seeking (Hamlin et al. 2008; Zavala et al. 2008a). Furthermore, drug context-induced ethanol-seeking is related to enhanced Fos protein expression in the ACC/PL (Dayas et al. 2007). While changes in immediate-early gene expression are rarely observed in saline-yoked control subjects exposed to cues or in drug-trained subjects not exposed to cues or levers (but see Hamlin et al. 2007), re-exposure to a distinct drug-paired context enhances Fos protein expression in the ACC/PL in rats with a history of passive cocaine, morphine, or nicotine treatment (Franklin and Druhan 2000; Schroeder et al. 2000, 2001; Schroeder and Kelley 2002). Thus, future studies will need to ascertain whether alterations in downstream signaling signify cue-induced incentive motivation or neuroplasticity resulting from drug exposure or behavioral experience, as each of these may influence the involvement of the prefrontal cortex in cue-induced drug seeking.
2.1.1 Anterior Cingulate and Prelimbic Cortex
Performing independent pharmacological manipulations of the ACC and PL has been technically challenging due to the location and proximity of these brain regions. Thus, despite putative differences in connectivity and function, the ACC and PL have not been consistently differentiated in experimental studies of the dorsomedial prefrontal cortex and, as a result, are discussed together here. These pharmacological studies provide direct evidence that the ACC/PL critically regulates cue-induced reinstatement of extinguished drug seeking. McLaughlin and See (2003) first demonstrated that tetrodotoxin (TTX)-induced inactivation of the ACC or PL significantly impairs CS-induced cocaine seeking. Subsequent studies employing lidocaine or baclofen plus muscimol (BM) infusions to achieve neural inactivation have further indicated that the PL mediates CS-induced motivation for methamphetamine and heroin (Hiranita et al. 2006; LaLumiere and Kalivas 2008; Rogers et al. 2008, but see Schmidt et al. 2005). Furthermore, dopamine and acetylcholine neurotransmission in the PL appears to be necessary for CS-induced reinstatement of cocaine and methamphetamine seeking, respectively (Ciccocioppo et al. 2001; Hiranita et al. 2006). In turn, the PL may initiate CS-induced drug seeking through glutamatergic innervation of the nucleus accumbens core (NAcore) given that BM-induced PL inactivation prevents heroin cue-induced drug seeking and concomitant glutamate release within the NAcore, while AMPA/kainate glutamate receptor antagonism in the NAcore abolishes CS-induced heroin seeking (LaLumiere and Kalivas 2008). Although drug-associated contexts engage a distinct neural circuitry relative to drug-paired CSs, TTX-induced ACC/PL inactivation similarly impairs both context-induced and CS-induced cocaine seeking, with the former depending on functional interaction between the ACC/PL and basolateral amygdala (Fuchs et al. 2005, 2007).
2.1.2 Infralimbic Cortex
To date, studies suggest that the IL plays a differential role in CS-induced motivation for different drugs of abuse. While the ACC/PL sends projections to the NAcore, a structure that promotes CS-induced reinstatement of cocaine seeking, the IL preferentially innervates the NAshell, which is not critical for this behavior (Sesack et al. 1989; McFarland and Kalivas, 2001; Fuchs et al. 2004a). Consistent with this, IL inactivation achieved through TTX or lidocaine infusions fails to alter CS-induced cocaine or methamphetamine seeking, respectively (McLaughlin and See, 2003; Hiranita et al. 2006). However, the neural circuitry underlying CS-induced reinstatement of heroin seeking may engage a wider neural network than that subserving cocaine or methamphetamine seeking. In support of this, BM-induced IL inactivation prevents CS-induced heroin seeking, an effect potentially mediated by cannabinoid 1 receptor stimulation (Rogers et al. 2008; Alvarez-Jaimes et al. 2008). Furthermore, self-administered heroin enhances AMPA receptor internalization in the IL, and this neural adaptation appears to facilitate CS-induced heroin seeking (Van der Oever et al. 2008). While the NAshell is involved in context-induced cocaine seeking, the IL does not mediate this behavior given that TTX inactivation of the IL fails to alter reinstatement elicited by a previously cocaine-paired context (Fuchs et al. 2005, 2008).
2.1.3 Orbitofrontal Cortex
The OFC may play a unique role in drug seeking given that damage to this structure produces irresponsibility, impulsivity, and perseveration in human drug users (Bechara et al. 1994). Moreover, it is a functionally heterogeneous brain region comprised of medial and lateral subregions. Fiber-sparing lesions or BM-induced inactivation of the medial OFC fail to alter CS- or context-induced cocaine seeking. In contrast, BM-induced inactivation of the lateral OFC, which is the putative functional homolog of the human medial OFC, attenuates both CS- and drug context-induced cocaine seeking (Gallagher et al. 1999; Fuchs et al. 2004b; Lasseter et al. 2008). Further, lesions of the lateral OFC made prior to self-administration training do not alter CS-induced cocaine seeking, but potentiate cocaine-context-induced reinstatement. One possible explanation for these seemingly discrepant findings is that OFC lesion-induced neural adaptations have different effects on cocaine seeking based on cue type (Fuchs et al. 2004b; Lasseter et al. 2008).
2.2 Relapse following Abstinence
While the previous sections discussed the role of the prefrontal cortex in cue-induced reinstatement of extinguished drug-seeking behavior, human drug users rarely undergo explicit extinction training. Because extinction training is an active learning process that induces neurobiological changes due to learning-induced neuroplasticity, different neural substrates may underlie drug seeking following extinction training versus drug-free abstinence periods (Self and Nestler 1998; Self et al. 2004). For example, while the ACC/PL critically regulates drug seeking following extinction training, its influence is diminished following abstinence (Fuchs et al. 2006). Moreover, the duration of the abstinence period may profoundly impact relapse behaviors given that enhanced drug seeking is observed following longer drug-free periods (Tran-Nguyen et al. 1998; Grimm et al. 2001).
2.2.1 Anterior Cingulate and Prelimbic Cortex
Enduring neural adaptations are present in the ACC/PL even after extended abstinence periods, which may be sufficient to facilitate cocaine seeking. After prolonged abstinence, exposure to a cocaine-associated context, discriminative stimuli, and/or CS produces robust cocaine seeking and enhances Fos and Arc expression in the ACC as well as Fos protein expression and dopamine transporter levels in the ACC/PL (Ciccocioppo et al. 2001; Grimm et al. 2002; Schwendt et al. 2007; Hearing et al. 2008). Similar regimens also decrease levels of regulator of G-protein signaling 4 in the ACC/PL, which are normalized following re-exposure to a drug-associated context (Schwendt et al. 2007). Fos-mediated cue-induced neuronal activation may involve AMPA glutamate receptor signaling in a subregion-specific manner within the prefrontal cortex because cue-induced cocaine seeking is correlated with significant Fos and GluR1 subunit co-expression in the ACC and Fos and GluR4 subunit co-expression in the IL (Zavala et al. 2007). Thus, cue-induced AMPA receptor-mediated signal transduction in the ACC and IL may involve alternate signaling pathways. In particular, AMPA receptor stimulation in the ACC may facilitate cocaine seeking following abstinence given that systemic AMPA receptor antagonism attenuates this behavior and decreases Fos protein expression in the ACC (Zavala et al. 2008b). Neuroplasticity that occurs in the prefrontal cortex during early abstinence may subsequently promote drug seeking. Consistent with this, acute brain derived neurotrophic factor (BDNF) administration into the ACC/PL after self-administration training disrupts cue-induced, as well as drug-primed, cocaine seeking following 6 days of abstinence and simultaneously enhances BDNF immunoreactivity and normalizes phospho-extracellular-related kinase (p-ERK) levels in the NAcore (Berglind et al. 2007).
2.2.2 Infralimbic cortex
The IL may facilitate cue-induced cocaine seeking following extended abstinence, even though it does not play a critical role in this behavior following extinction training, as discussed above. Re- exposure to cocaine-associated cues increases p-ERK levels in the IL after 30 days, but not 1 day, of abstinence (Koya et al. 2008). Furthermore, infusions of gamma-aminobutyric acid (GABA) agonists into the IL/PL transition area attenuate, while infusions of GABA antagonists enhance, this behavior following 30 days of abstinence (Koya et al., 2008). However, these effects may be partially mediated by the PL. In contrast, Peters et al. (2008a, 2008b) has reported that GABA agonist-induced IL neural inhibition is sufficient to reinstate cocaine seeking after extended extinction training and also facilitates spontaneous recovery. Hence, while the IL may promote drug seeking following abstinence, it may also be recruited during extinction training to inhibit the same behavior.
2.2.3 Orbitofrontal cortex
Drug-associated cues may recruit the OFC following extended drug-free periods in part via the stimulation of AMPA receptors. After abstinence, the OFC exhibits cue-induced Fos-mediated neuronal activation in conjunction with cocaine seeking, and systemic AMPA receptor antagonism attenuates cocaine seeking and decreases Fos protein expression in the OFC (Zavala et al. 2008b).
3 Drug-primed Relapse
Acute re-exposure to drugs of abuse precipitates drug craving and increases the probability of relapse in abstinent drug users (Jaffe et al. 1989). Similarly, preclinical studies verify that after self-administration and extinction training, an acute drug priming injection elicits robust reinstatement of drug seeking as well as renewal of extinguished conditioned place preference, both of which are thought to reflect motivation for drug. Numerous studies utilizing these animal models further demonstrate that drug-primed drug seeking engages the prefrontal cortex.
3.1 Anterior Cingulate and Prelimbic Cortex
The ACC/PL is critical for the ability of drug-priming injections to reinstate drug seeking for a variety of drugs of abuse, including cocaine, heroin, and methamphetamine (McFarland and Kalivas 2001; Capriles et al. 2003; Hiranita et al. 2006). For instance, ACC/PL inactivation induced by BM or TTX infusions impairs drug-primed heroin and cocaine seeking without producing nonspecific disruption in other goal-directed behaviors (McFarland and Kalivas 2001; Capriles et al. 2003; Rogers et al., 2008). Similarly, selective excitotoxic lesions of the PL attenuate cocaine-primed reinstatement of conditioned place preference (Zavala et al., 2003), while lidocaine-induced inactivation or nicotinic cholinergic receptor stimulation impairs methamphetamine-primed reinstatement (Hiranita et al. 2006).
Using functional disconnection techniques, McFarland and Kalivas (2001) have demonstrated that the ventral tegmental area, ACC/PL, NAcore, and ventral pallidum form a serial circuit that mediates cocaine-primed cocaine seeking. While similar circuitry mapping studies have not been conducted for other drugs of abuse, enhanced dopamine neurotransmission in the ACC/PL appears to initiate drug seeking in rats with a history of psychomotor stimulant self-administration. Infusions of dopamine, cocaine, or amphetamine into the ACC/PL can elicit cocaine seeking, while D1 or D2 dopamine receptor antagonists disrupt both cocaine-primed reinstatement of cocaine seeking and conditioned place preference (McFarland and Kalivas 2001; Park et al. 2002; Sanchez et al. 2003; Sun and Rebec 2005; but see Capriles et al. 2003).
Studies utilizing in vivo microdialysis suggest that glutamate input from the ACC/PL into the NAcore represents the critical pathway underlying drug-primed reinstatement of drug seeking. While drug priming injections substantially elevate glutamate release in the NAcore, BM-induced ACC/PL inactivation attenuates both cocaine- and heroin-primed drug seeking while simultaneously preventing the increase in glutamate release in the NAcore (McFarland et al. 2003; LaLumiere and Kalivas 2008).
3.2 Infralimbic Cortex
The IL appears to be involved in drug-primed reinstatement in a drug-dependent fashion. It does not mediate psychomotor stimulant-primed reinstatement given that cocaine-primed drug seeking is unimpaired by either BM- or TTX-induced IL inactivation, and methamphetamine-primed reinstatement is not altered by lidocaine infusions into the IL (McFarland and Kalivas 2001; Capriles et al. 2003; Hiranita et al. 2006). Interestingly, however, BM-induced IL inactivation impairs heroin-primed reinstatement of drug seeking, suggesting that drug-primed heroin seeking engenders wider prefrontal cortical recruitment than drug-primed cocaine seeking (Rogers et al. 2008).
3.3 Orbitofrontal Cortex
The involvement of the OFC in drug-primed reinstatement of cocaine seeking is unclear. TTX- or BM-induced inactivation of the OFC fails to attenuate cocaine-primed reinstatement (Capriles et al. 2003; Fuchs et al. 2004b) . In contrast, lesions of the lateral OFC, but not the medial OFC, increase cocaine-primed reinstatement in a perseverative manner (Fuchs et al. 2004b). This suggests that prolonged loss of lateral OFC output may potentiate cocaine seeking by prompting compensatory neuroadaptations in the drug-primed reinstatement circuitry (Fuchs et al. 2004b).
4 Stress-induced Relapse
Psychological stress plays an important role in the initiation and maintenance of drug use. Stress can produce drug craving in current cocaine users under laboratory conditions (Sinha et al. 1999). Similarly, acute stressors, such as footshock, restraint stress, food deprivation, and pharmacological manipulations, including corticotrophin-releasing factor, metyrapone, and yohimbine, reliably reinstate drug seeking in laboratory animals after prolonged drug-free periods (Shaham and Stewart 1995; Erb et al. 1996; for review, see Shaham et al. 2000). Both clinical and preclinical studies have implicated the prefrontal cortex in stress-induced relapse behaviors. However, perhaps due to fundamental differences between psychological and acute stress or methodological factors, human neuroimaging studies suggest stress-induced drug craving stems from diminished stress-induced frontal cortical activation in former drug users, while animals studies suggest stress-induced heroin seeking is correlated with enhanced PL Fos protein expression (Shalev et al. 2003; Sinha et al. 2005).
4.1 Anterior Cingulate and Prelimbic Cortex
Dopamine neurotransmission in the ACC/PL critically mediates stress-induced reinstatement. BM- or TTX-induced inactivation of, as well as dopamine receptor antagonism within, the ACC/PL or PL alone inhibits the ability of footshock and restraint stress to reinstate cocaine seeking or cocaine-conditioned place preference (Capriles et al. 2003; McFarland et al. 2003; Sanchez et al. 2003). Furthermore, it has been theorized that within the larger stress-induced reinstatement circuitry, glutamatergic input from the ACC/PL to the NAcore may initiate stress-induced reinstatement following analysis of inputs from the extended amygdala (McFarland et al. 2003).
Stressors are well-known to activate the central noradrenergic system, and this system may play a specific role in mediating drug seeking produced by acute stress. Systemic injections of alpha-2 adrenergic receptor agonists prevent footshock-induced reinstatement of cocaine seeking and inhibit stress-induced norepinephrine release within the ACC/PL (Erb et al. 2000). However, additional research is necessary to explore the direct involvement of ACC/PL norepinephrine release in this behavioral phenomenon.
4.3 Infralimbic Cortex
The IL does not appear to critically regulate stress-induced reinstatement of drug seeking. Neither BM- nor TTX-induced inactivation of the IL alters footshock-induced reinstatement of cocaine seeking (McFarland et al. 2004; Capiles et al. 2003). However, the possible involvement of the IL in the ability of stress to elicit other forms of drug-seeking behavior has yet to be explored.
4.4 Orbitofrontal Cortex
Dopamine in the OFC plays a critical role in footshock-induced drug seeking. Consistent with this, TTX-induced inactivation of the lateral OFC impairs footshock-induced reinstatement of cocaine seeking, while intra-OFC microinfusion of D1-like, but not D2-like, dopamine receptor antagonists have a similar effect on this behavior (Capriles et al. 2003).
5 Concluding Remarks
Research utilizing rodent models of drug relapse has demonstrated that prefrontal cortical subregions make distinct contributions to relapse behaviors. The ACC/PL exerts critical control over drug seeking elicited by drug-paired CSs, contextual stimuli, small amounts of drug, and stress following extinction training, but it exhibits diminished involvement in drug seeking following abstinence. Unlike the ACC/PL, the OFC does not appear to contribute critically to drug-primed reinstatement of drug seeking even though it facilitates cue-induced and stress-induced relapse behaviors. In further contrast to the ACC/PL, the IL appears to play a role in cue-induced drug seeking following abstinence whereas its involvement in cue-induced and drug-primed drug seeking following extinction training is limited to heroin-seeking behavior. Moreover, the IL does not appear to play a significant role in stress-induced relapse behaviors.
In addition to the evidence indicating that the functional integrity of the prefrontal cortex is necessary for various forms of drug seeking, intriguing molecular adaptations have been identified within the ACC, PL, OFC, and IL as well as other elements of the relapse circuitry in rats following passive drug exposure, drug-self administration, abstinence or extinction training, as well as in conjunction with drug-seeking behaviors (for review, see Kalivas and O'Brien 2008; Thomas et al. 2008). Thus, from an addiction treatment perspective, it will be imperative to ascertain whether these neuroadaptations have functional significance with respect to relapse behaviors.
Index of Abbreviations
- ACC
anterior cingulate cortex
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain derived neurotrophic factor
- BLA
basolateral amygdala
- BM
baclofen and muscimol
- BNST
bed nucleus of the stria terminalis
- CeA
central amygdaloid nucleus
- CS
conditioned stimulus
- DH
dorsal hippocampus dlCPu dorsolateral caudate-putamen
- GABA
gamma-aminobutyric acid
- IL
infralimbic cortex
- LTN
lateral tegmental nucleus
- NA
nucleus accumbens
- NAcore
core region of the nucleus accumbens
- NAshell
shell region of the nucleus accumbens
- NBm
nucleus basalis of Mynert
- OFC
orbitofrontal cortex
- p-ERK
phospho-extracellular-related kinase
- PL
prelimbic cortex
- RGS4
regulator of G-protein signaling 4
- TTX
tetrodotoxin
- VH
ventral hippocampus
- VP
ventral pallidum
- VTA
ventral tegmental area
References
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33(10):2483–93. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26(3):757–66. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, et al. Neural Systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, et al. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;169:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, et al. Limbic activation during cue-elicited cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA. 2001;98(4):1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, et al. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2007;363(1507):3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, et al. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61(8):979–89. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, et al. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107(4):523–29. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstatement cocaine-seeking after prolonged extinction and drug-free periods. Psychopharmacology (Berl) 1996;128(4):408–12. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Phil D, et al. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23(2):138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154(2):261–74. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149(1):24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12(6):2097–106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51(2):134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, et al. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004a;176(3-4):459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, et al. Differential involvement of the orbitofrontal cortex sub-regions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004b;24(29):6600–10. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmocology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–88. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su Z, et al. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Euro J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 2008;200(4):545–56. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19(15):6610–4. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–45. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, et al. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–88. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–70. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct funct. 2008;213:215–27. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, et al. Suppression of methamphetamine-seeking by nicotinic agonists. Proc Natl Acad Sci USA. 2006;103(22):8523–27. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KN, et al. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97(1):59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33(1):166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, et al. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.04.022. doi:10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie Xiaohu, et al. Effect of orbitofrontal cortex lesions and functional inactivation on context-induced and cocaine-primed on cocaine-seeking behaviors in rats. In prep. 2008 [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–77. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Bonson KR, Ernst M, et al. Brain imaging studies of cocaine abuse: implications for medication development. Crit Rev Neurobiol. 1999;13(3):227–42. doi: 10.1615/critrevneurobiol.v13.i3.10. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, et al. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19(3):1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, et al. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking in rats. Psychopharm (Berl) 2003;168(1-2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, et al. Fos protein expression and cocaine-seeking in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Bari AA, Jey AR, et al. Cocaine administered into the medial prefrontal cortex reinstates glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22(7):2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, et al. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008b;197(2):319–26. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–88. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA, et al. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68(5):641–48. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, et al. Manipulation of dopamine D1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Voorn P, Binnekade R, et al. Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. Eur J Neurosci. 2005;22(9):2347–56. doi: 10.1111/j.1460-9568.2005.04435.x. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Holahan MR, Landry CF, et al. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37(2):146–58. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–45. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Kelley AE. Conditioned Fos expression following morphine-paired contextual cue exposure is environment specific. Behav Neurosci. 2002;116(4):727–32. doi: 10.1037//0735-7044.116.4.727. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Hearing MC, See RE, et al. Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport. 2007;18(12):1261–65. doi: 10.1097/WNR.0b013e328240507a. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;1(1-2):49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11(5):648–57. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deuth AY, Roth RH, et al. Topographic organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoaglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharm. 1995;119:334–41. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Research Reviews. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L. The reinstatement model of drug relapse: history, methodology, and major findings. Psychopharmacology (Berl) 2003;168(1-2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Robarts P, Shaham Y, et al. Selective induction of c-Fos immunoreactivity in the prelimbic cortex during reinstatement of heroin seeking induced by acute food deprivation in rats. Behav Brain Res. 2003;145(1-2):79–88. doi: 10.1016/s0166-4328(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Mally S. Stress-induced craving and stress responses in cocaine dependent individuals. Psychopharm. 1999;142:343–51. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183(2):171–80. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, et al. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526(1-3):65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking in rats. Psychopharm. 2005;177:315–23. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008 May;154(2):327–42. doi: 10.1038/bjp.2008.77. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 2008;19(1):48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Wan Li K, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008 Aug 1; doi: 10.1038/nn.2165. 2008 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78(3):610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, et al. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Research. 2003;990:157–64. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, et al. Fos and Glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking in abstinent rats. Neuroscience. 2007;145:438–52. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, et al. Upregulation of the Arc expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking. Synapse. 2008a;62:421–31. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Browning JR, Dickey, et al. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur J Neurosci. 2008b;18:600–11. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]