Abstract
RATIONALE
The functional integrity of the dorsal hippocampus (DH) is necessary for drug context-induced reinstatement of cocaine seeking. However, the neuropharmacological mechanisms of this phenomenon are poorly understood.
OBJECTIVES
Given the known significance of group I metabotropic glutamate receptors (group I mGluRs), including the mGluR1 subtype, in drug-induced behaviors, the present study was designed to evaluate the contribution of mGluR1s in the DH to drug context-induced reinstatement of extinguished cocaine-seeking behavior.
METHODS
Sprague-Dawley rats were trained to lever press for unsignaled cocaine infusions in a distinct environmental context (cocaine-paired context) followed by extinction training in a distinctly different environmental context (extinction context). Using a counterbalanced within-subject testing design, rats were re-exposed to the cocaine-paired context or the extinction context while cocaine-seeking behavior (non-reinforced active lever pressing) was assessed. Prior to each test session, rats received bilateral microinfusions of the highly potent mGluR1-selective antagonist JNJ16259685 (0.6, 30 or 120 pg/0.5 µl per hemisphere) or 0.1 % DMSO vehicle into the DH or the overlying somatosensory cortex trunk region (SStr, anatomical control).
RESULTS
Intra-DH, but not intra-SStr, JNJ16259685 infusions dose-dependently attenuated drug context-induced reinstatement of cocaine seeking, without attenuating instrumental behavior in the extinction context, general motor activity, or food-reinforced instrumental behavior in control experiments.
CONCLUSIONS
Stimulation of mGluR1s in the DH is necessary for incentive motivational and/or memory processes that contribute to drug context-induced cocaine-seeking behavior. These findings indicate that the mGluR1 is an interesting target from an addiction treatment perspective.
Keywords: Cocaine, Context, Reinstatement, Doral hippocampus, Metabotropic glutamate receptor
Until recently, little attention has been focused on the contribution of the hippocampus to addictive behaviors, despite the fact that neural activation of this brain region correlates with self-reports of cue-induced craving in drug users (Sell et al. 2000; Wexler et al. 2001; Kilts et al. 2001; Schneider et al. 2001; Franklin et al. 2007). Furthermore, the functional integrity of the dorsal hippocampus (DH) is necessary for the expression of drug context-induced cocaine-seeking behavior in the reinstatement animal model of drug relapse (Fuchs et al. 2005). However, the neuropharmacological mechanisms of this phenomenon are poorly understood.
Group I metabotropic glutamate receptors (mGluRs) have been implicated in a variety of drug-induced and context-induced behaviors (McGeeham and Olive 2003; Gravius et al. 2006), even though it is unclear whether they play a role in drug context-induced cocaine-seeking behavior per se. Group I mGluRs include the mGluR1 and mGluR5 receptor subtypes, which regulate ionotropic glutamate receptor function, multiple forms of neural plasticity, and behavioral sensitization to various drugs of abuse, including cocaine (Bortolotto et al. 1990; Dravolina 2006; Kotlinska and Bochenski 2007). Specifically, stimulation of the mGluR5 subtype is necessary for the acquisition of cocaine-conditioned place preference, maintenance of ethanol self-administration, and the expression of cocaine-seeking and methamphetamine-seeking behaviors in response to an explicit drug-paired conditioned stimulus (CS; McGeehan and Olive 2003; Bespalov et al. 2005; Hodge et al. 2006; Backstrom and Hyytia 2006; 2007; Gass et al. 2008; 2009). Comparatively less is known about the contribution of the mGluR1 subtype to drug-induced behaviors due to the scarcity of selective mGluR1 antagonists. However, a recent report has demonstrated that systemic administration of the mGluR1 antagonist (3-ethyl-2-methyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)-methanone methanesulfonate (EMQMCM) attenuates CS-induced reinstatement of extinguished nicotine-seeking behavior (Dravolina et al. 2007).
The DH is characterized by the highest density of mGluR1s, besides the cerebellum (Masu et al. 1991; Shigemoto et al. 1992; Simonyi et al. 2005). Given that the DH is implicated in drug context-induced reinstatement of cocaine-seeking behavior (Fuchs et al. 2005) and mGluR1s are implicated in some cue-induced behaviors (Dravolina et al. 2007), the present study evaluated the hypothesis that stimulation of mGluR1s within the DH is necessary for drug context-induced reinstatement of cocaine-seeking behavior. To this end, the dose-dependent effects of the highly potent mGluR1 subtype-selective antagonist JNJ16259685 on context-induced reinstatement of cocaine-seeking behavior were assessed in rats. In order to discriminate the putative effects of JNJ16259685 on motivation and motor performance, we also assessed the effects of JNJ16259685 on non-reinforced instrumental responding in a non-cocaine-paired context, on preprandial and postprandial food-reinforced instrumental responding, as well as on motor behavior.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles-River, N = 58) weighing 250–275 g at the time of surgery, were individually housed under a reverse light/dark cycle. They were maintained on 20–25 g of rat chow per day, with water available ad libitum. The housing and treatment of the rats followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources, Commission on Life Sciences 1996), and the study protocol was approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Food training
In order to expedite the acquisition of cocaine self-administration, rats were first trained to lever press under a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Purina, Richmond, IN, USA) in standard sound-attenuated operant-conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA, USA) during a single 16-h overnight food training session. The chambers were equipped with two retractable levers and a food pellet dispenser between the levers. During the session, each lever press on one (active) lever resulted in delivery of one food pellet only. Lever presses on the second (inactive) lever had no programmed consequences. The contextual stimuli used for subsequent conditioning were not present during the food training session.
Surgery
Forty-eight hours after food training, rats were anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.3 mg/kg, i.p., respectively). Chronic indwelling catheters were constructed as described previously (Fuchs et al. 2007; 2008). The end of the catheter was inserted into the right jugular vein and was secured to surrounding tissue with suture. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. Immediately after the catheter surgery, stainless steel guide cannulae (26 G, Plastics One) were aimed at the DH (−3.4 mm anterioposterior, +/−3.1 mm mediolateral, −2.15 mm dorsoventral, relative to bregma) or the overlying trunk region of the somatosensory cortex (SStr: −3.4 mm anterioposterior, +/−3.1 mm mediolateral, −0.65 mm dorsoventral, relative to bregma), which served as an anatomical control region. Three stainless steel screws and dental acrylic anchored the cannulae. Stylets (Plastics One) and Tygon caps sealed the guide cannulae and catheters, respectively, in order to prevent occlusion. Rats were given 5 days for post-operative recovery before the start of the experiment.
To extend catheter patency, the catheters were flushed through once daily for 5 days following surgery with 0.1 ml of an antibiotic solution of cefazolin (100.0 mg/ml, Schein Pharmaceutical, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Baxter Healthcare, Deerfield, IL, USA). Thereafter, catheters were flushed with 0.1 ml of heparinized saline (10 U/ml) prior to each self-administration session and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline (70 U/ml) after each session. Catheter patency was periodically verified by infusing 0.1 ml of propofol (10 mg/ml, intravenous; Abbot Labs., North Chicago, IL, USA), an ultra short-acting barbiturate that produces rapid loss of muscle tone only when administered intravenously.
Cocaine self-administration training
Cocaine self-administration training was conducted during daily 2-h sessions on a minimum of 10 consecutive days during the rats’ dark cycle. The catheters were connected to liquid swivels (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One). The swivels were suspended above the operant conditioning chamber and were connected to infusion pumps via Tygon tubing (Coulbourn). Data collection and reinforcer delivery were controlled using Graphic State Notation software version 2.102 (Coulbourn). Rats (N = 48) were trained to press a lever under an FR 1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.15 mg/0.05 ml per infusion; National Institute on Drug Abuse, Research Triangle Park, NC, USA) with a 20-s timeout period. After each infusion, responses on this active lever had no programmed consequences during the 20-s timeout period. During the sessions, responses on a second, inactive lever had no programmed consequences but were recorded.
Self-administration training was conducted in operant-conditioning chambers that contained one of two distinctly different sets of multi-modal contextual stimuli as described before (Fuchs et al. 2008). Briefly, context 1 contained a continuous red houselight (0.4 fc brightness), intermittent pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine-scented air freshener strip, and wire mesh floor (26 cm × 27 cm). Context 2 contained an intermittent white stimulus light above the inactive lever (1.2 fc brightness; 2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener strip, and a slanted ceramic tile wall that bisected the bar floor (19 cm × 27 cm). Rats had no exposure to these contextual stimuli prior to cocaine self-administration training. These stimuli were presented throughout each session independent of responding, as in our previous studies (Fuchs et al. 2005; 2007; 2008). Assignment of rats to cocaine self-administration training in Context 1 versus Context 2 was random. Active lever presses resulted in 2-s activation of the infusion pump only. The pump was located outside of the sound attenuated chamber. Daily self-administration training sessions were continued until a rat reached the acquisition criterion (i.e., ≥ 10 infusions self-administered/session on a minimum of 10 training days).
Extinction and reinstatement testing
Rats received daily 2-h extinction training sessions in a context distinctly different from the one used for cocaine self-administration training on a minimum of seven consecutive days. During the extinction training sessions, responses were recorded on both levers, but had no programmed consequences. Rats were adapted to the intracranial infusion procedure prior to placement into the operant conditioning chamber on extinction day 4. To this end, injection cannulae were inserted bilaterally into the rat’s guide cannulae to a depth 1 mm below the tip of the guide cannulae. The injectors were left in place for 4 min, but no fluid was infused. Once rats reached the extinction criterion (≤ 25 active lever responses/session on at least 2 consecutive days), they received two test sessions in the previously cocaine-paired context and two test sessions in the extinction context. Five minutes prior to each test session, rats received bilateral microinfusions of one dose of the potent and selective mGluR1 antagonist JNJ16259685 (0.6, 30, or 120 pg/0.5 µl per hemisphere) or vehicle (0.1 % DMSO, 0.5 µl per hemisphere) into the DH or the SStr. Given that the behavioral effects of intracranially infused JNJ16259685 have not been previously examined, the antagonist dose range was derived from our pilot experiments testing the effects of 0.6–1200 pg/0.5 µl per hemisphere doses of JNJ16259685. Infusions were administered at a rate of 0.25 µl/min and injectors were left in the cannulae 1 min before and after the infusions. The order of testing in the previously cocaine-paired versus extinction contexts and treatment (JNJ16259685, vehicle) were counterbalanced based on mean active lever responding during the last three cocaine self-administration training days. Between test sessions, rats received additional extinction training sessions until they re-obtained the extinction criterion (described above). During each test session, responses on the active and inactive levers were recorded for 1 h, but had no programmed consequences.
Locomotor activity testing
To assess possible nonspecific effects of intra-DH JNJ16259685 treatment on general motor activity, the effects of intra-DH JNJ16259685 treatment and vehicle on locomotion were examined using a partial within-subjects design. Seventy-two hours after the last reinstatement test session, rats received bilateral microinfusions of a behaviorally active dose of JNJ16259685 (30 or 120 pg/0.5 µl per hemisphere) or vehicle (0.1 % DMSO, 0.5 µl per hemisphere) into the DH using the infusion procedures described above. Two tests were conducted per rat. Assignment to treatment groups and treatment order were random. Horizontal locomotor activity was measured in novel Plexiglas chambers (42 × 20 × 20 cm high), as described previously (Fuchs et al. 2007; 2008). The total number of photobeams interrupted by a rat moving in the chamber was recorded by a computerized activity system (San Diego Instruments, San Diego, CA, USA) during each 1-h test session.
Food-reinforced instrumental behavior
To assess possible nonspecific motor effects of JNJ16259685 on instrumental behavior per se, the effects of JNJ16259685 (30 or 120 pg/0.5 µl per hemisphere) and vehicle on food-reinforced lever pressing behavior were examined. Experimentally naïve rats (N = 18) received a single overnight food training session followed by stereotaxic surgery as described in the cocaine experiment. Some rats (N = 10, preprandial condition) were food-deprived overnight prior to the initial food training session and received 20–25 g of rodent chow after each training and test session, as in the cocaine experiment. Other rats (N = 8, postprandial condition) received free access to additional 100 food pellets (45 mg, Purina) in their home cages 1-h before each training and test session. The postprandial condition was included in order to elicit lever response rates more similar to those seen during the reinstatement test in the cocaine-trained groups. Rats in the preprandial and postprandial condition underwent daily 2-h food self-administration training sessions in context 1 or 2, modified to include a food pellet dispenser. Assignment of rats to food self-administration training in context 1 or 2 was random. Active lever presses resulted in the delivery of a single food pellet (45 mg, Purina) under an FR 1 reinforcement schedule with a 20-s timeout period. Inactive lever presses were recorded but had no programmed consequences. After responding stabilized (i.e., ≤ 20% variability in active lever responding across two consecutive sessions), three 1-h test sessions were conducted using a fully counterbalanced within-subjects test design. Five minutes before each test session, rats received bilateral microinfusions of a behaviorally active dose of JNJ16259685 (30 or 120 pg/0.5 µl per hemisphere) or vehicle (0.1 % DMSO, 0.5 µl per hemisphere) into the DH, using the infusion procedure described above. During the test sessions, active lever presses continued to be reinforced with food pellets under an FR 1 reinforcement schedule with a 20-s timeout period. Inactive lever presses were recorded but had no programmed consequences. Treatment order was counterbalanced based on mean food-reinforced responding during the last two food self-administration sessions that preceded the first test session. Between the test sessions, rats received a minimum of two additional food self-administration training sessions to re-establish less than 10% variability in baseline responding.
Histology
After the last experimental session, catheterized rats were fully anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.3 mg/kg, i.v., respectively); rats without catheters were anesthetized using ketamine hydrochloride and xylazine (199.8 and 3.9 mg/kg, i.p., respectively) Rats were then transcardially perfused using 1×phosphate-buffered saline (Fisher Scientific) and 10% formaldehyde solution (Sigma). The brains were dissected out and stored in 10% formaldehyde solution. The brains were sectioned in the coronal plane at a thickness of 75 µm using a vibratome. The sections were mounted onto gelatin-coated slides, and stained using cresyl violet (Kodak, Rochester, NY, USA). Cannula placements were determined using light microscopy. The most ventral portion of each cannula track was mapped onto schematics of appropriate plates from the rat brain atlas (Paxinos and Watson 1997).
Data analysis
To test for possible pre-existing differences between the treatment groups, cocaine-reinforced active and inactive lever presses, cocaine infusions, the total number of sessions needed to reach the extinction criterion, and extinction responding on the active lever and inactive lever were analyzed separately using analyses of variance (ANOVAs) with cannula location (DH, SStr) and subsequent treatment (JNJ16259685 doses, vehicle) as between-subjects factors and time (days) as the within-subjects factor, where appropriate. To test for a possible test order effect, non-reinforced active and inactive lever presses on the test days were analyzed separately using mixed-factorial ANOVA with testing order (extinction first, reinstatement first) as the between-subjects factor and testing context (extinction, cocaine-paired) as the within-subjects factor. To determine whether the vehicle data can be collapsed, non-reinforced active and inactive lever presses on the test days following intra-DH vehicle administration were analyzed separately using mixed-factorial ANOVA with additional treatment (JNJ16259685 doses) as the between-subjects factor and testing context (extinction, cocaine-paired) as the within-subjects factor.
To assess the effects of JNJ16259685 on lever responding on the test days, non-reinforced active and inactive lever presses were analyzed separately using mixed-factorial ANOVAs with treatment (JNJ16259685 doses, vehicle) as the between-subjects factor and testing context (extinction, cocaine-paired) as the within-subjects factor. To assess the effects of JNJ16259685 on locomotor activity, photobeam breaks were analyzed using a mixed-factorial ANOVA with treatment (JNJ16259685 doses, vehicle) as the between-subjects factor and time (20-min intervals) as the within-subjects factor. Food-reinforced active and inactive lever presses were analyzed separately using a repeated-measures one-way ANOVA with treatment (JNJ16259685 does, vehicle) as the factor. In all analyses, significant ANOVA main and interaction effects were further investigated using Tukey HSD tests. Alpha was set at 0.05. Only statistically significant effects are reported below.
RESULTS
Histology
A schematic illustrating cannula placements and photomicrographs showing representative cannula tracts are shown in Fig. 1. The target brain regions were defined as the DH and the overlying SStr. The most ventral point of the infusion cannula tracks was located within the DH (N = 36) or SStr (N = 12) bilaterally in all cocaine-trained rats and within the DH in all food-trained rats (N = 18). The resulting sample sizes per group are reported in the figure captions for figures 2 and 3. High power microscopy did not reveal any evidence of abnormal tissue damage (i.e., extensive cell loss or gliosis) at the infusion site.
Fig. 1.
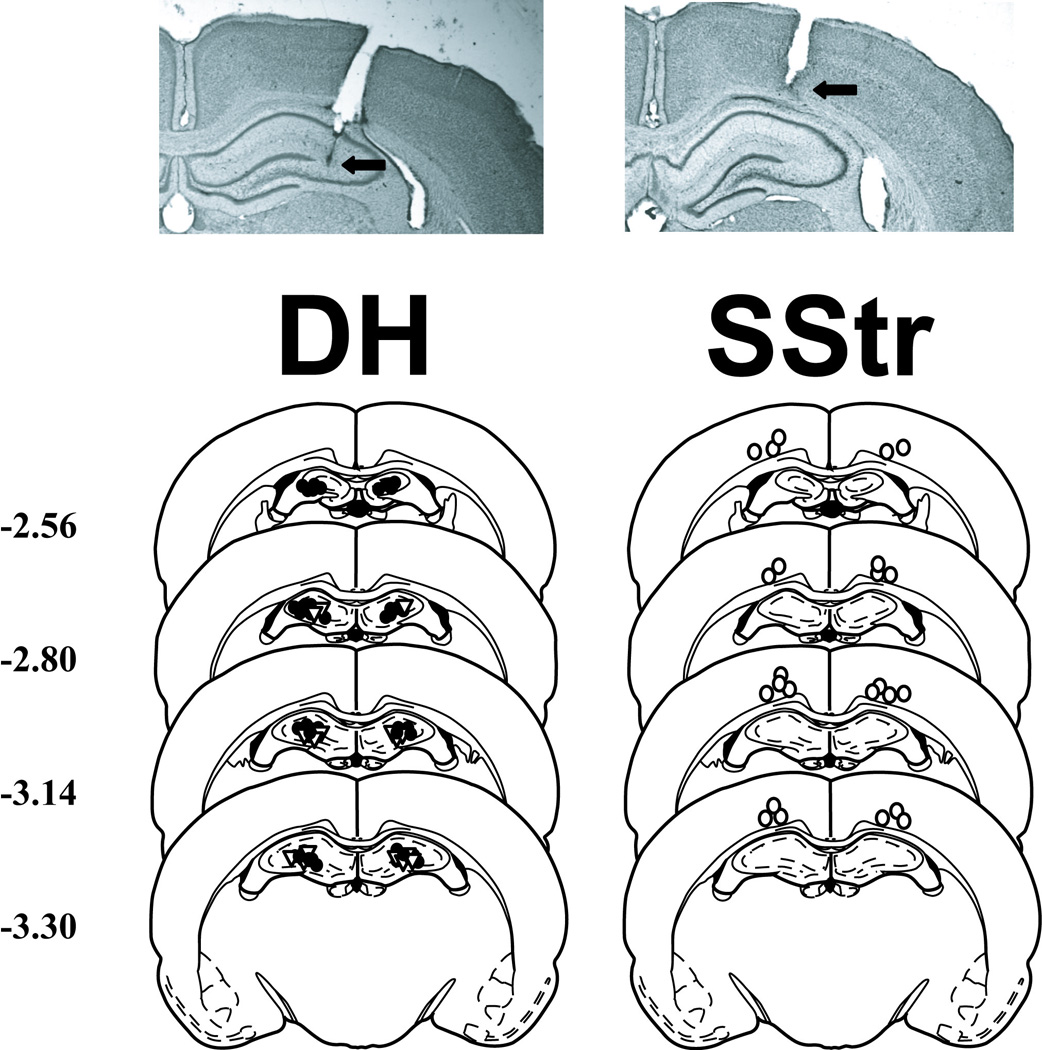
Schematic and photographic representation of injection cannula placements within the dorsal hippocampus (DH) and trunk region of the somatosensory cortex (SStr). Arrows identify the most ventral point of the infusion cannula tracts on each photomicrograph of representative cresyl violet-stained brain sections. Filled circles, open triangles, and open circles represent the most ventral point of the cannula tracts for rats in the DH-cannulated cocaine-trained, DH-cannulated food-trained, and SStr-cannulated cocaine-trained groups, respectively, on schematics from the rat brain atlas of Paxinos and Watson (1997). Numbers indicate the distance from bregma in millimeters.
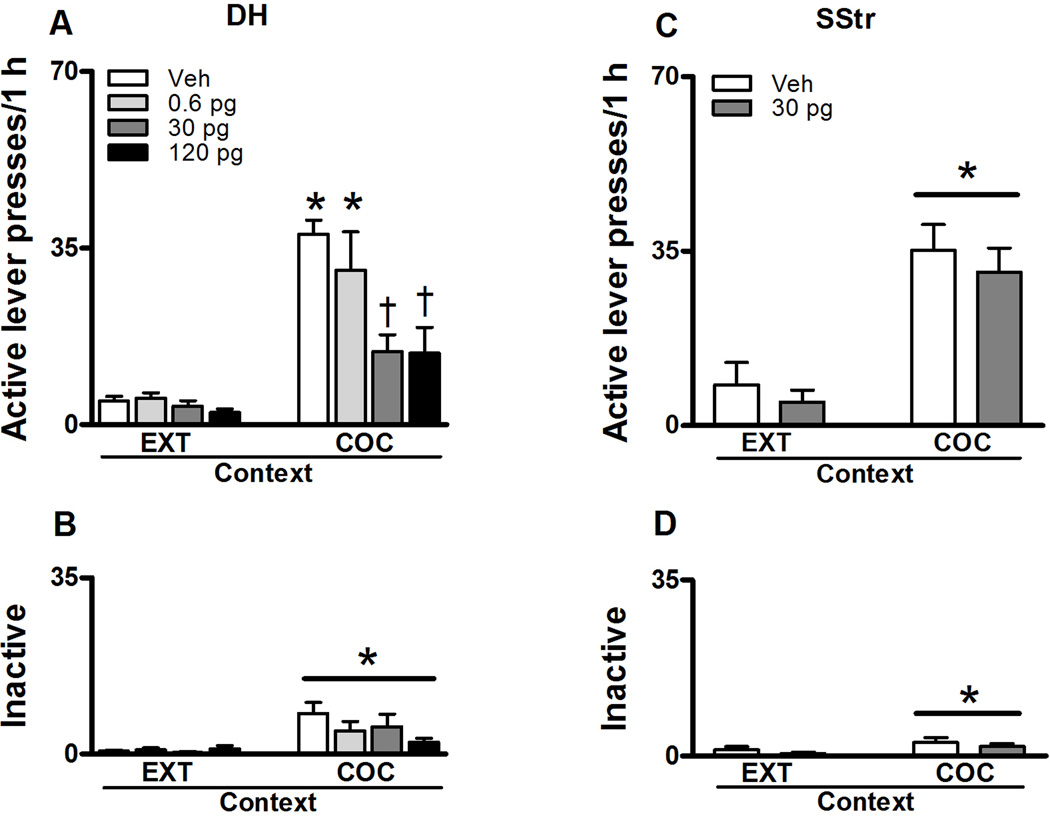
Fig. 2.
Non-reinforced active and inactive lever responses (mean/1 h ± SEM) during testing in the extinction (EXT) and previously cocaine-paired contexts (COC). JNJ16259685 or vehicle was infused bilaterally into the DH (A, B: N = 10–14/group) or the SStr (C, D: N = 12) before testing. The Asterisks represent a significant difference relative to responding in the extinction context (Panel A: ANOVA context simple main effect, Tukey test, p < 0.05; Panels B, C, D: ANOVA context main effect, p < 0.05). The Dagger represents a significant difference relative to the vehicle treatment (ANOVA treatment simple main effect, Tukey test, p < 0.05).
Fig. 3.
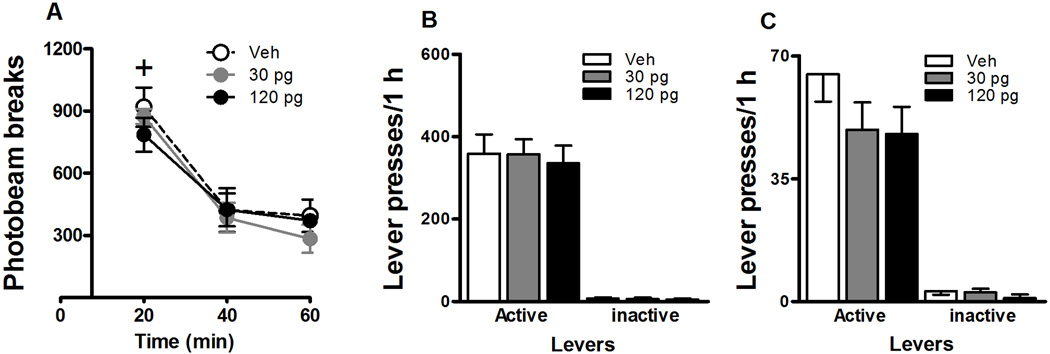
Locomotor activity (A; mean photobeam breaks/1 h ± SEM) in a novel context as well as preprandial food-reinforced instrumental behavior (B; mean lever presses/1 h ± SEM) and postprandial food-reinforced instrumental behavior (C; mean lever presses/1 h ± SEM) in contexts 1 or 2. JNJ16259685 or vehicle was administered bilaterally into the DH (Panel A: N = 10–14/group; Panel B: N = 10; Panel C: N = 8) before testing. Rats in the postprandial condition received 100 45-mg food pellets 1 h before each behavioral session. The Plus sign represents a significant difference relative to all other time points (ANOVA time simple main effect, Tukey test, p < 0.05).
Cocaine Self-administration
Both the DH- and SStr-cannulated groups exhibited stable responding on the active lever during the last three self-administration training days with a within-subject variability of < 10% in daily cocaine intake. Collapsed across groups, the mean numbers of active lever responses was 53.83 ± 4.87 and the mean daily cocaine intake (± SEM) was approximately 11.55 ± 1.50 mg/kg per session (23.10 ± 1.50 infusions), based on mean body weight. The DH- and SStr-cannulated groups did not differ in active or inactive lever responding during the last three days of cocaine self-administration training (all time and cannula location main and interaction effects, N.S.). There was also no pre-existing difference between the DH-cannulated groups that were subsequently assigned to receive JNJ16259685 at a dose of 0.6, 30, or 120 pg/hemisphere in active or inactive lever responding (all time and treatment main and interaction effects, N.S.).
Extinction
Upon the removal of cocaine reinforcement, active and inactive lever responding gradually declined in both DH- and SStr-cannulated groups (time main effects, F(6,276) = 7.277–21.625, p = 0.0001). There was no pre-existing difference between the DH- and SStr-cannulated groups in active and inactive lever responding during the first seven days of extinction training (all cannula location main and interaction effects, N.S.) and in the mean number of daily sessions (± SEM; 7.40 ± 0.08) needed to reach the extinction criterion. Separate ANOVAs indicated that active and inactive lever responding gradually declined to criterion in the three DH-cannulated groups that were assigned to receive different doses of JNJ16259685 on subsequent test days (time main effect, F(6,198) = 6.640–28.323, p = 0.0001). Furthermore, there was no pre-existing difference between these treatment groups in active or inactive lever responding during the first seven days of extinction training (all treatment main and interaction effects, N.S.) or in the mean number of daily sessions (± SEM) needed to reach the extinction criterion (7.30 ± 0.07).
Effects of intra-DH JNJ16259685 treatment on drug context-induced reinstatement of cocaine seeking
Exposure to the previously cocaine-paired context reinstated active lever responding in rats that received intra-DH or intra-SStr vehicle pretreatment regardless of treatment order and treatment history (Fig. 2). Separate 2 × 2 ANOVAs indicated no difference in active or inactive lever responding as a function of test order in the cocaine-paired context versus extinction context (all test order main or interaction effects, N.S.). Hence, data were collapsed across this variable. There was also no difference in active or inactive lever responding following vehicle treatment among the three DH-cannulated treatment groups that were assigned to receive different doses of JNJ16259685 on alternate test days (all subsequent treatment main or interaction effects, N.S.). Therefore, data were collapsed across these groups to form a single vehicle condition.
Intra-DH JNJ16259685 treatment differently altered active lever responding as a function of testing context, dose, and infusion site (Fig. 2a). Exposure to the cocaine-paired context increased active lever responding following intra-DH vehicle pre-treatment relative to responding in the extinction context (context main effect, F(1,68) = 61.758, p = 0.0001). The effects of intra-DH JNJ16259685 treatment were dose-dependent in the cocaine-paired context (treatment × context interaction effect, F(3,68) = 5.724, p = 0.001 and treatment main effect, F(3,68) = 7.541, p = 0.0001). Specifically, the 0.6 pg dose of JNJ16259685 administered into the DH failed to alter active lever responding in the extinction context or in the cocaine-paired context relative to vehicle. In contrast, the 30 or 120 pg doses of JNJ16259685 administered into the DH significantly decreased active lever responding in the cocaine-paired context (Tukey test, p < 0.05), without altering active lever responding in the extinction context, relative to vehicle. Furthermore, following intra-DH administration of the 30 or 120 pg doses of JNJ16259685, active lever responding in the cocaine-paired context was not significantly different from that in the extinction context. Exposure to the cocaine-paired context slightly increased responding on the inactive lever relative to responding in the extinction context (context main effect, F(1,68) = 9.766, p = 0.003, Fig. 2b). However, intra-DH administrations of JNJ16259685 failed to alter inactive lever responding in either the cocaine-paired or the extinction context (treatment and context main and treatment × context interaction effects, N.S.).
Effects of intra-SStr JNJ16259685 treatment on drug context-induced reinstatement of cocaine seeking
Exposure to the previously cocaine-paired context increased active lever responding following vehicle pre-treatment administered into the SStr relative to responding in the extinction context (context main effect, F(1,11) = 31.482, p = 0.0001, Fig. 2c). However, intra-SStr administrations of the 30 pg dose of JNJ16259685, a dose behaviorally effective in the DH, failed to alter active lever responding relative to vehicle in either context (treatment main and treatment × context interaction effects, N.S.). Similarly, exposure to the cocaine-paired context slightly increased responding on the inactive lever relative to responding in the extinction context (context main effect, F(1,11) = 7.275, p = 0.021, Fig. 2d). Furthermore, intra-SStr administrations of JNJ16259685 failed to alter inactive lever responding relative to vehicle in either context (treatment main and treatment × context interaction effects, N.S.).
Effects of intra-DH JNJ16259685 treatment on locomotor activity and food-reinforced instrumental behavior
Intra-DH administration of JNJ16259685 failed to alter locomotor activity in a novel context relative to vehicle (Fig. 3a). The number of photobeam breaks decreased across the three 20-min intervals of the motor activity test session (time main effect, F(2,48) = 54.601, p = 0.0001; interval 1 > 2–3, Tukey test, p < 0.05). Furthermore, the 30 or 120 pg doses of JNJ16259685 administered into the DH failed to alter the number of photobeam breaks relative to vehicle (treatment main and time × treatment interaction effects, N.S.).
Intra-DH administration of JNJ16259685 failed to alter preprandial (Fig. 3b) or postprandial (Fig. 3c) food-reinforced instrumental behavior in context 1 or 2, relative to vehicle. Vehicle-pretreated rats exhibited more active lever presses for food reinforcement in the preprandial condition (Mean ± SEM = 359.40 ± 47.09) than in the postprandial condition (Mean ± SEM = 64.75 ± 7.77). However, intra-DH administrations of the 30 or 120 pg doses of JNJ16259685 failed to alter preprandial or postprandial food-reinforced active (F(2,21–30) = 0.091–1.485, N.S.) or inactive lever responding (F(2,21–30) = 0.060–1.056, N.S.), relative to vehicle.
DISCUSSION
The present study, to our knowledge, demonstrates for the first time that the expression of drug context-induced instrumental cocaine-seeking behavior relies on glutamatergic neurotransmission in the DH via the stimulation of mGluR1s. Blockade of the mGluR1s in the DH by JNJ16259685, a highly selective mGluR1 receptor antagonist (Lavreysen et al. 2004b; Fukunaga et al. 2007), dose-dependently attenuated the expression of drug context-induced cocaine seeking in rats. Impairment in cocaine seeking was brain region specific, since administration of JNJ16259685 into the overlying SStr failed to alter this behavior. Also, impairment in cocaine seeking was not due to nonspecific effects on general activity or instrumental motor performance, since administration of behaviorally effective doses of JNJ16259685 into the DH failed to inhibit locomotor activity and preprandial or postprandial food-reinforced instrumental behavior. Furthermore, impairment in cocaine-seeking behavior was lever and context specific, since JNJ16259685 treatment failed to alter inactive lever responding in the previously cocaine-paired context or active lever responding in the extinction context. Taken together, these findings suggest that stimulation of mGluR1 receptors in the DH is necessary for drug context-induced cocaine-seeking behavior. It should be considered, however, that the hippocampus as well as mGluR1s have been implicated in memory processes (Maciejak et al. 2003; Simonyi et al. 2007; Sukhotina et al. 2008). For instance, systemic administration of the mGluR1 antagonist EMQMCM inhibits the expression of hippocampus-dependent contextual fear conditioning (Gravius et al. 2006). mGluR1s may therefore play a role in the recall or utilization of context-response-cocaine associations that are thought to contribute to drug-seeking behavior in our model (Fuchs et al. in press). Thus it is unclear whether mGluR1 stimulation in the DH mediates memory and/or motivational processes that contribute to this behavior.
The possible contribution of mGluR1 to drug-induced behavior has not been studied extensively. Study of mGluR1 function has been restricted until the recent availability of selective mGluR1 antagonists, including JNJ16259685, BAY36-7620, YM-230888, and EMQMCM (De Vry et al. 2001; Lavreysen et al. 2004b; Sevostianova and Danysz 2006; Kohara et al. 2007). In the present study, we utilized JNJ16259685, a highly potent non-competitive mGluR1 antagonist (in vitro IC50 < 19 nM) (Lavreysen et al. 2004b; Fukunaga et al. 2007). JNJ16259685 displays a greater than 1,000-fold selectivity for mGluR1 over mGluR5 in Ca2+ mobilization assays (Lavreysen et al. 2004b), it does not act as a positive allosteric modulator of other mGluRs subtypes (i.e., mGluR2, mGluR3, mGluR4, and mGluR6; Lavreysen et al. 2004b), and it has no affinity for NMDA or AMPA ionotropic glutamate receptors (in vitro IC50 > 10 µM) (Lavreysen et al. 2004b). Hence, the JNJ16259685-induced impairment in drug context-induced cocaine-seeking behavior in the present study was likely due to the selective blockade of mGluR1 in the DH. Nevertheless, given that the pharmacology of JNJ16259685 has not been extensively studied in vivo, it will be important to verify the receptor selectivity of this effect using selective mGluR1 agonists, once these become available.
Although mGluR1 antagonism in the DH selectively impairs drug context-induced cocaine-seeking behavior, mGluR1s have also been implicated in motor behavior and food-motivated goal-directed behavior. While, in the present study, administration of JNJ16259685 into the DH failed to alter the locomotor activity, systemic infusions of JNJ16259685 impair locomotor activity (Steckler et al. 2005; Besheer et al. 2008). These systemic effects of JNJ16259685 likely result from the inhibition of mGluR1s in brain regions other than the DH, such as cerebellum (Ichise et al. 2000; Nakao et al. 2007) which exhibits high mGluR1 density (Masu et al. 1991; Shigemoto et al. 1992; Lavreysen et al. 2004a) or the NAC (Besheer et al. 2009). The present findings suggested that mGluR1s in the DH do not play a critical role in the reinforcing effects of food; however, it remains to be investigated whether they contribute to context-induced motivation for natural reward. Systemic mGluR1 antagonism using EMQMCM attenuates explicit CS-induced reinstatement of food-seeking behavior (Dravolina et al. 2007). Thus, it is possible that intra-DH administration of JNJ16259685 would have a similar effect on context-induced reinstatement of food-seeking behavior. However, context-induced natural reward-seeking behavior is difficult to study in isolation due to the presence of response-contingent visual and olfactory stimuli associated with natural rewards. These sensory stimuli are established as CSs during training. They can suppress contextual conditioning due to overshadowing: a well documented interference with the conditioning of a stimulus (i.e., the context) because of the simultaneous presence of another stimulus (i.e., food-related CS) that is easier to condition (Domjan 1998). Additionally, they may alter the neural systems recruited to control food-seeking behavior. Consistent with a possible overshadowing effect, our attempt to examine the effects of intra-DH JNJ16259685 (30 pg/0.5 µl per hemisphere) on context-induced reinstatement of food-seeking behavior was unsuccessful (data not shown). In this experiment, all training and testing procedures were identical to those in the cocaine reinstatement experiments reported here except that a natural reinforcer (45-mg food pellets) was used. Under these experimental conditions, exposure to the previously food-paired context failed to reinstate extinguished food-seeking behavior following vehicle (8.25 ± 2.76 active lever presses) or JNJ16259685 administration into the DH (12.16 ± 2.76 active lever presses), relative to responding in the extinction context (9.68 ± 2.22 active lever presses).
The effect of JNJ16259685 on context-induced reinstatement of cocaine-seeking behavior appears to be mediated by inhibition of mGluR1 receptor signaling, but the exact hippocampal receptor subpopulations and possible receptor interactions that mediate this effect have yet to be identified. In vitro studies have shown that JNJ16259685 inhibits glutamate-induced inositol phosphate production, Ca2+ mobilization, and the generation of mGluR1-mediated EPSPs (Lavreysen et al. 2004b, Fukunaga et al. 2007). mGluR1s are preferentially expressed postsynaptically within the DH (Ferraguti et al. 2004; Ferraguti and Shigemoto 2006). While the expression of mGluR1s is largely uniform throughout the hippocampus proper, critical mGluR1 populations may be located postsynaptically on CA1 pyramidal neurons. Stimulation of these mGluR1s has direct excitatory effects on CA1 pyramidal neurons, resulting in depolarization and increased cell firing (Desai and Conn 1991; Mannaioni et al. 2001). However, mGluR1s are also located on GABAergic inhibitory interneurons within the hippocampus (van Hooft et al. 2000; Topolnik et al. 2005), and the stimulation of these receptors inhibits the CA3-CA1 synapse (Mannaioni et al. 2001). Therefore, in the present study, we are not able to ascribe the effects of JNJ16259685 on cocaine-seeking behaivor to the blockade of a specific mGluR1 subpopulation within the DH. Studies have revealed functional interactions between mGluR1 and NMDA receptors. Specifically, in vitro stimulation of mGluR1 potentiates NMDA receptor-mediated currents and accelerates NMDA receptor trafficking (Krieger et al. 2000; Lan et al. 2001; Skeberdis et al. 2001). Hence, although JNJ16259685 has no affinity for the NMDA receptor (Lavreysen et al. 2004b), functional interactions between mGluR1s and NMDA receptors might contribute to the effect of JNJ16259685 on cocaine-seeking behavior in the present study. Future studies examining the effects of a sub-threshold dose of JNJ16259685 co-administered with an NMDA receptor antagonist (e.g. MK-801) may provide insight into this question.
In summary, the main contribution of the present study is to demonstrate that stimulation of mGluR1s in the DH is critical for the expression of drug context-induced reinstatement of cocaine-seeking behavior. Thus, the mGluR1 is a potential target of pharmacotherapeutic prevention of drug relapse. As these findings imply, glutamatergic neurotransmission within the DH is critical for drug context-induced reinstatement of cocaine-seeking behavior, but the source of glutamate input has yet to be identified. Anatomical studies indicate that the hippocampus proper – including the DH and ventral hippocampus (VH) – receives sensory input via glutamatergic fibers from sensory association cortical areas, and projects reciprocally to the dorsomedial prefrontal cortex (dmPFC), which has also been identified as a mediator of context-induced reinstatement of cocaine-seeking behavior (Jay et al. 1992; Moser and Moser 1998; Fuchs et al. 2005; 2007). Using the functional disconnection technique, we have demonstrated that serial information processing by the basolateral amygdala (BLA) and DH is necessary for drug context-induced cocaine-seeking behavior (Fuchs et al. 2007). While cholinergic and noradrenergic neurotransmission within the BLA have been indicated in modulating hippocampal neuron activity (Dringenberg et al. 1996; Roozendaal et al. 1999), it remains to be determined whether glutamatergic input from the BLA to the DH contributes to drug context-induced cocaine-seeking behavior. Given that direct anatomical connections between the BLA and DH are sparse, interactions between these brain regions may occur via an intermediary, such as the lateral entorhinal cortex (Fuchs et al. 2007). Conversely, dense reciprocal glutamatergic connections exist between the BLA and the VH (van Groen and Wyss 1990), which also plays a role in context-induced cocaine-seeking behavior (Lasseter et al. 2009). However, it has yet to be determined whether the BLA and VH are functionally interdependent with respect to this behavior. It has been theorized that, upon drug-context exposure, the information from the DH, VH, and BLA is integrated by the dmPFC, which initiates the expression of drug context-induced cocaine-seeking behavior via inputs to the nucleus accumbens (NAC; Fuchs et al. 2008; Lasseter et al. in press). Alternatively, information from the DH, VH, BLA, and dmPFC may converge at the level of the NAC (van Groen and Wyss 1990; Amaral and Witter 1995; O’Donnell and Grace 1995; Groenewegen et al. 1999; Goto and O’Donnell 2002; Peleg-Raibstein and Feldon 2006; Fuchs et al. 2008; Lasseter et al. 2009), likely permitting greater hippocampal influence over cocaine-seeking behavior. Future studies will need to investigate the neuropharmacological mechanisms underlying the putative interactions between these subcircuits in order to better understand the role of the hippocampus in cocaine addiction and to advance drug relapse prevention.
ACKNOWLEDGMENTS
The authors thank Stephanie Kaszycki, KaiCee Ponds, Andrew Hamlet, Jennifer Shurney, and Chandler Sours for excellent technical assistance. This work was supported by National Institute on Drug Abuse (NIDA) grant R01 DA017673, a NIDA R01 grant supplement to promote diversity in health-related research (DA017673-S1), a University of North Carolina at Chapel Hill Junior Faculty Development Award, and the Mason and Linda Stephenson Faculty Award.
Footnotes
The authors do not have any conflicts of interest to disclose.
REFERENCE
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Los Angeles, CA: Academic; 1995. pp. 443–493. [Google Scholar]
- Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol. 2008;42:13–20. doi: 10.1016/j.alcohol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49:167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Fitzjohn SM, Collingridge GL. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol. 1999;9:299–304. doi: 10.1016/s0959-4388(99)80044-0. [DOI] [PubMed] [Google Scholar]
- Desai MA, Conn PJ. Excitatory effects of ACPD receptor activation in the hippocampus are mediated by direct effects on pyramidal cells and blockade of synaptic inhibition. J Neurophysiol. 1991;66:40–52. doi: 10.1152/jn.1991.66.1.40. [DOI] [PubMed] [Google Scholar]
- De Vry J, Horváth E, Schreiber R. Neuroprotective and behavioral effects of the selective metabotropic glutamate mGlu(1) receptor antagonist BAY 36–7620. Eur J Pharmacol. 2001;428:203–214. doi: 10.1016/s0014-2999(01)01296-1. [DOI] [PubMed] [Google Scholar]
- Domjan M. Brooks/Cole Stimulus control of behavior. 4th edn. California: Pacific Grove; 1998. The Principles of Learning and Behavior; pp. 216–250. [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 2006;187(4):397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Exp Brain Res. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Cobden P, Pollard M, Cope D, Shigemoto R, Watanabe M, Somogyi P. Immunolocalization of metabotropic glutamate receptor 1alpha (mGluR1alpha) in distinct classes of interneuron in the CA1 region of the rat hippocampus. Hippocampus. 2004;14:193–215. doi: 10.1002/hipo.10163. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Robertson DJ, Roberts DC, Vrana KE. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001;108:371–380. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov. Today Dis. Models. doi: 10.1016/j.ddmod.2009.03.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga I, Yeo CH, Batchelor AM. Potent and specific action of the mGlu1 antagonists YM-298198 and JNJ16259685 on synaptic transmission in rat cerebellar slices. Br J Pharmacol. 2007;151:870–876. doi: 10.1038/sj.bjp.0707286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Timing-dependent limbic-motor synaptic integration in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:13189–13193. doi: 10.1073/pnas.202303199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravius A, Barberi C, Schäfer D, Schmidt WJ, Danysz W. The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats-a comparison. Neuropharmacology. 2006;51:1146–1155. doi: 10.1016/j.neuropharm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;29(877):49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory Amino Acid Pathway from the Hippocampus to the Prefrontal Cortex. Contribution of AMPA Receptors in Hippocampo-prefrontal Cortex Transmission. Eur J Neurosci. 1992;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kohara A, Nagakura Y, Kiso T, Toya T, Watabiki T, Tamura S, Shitaka Y, Itahana H, Okada M. Antinociceptive profile of a selective metabotropic glutamate receptor 1 antagonist YM-230888 in chronic pain rodent models. Eur J Pharmacol. 2007;571:8–16. doi: 10.1016/j.ejphar.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M. Comparison of the effects of mGluR1 and mGluR5 antagonists on the expression of behavioral sensitization to the locomotor effect of morphine and the morphine withdrawal jumping in mice. Eur J Pharmacol. 2007;558:113–118. doi: 10.1016/j.ejphar.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Krieger P, Hellgren-Kotaleski J, Kettunen P, El Manira AJ. Interaction between metabotropic and ionotropic glutamate receptors regulates neuronal network activity. J Neurosci. 2000;20:5382–5391. doi: 10.1523/JNEUROSCI.20-14-05382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Zheng X, Bennett MV, Zukin RS. Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J Neurosci. 2001;21:6058–6068. doi: 10.1523/JNEUROSCI.21-16-06058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug self-administration and relapse. In: Self DW, Staley JK, editors. Behavioral Neuroscience of Addiction. Springer: (in press) Current Topics in Behavioral Neurosciences (Series Eds. Geyer M, Marsden C, and Ellenbroek B) [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Program No. 65.6. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Sub-region specific involvement of the ventral hippocampus in drug context-induced reinstatement of cocaine-seeking behavior in rats. 2009. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Pereira SN, Leysen JE, Langlois X, Lesage AS. Metabotropic glutamate 1 receptor distribution and occupancy in the rat brain: a quantitative autoradiographic study using [3H]R214127. Neuropharmacology. 2004a;46:609–619. doi: 10.1016/j.neuropharm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nóbrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004b;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Maciejak P, Taracha E, Lehner M, Szyndler J, Bidziński A, Skórzewska A, Wisłowska A, Zienowicz M, Płaźnik A. Hippocampal mGluR1 and consolidation of contextual fear conditioning. Brain Res Bull. 2003;62:39–45. doi: 10.1016/j.brainresbull.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nakao H, Nakao K, Kano M, Aiba A. Metabotropic glutamate receptor subtype-1 is essential for motor coordination in the adult cerebellum. Neurosci Res. 2007;57:538–543. doi: 10.1016/j.neures.2006.12.014. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd edn. Los Angeles, CA: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2007;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Hönig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Ngomba RT, Storto M, Catania MV, Miller LA, Youngs B, DiGiorgi-Gerevini V, Nicoletti F, Sun GY. Expression of groups I and II metabotropic glutamate receptors in the rat brain during aging. Brain Res. 2005;1043:95–106. doi: 10.1016/j.brainres.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Shelat PB, Dopheide MM, Coulibaly AP, Schachtman TR. Differential roles of hippocampal metabotropic glutamate receptors 1 and 5 in inhibitory avoidance learning. Neurobiol Learn Mem. 2007;88:305–311. doi: 10.1016/j.nlm.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Opitz T, Zheng X, Bennett MV, Zukin RS. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40:856–865. doi: 10.1016/s0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Steckler T, Lavreysen H, Oliveira AM, Aerts N, Van Craenendonck H, Prickaerts J, Megens A, Lesage AS. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology (Berl) 2005;179:198–206. doi: 10.1007/s00213-004-2056-7. [DOI] [PubMed] [Google Scholar]
- Sukhotina IA, Dravolina OA, Novitskaya Y, Zvartau EE, Danysz W, Bespalov AY. Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology (Berl) 2008;196:211–220. doi: 10.1007/s00213-007-0953-2. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Congar P, Lacaille JC. Differential regulation of metabotropic glutamate receptor- and AMPA receptor-mediated dendritic Ca2+ signals by presynaptic and postsynaptic activity in hippocampal interneurons. J Neurosci. 2005;25:990–1001. doi: 10.1523/JNEUROSCI.4388-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem. 2008;106:1780–1790. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]