Abstract
Among the food plants, the presence of melatonin in grapes (Vitis vinifera L.) deserves particular attention because of the production of wine, an alcoholic beverage of economic relevance and with putative healthy effects. Furthermore, melatonin isomers have been detected in wine too. Recently, one of these isomers has been identified as tryptophan-ethylester, a compound with the same molecular weight of melatonin. In this Commentary, we briefly comment the source(s) of tryptophan-ethylester in wine and the putative nutritional role(s).
Keywords: tryptophan, melatonin, melatonin isomers, tryptophan-ethylester, red wine, grapes
Introduction
The topic of melatonin in grape products began less than a decade ago, when it was detected, for the first time, in berry skin of Italian and France grapevine varieties (Vitis vinifera L. cv. Barbera, Cabernet Franc, Cabernet Sauvignon, Croatina, Marzemino, Merlot, Nebbiolo, and Sangiovese) grown in north-western Italy.1 Since then, indoleamine was reported in other grape tissues with varying levels according to both endogenous and exogenous factors, such as genetic traits, phenological stages, environmental and climatic conditions, and agricultural practices (Table 1). The presence of melatonin was also ascertained in red and white wine produced in different geographical areas (Table 1).
Table 1.
Melatonin content in grapes and wine.
| GRAPES | MELATONIN (NG G−1) | REFERENCES |
|---|---|---|
| Nebbiolo, Croatina, Barbera, Sangiovese, Marzemino, Cabernet Sauvignon, Merlot, Cabernet Franc (skin, Italy) | 0.005–0.96 | Iriti et al.1 |
| Malbec, Cabernet Sauvignon, Chardonnay (skin, Argentina) | 0.6–1.2 | Stege et al.22 |
| Merlot (whole berry, Canada) | 100,000–150,000 | Murch et al.23 |
| Merlot (skin, Italy) | 9.3–17.5 | Vitalini et al.24 |
| Merlot (seed, Italy) | 3.5–10 | Vitalini et al.24 |
| Merlot (flesh, Italy) | 0.2–3.9 | Vitalini et al.24 |
| Malbec (skin, Argentina) | 9–159 | Boccalandro et al.25 |
| Malbec (skin, Argentina) | 120–160 | Gomez et al.5 |
| Albana, Sangiovese (whole berry, Italy) | 1.2, 1.5 | Mercolini et al.26 |
| Malbec (skin, Argentina) | 440 | Gomez et al.6 |
| WINE | MELATONIN (NG ML−1) | REFERENCES |
| Albana, Sangiovese, Trebbiano (Italy) | 0.6, 0.4 | Mercolini et al.26,27 |
| Chardonnay, Malbec, Cabernet Sauvignon (Argentina) | 0.16–0.32 | Stege et al.22 |
| Groppello, Merlot (Italy) | 8.1, 5.2 | Vitalini et al.28 |
| Cabernet Sauvignon, Merlot, Syrah, Tempranillo, Tintilla de Rota, Petit Verdot, Prieto Picudo and Palomino Fino (Spain) | 5.1–420 | Rodriguez-Naranjo et al.4,29 |
In the field of melatonin research, the occurrence of melatonin isomers in nature represents an emerging topic.2 Isomers can be classified according to the position of the two side chains present in the indole ring of melatonin, the methoxy (M) group at position 5 and the N-acetylaminoethyl (A) group at position 3. Hypothetically, either one of these two side chains can be relocated to any one of the seven positions in the indole nucleus of melatonin to form isomers.3 In particular, different isomers were found in grape products, including red wine, even if their chemical structure has not been identified yet.4–7
Very recently, in the attempt to determine the conformation of the most abundant (putative) melatonin isomer detected in red wine, we have identified it as tryptophan-ethylester, a compound with the same molecular weight of melatonin (Fig. 1).8 In particular, the concentrations of tryptophan-ethylester and melatonin in wine were 84 and 3 ng mL−1, respectively.8 However, to date, the relationship between concentrations of melatonin and tryptophan-ethylester in grape products is still unknown: it seems that tryptophan-ethylester may arise from a pathway different from the melatonin biosynthetic route, possibly directly from tryptophan.
Figure 1.
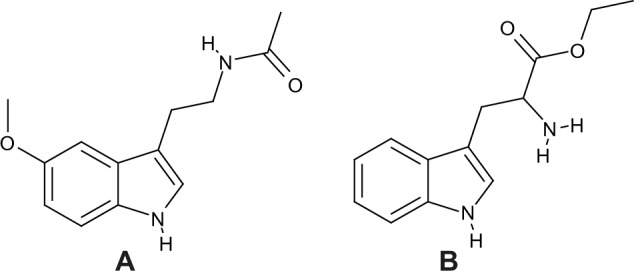
Chemical structure of (A) melatonin and (B) tryptophan-ethylester.
Amino acid esters readily cross cell membranes because of their lipophilicity and are subject to intracellular enzymatic hydrolysis, thus regenerating the native amino acids.9 Therefore, dietary tryptophan-ethylester, a lipid-soluble tryptophan derivative, may bypass defective gastrointestinal neutral amino acid transport and be metabolized to melatonin in enteroendocrine cells of the gastrointestinal mucosa. In a child with Hartnup disease (an autosomic recessive metabolic disorder affecting the absorption of nonpolar amino acids, particularly tryptophan), tryptophan-ethylester administration successfully corrected tryptophan deficiency state, and in vitro experiments demonstrated that ester was hydrolyzed by intestinal mucosa, liver, and kidney to provide tryptophan.10 More recently, in rats, tryptophan-ethylester, but not tryptophan, evoked a rapid and transient dose-dependent decrease in mean arterial pressure and heart rate and significantly promoted vasodilatation in small mesenteric arteries by blocking voltage-operated calcium channels on vascular smooth muscle cells.11 Noteworthy, to the best of our knowledge, it seems that gut microflora has not the capacity to synthesize tryptophan-ethylester or melatonin isomers.
However, the exact contribution of grapes to melatonin, melatonin isomers, and tryptophan-ethylester in wine has not entirely been elucidated yet, and a pivotal role of yeasts and, possibly, of bacteria in the production of these metabolites in wine has been suggested. In a pioneering paper, Sprenger and colleagues demonstrated that, in Saccharomyces cerevisiae, melatonin is synthesized and metabolized to other 5-methoxylated indoles (5-methoxytryptamine and 5-methoxytryptophol).12 In other yeast species, ie, Saccharomyces uvarum and S. cerevisiae var. bayanus, melatonin production in synthetic grapes must depend on growth conditions and medium, including tryptophan concentration.13 Though no information is available on tryptophan-ethylester, the ability of yeasts to enrich indoleamine-fermented foods and beverages different from wine is corroborated by a number of studies.14–19 In this view, these microorganisms may also contribute to the biosynthesis of tryptophan-ethylester in wine. Our preliminary results showed that high levels of tryptophan-ethylester are produced by yeasts in enological conditions.20
In conclusion, as the source(s) of tryptophan-ethylester in wine is(are) still unknown, we can only speculate on the putative nutritional role(s) of this compound: it may provide a pool of tryptophan able to cross the gastrointestinal tract and, possibly, the blood–brain barrier; then, de-esterified tryptophan-ethylester may be rapidly metabolized in target cells like enteroendocrine cells, which produce serotonin and melatonin, two paracrine and endocrine factors.21 However, we have to take into account that pharmacologically active tryptophan-ethylester concentrations (5–20 mg/kg and higher) are unlikely to be reached in wines, at least based on current knowledge; therefore, we solicit further studies to quantify tryptophan-ethylester in a range of wines and grapes.
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: MI. Analyzed the data: MI, IV. Wrote the first draft of the manuscript: MI. Contributed to the writing of the manuscript: MI, IV. Agree with manuscript results and conclusions: MI, IV. Jointly developed the structure and arguments for the paper: MI, IV. Made critical revisions and approved final version: MI, IV. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Iriti M, Rossoni M, Faoro F. Melatonin content in grape: myth or panacea? J Sci Food Agric. 2006;86:1432–8. [Google Scholar]
- 2.Tan D-X, Hardeland R, Manchester LC, et al. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J Pineal Res. 2012;53:113–21. doi: 10.1111/j.1600-079X.2012.00979.x. [DOI] [PubMed] [Google Scholar]
- 3.Diamantini G, Tarzia G, Spadoni G, D’Alpaos M, Traldi P. Metastable ion studies in the characterization of melatonin isomers. Rapid Commun Mass Spectrom. 1998;12(20):1538–42. [Google Scholar]
- 4.Rodriguez-Naranjo MI, Gil-Izquierdo A, Troncoso AM, Cantos E, Garcia-Parrilla MC. Melatonin: a new bioactive compound in wine. J Food Compost Anal. 2011;24:603–8. [Google Scholar]
- 5.Gomez FJ, Raba J, Cerutti S, Silva MF. Monitoring melatonin and its isomer in Vitis vinifera cv Malbec by UHPLC-MS/MS from grape to bottle. J Pineal Res. 2012;52(3):349–55. doi: 10.1111/j.1600-079X.2011.00949.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomez FJ, Hernández IG, Martinez LD, Silva MF, Cerutti S. Analytical tools for elucidating the biological role of melatonin in plants by LC-MS/MS. Electrophoresis. 2013;34(12):1749–56. doi: 10.1002/elps.201200569. [DOI] [PubMed] [Google Scholar]
- 7.Vitalini S, Gardana C, Simonetti P, Fico G, Iriti M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J Pineal Res. 2013;54:322–33. doi: 10.1111/jpi.12028. [DOI] [PubMed] [Google Scholar]
- 8.Gardana C, Iriti M, Stuknytė M, De Noni I, Simonetti P. ‘Melatonin isomer’ in wine is not an isomer of the melatonin but tryptophan-ethylester. J Pineal Res. 2014;57(4):435–41. doi: 10.1111/jpi.12183. [DOI] [PubMed] [Google Scholar]
- 9.Reeves JP. Accumulation of amino acids by lysosomes incubated with amino acid methyl esters. J Biol Chem. 1979;254(18):8914–21. [PubMed] [Google Scholar]
- 10.Jonas AJ, Butler IJ. Circumvention of defective neutral amino acid transport in Hartnup disease using tryptophan ethyl ester. J Clin Invest. 1989;84(1):200–4. doi: 10.1172/JCI114141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadhav A, Liang W, Balsevich J, et al. L-tryptophan ethyl ester dilates small mesenteric arteries by inhibition of voltage-operated calcium channels in smooth muscle. Br J Pharmacol. 2012;166(1):232–42. doi: 10.1111/j.1476-5381.2011.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprenger J, Hardeland R, Fuhrberg B, Han SZ. Melatonin and other 5-methoxylated indoles in yeast: presence in high concentrations and dependence on tryptophan availability. Cytologia. 1999;64:209–13. [Google Scholar]
- 13.Rodriguez-Naranjo MI, Torija MJ, Mas A, Cantos-Villar E, Garcia-Parrilla Mdel C. Production of melatonin by Saccharomyces strains under growth and fermentation conditions. J Pineal Res. 2012;53(3):219–24. doi: 10.1111/j.1600-079X.2012.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Mena P, Gil-Izquierdo Á, Moreno DA, Martí N, García-Viguera C, Assessment of the melatonin production in pomegranate wines LWT Food Sci Technol. 2012;47(1):13–8. [Google Scholar]
- 15.Garcia-Moreno H, Calvo JR, Maldonado MD. High levels of melatonin generated during the brewing process. J Pineal Res. 2013;55(1):26–30. doi: 10.1111/jpi.12005. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Pachón MS, Medina S, Herrero-Martín G, et al. Alcoholic fermentation induces melatonin synthesis in orange juice. J Pineal Res. 2014;56(1):31–8. doi: 10.1111/jpi.12093. [DOI] [PubMed] [Google Scholar]
- 17.Kocadağlı T, Yılmaz C, Gökmen V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014;153:151–6. doi: 10.1016/j.foodchem.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Yılmaz C, Kocadağlı T, Gökmen V. Formation of melatonin and its isomer during bread dough fermentation and effect of baking. J Agric Food Chem. 2014;62(13):2900–5. doi: 10.1021/jf500294b. [DOI] [PubMed] [Google Scholar]
- 19.Vigentini I, Gardana C, Foschino R, et al. Production of melatonin and its isomers by yeasts and effects of tryptophan supplementation; Conference of the Italian Society of Human Nutrition (SINU); Florence. 2013. [Google Scholar]
- 20.Vigentini I, Gardana C, Fracassetti D, et al. Yeast contribution to melatonin, melatonin isomers and tryptophan-ethylester during alcoholic fermentation of grape musts. J Pineal Res. 2015 doi: 10.1111/jpi.12223. [DOI] [PubMed] [Google Scholar]
- 21.Konturek SJ, Konturek PC, Brzozowska I, et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT) J Physiol Pharmacol. 2007;58(3):381–405. [PubMed] [Google Scholar]
- 22.Stege PW, Sombra LL, Messina G, Martinez LD, Silva MD. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis. 2010;31:2242–8. doi: 10.1002/elps.200900782. [DOI] [PubMed] [Google Scholar]
- 23.Murch SJ, Hall BA, Le CH, Saxena PK. Changes in the levels of indoleamine phytochemicals during véraison and ripening of wine grapes. J Pineal Res. 2010;49:95–100. doi: 10.1111/j.1600-079X.2010.00774.x. [DOI] [PubMed] [Google Scholar]
- 24.Vitalini S, Gardana C, Zanzotto A, et al. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J Pineal Res. 2011;51:331–7. doi: 10.1111/j.1600-079X.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 25.Boccalandro HE, Gonzáles CV, Wunderlin DA, Silva MF. Melatonin levels, determined by LC-ESI-MS/MS, deeply fluctuate during the day in Vitis vinifera cv Malbec. Evidences for its antioxidant role in fruits. J Pineal Res. 2011;51:226–32. doi: 10.1111/j.1600-079X.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 26.Mercolini L, Mandrioli R, Raggi MA. Content of melatonin and other antioxidants in grape related foodstuffs: measurement using a MEPS-HPLC-F method. J Pineal Res. 2012;55:21–8. doi: 10.1111/j.1600-079X.2011.00967.x. [DOI] [PubMed] [Google Scholar]
- 27.Mercolini L, Addolorata Saracino M, Bugamelli F, et al. HPLC-F analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J Sep Sci. 2008;31:1007–14. doi: 10.1002/jssc.200700458. [DOI] [PubMed] [Google Scholar]
- 28.Vitalini S, Gardana C, Zanzotto A, et al. From vineyard to glass: agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J Pineal Res. 2011;51:278–85. doi: 10.1111/j.1600-079X.2011.00887.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Naranjo MI, Gil-Izquierdo A, Troncoso AM, Cantos-Villar E, Garcia-Parrilla MC. Melatonin is synthesized by yeast during alcoholic fermentation in wines. Food Chem. 2011;126:1608–13. doi: 10.1016/j.foodchem.2010.12.038. [DOI] [PubMed] [Google Scholar]


