Highlights
-
•
Neural correlates of 6-month-old infants’ detection of pro-social agents.
-
•
ERP component P400 over posterior temporal areas index social valence.
-
•
First non-behavioral demonstration of pro-social preferences in young infants.
Keywords: ERP, P400, Prosocial, Empathy, Infant, EEG
Abstract
The current study is the first to investigate neural correlates of infants’ detection of pro- and antisocial agents. Differences in ERP component P400 over posterior temporal areas were found during 6-month-olds’ observation of helping and hindering agents (Experiment 1), but not during observation of identically moving agents that did not help or hinder (Experiment 2). The results demonstrate that the P400 component indexes activation of infants’ memories of previously perceived interactions between social agents. This leads to suggest that similar processes might be involved in infants’ processing of pro- and antisocial agents and other social perception processes (encoding gaze direction, goal directed grasping and pointing).
1. Introduction
Pioneering work by Premack and Premack (1997) demonstrated that 1-year-old infants attribute goals to animated agents helping or hindering each other, suggesting that these events are valued as positive and negative by the infants. This initial finding has been replicated and extended in several ways. Infants from 3 months of age express different preferences for animated agents that either help (pro-social) or hinder (anti-social) another geometric shape from climbing a hill (Hamlin et al., 2010). At this age infants look longer toward the helper than the hinderer, the effect being driven by a tendency to avoid looking at the anti-social agent. At 6 months, once infants’ own manual capabilities have developed, infants systematically reach for pro-social agents when given a choice between a helper and a hinderer (Hamlin et al., 2007).
These initial pro-social preferences are more complex during the second half of the first year, being influenced by the social context in which an interaction occurs. Eight-month-olds prefer agents that act positively toward pro-social others and negatively toward anti-social others (Hamlin et al., 2011) whereas 9-month-olds prefer agents that treat similar others well and dissimilar others poorly (Hamlin et al., 2013a). At 1 year of age, infants not only attribute dispositional states to these interacting geometric shapes (Kuhlmeier et al., 2003) but also predict that agents will seek out others that have previously helped them (Fawcett and Liszkowski, 2012).
Scarf et al. (2012) have criticized the interpretation made by Hamlin et al. (2007). Instead of focusing on helping and hindering actions, Scarf et al. (2012) argue that infants prefer agents that are associated with positive events. Although this was a reasonable criticism of the original study this alternative interpretation cannot account for the conceptual replications of this method that have been performed using different stimuli (Hamlin et al., 2013b, Hamlin and Wynn, 2011), nor can it account for the subsequent more complex findings that infants’ prosocial preferences are not rigid but depend on context (Hamlin et al., 2011, Hamlin et al., 2013a, Hamlin et al., 2013b). In sum, there is currently substantial support for the notion that infants are able to interpret the interaction of animate agents as pro- or anti-social and that they use this information to guide their attention and reaching actions (for a review see Hamlin, 2013, Hamlin, 2014).
Several different interpretations have been proposed to account for infants’ evaluation of actions’ social valence. One possibility is that early-emerging mentalistic processes such as theory of mind and perspective taking (Kovacs et al., 2010, Onishi and Baillargeon, 2005, Southgate et al., 2007) mediate infants’ pro-social preferences (Hamlin et al., 2013b). In fact, Hamlin et al. (2013b) suggest, based on modeling of empirical data, that 10-month-olds’ pro-social preferences might involve second-order mental-state representations. That is, the goal of one agent relates to the intention of another (here referred to as the mentalistic account).
In adults and children the temporal parietal junction (TPJ), and with increased age the pre-frontal cortex (Kobayashi et al., 2007), are often implicated in mentalistic processes (Van Overwalle and Baetens, 2009), along with activation in the superior temporal sulcus (STS; Decety, 2011, Decety and Howard, 2013, Moll et al., 2002). Based on neuropsychological investigations of empathy conducted with adults and older children, alternative accounts are also possible. It is conceivable that some pro-social preferences are governed by lower level social perception processes, which relate actions to goals without the need for mentalizing (here referred to as the social perception account). Several indications suggest that these processes are organized by the STS without necessary involvement of higher cognitive functions. Adults showed more activation of the STS for animated geometrical shapes that appeared to interact in an intentional manner when compared to random movements, the activity being related to participant's ratings of intentionality (Castelli et al., 2000). In children, a recent fMRI study demonstrated that the STS (as one of several areas) is sensitive to perceived intentional harm in others (Decety et al., 2012). On a larger scale the STS is sensitive to body movement and goal directed actions such as reaching and looking, in addition to being sensitive to faces and emotional expressions in general (Allison et al., 2000).
The common denominator of the mentalistic and social perception accounts of processing of socially valenced actions is therefore the involvement of the STS. In infants it has been argued that the P400 ERP component is an index of STS activity (Gredebäck et al., 2010). There are several lines of enquiry that support this assumption. First of all, the P400 is often described as an infant version of the adult N170–N200 (along with the infant N290; de Haan et al., 2002, Nelson et al., 2006). This is based on studies that demonstrate infant P400 and adult N170–N200 in response to identical stimuli (Gredebäck et al., 2010, Senju et al., 2006) and the observation that similar manipulations alter the amplitude of infant P400 and adult N170–N200 components (Csibra et al., 2008). The N170–N200 in turn has been directly related to the STS via source localization and joint EEG, fMRI measures (Puce et al., 1998, Itier and Taylor, 2004, Dalrymple et al., 2011). On the basis of these arguments, and the observation that both accounts involve STS activity, we hypothesize that an infant P400 ERP component indexes processing of actions’ social valence.
No study has investigated the neural correlates of pro-social preferences in young infants. However, a few studies have demonstrated that the infant ERP component P400 is related to processing of goal directed actions such as grasping (Bakker et al., in press) and pointing (Gredebäck et al., 2010, Melinder et al., in press), to emotional processing (Leppanen et al., 2007), biological motion (Reid et al., 2006) and gaze direction (Senju et al., 2006). In this context it is important to note that P400 amplitudes are larger for functional and goal directed actions (reaching for, or pointing toward objects, looking at interesting sights) than control stimuli that lack these object directed properties.
In this study we examine the neural correlates of infants’ pro-social preferences by measuring EEG and target ERP components hypothesized to be sensitive to pro- and anti-social agents in the hill-climber paradigm (Hamlin et al., 2007). More specifically, 6-month-old infants were presented with two scenarios. In Experiment 1 one agent helps another agent (ball with eyes) to reach the top of a hill, whereas a different agent hinders the circular agent from reaching the top of a hill. In Experiment 2 one agent pushes up an inanimate ball (without eyes) to the top of the hill and another agent pushed the ball down the hill. Following these scenarios infants were presented with repeated images of the two agents that helped or hindered in Experiment 1 and moved up vs down in Experiment 2 (only one image was presented on each trial) and ERP components for these images were analyzed (for similar designs see Kaduk et al., 2013, Parise et al., 2008).
We hypothesize that P400 amplitudes will differ between pro- and anti-social agents (Experiment 1). Furthermore, given that prior studies have demonstrated larger amplitudes of P400 for congruent than incongruent pointing (Gredebäck et al., 2010) and gaze direction (Senju et al., 2006), as well as for upright over inverted biological motion point-light displays (Reid et al., 2006), we predict a larger P400 in response to agents that previously have helped over agents that previously hindered others. The hypothesized common denominator of previously and currently investigated social stimuli is a larger P400 amplitude for functional and typical social behavior (pointing to objects, looking at interesting sights, and agents that help others). No difference in P400 amplitudes is expected in Experiment 2 where the agent that is being helped and/or hindered is replaced by an inanimate ball.
We also analyzed the Nc component. This mid-latency component occurs approximately 300–700 ms after stimulus onset and is most prominent at fronto-central electrodes. It reflects attentional orienting to salient stimuli (Courchesne et al., 1981) and/or a general attentional arousal (Richards, 2003), as it is larger to infrequent than frequent stimuli (e.g. Courchesne et al., 1981) and is larger during periods of sustained attention (Richards, 2003). We examined the Nc in order to preclude attention and other lower level accounts that may provide an alternative explanation for differences found between conditions in the P400.
2. Experiment 1
2.1. Methods
2.1.1. Participants
Fourteen 6-month-old infants (eight female; mean age 6 months, 3 days, range 5 months, 27 days to 6 months, 12 days) were included in the final analysis. An additional 11 infants (five female) were excluded since they did not pass the inclusion criteria of at least 10 artifact free trials per stimulus set (helper/hinderer). None of these infants participated in Experiment 2. Parents signed a consent form prior to participation and received a gift certificate (100 SEK). The local ethical committee, in accordance with the declaration of Helsinki, approved the study.
2.1.2. Stimuli and procedure
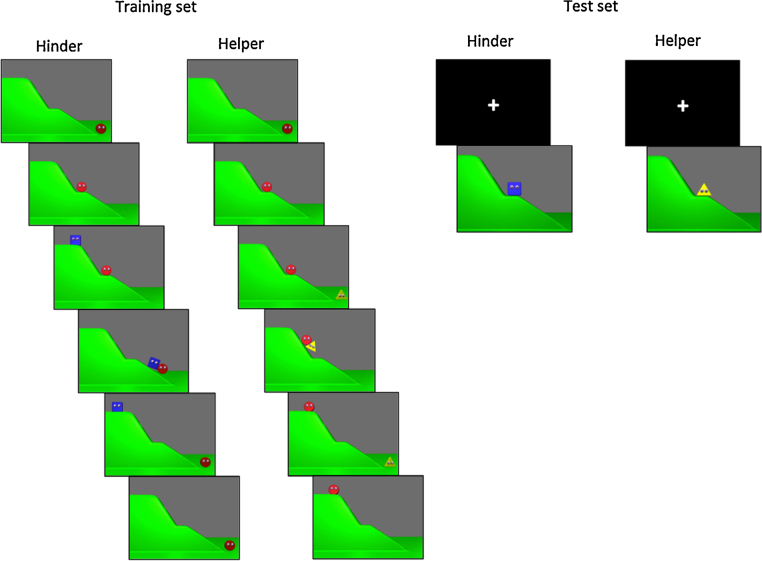
Each participating infant was presented with two sets of stimuli, one training set and one test set, created in Blender, an open source 3D animation environment (www.blender.org). The training set included six movies (three helper and three hinderer) in which a protagonist (a red ball with eyes) attempted and failed to climb a hill. In each movie the protagonist made three failed attempts to climb the hill after which the protagonist was either helped or hindered by another geometric shape (a blue square or a yellow triangle with eyes, see Fig. 1). The movies were presented in a counterbalanced order (helper or hinderer first), with square and triangle randomly assigned to be helper or hinderer. Each movie was 14 s long and was preceded by a black screen (duration 3 s prior to first movie, 2 s prior to all remaining movies). The total duration of the entire training session was 97 s (judged by the authors to be the maximum amount of time that infants would attend to the training stimuli and the subsequent test stimuli).
Fig. 1.
Stimuli used in training and test set of Experiment 1. Note that the identity of the helper (here a triangle) and the hinderer (here a square) was randomized across participants. (For interpretation of the references to color in this sentence, the reader is referred to the web version of the article.)
The hinderer movies started with a still image of the hill (1000 ms). The protagonist emerged from the right, climbed the first half of the hill and then bounced up and down three times (after which 4000 ms were elapsed since movie start). The protagonist then tried unsuccessfully to climb the steeper second half of the hill. These motions were intended to establish the protagonist as an agent in need of assistance because it was unable to reach its goal. Movements of the protagonist during these events were accompanied by a sound highlighting the movements of the ball. Following these initial events the hinderer entered from the top of the hill (7700 ms elapsed) and pushed the protagonist all the way down the hill before returning to its initial position and disappearing (13 100 ms). The movie ended with a stationary picture depicting the protagonist at the bottom of the hill. In total the movie lasted 14 s. The helper movies had an identical timing and overall structure, except that the helper appeared from below and helped the protagonist by pushing it up the hill. As the helper moved back down the hill, the protagonist bounced three times on the hilltop. This movement ended just before the helper disappeared from the screen at the bottom of the hill. The helper and hinderer had moving eyes and gazed at the protagonist as they interacted with it. The protagonist's eyes always fixated its goal at the top of the hill. In each movie the helper and hinderer were accompanied by different sounds highlighting their movements, with a slightly lower pitched sound always accompanying the triangle and a slightly higher pitched sound accompanying the square.
The test set of 40 trials randomly alternated still images of the helper and hinderer (20 trials each), positioned at the middle of the hill. Each trial consisted of a black picture with a fixation-cross (duration 1200–1400 ms) followed by an image of the helper or hinderer together with its corresponding sound (image duration 400 ms, sound duration 250 ms). This target stimulus was then followed by the next fixation cross and the next stimulus image.
In order to increase participants’ opportunity to process the training material, participating families were sent internet links to the training set and asked to watch it together with their child on the two consecutive days immediately prior to the experiment day. Parents were instructed to watch the movies in full screen mode in a quiet environment without distractions. Parents were also informed that presenting the movies more times might reduce their infant's attention to the stimuli once in the lab. All parents reported that they had followed these instructions when asked on arrival to the lab.
During the lab session participants once more viewed the training set and directly thereafter the test set. The study was a within subject design, as such each infant was presented with both helper and the hinderer trials. Once the test set was completed, or if infants lost attention to the screen, the training set was presented once more followed by the test set. The stimuli were repeated until participants stopped attending. On average infants attended to 48 trials (range 24–59) of the test stimuli depicting the helper and 48 trials (range 24–58) of the test stimuli depicting the hinderer.
2.1.3. EEG recording and analysis
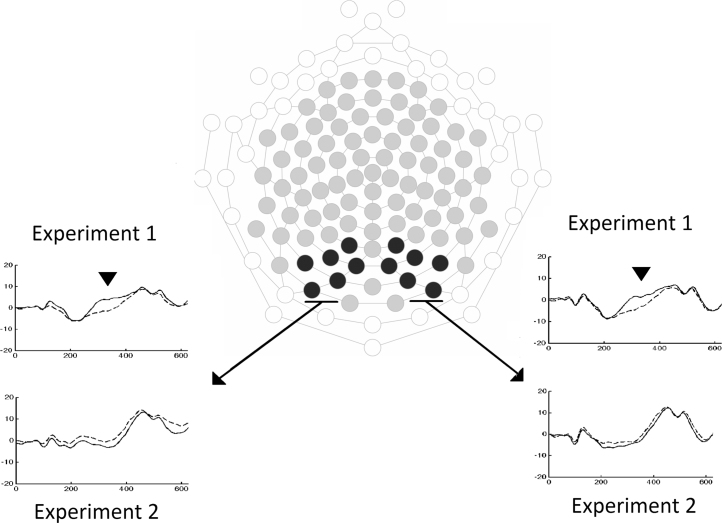
Age appropriate 128-channel Geodesic Sensor Nets (HCGSN 130; EGI, Eugene, OR) were used to record EEG signals. The signal (vertex referenced) was sampled at 250 Hz, amplified by EGI Net amplifier (GES 300 Amp; EGI, Eugene, OR) and stored for off-line analysis. Continuous EEG data were digitally filtered (0.3–30 Hz) and segmented from 200 ms prior to the appearance of the agent to 1000 ms after the agent's appearance. The electrodes from the most anterior and posterior area were not included in the final analysis due to high noise caused by poor contact with the scalp. In total, 38 electrodes were excluded from the analyses (see Fig. 2). On remaining 90 electrodes artifacts within trials were initially defined as channels with more than 200 μV change in amplitude. The data were then manually checked for artifacts. On average 21 helper trials per infant (range 13–34) and 20 hinderer trials (range 10–30) were included in the final analysis. This data were baseline corrected (average amplitude of 200 ms prior to appearance of agent) individually for each trial. Segments were then re-referenced (average reference) and aggregated to individual averages for each trial type (helper/hinderer). Individual averages and the grand average ERPs were visually inspected and electrodes in locations related to posterior temporal cortex (P400) were further examined (see Fig. 2).
Fig. 2.
Denotes channels used to analyze P400 (black circles), channels included in the analysis (gray circles) and excluded channels (white circles). Grand average ERP data are shown for selected channels for the first 600 ms after the onset of the test stimulus (image of helper/hinderer in Experiment 1 agent moving up or down in Experiment 2). Black triangles mark significant differences between helper and hinder trials. Solid lines represent helper in Experiment 1 and agent moving up in Experiment 2, dashed lines represent hinderer in Experiment 1 and agent moving down in Experiment 2.
Channels previously used to assess the P400 component over posterior temporal areas in infancy (Gredebäck et al., 2010) were also investigated in the present study (left channel numbers; 59, 60, 61, 65, 66, 67, and right channel numbers; 77, 78, 84, 85, 90, 91). In addition ERP component Nc (channel numbers 5, 6, 7, 12, 13, 20, 29, 104, 105, 106, 111, 112, 118) was analyzed. Analyses were based on the average amplitude of these channels and a time interval ranging from 250 to 400 ms after the appearance of the helper or hinderer on the screen (in the test set) for P400. The Nc was investigated using the average amplitude of the above-mentioned channels during a time range from 400 to 600 ms after the appearance of the helper or hinderer. Amplitude data for P400 was aggregated over electrodes and entered as the dependent variable in a General Linear Model (GLM) with agent (helper, hinderer) and hemisphere (left, right) as predictors and complemented with non-parametric Wilcoxon Matched Pairs Test (aggregated over hemisphere). Amplitude data for Nc were aggregated over channels and entered as the dependent variable in a repeated measure t-test with agent as independent variable and complemented with non-parametric Wilcoxon Matched Pairs Test. All data sets were checked for outliers (±3 z-score), however, no outlier channels were detected.
2.2. Results
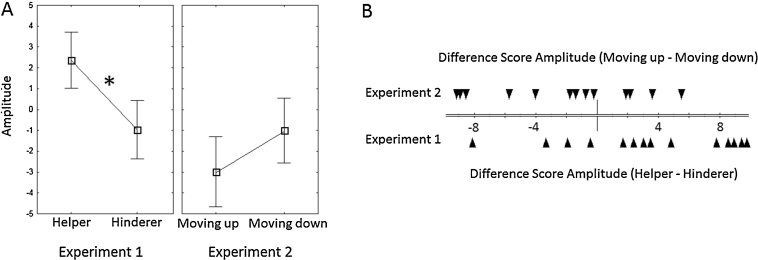
The amplitude of P400 differed significantly between the helper (2.36 μV, SE = 1.35) and hinderer (−0.96 μV, SE = 1.4), F(1,13) = 5.27, p = .039, (see Fig. 2, Fig. 3). No significant main effect of hemisphere (left and right), F(1,13) = 1.09, p = .31, or interaction effect between hemisphere and agent, F(1,13) = 0.002, p = .96, was observed. A Wilcoxon Matched Pairs Test demonstrated a significant effect of trial type, W(14) = 20, p = .04 for the P400. At the same time no differences between helper (−3.82 μV, SE = 1.05) and hinderer (−1.98 μV, SE = 1.12) trials were observed for the Nc, t(13) = 1.33, p = .21. A Wilcoxon Matched Pairs Test confirmed no significant effect of trial type, W(14) = 34, p = .24 for the Nc component.
Fig. 3.
Average amplitude of the P400 component, separate for the two trial types (helper, hinderer) in Experiments 1 and (moving-up, moving-down agent) 2 with asterisks indicating differences between helping and hindering trials at p < .05 and error bars representing SE (A). Individual amplitude difference scores (helper–hinderer; moving up–moving down agent) separate for each experiment (B).
2.3. Discussion
Experiment 1 demonstrates differential processing of pro- and anti-social agents, expressed as larger P400 amplitudes for agents that previously helped than agents that previously hindered another agent. No differences were observed in ERP component Nc. One important caveat is the possibility that reported effects reflects superficial properties of the stimuli (e.g. upward and downward motion) and not of social valence. In order to control for this Experiment 2 presented infants with highly similar events in which the agent being helped or hindered is replaced with a ball. Thus, the helping agent in Experiment 1 is now moving a ball (with no eyes) up a hill and the prior hindering agent from Experiment 1 now moves a ball down the hill.
ERP components reflecting evaluations of social valence should no longer differentiate between the two trial types in Experiment 2, because the red ball is no longer established as an agent with goals and can therefore no longer be helped or hindered. At the same time ERP components resulting from other properties of the stimuli such as superficial perceptual or attentional differences should remain or be enhanced. Along these lines we hypothesize that differences in amplitude of P400 between helper and hinderer should disappear when comparing upward and downward motion in Experiment 2.
3. Experiment 2
3.1. Methods
3.1.1. Participants
Fourteen 6-month-old infants (six female; mean age 6 months, 3 days, range 5 months, 21 days to 6 months, 12 days) were included in the final analysis. An additional 10 infants (five females) were excluded since they did not pass the inclusion criterion of at least 10 artifact free trials per trial type. General procedures were conducted as in Experiment 1.
3.1.2. Stimuli and procedure
The general properties of the stimuli remained the same as described in Experiment 1 above. The test stimuli and procedure were identical. The main difference between the two training stimuli sets was the ball's absence of eyes and self-propulsion. Thus the ball was inanimate and moved only when being pushed up (move up movies) or down (move down movies) the hill. The movements of the two agents as they pushed the ball up and down the hill were identical to in Experiment 1. The duration of each movie was 7 s – movies were shorter because they lacked the initial phase establishing the ball's attempt to climb the hill. Before the first movie a 3 s black screen was presented. For the remaining movies the duration of the black screen was 2 s. The total presentation time of the entire training session was 55 s long. The movies were presented in a counterbalanced order, with the two agents (square and triangle) randomly assigned to movie types (move up or move down).
The move down movies started with a still image of the hill with the ball located at the middle of the hill (1000 ms). The agent moving down then appeared from above, pushed the ball down the hill, and moved up the hill and left the screen using identical motions and timings to the hinderer in Experiment 1. The movie ended with a still picture containing the ball stationary at the bottom of the hill (600 ms). The move up movies had an identical timing and overall structure, but here the agent moving up appeared from the bottom, pushed the ball up the hill, using identical motions and timings to the helper in Experiment 1. All other aspects of the training set, the test set, and the experimental design were identical to Experiment 1, including sounds associated with the different agents. On average infants attended to 49 trials (range 30–87) of the test stimuli depicting the move-up agent and 50 trials (range 30–70) of the test stimuli depicting the move-down agent.
3.1.3. EEG recording and analysis
The data reduction procedure was identical to that described under Experiment 1. On average 20 move-up agent trials per infant (range 11–47) and 21 move-down agent trials (range 10–45) were included in the final analysis.
3.2. Results
The amplitude of P400 did not differ between the two trial types; move-up agent (−2.98 μV, SE = 1.68) and move-down agent (−1.0 μV, SE = 1.56), F(1,13) = 2.48, p = .14 (Fig. 2, Fig. 3). No significant main effects of hemisphere (left and right), F(1,13) = .006, p = .94, or interaction effects between hemisphere and agent, F(1,13) = 1.94, p = .18, were observed. A Wilcoxon Matched Pairs Test demonstrated no significant effect of trial type, W(14) = 31, p = .18 for the P400. In addition the results demonstrate a significant Nc component, t(13) = 2.74, p = .002, with a more negative Nc amplitude for the moving down agent (−4.83 μV, SE = 1.79) when contrasted to the moving up agent (−2.74 μV, SE = 1.38). The Wilcoxon Matched Pairs Test further confirmed the significant effect of trial type, W(14) = 15, p = .02.
3.3. Discussion
Experiment 2 did not find differences between amplitudes in ERP component P400 when comparing agents moving an inanimate ball up or down a hill. There is therefore no evidence for a P400 effect when the social valence dimension is removed, suggesting that P400 in the current context is modulated by degree of prosociality. Instead Experiment 2 demonstrates differences between upward and downward moving agents in the Nc component often assumed to index attention (Richards, 2003). As no such effect was demonstrated in Experiment 1, this is likely not related to the core question at hand and might reflect attentional difference with respect to low-level visual properties of the stimuli used in Experiment 2. Apparently these aspects of the stimuli become more salient when the social interaction and the helping or hindering is removed.
4. General discussion
Six-month-old infants’ neural activity, as measured by the ERP component P400, differed when observing agents that previously helped and agents that previously hindered others. The P400 amplitude differences did not manifest themselves in Experiment 2, where upward and downward moving agents had no pro- or antisocial connotations. This is the first study that relates detection of prosociality to a specific ERP component and the first to demonstrate that infant P400 amplitudes are mediated by an agent's history of helping or hindering others (for prior work on EEG oscillations in 14-month-olds in the context of prosocial behavior see Paulus et al., 2013).
The current finding appears consistent with behavioral data at 6 months of age. Prior work has documented overt preferences for helping over hindering agents, measured by both looking times and preferential reaching (Hamlin et al., 2007, Hamlin et al., 2010) at the same age. We did not measure infants’ behavioral responses to the stimuli, but it should be noted that the current stimuli very closely resemble the stimuli used in previous studies to demonstrate behavioral preferences for pro-social agents and avoidance of antisocial agents in infants of the same age (Hamlin et al., 2007). It is therefore very likely that the neural correlates of social valence processing demonstrated here represent the first stages of the neural process leading to infants’ expression of pro-social preferences.
The findings are also consistent with the few prior studies that have indexed social perception using the P400 component. The same ERP component that here indexes social valence has previously been documented to index social perception of various stimuli, including goal directed grasping (Bakker et al., in press), pointing (Gredebäck et al., 2010, Melinder et al., in press) and gaze direction (Senju et al., 2006). In those studies functional and goal directed actions resulted in larger amplitudes than non-goal directed and/or non-functional events. We suggest that the current results fit in with this interpretation under the assumption that 6-month-olds are more readily attuned to process positive compared to negative valenced social actions. This processes is indexed by ERP component P400. This assumption receives support from the observation that across a variety of social modalities, infants in their first 6 months devote more attention to positive stimuli, with a bias toward negativity developing later (Vaish et al., 2008). Furthermore, specifically in the hill climber paradigm young infants devote more visual attention to the helper than the hinderer (Hamlin et al., 2010). However, this latter study also demonstrates that 3-month-olds discriminate a hinderer from a neutral actor but not a helper from a neutral actor. Together, these findings are not yet fully interpretable. What is clear however is that the infant P400 response is general to a broad range of social stimuli including not only the discrimination of more or less functional actions but also the discrimination of valenced social actions. The P400 component not only indexes social perceptual processes that relate one person's actions to immediate goals (Bakker et al., in press, Gredebäck et al., 2010, Senju et al., 2006) but also to interactions between social agents. Though the current stimuli belong to a somewhat different class of social events it is possible that current P400 response maps the same underlying neural networks dedicated to other forms of social perception.
One design difference between this study and previous studies of the infant P400 reveals a new aspect of the P400. In previous studies, condition differences were directly perceivable in the test stimuli, for example because a pointing gesture was congruent or incongruent with an object location displayed a fraction of a second previously. In this study, however, there were no differences in the directly perceivable properties of the test stimuli (the agents themselves). Rather, agents differed in behavior displayed during the training stimuli, which were presented separately. Most infants saw a full set of 40 test trials, which lasted 68 s, meaning test trials were presented on average 34 s after a training stimulus. Different responses to different agents based on their previous history of social interaction imply that infants encode in memory attributions of valence to specific agents (Hamlin et al., 2013a). The current results indicate that the infant P400 can index not only immediate evaluations of social stimuli but also activation of memory of properties associated with particular agents.
It is difficult to connect ERP components to specific spatial locations on the cortex, in the absence of source localization. However, with respect to P400, there is a strong candidate area that has previously been associated with this neural marker. Nelson et al. (2006) argue that the infant P400 is functionally similar to the adult N170 component (see also Csibra et al., 2008, de Haan et al., 2003, Nelson et al., 2006). In turn, the adult N170 component has been connected with the STS using both source localization of ERP data (Itier and Taylor, 2004) and joint recordings of ERP and fMRI (Dalrymple et al., 2011). One possibility, that requires further study before firm conclusions can be made, is that the P400 component demonstrated for pro- and antisocial agents in 6-month-olds originates from processes similar to those underlying other forms of social perception, namely STS activity (Gredebäck et al., 2010).
The aim of this study was not to directly compare the mentalistic and non-mentalistic accounts for social valence detection described in the introduction, as there are many aspects of neural activity that are not accessible via ERP techniques. We hypothesized a P400 component because both these accounts involve STS activity, which the infant P400 has been argued to reflect. However, we note that visual inspection of ERP data did not demonstrate any other ERP components selectively processing helping and hindering (Experiment 1) but not agents moving up or down (Experiment 2). As such, no clear evidence of a larger network involving the pre-frontal cortex can be found in the current study. The current findings therefore provide no support for the hypothesis that second-order mentalization lies behind prosocial preferences at 6-months of age, although such processes have been implicated in older infants (Hamlin et al., 2013b). Although undetected frontal correlates cannot be ruled out, a parsimonious account of 6-month-olds’ prosocial preferences based on the current data is that they rely on a network restricted to posterior-temporal areas and the STS, as suggested by the non-mentalistic account. One possibility is that STS activation is supported by anthropomorphizing processes in the amygdala (Heberlein and Adolphs, 2004) that transform moving geometric shapes into perceived agents with intentions and goals.
5. Conclusions
We demonstrated that the P400 component not only indexes social perceptual processes that relate one person's actions to immediate goals (Bakker et al., in press, Gredebäck et al., 2010, Senju et al., 2006) but also activation of the memory of social agents’ previous pro- and antisocial actions. Similarities between the current results and prior studies targeting social perception suggest that similar mechanisms might be involved in processing both goal directed actions (pointing and gaze), biological motion, and collaborative interactions between non-human agents (helping). When it comes to social valence these processes appear functional at 6 months of age.
Conflict of interest
No conflicts of interest to report.
Acknowledgments
The paper was supported by ERC-StG grant CACTUS (312292) and Marie Curie ITN ACT (289404).
Footnotes
Available online 28 January 2015
References
- Allison T., Puce A., McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Bakker M., Daum M.M., Handl A., Gredebäck G. Neural correlate of action perception at the onset of functional grasping. Soc. Cogn. Affect. Neurosci. 2015 doi: 10.1093/scan/nsu119. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Happe F., Frith U., Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Ganz L., Norcia A.M. Event-related brain potentials to human faces in infants. Child Dev. 1981;52:804–811. [PubMed] [Google Scholar]
- Csibra G., Kushnerenko E., Grossman T. Electrophysiological methods in studying infant cognitive development. In: Nelson C.A., Luciana M., editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2008. pp. 247–262. [Google Scholar]
- Dalrymple K.A., Oruc I., Duchaine B., Pancaroglu R., Fox C.J., Iaria G. The anatomic basis of the right face-selective N170 IN acquired prosopagnosia: a combined ERP/fMRI study. Neuropsychologia. 2011;49(9):2553–2563. doi: 10.1016/j.neuropsychologia.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Decety J. Dissecting the neural mechanisms mediating empathy. Emot. Rev. 2011;3(1):92–108. [Google Scholar]
- Decety J., Howard L.H. The role of affect in the neurodevelopment of morality. Child Dev. Perspect. 2013;7(1):49–54. [Google Scholar]
- Decety J., Michalska K.J., Kinzler K.D. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study [Research Support, U.S. Gov’t, Non-P.H.S.] Cereb. Cortex. 2012;22(1):209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- de Haan M., Johnson M.H., Halit J. Development of face-sensitive event-related potentials during infancy: a review. Int. J. Psychophysiol. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M., Pascalis O., Johnson M.H. Specialization of neural mechanisms underlying face recognition in human infants. J. Cogn. Neurosci. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Fawcett C., Liszkowski U. Infants anticipate others’ social preferences. Infant Child Dev. 2012;21(3):239–249. [Google Scholar]
- Gredebäck G., Melinder A., Daum M. The development and neural basis of pointing comprehension. Soc. Neurosci. 2010;5(5–6):441–450. doi: 10.1080/17470910903523327. [DOI] [PubMed] [Google Scholar]
- Hamlin J.K. Moral judgment and action in preverbal infants and toddlers: evidence for an innate moral core. Curr. Dir. Psychol. Sci. 2013;22(3) [Google Scholar]
- Hamlin J.K. The case for social evaluation in preverbal infants: gazing toward one's goal drives infants’ preferences for helpers over hinderers in the hill paradigm. Front. Psychol. 2014;5:1563. doi: 10.3389/fpsyg.2014.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J.K., Mahajan N., Liberman Z., Wynn K. Not like me = bad: infants prefer those who harm dissimilar others. Psychol. Sci. 2013;24(4):589–594. doi: 10.1177/0956797612457785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J.K., Ullman T., Tenenbaum J., Goodman N., Baker C. The mentalistic basis of core social cognition: experiments in preverbal infants and a computational model. Dev. Sci. 2013;16(2):209–226. doi: 10.1111/desc.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J.K., Wynn K., Bloom P. Social evaluation by preverbal infants. Nature. 2007;450(22):557–560. doi: 10.1038/nature06288. [DOI] [PubMed] [Google Scholar]
- Hamlin J.K., Wynn K., Bloom P. Three-month-olds show a negativity bias in their social evaluations. Dev. Sci. 2010;13(6):923–929. doi: 10.1111/j.1467-7687.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J.K., Wynn K. Young infants prefer pro-social to antisocial others. Cogn. Dev. 2011;26(1):30–39. doi: 10.1016/j.cogdev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J.K., Wynn K., Bloom P., Mahajan N. How infants and toddlers react to antisocial others. Proc. Natl. Acad. Sci. U. S. A. 2011;108(50):19931–19936. doi: 10.1073/pnas.1110306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A.S., Adolphs R. Impaired spontaneous anthropomorphizing despite intact perception and social knowledge. Proc. Natl. Acad. Sci. U. S. A. 2004;101(19):7487–7491. doi: 10.1073/pnas.0308220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier R.J., Taylor M.J. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15(8):1261–1265. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- Kaduk K., Elsner B., Reid V. Discrimination of animate and inanimate motion in 9-month-old infants: an ERP study. Dev. Cogn. Neurosci. 2013;6:14–22. doi: 10.1016/j.dcn.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C., Glover G.H., Temple E. Children's and adults’ neural bases of verbal and nonverbal ‘theory of mind’. Neuropsychologia. 2007;45(7):1522–1532. doi: 10.1016/j.neuropsychologia.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs A.M., Teglas E., Endress A.D. The social sense: susceptibility to others’ beliefs in human infants and adults. Science. 2010;330(6012):1830–1834. doi: 10.1126/science.1190792. [DOI] [PubMed] [Google Scholar]
- Kuhlmeier V., Wynn K., Bloom P. Attribution of dispositional states by 12-month-olds. Psychol. Sci. 2003;14(5):402–408. doi: 10.1111/1467-9280.01454. [DOI] [PubMed] [Google Scholar]
- Leppanen J.M., Moulson M.C., Vogel-Farley V.K., Nelson C.A. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinder A.M., Konijnenberg C., Hermansen T., Daum M., Gredebäck G. The developmental trajectory of pointing perception in the first year of life. Exp. Brain Res. 2015 doi: 10.1007/s00221-014-4143-2. (in press) [DOI] [PubMed] [Google Scholar]
- Moll J., de Oliveira-Souza R., Bramati I.E., Grafman J. Functional networks in emotional moral and nonmoral social judgments. Neuroimage. 2002;16(3):696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Nelson C.A., Moulson M.C., Richmond J. How does neuroscience inform the study of cognitive development? Hum. Dev. 2006;49:260–272. [Google Scholar]
- Onishi K.H., Baillargeon R. Do 15-month-old infants understand false beliefs? Science. 2005;308:255–258. doi: 10.1126/science.1107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise E., Reid V.M., Stets M., Striano T. Direct eye contact influences the neural processing of objects in 5-month-old infants. Soc. Neurosci. 2008;3(2):141–150. doi: 10.1080/17470910701865458. [DOI] [PubMed] [Google Scholar]
- Paulus M., Kühn-Popp N., Licata M., Sodian B., Meinhardt J. Neural correlates of pro-social behavior in infancy: different neurophysiological mechanisms support the emergence of helping and comforting. Neuroimage. 2013;66:522–530. doi: 10.1016/j.neuroimage.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Puce A., Allison T., Bentin S., Gore J.C., McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J. Neurosci. 1998;18(6):2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D., Premack A.J. Infants attribute value± to the goal-directed actions of self-propelled objects. J. Cogn. Neurosci. 1997;9(6):848–856. doi: 10.1162/jocn.1997.9.6.848. [DOI] [PubMed] [Google Scholar]
- Reid V., Hoehl S., Striano T. The perception of biological motion by infants: an event-related potential study. Neurosci. Lett. 2006;395:211–214. doi: 10.1016/j.neulet.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Richards J.E. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Dev. Sci. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarf D., Imuta K., Colombo M., Hayne H. Social evaluation or simple association? Simple associations may explain moral reasoning in infants. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A., Johnson M.H., Csibra G. The development and neural basis of referential gaze perception. Soc. Neurosci. 2006;1(3–4):220–234. doi: 10.1080/17470910600989797. [DOI] [PubMed] [Google Scholar]
- Southgate V., Senju A., Csibra G. Action anticipation through attribution of false belief by 2-year-olds. Psychol. Sci. 2007;18(7):587–592. doi: 10.1111/j.1467-9280.2007.01944.x. [DOI] [PubMed] [Google Scholar]
- Vaish A., Grossmann T., Woodward A. Not all emotions are created equal: the negativity bias in social-emotional development. Psychol. Bull. 2008;134(3):383–403. doi: 10.1037/0033-2909.134.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]