Abstract
Background
The aim of this study was to determine whether MSC are excellent materials for MSCs transplantation in the treatment of osteoporosis.
Material/Methods
We studied normal, osteoporosis, and TERT-transfected MSC from normal and osteoporosis rats to compare the proliferation and osteogenic differentiation using RT-PCR and Western blot by constructing an ovariectomized rat model of osteoporosis (OVX). The primary MSC from model rats were extracted and cultured to evaluate the proliferation and differentiation characteristics.
Results
MSCs of osteoporosis rats obviously decreased in proliferation ability and osteogenic differentiation compared to that of normal rats. In contrast, in TERT-transfected MSC, the proliferation and differentiation ability, and especially the ability of osteogenic differentiation, were significantly higher than in osteoporosis MSC.
Conclusions
TERT-transfected MSCs can help osteoporosis patients in whom MSC proliferation and osteogenic differentiation ability are weak, with an increase in both bone mass and bone density, becoming an effective material for autologous transplantation of MSCs in further treatment of osteoporosis. However, studies are still needed to prove the in vivo effect, biological safety, and molecular mechanism of TERT-osteoporosis treatment. Additionally, because the results are from an animal model, more research is needed in generalizing rat model findings to human osteoporosis patients.
MeSH Keywords: Cell Proliferation, Mesenchymal Stromal Cells, Osteoporosis, RANK Ligand, Therapeutics
Background
Osteoporosis is an osteometabolic disease, characterized by low bone mass and micro-architectural deterioration of bone tissue, enhanced bone fragility, and increased fracture risk [1], which has become a major threat to health of the elderly people in aging societies. There are about 200 million people worldwide with this disease. In the United States in 2014 its prevalence reached 14 million [2]. Osteoporosis can be primary or secondary to various conditions such as hypogonadism, hyperthyroidism, skeletal metastases, multiple myeloma, anticonvulsants, corticosteroids, and alcohol abuse [3]. Furthermore, 3 types of primary osteoporosis are classified in consideration of the pathogenic factors: menopause, senile, and idiopathic osteoporosis.
Pharmacological strategies such as the use of antiresorptive and anabolic agents that may increase bone mineral density (BMD) and reduce the risk of osteoporotic fractures can be rather expensive. However, general measures of prevention and treatment, such as calcium and vitamin D supplementation, guidance for fall prevention, and the practice of specific physical exercises, can be instituted before the manifestation of the disease and may promote other health benefits [4].
Marrow mesenchymal stem cells (MSCs), distributed in bone marrow with strong potential for self-renewal, can differentiate into various cells such as osteoblasts, fibroblasts, chondrocytes, adipocytes, myocytes, and pluripotent stem cells [5–7]. In addition to the manageable separation and extraction, the potential multi-directional differentiation of MSCs has made it an outstanding material for tissue repair, immune regulation, and cell therapy [7–12]. Primary osteoporosis generally results from senescence and abnormal function of MSCs. Accordingly, transplanting healthy MSC to promote bone formation, which can enhance the ability of osteogenic differentiation and increase the number of osteoblasts, is becoming a potential therapeutic approach for the treatment of osteoporosis.
Traditionally, transplantation of MSC can be divided into autograft and allograft, with different methods like intravenous injection, local support fixed transplantation, and bone marrow cavity injection. Ichioka et al. [13] found that transplantation of allogeneic MSC in SAMP6 mice by bone marrow cavity injection resulted in replacement of blood and lymph systems with donor cells, which can increase density of the entire trabecular bone. Moreover, the bone mineral density, as well as the regulating hormone of bone remodeling and cytokine in vivo, was shown to be equal to that of healthy mice and bone absorption was reduced. Zhou et al. [14] constructed autologous MSC from OVX rabbits in calcium alginate gels and then transplanted them into distal femurs. Eight weeks after implantation, more bone apposition was found in the MSC-alginate-treated group, trabecular thickness increased, microstructures improved with newly-formed osteoids, and trabecular thickness was enhanced. In addition, biomechanical testing revealed stronger stiffness in the MSC-alginate treatment group. Ultimately, their study showed that transplantation of MSC can help to strengthen osteoporotic bone in rabbits.
Although transplantation of MSC can help improve local or systemic osteoporosis status, the amplification of MSC is an obstacle to this method and the culture of MSC in vitro is difficult, with subculture senescence [15–17]. Immune rejection after transplantation can reduce proliferation and activity of MSC in vivo [18,19]. There is still much controversy on the treatment effect of MSC transfection. The key problems of MSC transplantation in the treatment of osteoporosis are improving the proliferating ability of MSC in vitro to increase the amount available for cell transplantation, deferring MSC senescence to promote osteoblast differentiation, and weakening the immune rejection to improve the treatment effect. Zhang et al. [20,21] investigated the feasibility of increasing endosteal bone formation in mice by ex vivo gene therapy with MSC transduced with an MLV-based retroviral vector to express human bone morphogenetic protein 4 (BMP4). Various studies have indicated that transplantation of autologous or allogeneic MSCs or genetically modified MSC that effectively increases the bone mass and bone density and improves local osteoporosis is a fundamentally new potential therapeutic method for treatment of osteoporosis. However, there are still many issues to resolve and the use of MSC transplantation in the treatment of osteoporosis is still in the initial stage.
In the present research, we compared the proliferative ability and osteogenic differentiation of MSC from normal rats, osteoporosis rats, and TERT-transfection treated rats using RT-PCR and Western blot analysis. We hope that this research will provide basic contributions to a new potential therapeutic approach for the treatment of osteoporosis.
Material and Methods
Cells and animals
We grew 293T cells in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). Rat models of osteoporosis were prepared by the following surgical operation: 14 eligible females SD rats (6–9 months) were randomly grouped into an ovariectomized (OVX) group and a sham group. Ovaries of the rats in the OVX group were removed and the same surgical processes were performed without ovary removal on the rats in the sham group. Three months after the operation, a dual-energy X ray absorptiometry instrument was used to measure bone density in unilateral femurs of the rats. At the same time, pathological analysis of femur sections was performed to confirm the establishment of the osteoporosis rat models. The uterus was compared and weighing as well.
Primary culture and growth curve of MSCs
Rats of the OVX and sham groups were sacrificed to access long extremity bones under aseptic conditions. The bone marrow cavity was opened and flushed with PBS in a culture dish leaning at a 30 degree angle at 4°C. Removing the bone fragments, blood clots and flab, phosphate-buffered saline solution (PBS) containing MSCs were was centrifuged for 3 min at 560 g. We discarded supernatant, supplemented complete medium were supplemented, and cells were incubated at 37°C and 5% CO2. Medium was changed at 24 h, 48 h and 72 h, respectively. The cells were passaged when the density was above 70%.
Expression of cell surface antigen
According to the minimal criteria to define MSC proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT), MSC must express CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19, and HLA-DR surface molecules [22,23]. After a comprehensive consideration of the specific differences between humans and mice, CD29, CD44, CD34, CD45, and HSC surface molecules were selected for detection of MSC.
After digestion and centrifugation, MSC were harvested and resuspended at a concentration of 3.0×106/ml in buffer. We incubated 100 μl of cell suspension of each sample at 4°C in the dark for 30 min with the corresponding antibody and labeled them carefully after mixing. Cells were washed with 2 ml buffer, resuspended with 400 μl buffer, and detected by use of a FACSCalibur flow cytometer.
Osteoinduction and alizarin red staining of MSCs
We cultured MSC to 90% confluency, and digested and centrifuged them at 244 g for 4 min. After discarding the supernatant and resuspending, the cells (3.1×103/cm2) were seeded in 6-well culture plates and incubated at 37°C in 5% CO2 for 24 h. After growing to 50% confluency, MSC were washed with phosphate-buffered solution (PBS) and 2 ml medium was added. The medium was changed every 2 days. On the tenth day, some of the cells were digested and centrifuged to analyze the gene expression by PCR and Western blot. At 21 days after induction and discarding the culture medium, MSC were fixed by formaldehyde and stained with alizarin red for observation of calcified nodules.
Construction of TERT lentiviral vector
We transfected 293T cells in 10-cm plates with 4.0 μg of pLV/helper-SL3, pLV/helper-SL4, pLV/helper-SL5, and target plasmid using the Lipofectamine 2000 transfection reagent according to the protocol of manufacturer. After 24 h, the transfection media were replaced by fresh DMEM supplemented with 0.1% FBS and 1% bovine serum albumin, and 293T cells were further incubated at 37°C for 48 h. The supernatants were then harvested after 72 h and filtered to collect the viruses. The available viruses were titered and stored at −80°C.
Construction of stable transfected MSCs
MSC (2.5×104/cm2) were seeded in 6-well tissue culture dishes until completely adherent. After thoroughly rinsing with 1×PBS, we added fresh MSC culture medium (1 ml/well) mixed with 30 μl of Lenti-Tert-eGFP and Lenti-eGFP in 2 wells. Infected cells were incubated for 6 h at 37°C, 5% CO2, and 95% relative humidity and then washed twice in 1×PBS. Finally, the transfected MSCs were cultured for 48 h with complete medium at 37°C, 5% CO2, and 95% relative humidity. To confirm the effect of transfection, cells were observed by fluorescence microscopy.
Validation of gene expression difference
Total RNA was extracted from plasmid-transfected MSCs grown to 90% confluency by using RNAbee reagent (TEL-TEST, Inc.) according to the manufacturer’s protocol. The total RNA was cleared of plasmid DNA contamination by incubation for 30 min at 37°C with DNase I, which was then inactivated by heating to 85°C for 15 min, then 1 μg of total RNA was incubated with Oligo-dT and random primer at 70°C for 5 min. Reverse transcription was continually conducted according to the manufacturer’s protocol for the SuperScript III first-strand synthesis system for reverse transcriptase PCR (RT-PCR) (Invitrogen). The available cDNA were stored at −20°C.
RT-PCR amplification was carried out with the following reaction system: 25 μl of 2×Es Taq MasterMix (CWBIO, China), 5 μl cDNA, 1 μl upstream and downstream primer, and DEPC up to 50 μl. Real-time PCR was carried out in a 20-μl reaction mixture with gene-specific primers of Actin, Oct4, OCN, BSP, GAPDH, and TERT (Table 1), using SYBR green DNA dye (Invitrogen). The PCR conditions were 50°C for 2 min, 95°C for 2 min, and 45 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s. The differences in gene expression were further detected by Western blot analysis using specific antibody that shows specific protein bands in plasmid-transfected MSCs, indicating that eGFP was efficiently cleaved by the 2A peptide.
Table 1.
Primer sequences of RT-PCR and real-time PCR.
| Genes | Primer sequences |
|---|---|
| Actin | F 5′-AGACCTTCAACACCCCAGC-3′ |
| F 5′-GTCACGCACGATTTCCCT-3′ | |
| Oct 4 | F 5′-GGACACCTGGCTTCAGACTT-3′ |
| R 5′-ATCCCTCCACAGAACTCGTATG-3′ | |
| OCN | F 5′-ACAAGTCCCACACAGCAACTC-3′ |
| R 5′-CCAGGTCAGAGAGGCAGAAT-3′ | |
| BSP | F 5′-AAAGAGCAGCACGGTTGAGTAT-3′ |
| R 5′-CTGACCCTCGTAGCCTTCATAG-3′ | |
| GAPDH | F 5′- CAAGGATACTGAGAGCAAGAGA -3′ |
| R 5′- AGGCCCCTCCTGTTGTTAT -3′ | |
| TERT | F 5′- CCAGTATGCAGTGGTTCAGA -3′ |
| R 5′- CTCGATGACAACAGAGTTCCT -3′ |
Statistical analysis
Gray analysis of all gel maps was performed by Scion Image analysis software (IBM, PC, USA). All data analysis was performed using IBM SPSS statistics 21 (IBM PC, USA). All assays were performed a minimum of 2 times in repetitions, and in each repetition there were 14 donor mice. To determine whether results were statistically significant, the paired t-test was used. For multiple comparisons, 2-way ANOVA was performed followed by a post hoc Bonferroni test. p<0.05 was considered significant.
Results
Constructing a rat model of osteoporosis
A total of 14 rats were separated into an OVX group in which ovary removal was performed, and a sham group on in which the same operation was undertook performed but ovary ovaries were not removed. Three months later, the body weight, uterine weight, bone mineral density, and other aspects changed significantly (Table 2). Comparing Compared to the rats in sham group, both body weight and ovary weight were reduced ovary removal. Tissue pathology inspection of femur bone showed cavities with obvious bone loss, indicating that the rat model of osteoporosis was established successfully (Figure 1).
Table 2.
Basic characteristics of OVX and sham rats.
| Group | NO. | Body weight before operation (g) | Body weight after operation (g) | Uterus index (%) | Bone density (g/cm2) |
|---|---|---|---|---|---|
| OVX | 7 | 443.14±40.27 | 417.29±33.20** | 0.039±0.010* | 0.199±0.006* |
| Sham | 7 | 434.29±45.42 | 430.42±36.23* | 0.058±0.006 | 0.238±0.015 |
Data are expressed as the mean ±SD. PS:
indicates significant difference between the OVX and the sham groups, P<0.05;
indicates significant difference between the preoperative and the postoperative weights of the OVX group, P<0.05.
Figure 1.
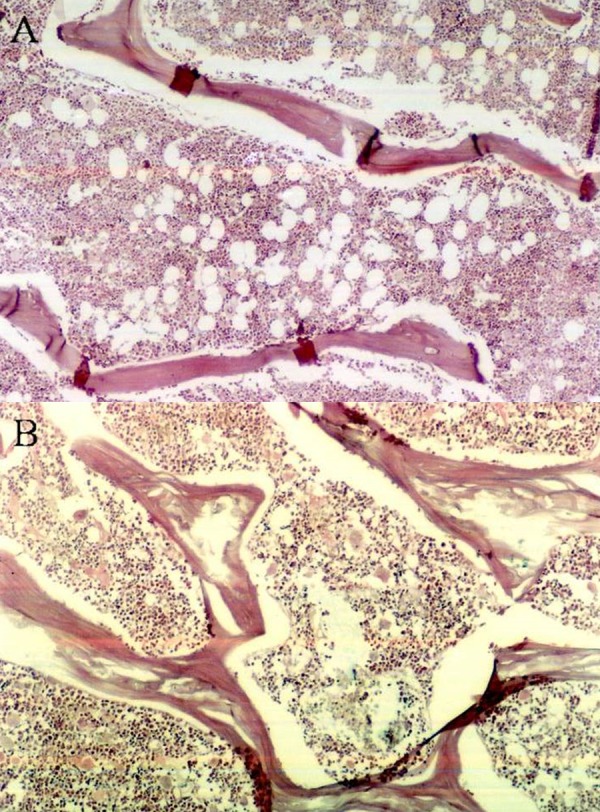
Bone histopathologic examination of the femurs of OVX and Sham rats. PS: (A) OVX group; (B) sham group.
Comparison of normal and osteoporosis MSCs
Observed by microscope, cell morphology of MSCs from normal and ovariectomized osteoporosis rats were significantly different (Figure 2). Surface antigen expression of cultured adherent cells was analyzed with flow cytometry. We could found that among surface antigens of the primary cells extracted from diaphysis of long bones in the OVX and sham group, expression of bone marrow mesenchymal stem cell antigen CD29 and CD44 were positive, and hematopoietic stem cell (HSC) antigens CD34 and, CD45 were negative. As shown in Table 3, the positive rates of CD29 and CD44 of cells in OVX group were 99.86% and 90.04%, respectively; and the positive rate of CD34 and CD45 were both less than 1%, as. So were the results in the sham group. All these results show that the primary cells were bone marrow mesenchymal stem cells.
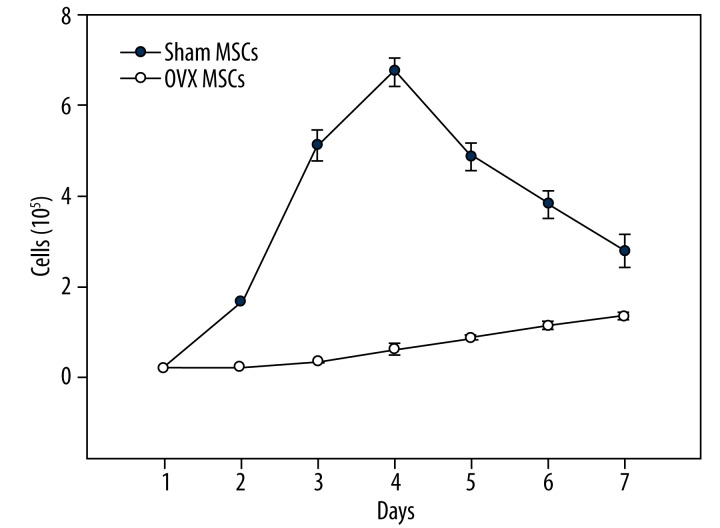
Figure 2.
The grouth curve of OVX MSCs and normal MSCs.
Table 3.
Flow cytometry analysis of cells from both groups about CD 29, CD44, CD 34, CD45.
| CD 29(%) | CD 34(%) | CD 44(%) | CD 45(%) | |
|---|---|---|---|---|
| OVX | 99.86 | 0.54 | 90.04 | 0.43 |
| Sham | 99.86 | 0.64 | 99.47 | 0.35 |
Flow cytometry analysis of cells from both groups about CD 29, CD44, CD 34, CD45. Values in %, n=7 per groups. According to the cell growth curve (Figure 2), proliferation rate of MSCs in OVX group was lower than that in sham group (P<0.05). On the forth day, the total cell number of normal MSCs was up to 6.75±0.31×105, but osteoporosis MSCs proliferated continuously at a much slower rate during the whole culture period (7 days).
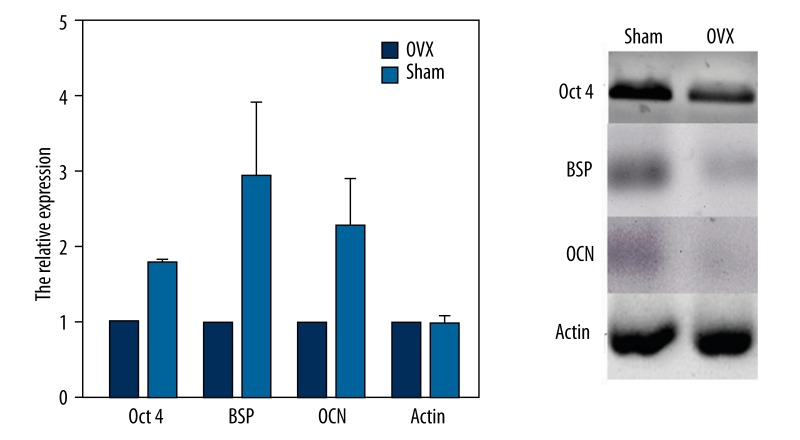
Oct4 was selected to evaluated pluripotency of MSCs. Cultured for 14 days, cDNA of MSCs were extracted to detect the gene expression of Oct4 by RT-PCR. The expression of osteoporosis MSCs significantly decreased compared to that of normal MSCs in the sham group (P<0.05) (Figure 3). Ten days after osteogenic differentiation, the expressions of BSP and OCN were tested by RT-PCR. Similarly, the expression of BSP and OCN was lower in osteoporosis MSCs than in normal MSCs (P<0.05). According to these results, we conclude that differentiation ability of osteoporosis MSCs significantly decreased before and after osteogenic differentiation.
Figure 3.
Differental gene expression between OVX and Sham MSCs.
At 21 days after osteogenic differentiation, microscopy revealed calcific nodules stained by alizarin red (Figure 4). The fact that nodule number was far lower in the OVX group than in the sham group further confirmed the decreased osteogenic differentiation ability in osteoporosis MSCs.
Figure 4.
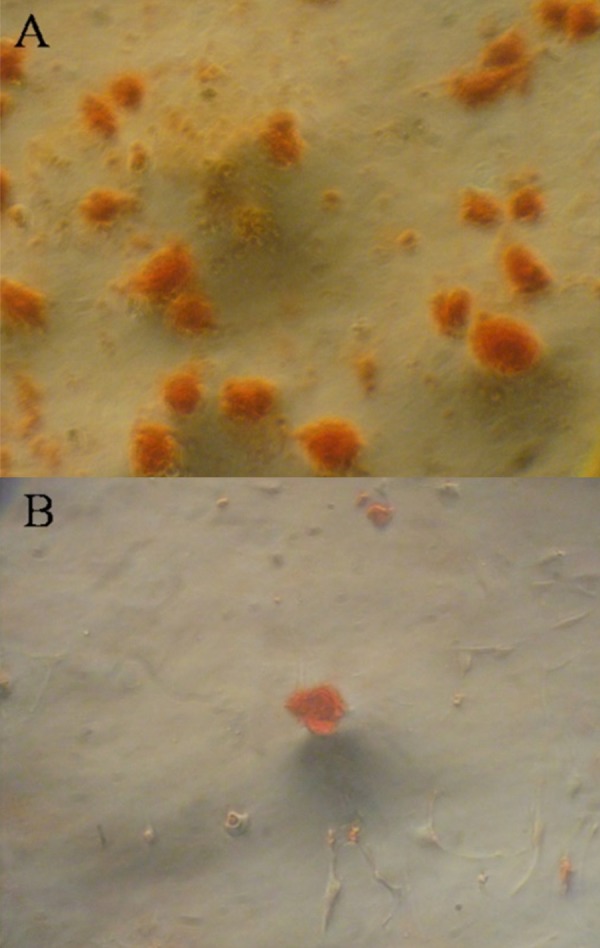
(A, B) OVX and sham MSCs stained by Alizarin red after osteogenic differentiation.
Construction and detection characteristics of stable transfected MSCs
Cell lesions and green-fluorescence could be observed in successfully transfected 293T cells (Figure 5). The virus titers of pLV.ExBi-CMV-TERT-IRES-eGFP and pLV.ExSi. CMV-eGFP were 7.3×107 TU/ml and 2.5×108TU/ml, respectively.
Figure 5.
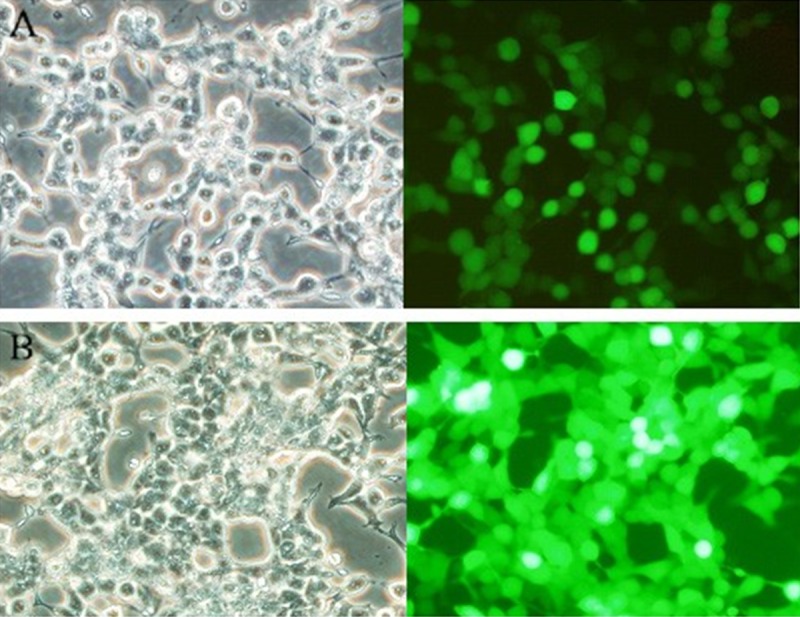
Plasmid transfected 293T cells after 48 h. PS: (A) pLV.ExBi-CMV-TERT-IRES-eGFP; (B) pLV.ExSi. CMV-eGFP.
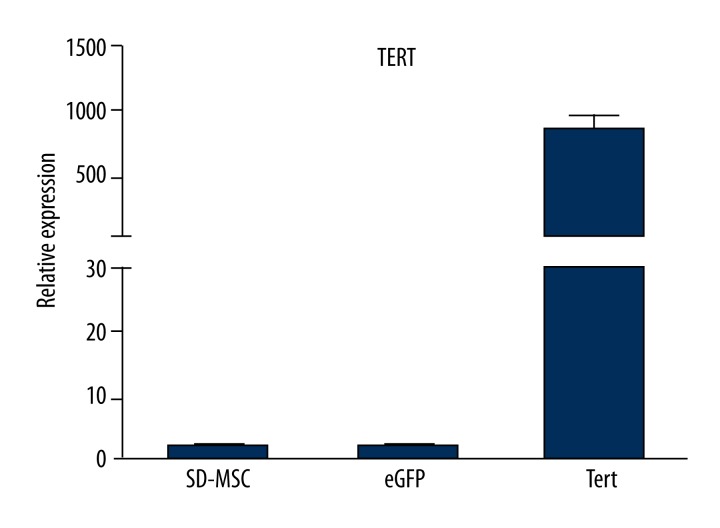
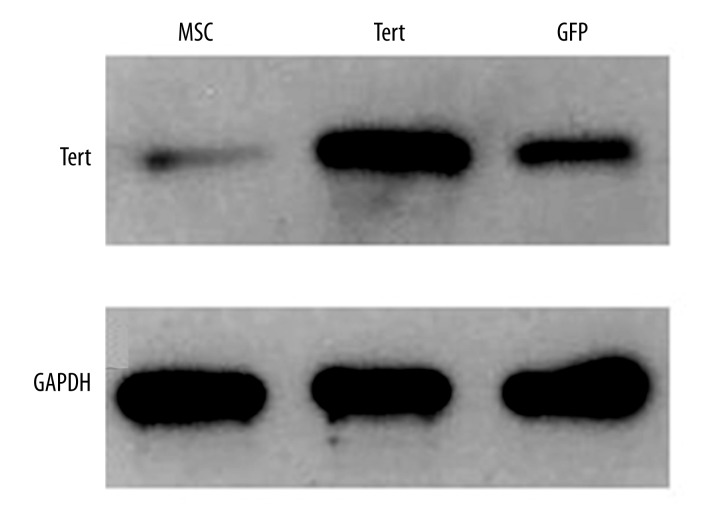
MSCs were seeded into a 6-well plate (2.5×104/cm2) and infected with 30 μl of TERT and simple lentiviruses expressing GFP. After transfection for 48 h, the transfection efficiency of TERT was about 70% and the fluorescence expression was weak, but the GFP transfection rate reached 95% with strong fluorescence expression (Figure 6). There was no significant difference in shape of MSCs appearing after the transfection. Total RNA of successfully transfected TERT-opMSCs were extracted for the expression of TERT gene with realtime PCR. As shown in Figure 7, the relative expression of TERT in TERT-opMSCs was significantly higher than that of opMSCs and GFP-transfected opMSCs (P<0.05). The total proteins were also extracted to authenticate TERT expression at the protein level using Western blot analysis (Figure 8). Among the 3 groups, expression of TERT in cells from the TERT-transfected group was significantly higher than that of the other 2 groups (P<0.05). It can be concluded that the expressions of TERT were up-regulated in osteoporosis MSCs cells at both RNA and protein levels.
Figure 6.
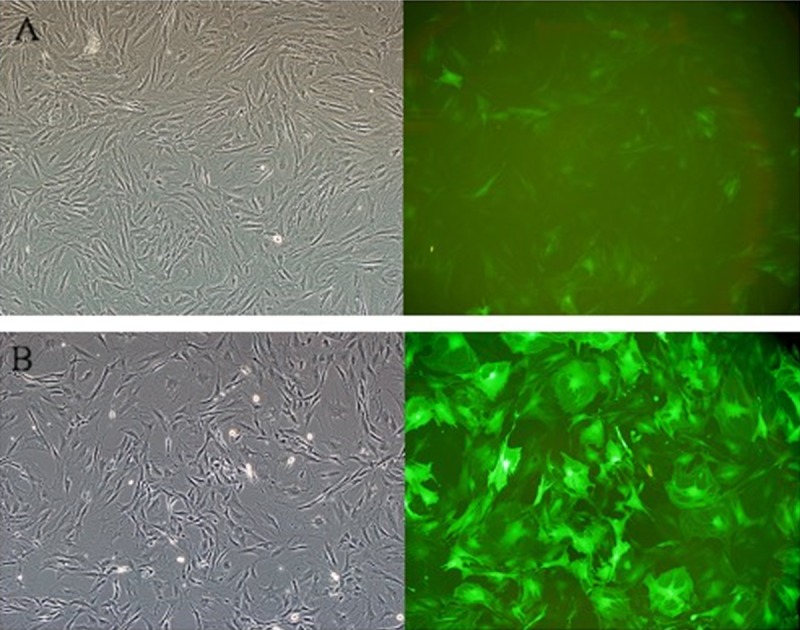
Lentiviral vector transfected osteoporosis MSCs. PS: (A) TERT-opMSCs; (B) GFP-opMSCs.
Figure 7.
TERT expression differences in 3 groups examined by real time PCR.
Figure 8.
TERT expression differences in 3 groups examined by Western blot.
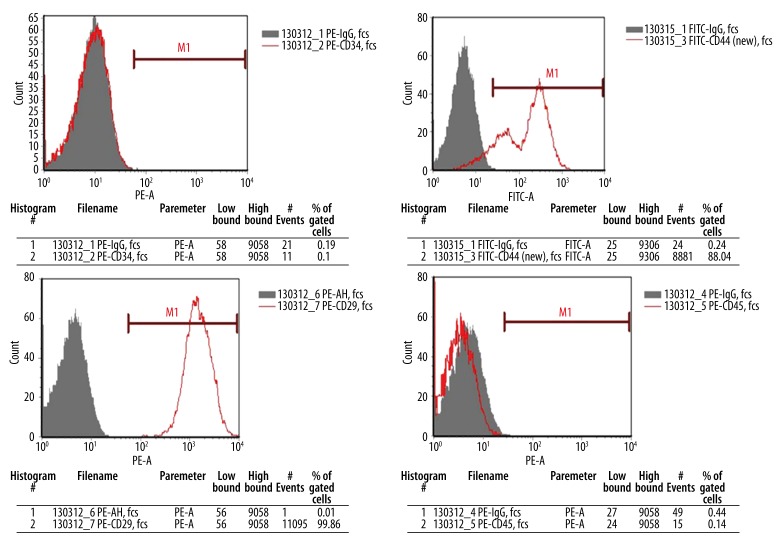
Cell surface antigens of TERT-opMSCs with stably-expressed TERT were identified by flow cytometry. The positive rates of CD29 and CD 44 were 99.86% and 88.04%, respectively, in TERT-opMSCs; and the rates of CD34 and CD45 were 0.10% and 0.14%, respectively (Figure 9). This suggests that virus infection made TERT stably expressed without surface marker changing on the op-MSCs, corresponding to the characteristics of MSCs surface antigens.
Figure 9.
Flow cytometry analysis of TERT-opMSCs.
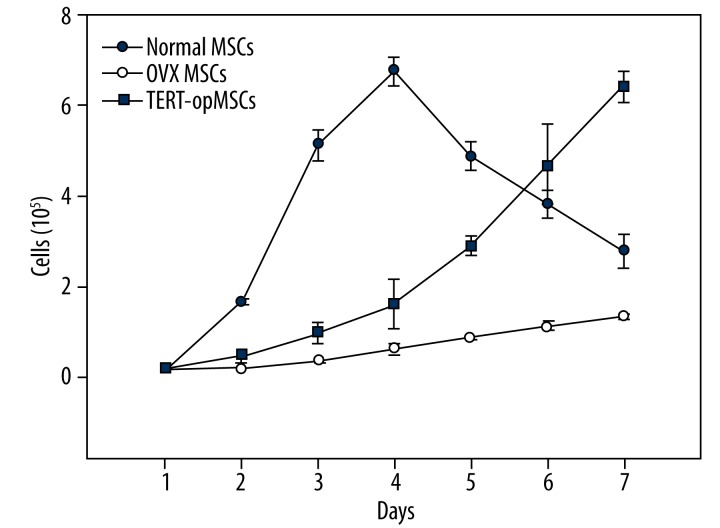
As Figure 10 shows, although the growth curves of TERT-opMSCs and opMSCs without transfection were similar, the overall growth rate of TERT-opMSCs was significantly higher and approached to the growth peak of normal MSCs at the end of the experimental period. The proliferation of opMSCs increased after TERT transfection.
Figure 10.
The grouth curve of 3 groups.
At 21 days after differentiation-inducing, TERT-opMSCs were stained with alizarin red (Figure 11). Calcific nodules were observable and the amount of TERT-opMSCs was higher than that of opMSCs, closer to normal MSCs. We could conclude that the significantly decreased osteogenic differentiation of osteoporosis MSCs could be was improved by TERT transfection, although the differentiation ability of opMSCs was still slightly weaker than in normal MSCs.
Figure 11.
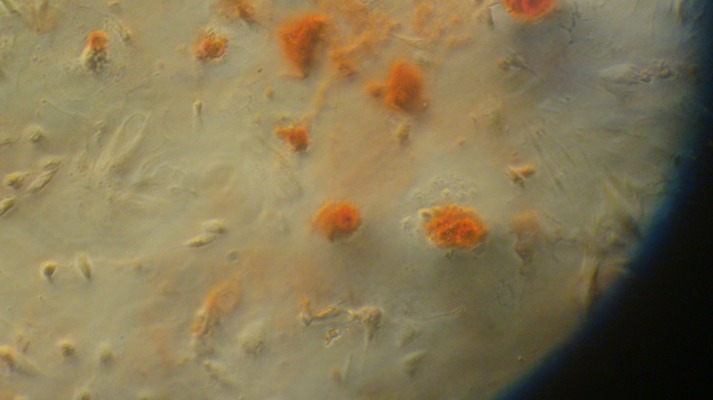
TERT-opMSCs stained by Alizarin red after osteogenic differentiation culture.
Discussion
Recent studies indicate that the main cause of osteoporosis is the imbalance between osteoblasts and osteoclasts in bone remodeling, which leads to decreased quantity and activity of osteoblasts [24] but increased function of osteoclasts [25], resulting in bone re-sorption increase and bone mass reduction. It has also been shown that the senescence of bone marrow mesenchymal stem cells (decrease in proliferation and differentiation) is one of the main forms of pathogenesis in primary osteoporosis [21,26–28]. Decreased stem cell ability to proliferate and differentiate caused by aging, together with osteogenic cells decrease, bone mineral matrix reduction, adipogenic differentiation, increased adipose tissue, eventually lead to osteoporosis [29–32]. Moreover, MSCs gradually lose potential to engage in multiple differentiation with increased age in vivo; however, the transplanted MSCs always had specific cell differentiation potential. Separation and extraction of bone marrow mesenchymal stem cells are easy to perform. MSC has become outstanding material for tissue repair, cell therapy, and immune regulation due to its potential for multi-directional differentiation [33]. Because most primary osteoporosis is associated with senescence and dysfunction of bone marrow mesenchymal stem cells, transplanting health MSCs to enhance the osteogenic differentiation increases the number of osteoblasts and promotes bone formation, making it a potential therapeutic approach for the treatment of osteoporosis.
Unfortunately, although studies on MSC-transplanting treatment of osteoporosis achieved a certain therapeutic effect, the number of MSCs decreased generally after being transplanted in vivo [34], contributing only to a local short-term effect. However, studies found that the modified MSCs, with strong ability of proliferation and differentiation in vitro, can exist in the recipient after transplantation for a long time. However, there is concern that tumors will form or that heterotopic ossification will occur, and that the osteogenesis process will be excessive. There is also a concern regarding long-term biological safety. Obviously, safety concerns are essential in use of immortalized cell transplantation in humans and removing the immortalized gene to prevent tumor formation. Researches on when and how to remove the immortalized gene are at preliminary stages without any certain conclusions.
Considering the similarity of rat and human genomes and that the rat pathologic models, such as bone defect model [35], cerebral infarction model [36] and myocardial ischemia model [37], have been widely used to replace patients in initial experiments. Additionally, ovariectomized pigs and dogs rarely develop osteopenia [38,39]. In contrast, OVX rats are similar to humans in their cancellous bone remodeling mechanism, as well as the periosteum and cortical bone formation pattern were similar to human. We also selected rats as the experimental materials subjects in the present study. The osteoporosis rat model was established successfully three 3 months after surgery. There were some problems in initial culture of extracted primary cells, such as slow cell proliferation, long generation time, and insufficient cell number. Finally, it was discovered that these are normal manifestations in osteoporosis MSCs.
Compared to MSCs from the sham group, growth rate of MSCs from rats in the OVX group were obviously slowed. In the whole chromosome replication process, telomerase reverse transcriptase (TERT) can improve the activity of telomerase catalytic and promote the extension of telomeres. However, in MSCs, the expression of TERT was minimal [40–42], causing a loss of telomerase activity so that telomere length shortens with cell division, eventually leads to cell senescence of mesenchymal stem cells after in vitro subculture [43]. Research shows that few or no clones of MSCs without telomere shortening keep their ability to proliferate and differentiate in vivo, and that cultured MSCs maintained telomere length in vitro. Accordingly, transplantation of the MSCs can help regenerate or self-update older individuals.
Compared with common MSCs, telomerase activity and length of MSCs transfected with TERT were higher with stronger ability of proliferation and, slow generation time. At the same time, differentiation abilities of TERT-MSCs cells, such as differentiation of osteogenic, fibroblast, and neuronal differentiation were increased compared to the ordinary MSCs [44,45]. Saeed H et al. [46] found that 32 weeks after Terc gene (TERT) knockout, whole bone mineral content (BMC) and total density (BMD) decreased 13% and 23%, respectively. Osteogenic ability of MSCs and osteoblasts from gene knockout mice decreased, cell senescence accelerated, but osteoclast function was not affected, thereby causing osteoporosis bone loss. It was also found that, after TERT transfection, the apoptosis rate of fibroblasts decreased and cellular lifespan extended [47,48]. In order to improve the ability of proliferation and differentiation of MSCs osteoporosis, TERT transfection to osteoporosis MSCs was first applied to enhance the proliferation and osteogenic differentiation ability of opMSCs in vitro to provide excellent material for autologous transplantation of the osteoporosis treatment.
Through the exogenous transplantation of TERT, researchers have established multi-stable immortalized cell lines with original cell characteristics and the normal phenotype and karyotype. Many studies have confirmed that after transfection of exogenous TERT inducing cell immortalization, the expression of p16 was inactive but expressions of p53 and p21 were normal [49], which regulates oncogene and DNA damage, contributing to stable proliferation without emergence of tumors.
We found that TERT-opMSCs possessed higher growth rate and shorter generation time than opMSCs before transfection in same proliferating culture time, similar to other studies. However compared with other research, long culture period (20 generations) of TERT-opMSCs was slightly shorter, which influenced the observation of biological safety. Further studies are needed.
Except for the influence of TERT on cell proliferation, there are more and more studies suggested that the telomerase activity may also contribute to maintaining cellular biological characteristics and function, even though the molecular mechanism is still not clear. Previous studies revealed more sensitive and rapid responses of TERT-MSC towards differentiation signals in vitro than senile cells. When cultured in vitro and in vivo, human fetal liver cells with high telomerase activity extended the proliferating lifespan with liver cell properties retained. In MSCs, ectopic expression of TERT can increase specific gene (BSP and OCN) expression of osteoblasts. Similarly, after the 21-day osteogenic differentiation of TERT-opMSCs, the amount of calcified nodules in TERT-opMSCs approached that of normal MSCs, and significantly above that of opMSCs. This result confirmed that although osteogenic differentiation of osteoporosis MSCs significantly decreased, the trend could be reversed through TERT transfection; osteogenic differentiation of opMSCs was clearly improved, but were a little weaker than normal MSCs. These results were also supported by many other experimental results.
Conclusions
This study on osteogenic proliferation of TERT-transfected osteoporosis MSCs provides a new idea and method for the treatment of osteoporosis. Although this approach has not been actually applied actually due to the being exploratory stage, autologous transplantation of TERT-opMSCs for the treatment of osteoporosis can may be a safe and reliable therapeutic method indeed,. Further research is needed on the biological safety and curative effect of TERT-opMSCs in treating osteoporosis.
Footnotes
Source of support: Departmental sources
References
- 1.Christian AT, Pattee MS, Attix CM, et al. Detection of DNA point mutations and mRNA expression levels by rolling circle amplification in individual cells. Proc Natl Acad Sci USA. 2001;98:14238–43. doi: 10.1073/pnas.251383598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Bandeira F, Costa AG, Soares Filho MA, et al. Bone markers and osteoporosis therapy. Arq Bras Endocrinol Metabol. 2014;58:504–13. doi: 10.1590/0004-2730000003384. [DOI] [PubMed] [Google Scholar]
- 4.Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2013;2013:741639. doi: 10.1155/2013/741639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adegani FJ, Langroudi L, Arefian E, et al. A comparison of pluripotency and differentiation status of four mesenchymal adult stem cells. Mol Biol Rep. 2013;40:3693–703. doi: 10.1007/s11033-012-2445-7. [DOI] [PubMed] [Google Scholar]
- 6.Ngo MA, Muller A, Li Y, et al. Human mesenchymal stem cells express a myofibroblastic phenotype in vitro: comparison to human cardiac myofibroblasts. Mol Cell Biochem. 2014;392:187–204. doi: 10.1007/s11010-014-2030-6. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo ME, Avanzini MA, Perotti C, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol. 2007;211:121–30. doi: 10.1002/jcp.20911. [DOI] [PubMed] [Google Scholar]
- 9.Gimeno MJ, Maneiro E, Rendal E, et al. Cell therapy: a therapeutic alternative to treat focal cartilage lesions. Transplant Proc. 2005;37:4080–83. doi: 10.1016/j.transproceed.2005.09.122. [DOI] [PubMed] [Google Scholar]
- 10.Kim N, Im KI, Lim JY, et al. Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice. Ann Hematol. 2013;92:1295–308. doi: 10.1007/s00277-013-1796-z. [DOI] [PubMed] [Google Scholar]
- 11.Sensebe L, Krampera M, Schrezenmeier H, et al. Mesenchymal stem cells for clinical application. Vox Sang. 2010;98:93–107. doi: 10.1111/j.1423-0410.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 12.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526–39. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichioka N, Inaba M, Kushida T, et al. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells. 2002;20:542–51. doi: 10.1634/stemcells.20-6-542. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Goh J, Das De S, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12:1753–61. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 15.Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep. 2014;12:41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- 16.Bidwell JP, Alvarez MB, Hood M, Jr, Childress P. Functional impairment of bone formation in the pathogenesis of osteoporosis: the bone marrow regenerative competence. Curr Osteoporos Rep. 2013;11:117–25. doi: 10.1007/s11914-013-0139-2. [DOI] [PubMed] [Google Scholar]
- 17.Pino AM, Rosen CJ, Rodriguez JP. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res. 2012;45:279–87. doi: 10.4067/S0716-97602012000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atoui R, Chiu RC. Immune responses after mesenchymal stem cell implantation. Methods Mol Biol. 2013;1036:107–20. doi: 10.1007/978-1-62703-511-8_10. [DOI] [PubMed] [Google Scholar]
- 19.Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann NY Acad Sci. 2007;1106:272–78. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XS, Linkhart TA, Chen ST, et al. Local ex vivo gene therapy with bone marrow stromal cells expressing human BMP4 promotes endosteal bone formation in mice. J Gene Med. 2004;6:4–15. doi: 10.1002/jgm.477. [DOI] [PubMed] [Google Scholar]
- 21.Khan E, Abu-Amer Y. Activation of peroxisome proliferator-activated receptor-gamma inhibits differentiation of preosteoblasts. J Lab Clin Med. 2003;142:29–34. doi: 10.1016/S0022-2143(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–95. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 24.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coipeau P, Rosset P, Langonne A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11:584–94. doi: 10.1080/14653240903079385. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–43. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon MJ, Kim JA, Kwon SH, et al. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–77. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 28.Astudillo P, Rios S, Pastenes L, et al. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem. 2008;103:1054–65. doi: 10.1002/jcb.21516. [DOI] [PubMed] [Google Scholar]
- 29.Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2002;277:46226–32. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez JP, Garat S, Gajardo H, et al. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–23. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Qiu W, Andersen TE, Bollerslev J, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 32.Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–29. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahara H, Misawa H, Hayashi T, et al. Bone repair by transplantation of hTERT-immortalized human mesenchymal stem cells in mice. Transplantation. 2009;88:346–53. doi: 10.1097/TP.0b013e3181ae5ba2. [DOI] [PubMed] [Google Scholar]
- 34.Ikehara S. A new bone marrow transplantation method for stem cell disorders. Ann NY Acad Sci. 2009;1173:774–80. doi: 10.1111/j.1749-6632.2009.04644.x. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal AK, Dhumal RV, Ghosh S, et al. Bone healing evaluation of nanofibrous composite scaffolds in rat calvarial defects: a comparative study. J Biomed Nanotechnol. 2013;9:2073–85. doi: 10.1166/jbn.2013.1736. [DOI] [PubMed] [Google Scholar]
- 36.Rezazadeh H, Hoseini Kahnuee M, Roohbakhsh A, et al. Neuroprotective consequences of postconditioning on embolic model of cerebral ischemia in rat. Iran J Basic Med Sci. 2013;16:144–49. [PMC free article] [PubMed] [Google Scholar]
- 37.Caliskan A, Yavuz C, Karahan O, et al. Iloprost reduces myocardial edema in a rat model of myocardial ischemia reperfusion. Perfusion. 2014;29:260–64. doi: 10.1177/0267659113514472. [DOI] [PubMed] [Google Scholar]
- 38.Kragstrup J, Richards A, Fejerskov O. Effects of fluoride on cortical bone remodeling in the growing domestic pig. Bone. 1989;10:421–24. doi: 10.1016/8756-3282(89)90073-2. [DOI] [PubMed] [Google Scholar]
- 39.Grynpas MD, Acito A, Dimitriu M, et al. Changes in bone mineralization, architecture and mechanical properties due to long-term (1 year) administration of pamidronate (APD) to adult dogs. Osteoporos Int. 1992;2:74–81. doi: 10.1007/BF01623840. [DOI] [PubMed] [Google Scholar]
- 40.Ueda Y, Inaba M, Takada K, et al. Induction of senile osteoporosis in normal mice by intra-bone marrow-bone marrow transplantation from osteoporosis-prone mice. Stem Cells. 2007;25:1356–63. doi: 10.1634/stemcells.2006-0811. [DOI] [PubMed] [Google Scholar]
- 41.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–96. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 42.Zhao YM, Li JY, Lan JP, et al. Cell cycle dependent telomere regulation by telomerase in human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2008;369:1114–19. doi: 10.1016/j.bbrc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, DiGirolamo CM, Navarro PA, et al. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp Cell Res. 2004;294:1–8. doi: 10.1016/j.yexcr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Tsai CC, Chen CL, Liu HC, et al. Overexpression of hTERT increases stem-like properties and decreases spontaneous differentiation in human mesenchymal stem cell lines. J Biomed Sci. 2010;17:64. doi: 10.1186/1423-0127-17-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakahara H, Misawa H, Hayashi T, et al. Bone repair by transplantation of hTERT-immortalized human mesenchymal stem cells in mice. Transplantation. 2009;88:346–53. doi: 10.1097/TP.0b013e3181ae5ba2. [DOI] [PubMed] [Google Scholar]
- 46.Saeed H, Abdallah BM, Ditzel N, et al. Telomerase-deficient mice exhibit bone loss owing to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. J Bone Miner Res. 2011;26:1494–505. doi: 10.1002/jbmr.349. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Zhang ZZ, Zhou TY, et al. [Effect of nanosize delivery system for ASODN against hTERT on the expression of telomerase in the esophageal cancer EC9706 cells]. Zhonghua Zhong Liu Za Zhi. 2008;30:566–72. [in Chinese] [PubMed] [Google Scholar]
- 48.Zhang Y, Ma H, Liu SL, et al. [Effects of human telomerase reverse transcriptase promoter and survivin promoter in targeted tumor gene therapy]. Zhonghua Yi Xue Za Zhi. 2008;88:475–79. [in Chinese] [PubMed] [Google Scholar]
- 49.Tao Q, Lv B, Qiao B, et al. Immortalization of ameloblastoma cells via reactivation of telomerase function: Phenotypic and molecular characteristics. Oral Oncol. 2009;45:e239–44. doi: 10.1016/j.oraloncology.2009.08.007. [DOI] [PubMed] [Google Scholar]